Microfluidic Obstacle Arrays Induce Large Reversible Shape Change in Red Blood Cells
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
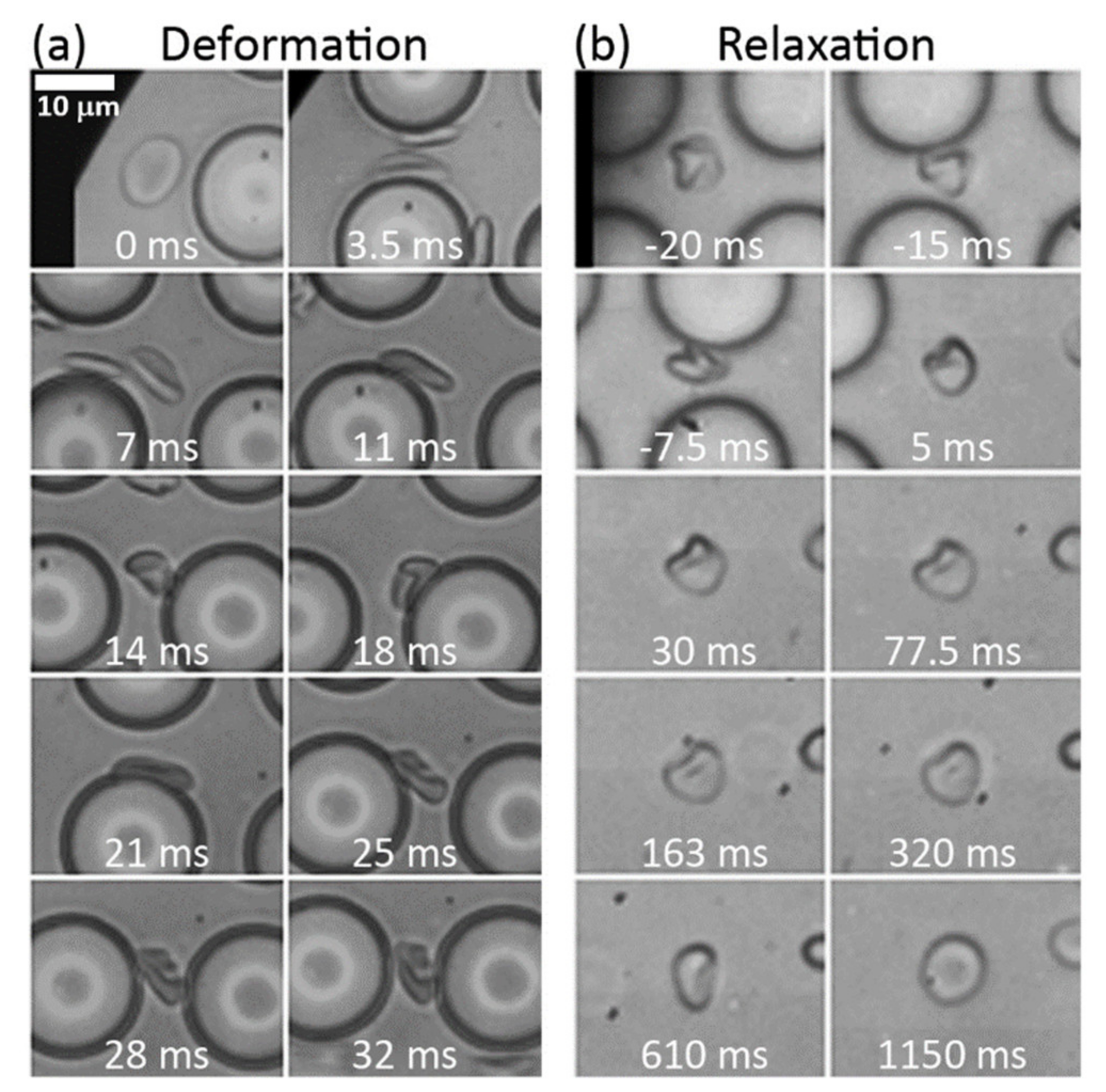
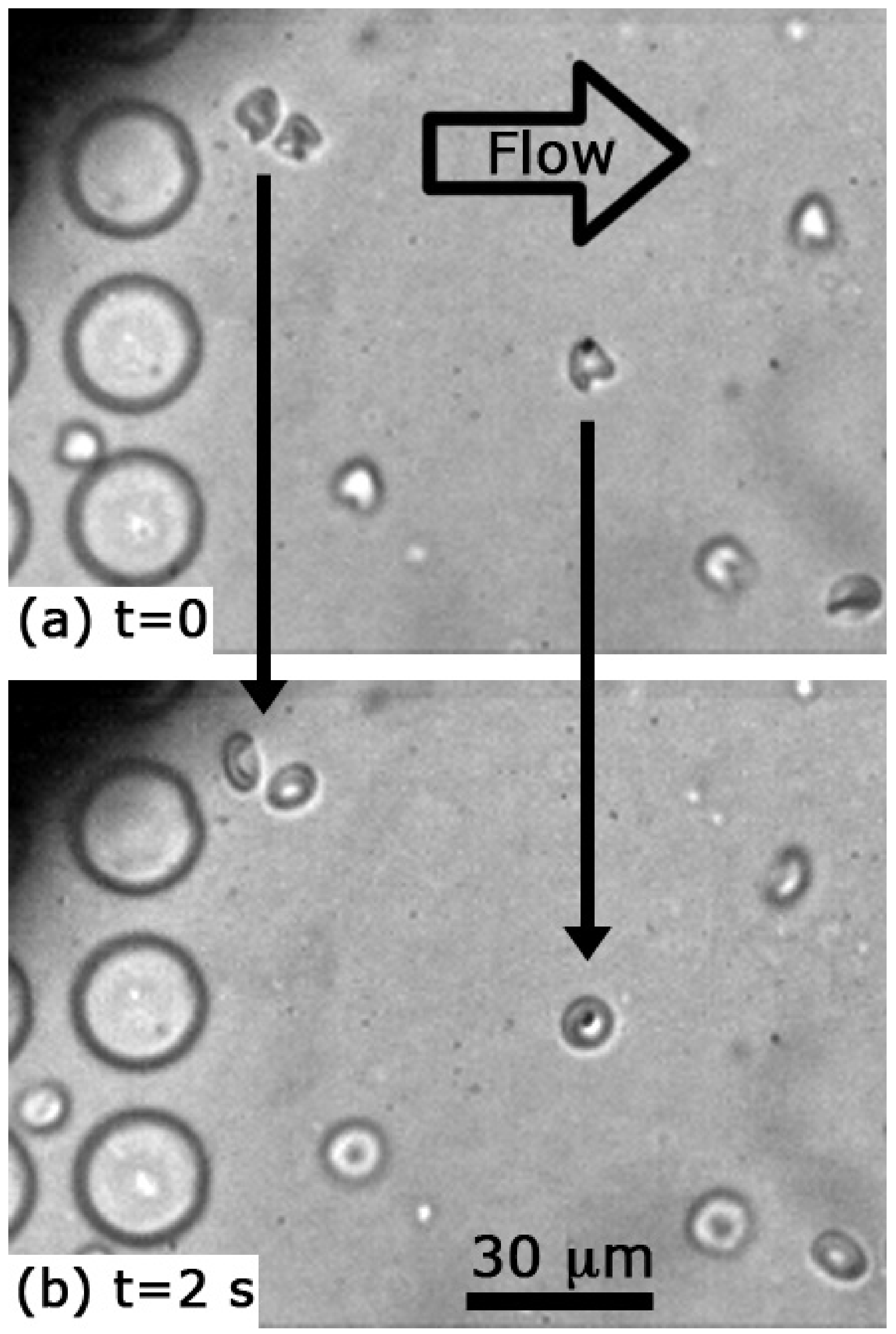
3.1. Cell Shapes and Size
3.2. Deformation Rates
3.3. Relaxation Rates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chen, B.; Guo, F.; Xiang, H. Visualization study of motion and deformation of red blood cells in a microchannel with straight, divergent and convergent sections. J. Biol. Phys. 2011, 37, 429–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Noguchi, H.; Gompper, G. Shape transitions of fluid vesicles and red blood cells in capillary flows. Proc. Natl. Acad. Sci. USA 2005, 102, 14159–14164. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Forsyth, A.M.; Stone, H.A. Red blood cell dynamics: From cell deformation to ATP release. Integr. Biol. 2011, 3, 972–981. [Google Scholar] [CrossRef]
- Yoon, Y.-Z.; Kotar, J.; Yoon, G.; Cicuta, P. The nonlinear mechanical response of the red blood cell. Phys. Biol. 2008, 5, 036007. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, K.; Sekizuka, E.; Oshio, C.; Minamitani, H. Direct measurement of erythrocyte deformability in diabetes mellitus with a transparent microchannel capillary model and high-speed video camera system. Microvasc. Res. 2001, 61, 231–239. [Google Scholar] [CrossRef]
- Dodson, W.R.; Dimitrakopoulos, P. Oscillatory tank-treading motion of erythrocytes in shear flows. Phys. Rev. E 2011, 84, 011913. [Google Scholar] [CrossRef]
- Basu, H.; Dharmadhikari, A.K.; Dharmadhikari, J.A.; Sharma, S.; Mathur, D. Tank treading of optically trapped red blood cells in shear flow. Biophys. J. 2011, 101, 1604–1612. [Google Scholar] [CrossRef]
- Abkarian, M.; Faivre, M.; Viallat, A. Swinging of red blood cells under shear flow. Phys. Rev. Lett. 2007, 98, 188302. [Google Scholar] [CrossRef]
- Beech, J.P.; Holm, S.H.; Adolfsson, K.; Tegenfeldt, J.O. Sorting cells by size, shape and deformability. Lab Chip 2012, 12, 1048–1051. [Google Scholar] [CrossRef]
- Bessis, M. Living Blood Cells and Their Ultrastructure; Springer: Berlin/Heidelberg, Germany, 1973. [Google Scholar]
- Li, J.; Lykotrafitis, G.; Dao, M.; Suresh, S. Cytoskeletal dynamics of human erythrocyte. Proc. Natl. Acad. Sci. USA 2007, 104, 4937–4942. [Google Scholar] [CrossRef]
- Mauer, J.; Mendez, S.; Lanotte, L.; Nicoud, F.; Abkarian, M.; Gompper, G.; Fedosov, D.A. Flow-induced transitions of red blood cell shapes under shear. Phys. Rev. Lett. 2018, 121, 118103. [Google Scholar] [CrossRef]
- Bento, D.; Rodrigues, R.O.; Faustino, V.; Pinho, D.; Fernandes, C.S.; Pereira, A.I.; Garcia, V.; Miranda, J.M.; Lima, R. Deformation of red blood cells, air bubbles, and droplets in microfluidic devices: Flow visualizations and measurements. Micromachines 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Inglis, D.W.; Morton, K.J.; Lawrence, D.A.; Huang, L.R.; Chou, S.Y.; Sturm, J.C.; Austin, R.H. Deterministic hydrodynamics: Taking blood apart. Proc. Natl. Acad. Sci. USA 2006, 103, 14779–14784. [Google Scholar] [CrossRef] [PubMed]
- Zeming, K.K.; Vernekar, R.; Chua, M.T.; Quek, K.Y.; Sutton, G.; Krüger, T.; Kuan, W.S.; Han, J. Inflammation biomarkers: Label-free biophysical markers from whole blood microfluidic immune profiling reveal severe immune response signatures. Small 2021, 17, 2170051. [Google Scholar] [CrossRef]
- Zeming, K.K.; Salafi, T.; Chen, C.-H.; Zhang, Y. Asymmetrical deterministic lateral displacement gaps for dual functions of enhanced separation and throughput of red blood cells. Sci. Rep. 2016, 6, 22934. [Google Scholar] [CrossRef]
- Inglis, D.W. A method for reducing pressure-induced deformation in silicone microfluidics. Biomicrofluidics 2010, 4, 026504. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Shire, S.J.; Kalonia, D.S. Viscosity analysis of high concentration bovine serum albumin aqueous solutions. Pharm. Res. 2011, 28, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.M. Shape memory of human red blood cells. Biophys. J. 2004, 86, 3304–3313. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Pries, A.R.; Secomb, T.W.; Gaehtgens, P. Design principles of vascular beds. Circ. Res. 1995, 77, 1017–1023. [Google Scholar] [CrossRef]
- Leverett, L.B.; Hellums, J.D.; Alfrey, C.P.; Lynch, E.C. Red blood cell damage by shear stress. Biophys. J. 1972, 12, 257–273. [Google Scholar] [CrossRef]
- Markou, C.P.; Marzec, U.M.; Chinn, J.A.; Hirt, F.; Hanson, S.R. Shear induced platelet activation. In Proceedings of the First Joint BMES/EMBS Conference. 1999 IEEE Engineering in Medicine and Biology 21st Annual Conference and the 1999 Annual Fall Meeting of the Biomedical Engineering Society (Cat. N), Atlanta, GA, USA, 13–16 October 1999; Volume 2, p. 770. [Google Scholar] [CrossRef]
- Henry, E.; Holm, S.H.; Zhang, Z.; Beech, J.P.; Tegenfeldt, J.O.; Fedosov, D.A.; Gompper, G. Sorting cells by their dynamical properties. Sci. Rep. 2016, 6, 34375. [Google Scholar] [CrossRef] [PubMed]
- Namvar, A.; Blanch, A.J.; Dixon, M.W.; Carmo, O.M.S.; Liu, B.; Tiash, S.; Looker, O.; Andrew, D.; Chan, L.-J.; Tham, W.-H.; et al. Surface area-to-volume ratio, not cellular viscoelasticity, is the major determinant of red blood cell traversal through small channels. Cell. Microbiol. 2021, 23, e13270. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Best, C.A.; Kuriabova, T.; Henle, M.L.; Feld, M.S.; Levine, A.J.; Popescu, G. Measurement of the nonlinear elasticity of red blood cell membranes. Phys. Rev. E 2011, 83, 051925. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.G.; Wortis, M.; Ranjan, M. Lipid bilayers and red blood cells. In Soft Matter, Volume 4: Lipid Bilayers and Red Blood Cells; Gompper, G., Schick, M., Eds.; Wiley: Hoboken, NJ, USA, Chapter 2; 2006; pp. 83–146. [Google Scholar]
- Evans, E.A. Structure and deformation properties of red blood cells: Concepts and quantitative methods. In Methods in Enzymology; Biomembranes Part T; Academic Press: Cambridge, MA, USA, 1989; Volume 173, pp. 3–35. [Google Scholar] [CrossRef]
- Engelhardt, H.; Gaub, H.; Sackmann, E. Viscoelastic properties of erythrocyte membranes in high-frequency electric fields. Nature 1984, 307, 378–380. [Google Scholar] [CrossRef]
- Hochstetter, A.; Vernekar, R.; Austin, R.H.; Becker, H.; Beech, J.P.; Fedosov, D.A.; Gompper, G.; Kim, S.-C.; Smith, J.T.; Stolovitzky, G.; et al. Deterministic lateral displacement: Challenges and perspectives. ACS Nano 2020, 14, 10784–10795. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inglis, D.W.; Nordon, R.E.; Beech, J.P.; Rosengarten, G. Microfluidic Obstacle Arrays Induce Large Reversible Shape Change in Red Blood Cells. Micromachines 2021, 12, 783. https://doi.org/10.3390/mi12070783
Inglis DW, Nordon RE, Beech JP, Rosengarten G. Microfluidic Obstacle Arrays Induce Large Reversible Shape Change in Red Blood Cells. Micromachines. 2021; 12(7):783. https://doi.org/10.3390/mi12070783
Chicago/Turabian StyleInglis, David W., Robert E. Nordon, Jason P. Beech, and Gary Rosengarten. 2021. "Microfluidic Obstacle Arrays Induce Large Reversible Shape Change in Red Blood Cells" Micromachines 12, no. 7: 783. https://doi.org/10.3390/mi12070783
APA StyleInglis, D. W., Nordon, R. E., Beech, J. P., & Rosengarten, G. (2021). Microfluidic Obstacle Arrays Induce Large Reversible Shape Change in Red Blood Cells. Micromachines, 12(7), 783. https://doi.org/10.3390/mi12070783







