Implantable Optrode Array for Optogenetic Modulation and Electrical Neural Recording
Abstract
1. Introduction
2. Fabrication
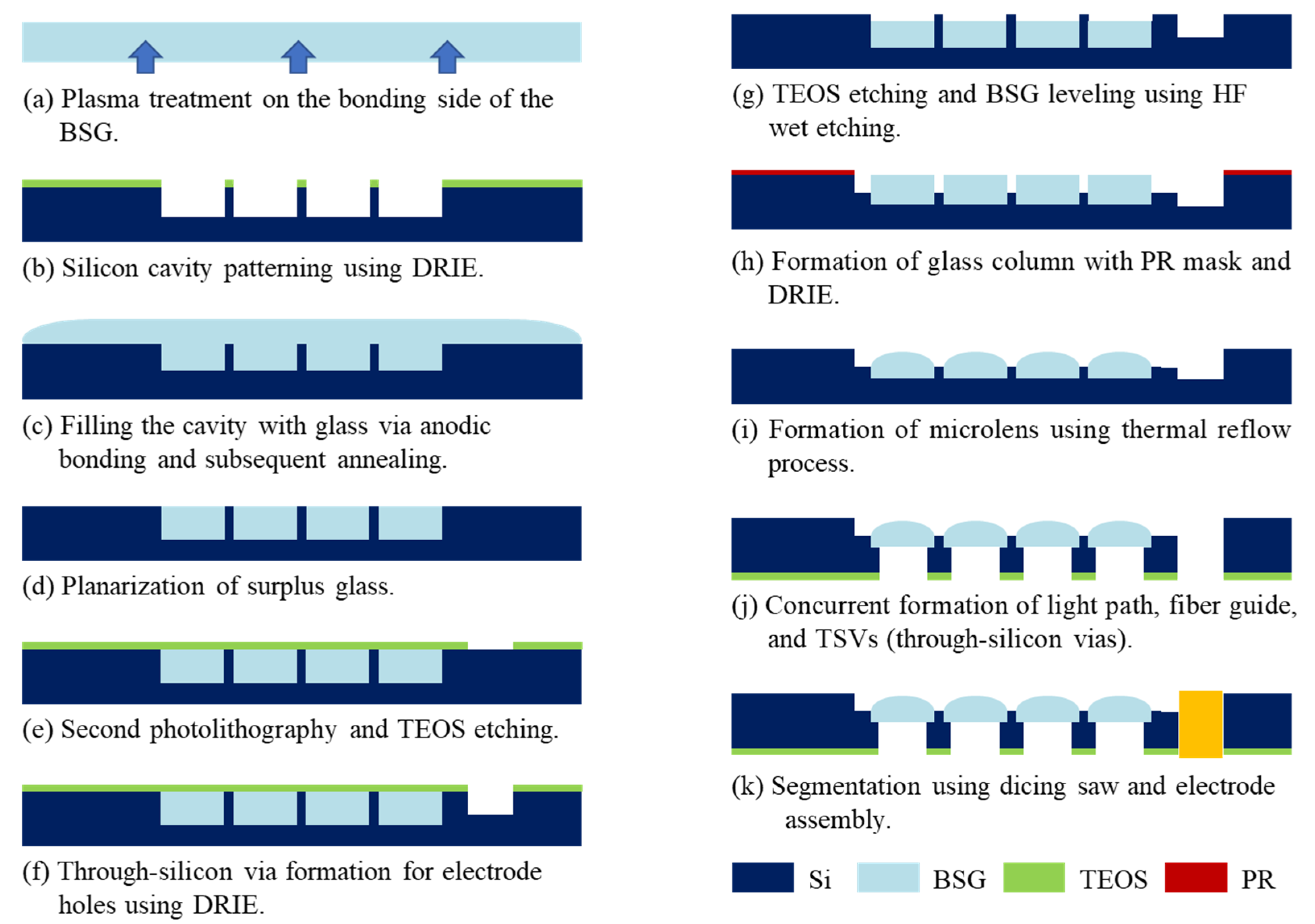
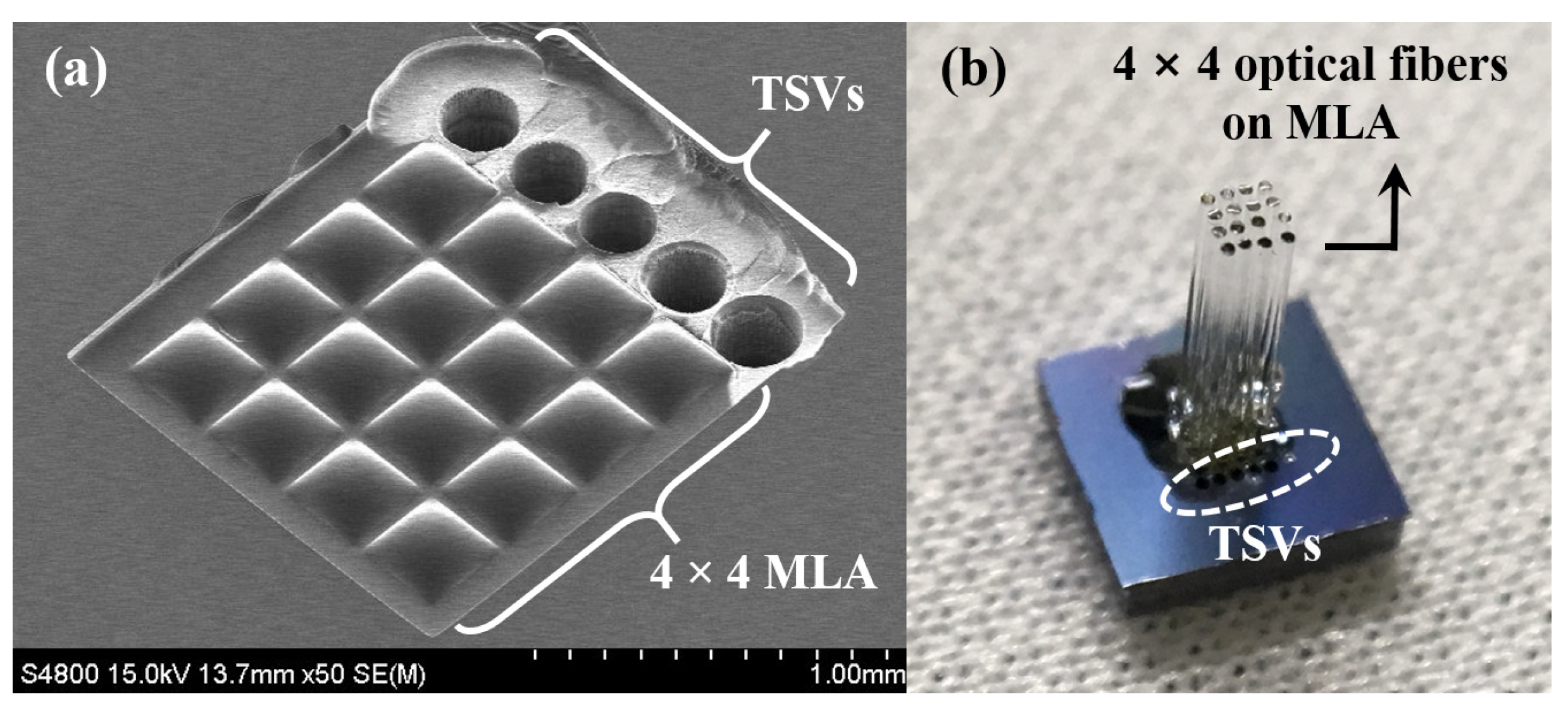
2.1. Microlens Array and Through-Silicon via Fabrication
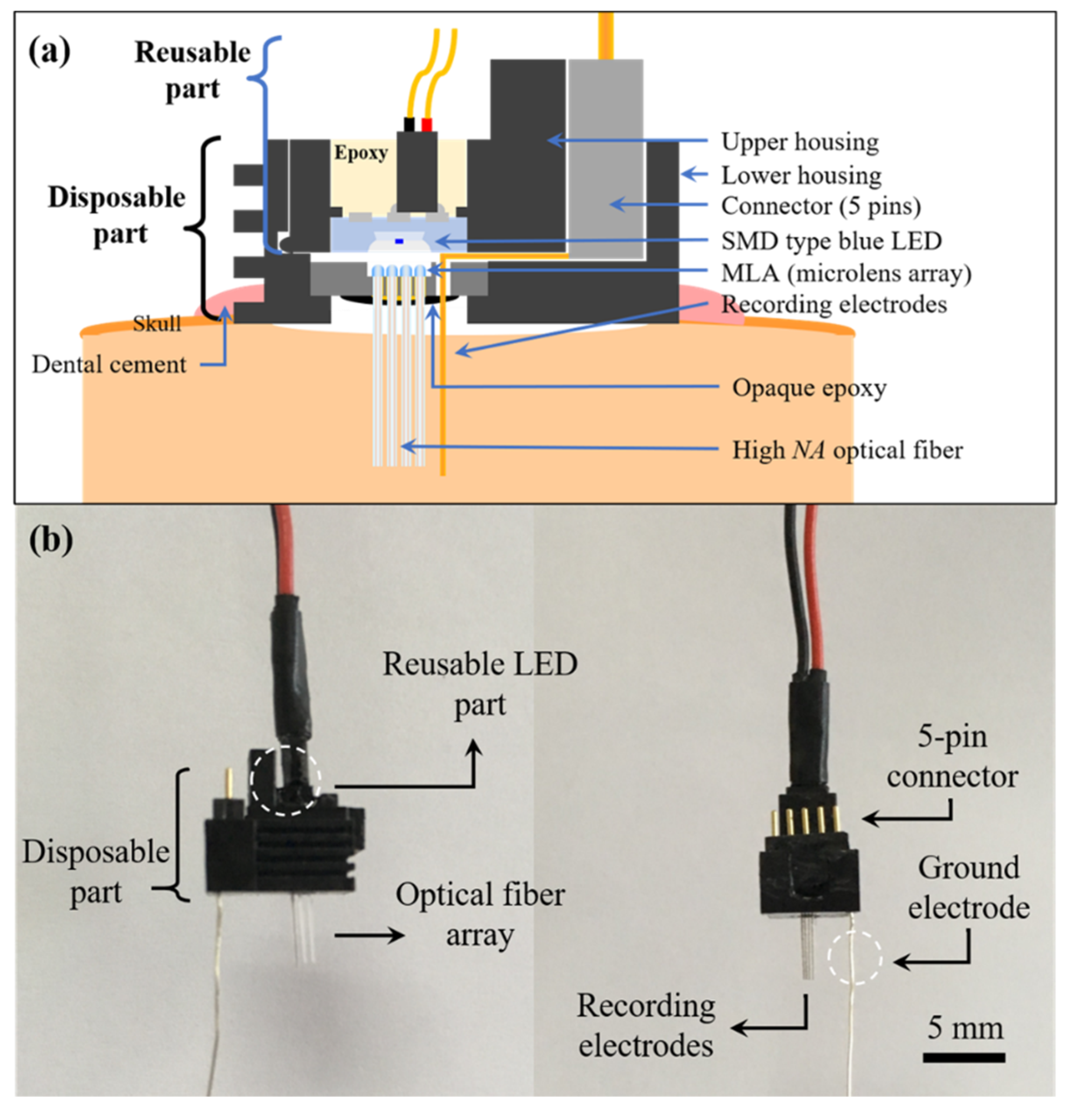
2.2. MEMS Optrode Array Assembly
3. Experimental Results
3.1. Optical Characterization
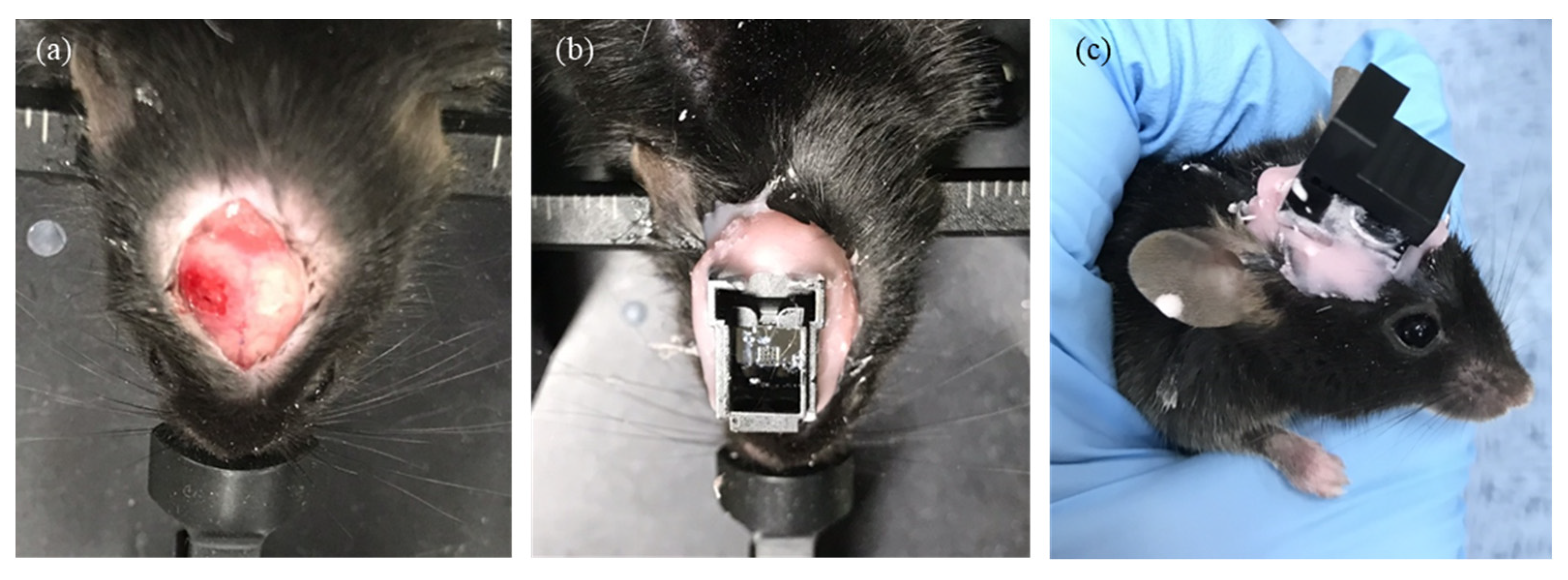
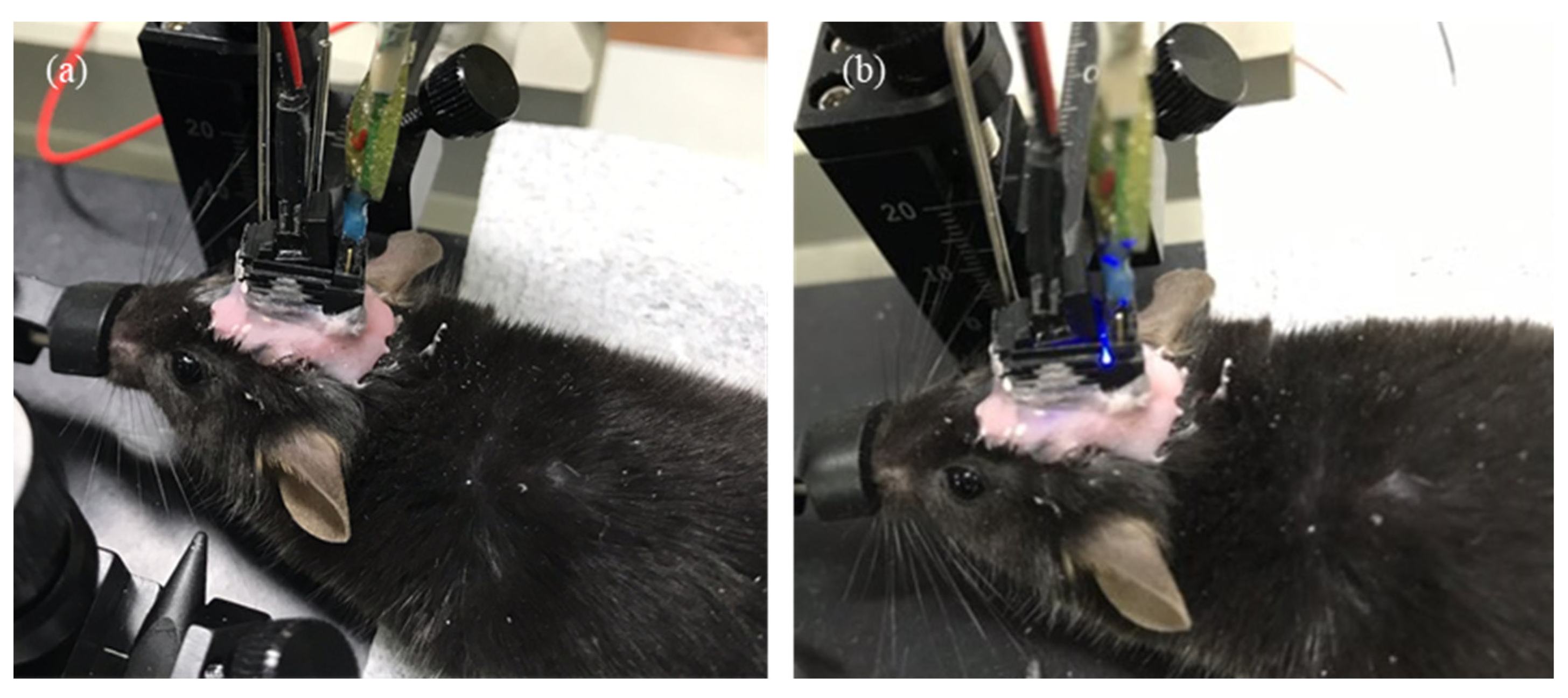
3.2. In Vivo Animal Experiment Protocol
3.3. Optogenetic and Electrophysiological Experiments
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Balasubramaniam, S.; Wirdatmadja, S.A.; Barros, M.T.; Koucheryavy, Y.; Stachowiak, M.; Jornet, J.M. Wireless Communications for Optogenetics-Based Brain Stimulation: Present Technology and Future Challenges. IEEE Commun. Mag. 2018, 56, 218–224. [Google Scholar] [CrossRef]
- Bedbrook, C.N.; Yang, K.K.; Robinson, J.E.; Mackey, E.D.; Gradinaru, V.; Arnold, F.H. Machine learning-guided channelrhodopsin engineering enables minimally invasive optogenetics. Nat. Methods 2019, 16, 1176–1184. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Optogenetics. Nat. Methods 2011, 8, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, P.; Veladi, H.; Pakdel, F.G. Optogenetics, Tools and Applications in Neurobiology. J. Med. Signals Sens. 2017, 7, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Piatkevich, K.D.; Murdock, M.H.; Subach, F.V. Advances in Engineering and Application of Optogenetic Indicators for Neuroscience. Appl. Sci. 2019, 9, 562. [Google Scholar] [CrossRef]
- Sasaki, Y.; Oshikawa, M.; Bharmoria, P.; Kouno, H.; Hayashi-Takagi, A.; Sato, M.; Ajioka, I.; Yanai, N.; Kimizuka, N. Near-Infrared Optogenetic Genome Engineering Based on Photon-Upconversion Hydrogels. Angew. Chem. Int. Ed. Engl. 2019, 58, 17827–17833. [Google Scholar] [CrossRef]
- Zhang, Y.; Castro, D.C.; Han, Y.; Wu, Y.; Guo, H.; Weng, Z.; Xue, Y.; Ausra, J.; Wang, X.; Li, R.; et al. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc. Natl. Acad. Sci. USA 2019, 116, 21427–21437. [Google Scholar] [CrossRef]
- Bernstein, J.G.; Boyden, E.S. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn. Sci. 2011, 15, 592–600. [Google Scholar] [CrossRef]
- Anikeeva, P.; Andalman, A.S.; Witten, I.; Warden, M.; Goshen, I.; Grosenick, L.; Gunaydin, L.A.; Frank, L.M.; Deisseroth, K. Optetrode: A multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 2011, 15, 163–170. [Google Scholar] [CrossRef]
- Aravanis, A.M.; Wang, L.P.; Zhang, F.; Meltzer, L.A.; Mogri, M.Z.; Schneider, M.B.; Deisseroth, K. An optical neural interface: In vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 2007, 4, S143–S156. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, A.V.; Owen, S.F.; Kreitzer, A.C. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain Res. 2013, 1511, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Shin, G.; McCall, J.G.; Al-Hasani, R.; Norris, A.; Xia, L.; Brenner, D.S.; Noh, K.N.; Bang, S.Y.; Bhatti, D.L.; et al. Stretchable multichannel antennas in soft wireless optoelectronic implants for optogenetics. Proc. Natl. Acad. Sci. USA 2016, 113, E8169–E8177. [Google Scholar] [CrossRef]
- Pashaie, R.; Anikeeva, P.; Lee, J.H.; Prakash, R.; Yizhar, O.; Prigge, M.; Chander, D.; Richner, T.J.; Williams, J. Optogenetic brain interfaces. IEEE Rev. Biomed. Eng. 2014, 7, 3–30. [Google Scholar] [CrossRef]
- Zhang, J.; Laiwalla, F.; Kim, J.A.; Urabe, H.; Van Wagenen, R.; Song, Y.K.; Connors, B.W.; Zhang, F.; Deisseroth, K.; Nurmikko, A.V. Integrated device for optical stimulation and spatiotemporal electrical recording of neural activity in light-sensitized brain tissue. J. Neural Eng. 2009, 6, 055007. [Google Scholar] [CrossRef]
- Royer, S.; Zemelman, B.V.; Barbic, M.; Losonczy, A.; Buzsáki, G.; Magee, J.C. Multi-array silicon probes with integrated optical fibers: Light-assisted perturbation and recording of local neural circuits in the behaving animal. Eur. J. Neurosci. 2010, 31, 2279–2291. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wagner, F.; Borton, D.A.; Zhang, J.; Ozden, I.; Burwell, R.D.; Nurmikko, A.V.; van Wagenen, R.; Diester, I.; Deisseroth, K. Integrated device for combined optical neuromodulation and electrical recording for chronic in vivo applications. J. Neural Eng. 2012, 9, 016001. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Song, Y.; Xu, S.; Dai, Y.; Wang, Y.; Lu, B.; Xie, J.; Wang, H.; Cai, X. In Vivo Optogenetic Modulation with Simultaneous Neural Detection Using Microelectrode Array Integrated with Optical Fiber. Sensors 2020, 20, 4526. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, S.B.; Ribeiro, J.F.; Silva, A.F.; Costa, R.M.; Correia, J.H. Design and manufacturing challenges of optogenetic neural interfaces: A review. J. Neural Eng. 2017, 14, 041001. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, J.H.; Lee, H.; Kim, Y.K.; Jun, S.B.; Lee, S.H.; Ji, C.H. Multi-wavelength light emitting diode-based disposable optrode array for in vivo optogenetic modulation. J. Biophotonics 2019, 12, e201800343. [Google Scholar] [CrossRef]
- Schwaerzle, M.; Paul, O.; Ruther, P. Compact silicon-based optrode with integrated laser diode chips, SU-8 waveguides and platinum electrodes for optogenetic applications. J. Micromech. Microeng. 2017, 27, 065004. [Google Scholar] [CrossRef]
- Sim, J.Y.; Haney, M.P.; Park, S.I.; McCall, J.G.; Jeong, J.W. Microfluidic neural probes: In vivo tools for advancing neuroscience. Lab Chip 2017, 17, 1406–1435. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Lee, H.J.; Kim, J.; Shin, H.; Choi, N.; Lee, C.J.; Yoon, E.S.; Yoon, E.; Wise, K.D.; Kim, T.G.; et al. In vivo optical modulation of neural signals using monolithically integrated two-dimensional neural probe arrays. Sci. Rep. 2015, 5, 15466. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Lee, H.; Jun, S.B.; Kim, Y.-K.; Ji, C.-H. Disposable MEMS optrode array integrated with single LED for neurostimulation. Sens. Actuators A Phys. 2018, 273, 276–284. [Google Scholar] [CrossRef]
- Chen, C.H.; McCullagh, E.A.; Pun, S.H.; Mak, P.U.; Vai, M.I.; Mak, P.I.; Klug, A.; Lei, T.C. An Integrated Circuit for Simultaneous Extracellular Electrophysiology Recording and Optogenetic Neural Manipulation. IEEE Trans. Biomed. Eng. 2017, 64, 557–568. [Google Scholar] [CrossRef]
- Clements, I.P.; Gnade, A.G.; Rush, A.D.; Patten, C.D.; Twomey, M.C.; Kravitz, A.V. Miniaturized LED sources for in vivo optogenetic experimentation. In Proceedings of the Optogenetics: Optical Methods for Cellular Control, San Francisco, CA, USA, 8 March 2013; p. 85860X. [Google Scholar]
- Rossi, M.A.; Go, V.; Murphy, T.; Fu, Q.; Morizio, J.; Yin, H.H. A wirelessly controlled implantable LED system for deep brain optogenetic stimulation. Front. Integr. Neurosci. 2015, 9, 8. [Google Scholar] [CrossRef][Green Version]
- Wentz, C.T.; Bernstein, J.G.; Monahan, P.; Guerra, A.; Rodriguez, A.; Boyden, E.S. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. J. Neural Eng. 2011, 8, 046021. [Google Scholar] [CrossRef]
- Arias-Gil, G.; Ohl, F.W.; Takagaki, K.; Lippert, M.T. Measurement, modeling, and prediction of temperature rise due to optogenetic brain stimulation. Neurophotonics 2016, 3, 045007. [Google Scholar] [CrossRef]
- Senova, S.; Scisniak, I.; Chiang, C.C.; Doignon, I.; Palfi, S.; Chaillet, A.; Martin, C.; Pain, F. Experimental assessment of the safety and potential efficacy of high irradiance photostimulation of brain tissues. Sci. Rep. 2017, 7, 43997. [Google Scholar] [CrossRef]
- Yoo, S.; Ji, C.-H.; Jin, J.-Y.; Kim, Y.-K. Suppression of surface crystallization on borosilicate glass using RF plasma treatment. Appl. Surf. Sci. 2014, 316, 484–490. [Google Scholar] [CrossRef]
- Antonenko, D.; Kulzow, N.; Cesarz, M.E.; Schindler, K.; Grittner, U.; Floel, A. Hippocampal Pathway Plasticity Is Associated with the Ability to Form Novel Memories in Older Adults. Front. Aging Neurosci. 2016, 8, 61. [Google Scholar] [CrossRef]
- Booker, S.A.; Vida, I. Morphological diversity and connectivity of hippocampal interneurons. Cell Tissue Res. 2018, 373, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Hynds, R.E. Divergent cellular pathways of hippocampal memory consolidation and reconsolidation. Hippocampus 2013, 23, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.E.; Grace, A.A. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron 2005, 46, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Preston, A.R.; Eichenbaum, H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 2013, 23, R764–R773. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Hosoya, A.; Yahagi, K.; Ferecsko, A.S.; Yaguchi, K.; Sik, A.; Itakura, M.; Takahashi, M.; Hirase, H. Hippocampal CA3 and CA2 have distinct bilateral innervation patterns to CA1 in rodents. Eur. J. Neurosci. 2012, 35, 702–710. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.; Lee, Y.; Ryu, D.; Cho, Y.K.; Lee, Y.; Jun, S.B.; Ji, C.-H. Implantable Optrode Array for Optogenetic Modulation and Electrical Neural Recording. Micromachines 2021, 12, 725. https://doi.org/10.3390/mi12060725
Jeon S, Lee Y, Ryu D, Cho YK, Lee Y, Jun SB, Ji C-H. Implantable Optrode Array for Optogenetic Modulation and Electrical Neural Recording. Micromachines. 2021; 12(6):725. https://doi.org/10.3390/mi12060725
Chicago/Turabian StyleJeon, Saeyeong, Youjin Lee, Daeho Ryu, Yoon Kyung Cho, Yena Lee, Sang Beom Jun, and Chang-Hyeon Ji. 2021. "Implantable Optrode Array for Optogenetic Modulation and Electrical Neural Recording" Micromachines 12, no. 6: 725. https://doi.org/10.3390/mi12060725
APA StyleJeon, S., Lee, Y., Ryu, D., Cho, Y. K., Lee, Y., Jun, S. B., & Ji, C.-H. (2021). Implantable Optrode Array for Optogenetic Modulation and Electrical Neural Recording. Micromachines, 12(6), 725. https://doi.org/10.3390/mi12060725





