Carbon Dioxide Sensor Module Based on NDIR Technology
Abstract
:1. Introduction
2. Theory
3. Design and Fabrication of Sensor Module
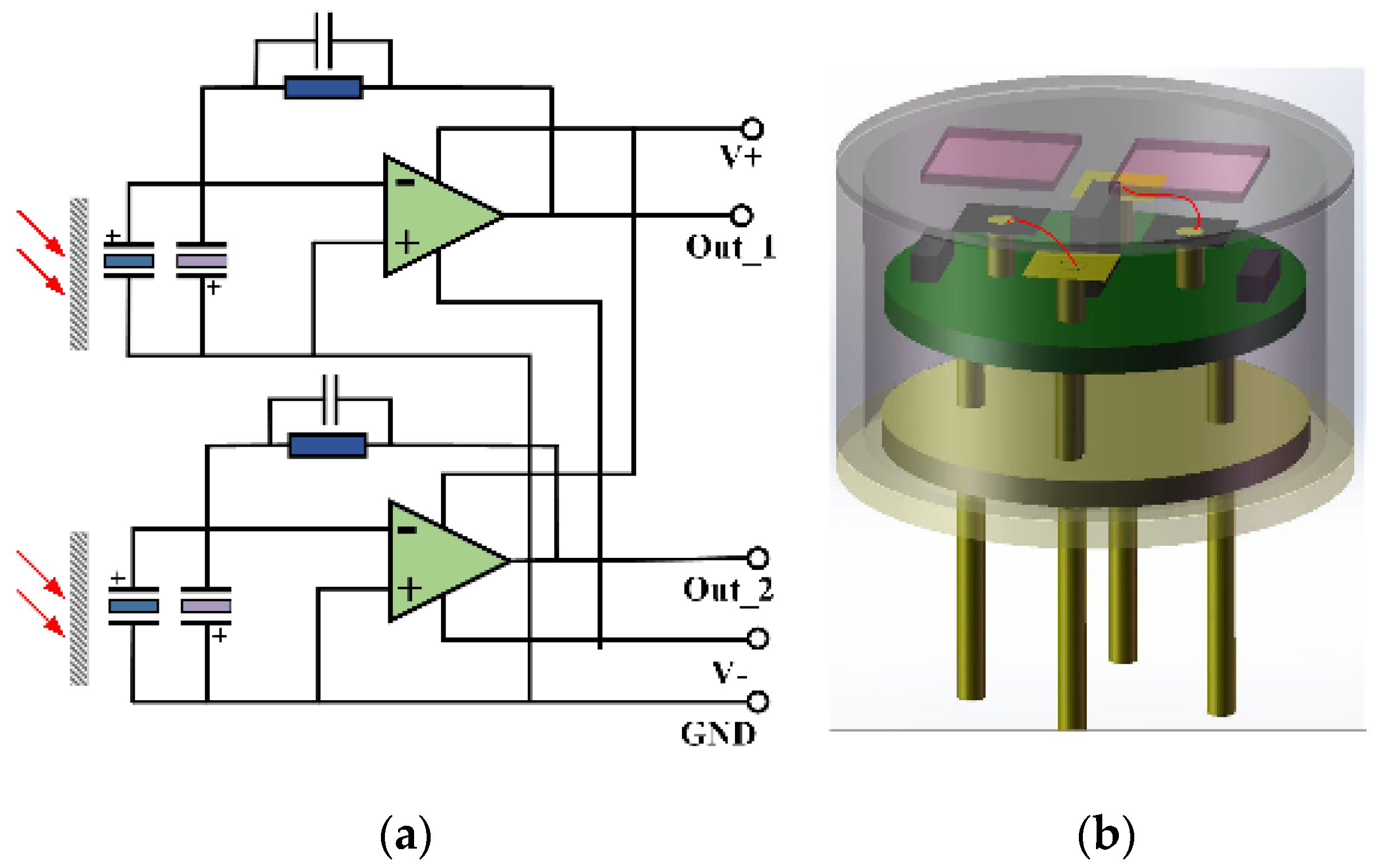
3.1. Preparation and Integration of Detector
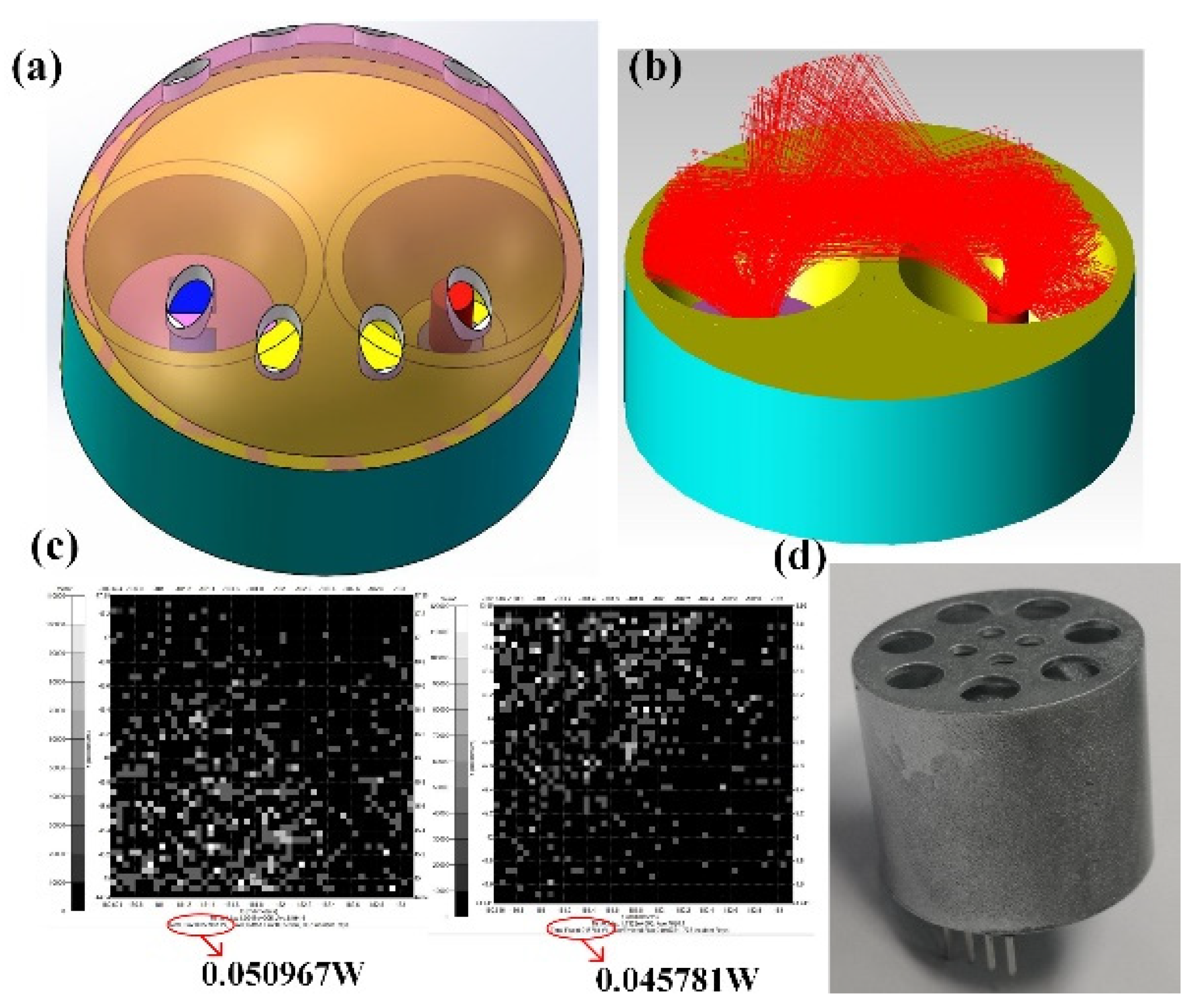
3.2. Optical Gas Chamber
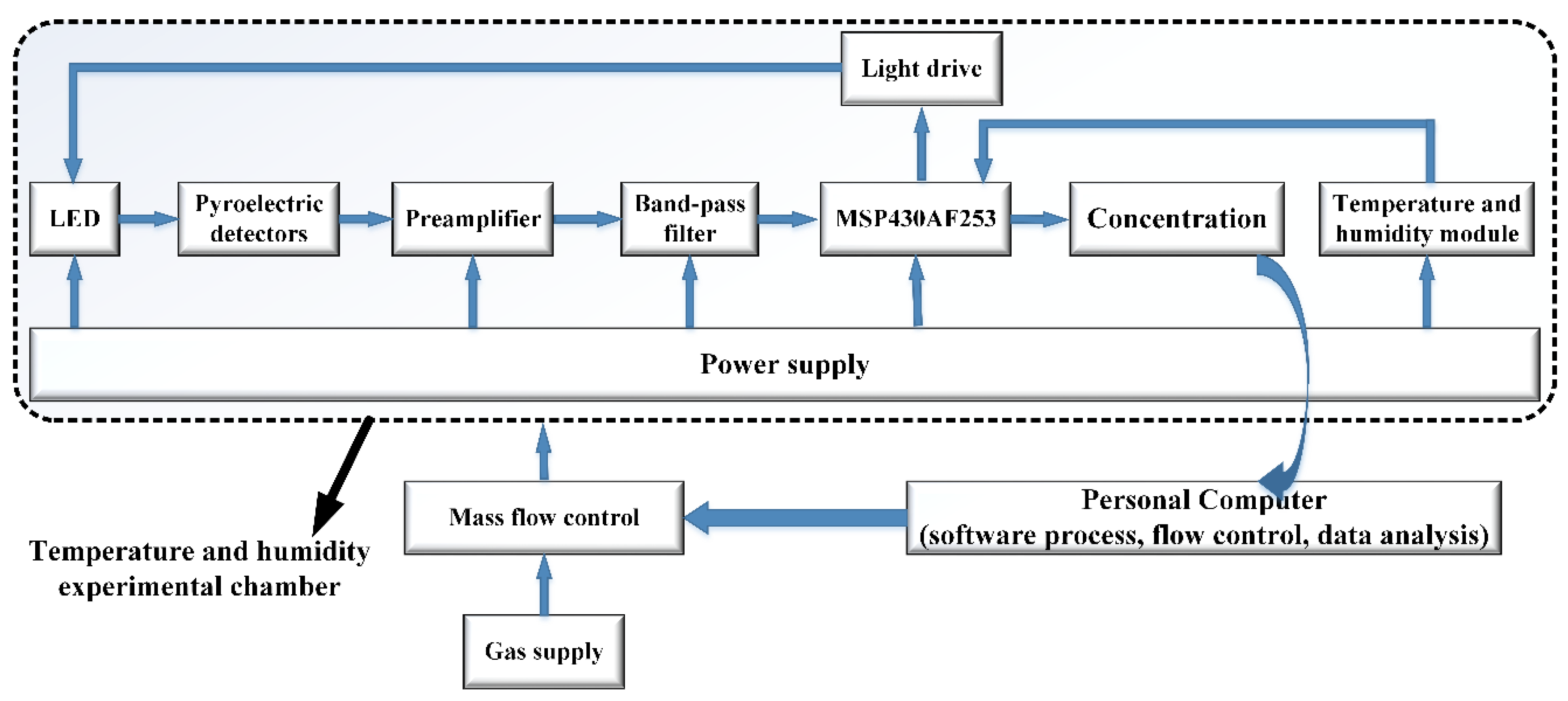
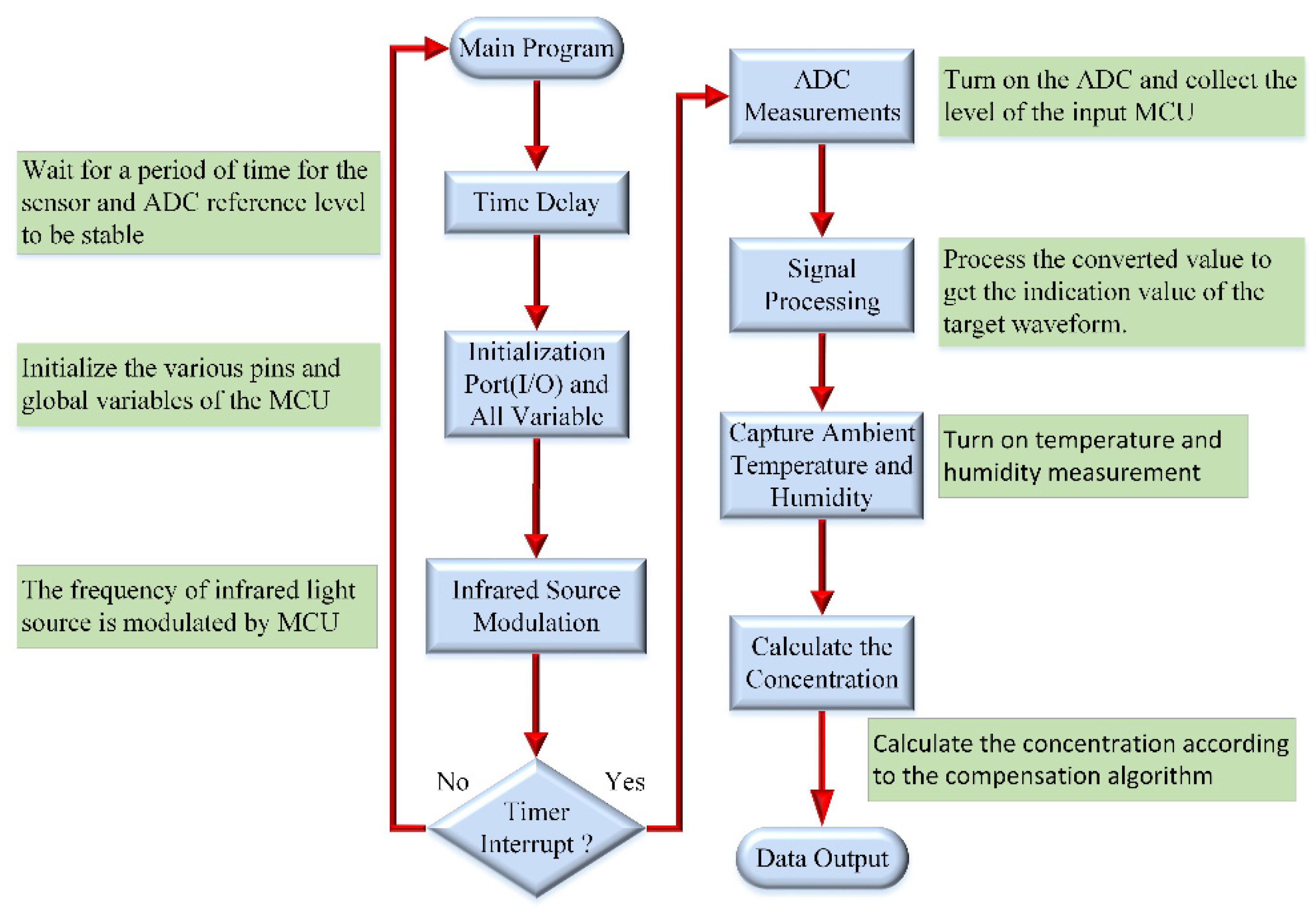
3.3. Detection System and Software
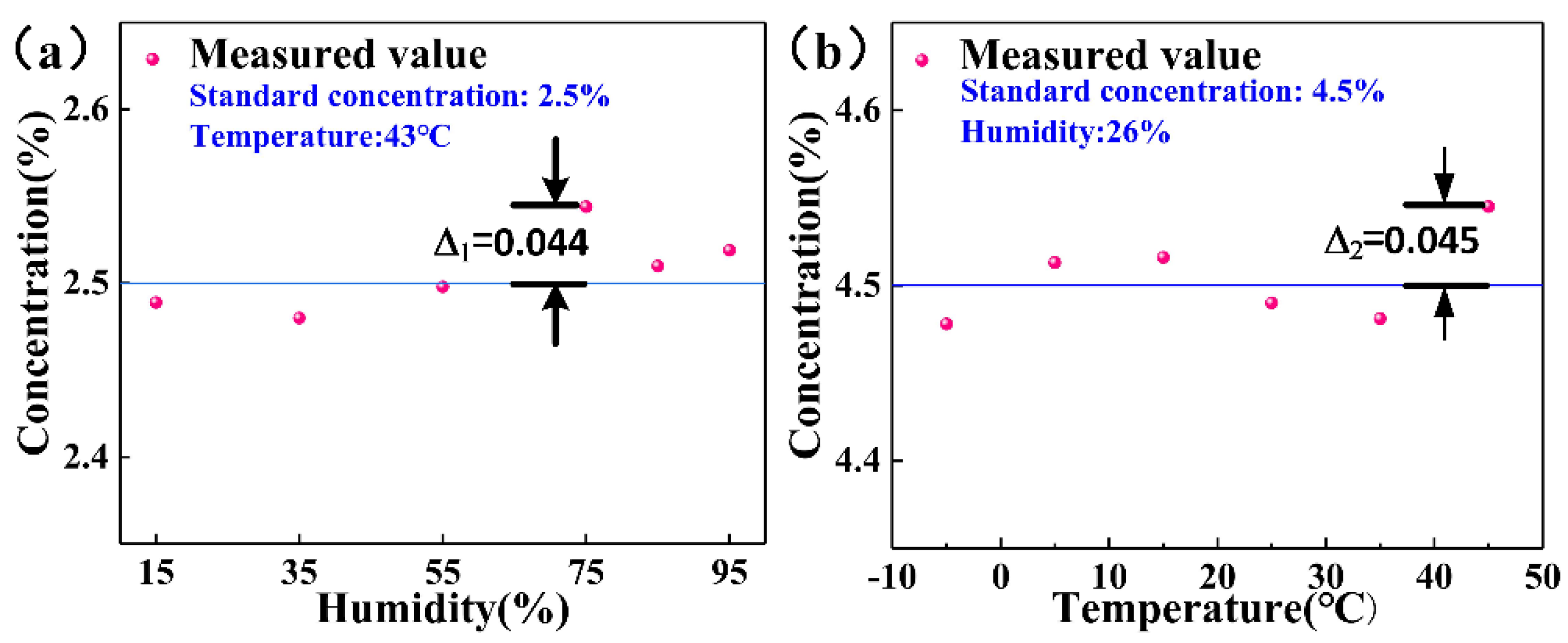
4. Method of Calibration and Compensation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gutmacher, D.; Hoefer, U.; Wöllenstein, J. Gas sensor technologies for fire detection. Sens. Actuators B Chem. 2012, 175, 40–45. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable sensors for ubiquitous monitoring of gases in the era of internet of things and industrial internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Betty, C.A.; Sipra, C.; Girija, K.G. Reliability studies of highly sensitive and specific multi-gas sensor based on nanocrystalline SnO2 film. Sens. Actuators B Chem. 2014, 193, 484–491. [Google Scholar] [CrossRef]
- Dinh, T.-V.; Choi, I.-Y.; Son, Y.-S.; Kim, J.-C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Goldenstein, C.S.; Spearrin, R.M.; Jeffries, J.B.; Hanson, R.K. Infrared laser-absorption sensing for combustion gases. Prog. Energy Combust. Sci. 2017, 60, 132–176. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Ren, W.; Liu, X. Design and fabrication of micromachined pyroelectric infrared detector array using lead titanate zirconate (PZT) thin film. Ceram. Int. 2017, 43, S464–S469. [Google Scholar] [CrossRef]
- Perez, A.O.; Bierer, B.; Scholz, L.; Wöllenstein, J.; Palzer, S. A wireless gas sensor network to monitor indoor environmental quality in schools. Sensors 2018, 18, 4345. [Google Scholar] [CrossRef] [Green Version]
- Metsälä, M. Optical techniques for breath analysis: From single to multi-species detection. J. Breath Res. 2018, 12, 027104. [Google Scholar] [CrossRef]
- Tomberg, T.; Vainio, M.; Hieta, T.; Halonen, L. Sub-parts-per-trillion level sensitivity in trace gas detection by cantilever-enhanced photo-acoustic spectroscopy. Sci. Rep. 2018, 8, 1–7. [Google Scholar]
- Zhang, T.; Huang, H.; Chen, R. Wet chemical etching process of BST thin films for pyroelectric infrared detectors. Ferroelectrics 2010, 410, 137–144. [Google Scholar] [CrossRef]
- Liang, W.F.; Lu, Y.; Wu, J.; Gao, H.; Li, M. Application of LiTaO3 pyroelectric crystal for pulsed neutron detection. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2016, 827, 161–164. [Google Scholar] [CrossRef]
- Tang, F.; Long, S.; Yang, X.; Yang, M.; Quan, J.; Lin, S.; Ma, D.; Zhu, Y.; Wang, B. Optimization of pyroelectric figures of merit via magnesia doping in lithium tantalate single crystal. J. Phys. D Appl. Phys. 2018, 51, 395101. [Google Scholar] [CrossRef]
- Liang, Z.; Li, S.; Liu, Z.; Jiang, Y.; Li, W.; Wang, T.; Wang, J. High responsivity of pyroelectric infrared detector based on ultra-thin (10 μm) LiTaO3. J. Mater. Sci. Mater. Electron. 2015, 26, 5400–5404. [Google Scholar] [CrossRef]
- Dong, M.; Zheng, C.; Miao, S.; Zhang, Y.; Du, Q.; Wang, Y.; Tittel, F.K. Development and measurements of a mid-infrared multi-gas sensor system for CO, CO2 and CH4 detection. Sensors 2017, 17, 2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhter, F.; Alahi, M.E.E.; Siddiquei, H.R.; Gooneratne, C.P.; Mukhopadhyay, S.C. Graphene Oxide (GO) Coated Impedimetric Gas Sensor for Selective Detection of Carbon dioxide (CO2) with Temperature and Humidity Compensation. IEEE Sens. J. 2020, 21, 4241–4249. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rahbarpour, S.; Hosseini-Golgoo, S.-M.; Jamaati, H. Reducing the destructive effect of ambient humidity variations on gas detection capability of a temperature modulated gas sensor by calcium chloride. Sens. Actuators B Chem. 2021, 331, 129091. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, P.; Cao, W.; Li, X.; Lai, J. High-sensitivity ethylene gas sensor based on NDIR and dual-channel lock-in amplifier. Optik 2020, 223, 165630. [Google Scholar] [CrossRef]
- Yi, S. Temperature compensation methods of nondispersive infrared CO2 gas sensor with dual ellipsoidal optical waveguide. Sens. Mater 2017, 29, 243–252. [Google Scholar]
- Tan, Q.; Tang, L.; Yang, M.; Xue, C.; Zhang, W.; Liu, J.; Xiong, J. Three-gas detection system with IR optical sensor based on NDIR technology. Opt. Lasers Eng. 2015, 74, 103–108. [Google Scholar] [CrossRef]
- van den Bossche, M.; Rose, N.T.; De Wekker, S.F.J. Potential of a low-cost gas sensor for atmospheric methane monitoring. Sens. Actuators B Chem. 2017, 238, 501–509. [Google Scholar] [CrossRef]
- Grangeat, P.; Gharbi, S.; Accensi, M.; Grateau, H. First Evaluation of a transcutaneous Carbon Dioxide Monitoring Wristband Device during a cardiopulmonary Exercise Test. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar]
- Grangeat, P.; Gharbi, S.; Koenig, A.; Comşa, M.-P.; Accensi, M.; Grateau, H.; Ghaith, A.; Chacaroun, S.; Doutreleau, S.; Vergès, S. Evaluation in healthy subjects of a transcutaneous carbon dioxide monitoring wristband during hypo and hypercapnia conditions. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020. [Google Scholar]
- Tan, Q.; Zhang, W.; Xue, C.; Xiong, J.; Ma, Y.; Wen, F. Design of mini-multi-gas monitoring system based on IR absorption. Opt. Laser Technol. 2008, 40, 703–710. [Google Scholar] [CrossRef]
- Ishak, B.J.C. Spectra of Atoms and Molecules, 3rd ed.; Bernath, P.F., Ed.; Oxford University Press on Demand: Oxford, UK, 2017; Volume 58, pp. 201–202. [Google Scholar] [CrossRef]
- Jun, L.; Qiulin, T.; Wendong, Z.; Chenyang, X.; Tao, G.; Jijun, X. Miniature low-power IR monitor for methane detection. Measurement 2011, 44, 823–831. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Panjwani, D.; Yesiltas, M.; Nath, J.; Maukonen, D.E.; Rezadad, I.; Smith, E.M.; Peale, R.E.; Hirschmugl, C.; Sedlmair, J.; Wehlitz, R.; et al. Patterning of oxide-hardened gold black by photolithography and metal lift-off. Infrared Phys. Technol. 2014, 62, 94–99. [Google Scholar] [CrossRef]
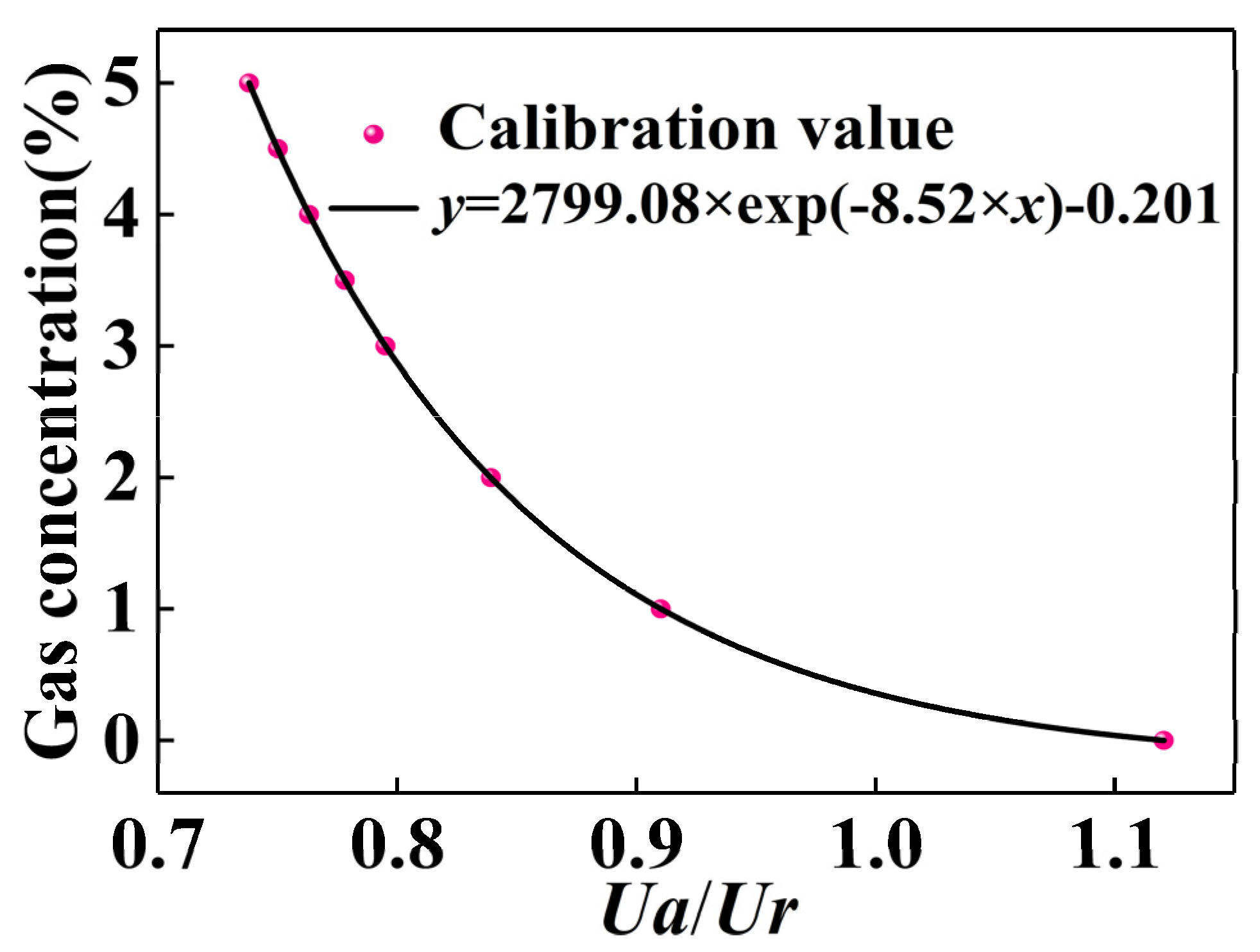

| CO2(%) | Ua/Ur |
|---|---|
| 0 | 1.120 |
| 1 | 0.910 |
| 2 | 0.839 |
| 3 | 0.795 |
| 3.5 | 0.778 |
| 4.0 | 0.763 |
| 4.5 | 0.750 |
| 5 | 0.738 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; He, Y.; Zhang, Q.; Zhang, L. Carbon Dioxide Sensor Module Based on NDIR Technology. Micromachines 2021, 12, 845. https://doi.org/10.3390/mi12070845
Zhou L, He Y, Zhang Q, Zhang L. Carbon Dioxide Sensor Module Based on NDIR Technology. Micromachines. 2021; 12(7):845. https://doi.org/10.3390/mi12070845
Chicago/Turabian StyleZhou, Libing, Yaoyi He, Qing Zhang, and Lei Zhang. 2021. "Carbon Dioxide Sensor Module Based on NDIR Technology" Micromachines 12, no. 7: 845. https://doi.org/10.3390/mi12070845
APA StyleZhou, L., He, Y., Zhang, Q., & Zhang, L. (2021). Carbon Dioxide Sensor Module Based on NDIR Technology. Micromachines, 12(7), 845. https://doi.org/10.3390/mi12070845





