Recent Progress on Plant-Inspired Soft Robotics with Hydrogel Building Blocks: Fabrication, Actuation and Application
Abstract
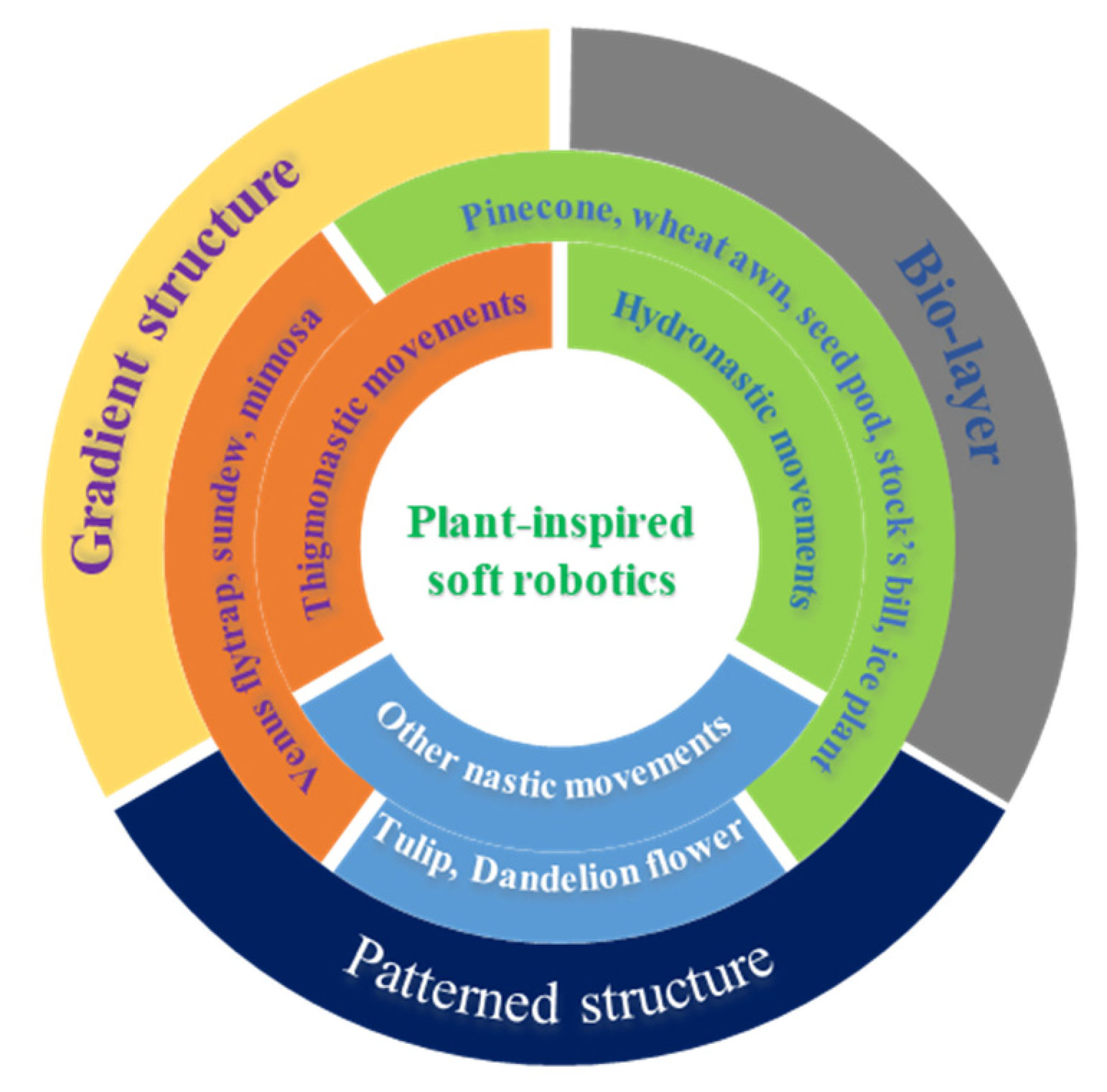
1. Introduction
2. Nastic Movements of Plants
2.1. Hydronastic Movements and Actuation Mechanisms
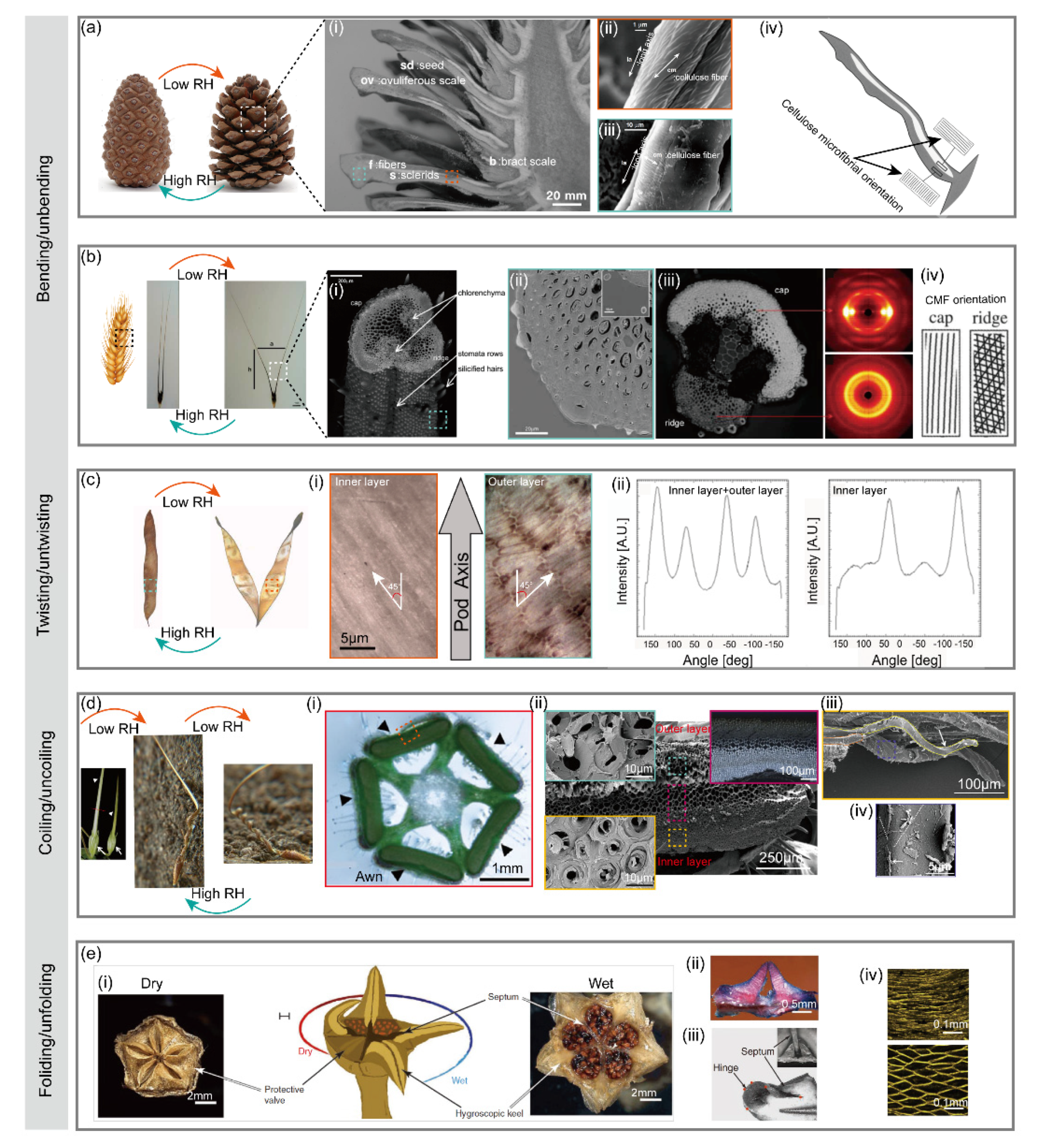
2.1.1. Pinecone
2.1.2. Wheat Awn
2.1.3. Seed Pod
2.1.4. Stork’s Bill
2.1.5. Ice Plant
2.2. Thigmonastic Movements and Actuation Mechanisms
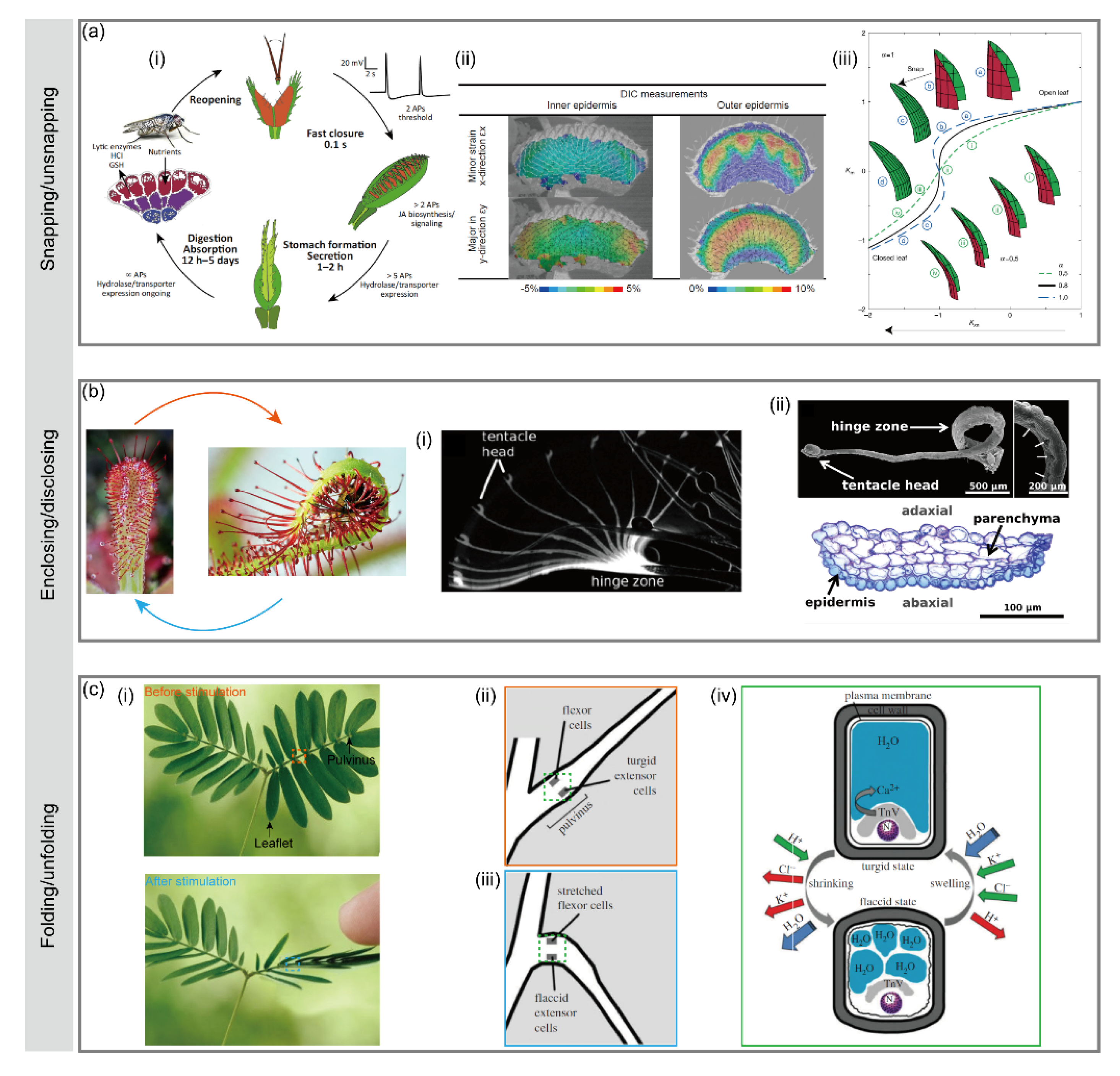
2.2.1. Venus Flytrap
2.2.2. Sundew
2.2.3. Mimosa
3. Plant-Inspired, Hydrogel-Based Soft Robotics
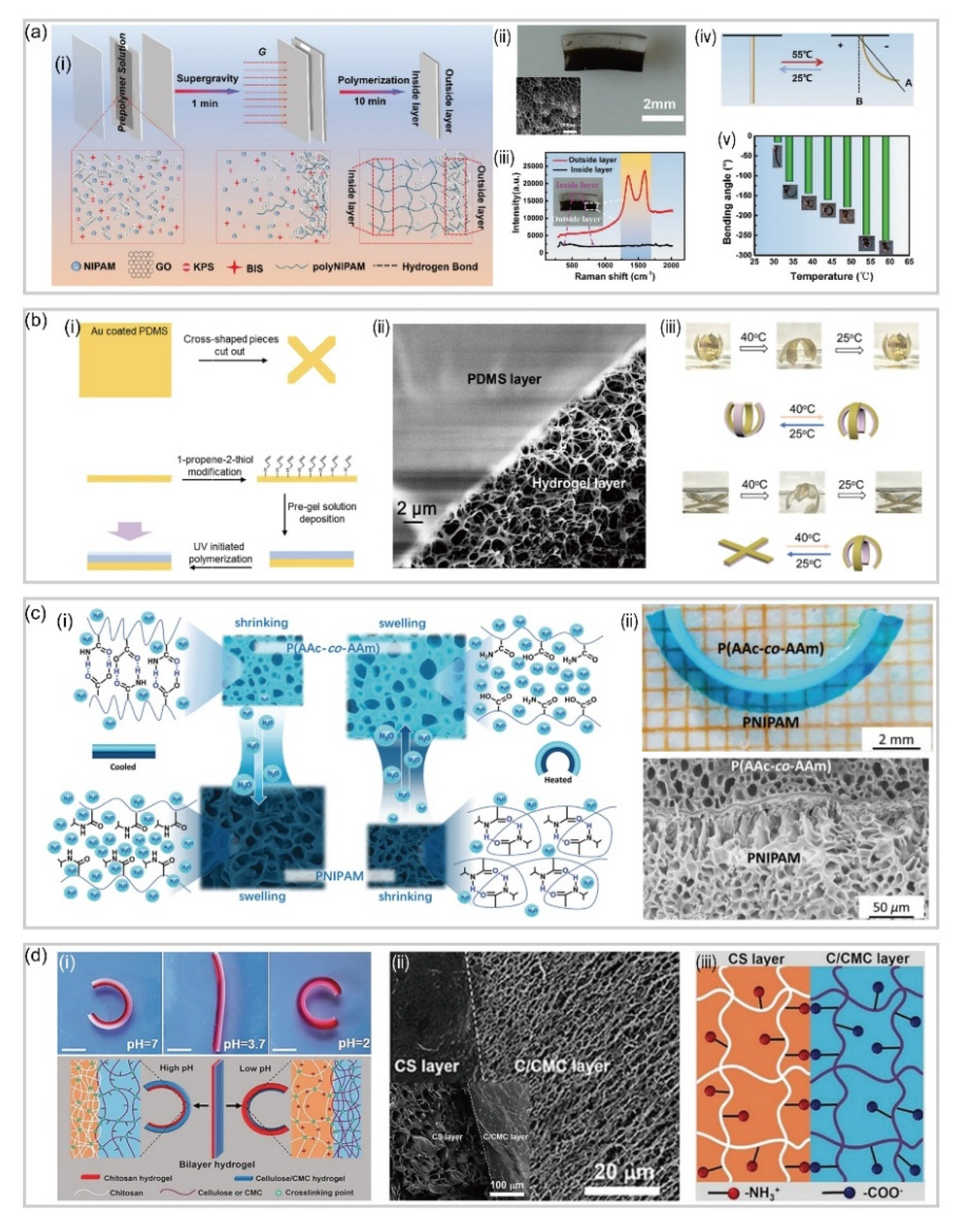
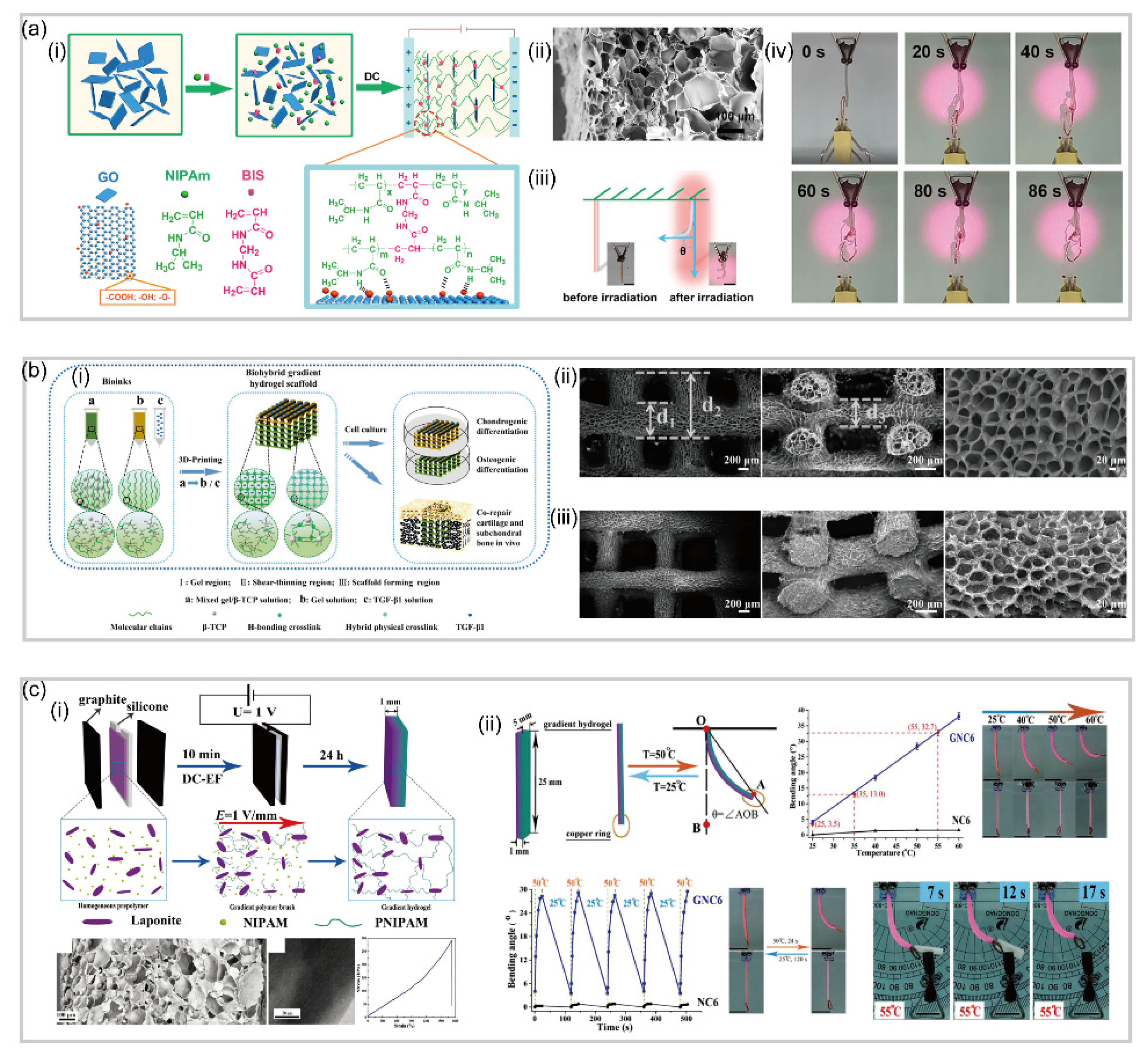
3.1. Bilayer Hydrogel-Based Soft Robotics
3.2. Gradient Hydrogel-Based Soft Robotics
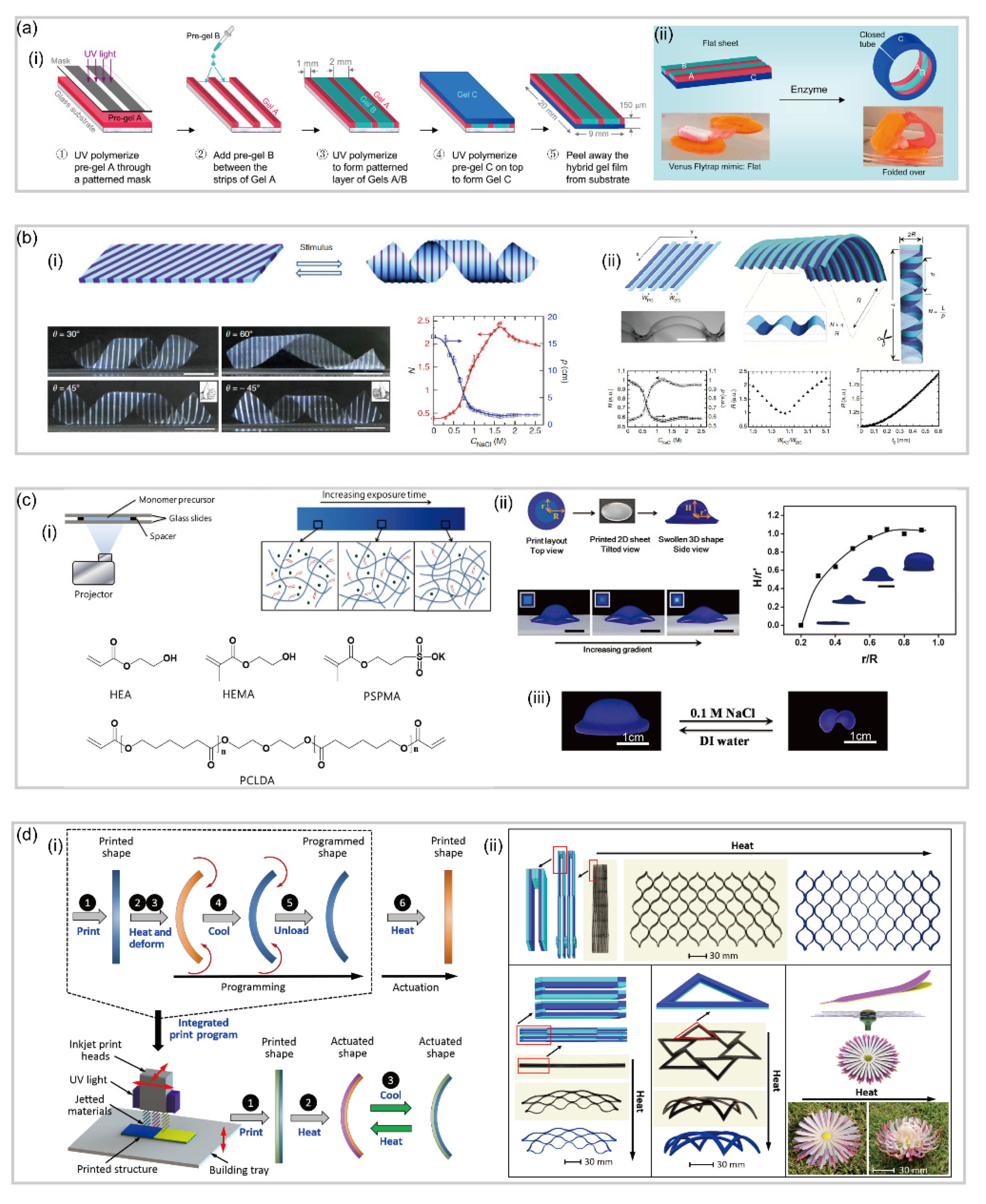
3.3. Patterned Hydrogel-Based Soft Robotics
4. Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ospovat, D. The Development of Darwin’s Theory: Natural History, Natural Theology, and Natural Selection, 1838–1859; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Somveille, M.; Rodrigues, A.S.; Manica, A. Why do birds migrate? A macroecological perspective. Glob. Ecol. Biogeogr. 2015, 24, 664–674. [Google Scholar] [CrossRef]
- Hansen, L.P.; Jonsson, N.; Jonsson, B. Oceanic migration in homing Atlantic salmon. Anim. Behav. 1993, 45, 927–941. [Google Scholar] [CrossRef]
- Skotheim, J.M.; Mahadevan, L. Physical limits and design principles for plant and fungal movements. Science 2005, 308, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Koller, D.; Van Volkenburgh, E. The Restless Plant; Harvard University Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Forterre, Y. Slow, fast and furious: Understanding the physics of plant movements. J. Exp. Bot. 2013, 64, 4745–4760. [Google Scholar] [CrossRef] [PubMed]
- Brauner, L. Tropisms and nastic movements. Annu. Rev. Plant Biol. 1954, 5, 163–182. [Google Scholar] [CrossRef]
- Kiss, J.Z. Up, down, and all around: How plants sense and respond to environmental stimuli. Proc. Natl. Acad. Sci. USA 2006, 103, 829–830. [Google Scholar] [CrossRef]
- Dawson, C.; Vincent, J.F.; Rocca, A.-M. How pine cones open. Nature 1997, 390, 668. [Google Scholar] [CrossRef]
- Elbaum, R.; Zaltzman, L.; Burgert, I.; Fratzl, P. The role of wheat awns in the seed dispersal unit. Science 2007, 316, 884–886. [Google Scholar] [CrossRef]
- Harrington, M.J.; Razghandi, K.; Ditsch, F.; Guiducci, L.; Rueggeberg, M.; Dunlop, J.W.C.; Fratzl, P.; Neinhuis, C.; Burgert, I. Origami-like unfolding of hydro-actuated ice plant seed capsules. Nat. Commun. 2011, 2, 337. [Google Scholar] [CrossRef]
- Forterre, Y.; Skotheim, J.M.; Dumais, J.; Mahadevan, L. How the Venus flytrap snaps. Nature 2005, 433, 421–425. [Google Scholar] [CrossRef]
- Nakamura, Y.; Reichelt, M.; Mayer, V.E.; Mithöfer, A. Jasmonates trigger prey-induced formation of ‘outer stomach’in carnivorous sundew plants. Proc. Royal Soc. B 2013, 280, 20130228. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Sehgal, S.; Mishra, A.; Gupta, R. Mimosa pudica L. (Laajvanti): An overview. Pharmacogn. Rev. 2012, 6, 115. [Google Scholar]
- Van Doorn, W.G.; Van Meeteren, U. Flower opening and closure: A review. J. Exp. Bot. 2003, 54, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Laschi, C.; Trimmer, B. Soft robotics: A bioinspired evolution in robotics. Trends Biotechnol. 2013, 31, 287–294. [Google Scholar] [CrossRef]
- Fratzl, P.; Barth, F.G. Biomaterial systems for mechanosensing and actuation. Nature 2009, 462, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Coyle, S.; Majidi, C.; LeDuc, P.; Hsia, K.J. Bio-inspired soft robotics: Material selection, actuation, and design. Extreme Mech. Lett. 2018, 22, 51–59. [Google Scholar] [CrossRef]
- Lee, Y.; Song, W.J.; Sun, J.Y. Hydrogel soft robotics. Mater. Today Phys. 2020, 15, 100258. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Stano, G.; Percoco, G. Additive manufacturing aimed to soft robots fabrication: A review. Extreme Mech. Lett. 2021, 42, 101079. [Google Scholar] [CrossRef]
- Ionov, L. Biomimetic hydrogel-based actuating systems. Adv. Funct. Mater. 2013, 23, 4555–4570. [Google Scholar] [CrossRef]
- Van der Pijl, L. Principles of Dispersal; Springer: Berlin, Germany, 1982. [Google Scholar]
- Cousens, R.; Dytham, C.; Law, R. Dispersal in Plants: A Population Perspective; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Burgert, I.; Fratzl, P. Actuation systems in plants as prototypes for bioinspired devices. Philos. Trans. R. Soc. A 2009, 367, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Harlow, W.M.; Côté, W.A.; Day, A.C. The opening mechanism of pine cone scales. J. For. Res. 1964, 62, 538–540. [Google Scholar] [CrossRef]
- Erb, R.M.; Sander, J.S.; Grisch, R.; Studart, A.R. Self-shaping composites with programmable bioinspired microstructures. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reyssat, E.; Mahadevan, L. Hygromorphs: From pine cones to biomimetic bilayers. J. R. Soc. Interface 2009, 6, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Emons, A.M.C.; Mulder, B.M. How the deposition of cellulose microfibrils builds cell wall architecture. Trends Plant Sci. 2000, 5, 35–40. [Google Scholar] [CrossRef]
- Burgert, I.; Fratzl, P. Plants control the properties and actuation of their organs through the orientation of cellulose fibrils in their cell walls. Integr. Comp. Biol. 2009, 49, 69–79. [Google Scholar] [CrossRef]
- Abraham, Y.; Tamburu, C.; Klein, E.; Dunlop, J.W.; Fratzl, P.; Raviv, U.; Elbaum, R. Tilted cellulose arrangement as a novel mechanism for hygroscopic coiling in the stork’s bill awn. J. R. Soc. Interface 2012, 9, 640–647. [Google Scholar] [CrossRef]
- Armon, S.; Efrati, E.; Kupferman, R.; Sharon, E. Geometry and mechanics in the opening of chiral seed pods. Science 2011, 333, 1726–1730. [Google Scholar] [CrossRef]
- Forterre, Y.; Dumais, J. Generating Helices in Nature. Science 2011, 333, 1715–1716. [Google Scholar] [CrossRef]
- Aßhoff, S.J.; Lancia, F.; Iamsaard, S.; Matt, B.; Kudernac, T.; Fletcher, S.P.; Katsonis, N. High-Power Actuation from Molecular Photoswitches in Enantiomerically Paired Soft Springs. Angew. Chem. Int. Ed. 2017, 56, 3261–3265. [Google Scholar] [CrossRef]
- Ghafouri, R.; Bruinsma, R. Helicoid to spiral ribbon transition. Phys. Rev. Lett. 2005, 94, 138101. [Google Scholar] [CrossRef] [PubMed]
- Abraham, Y.; Elbaum, R. Hygroscopic movements in Geraniaceae: The structural variations that are responsible for coiling or bending. New Phytol. 2013, 199, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, D.; Hotton, S.; Dumais, J. The mechanics of explosive dispersal and self-burial in the seeds of the filaree, Erodium cicutarium (Geraniaceae). J. Exp. Biol. 2011, 214, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Abraham, Y.; Dong, Y.; Aharoni, A.; Elbaum, R. Mapping of cell wall aromatic moieties and their effect on hygroscopic movement in the awns of stork’s bill. Cellulose 2018, 25, 3827–3841. [Google Scholar] [CrossRef]
- Almeida, A.P.C.; Querciagrossa, L.; Silva, P.E.S.; Gonçalves, F.; Canejo, J.P.; Almeida, P.L.; Godinho, M.H.; Zannoni, C. Reversible water driven chirality inversion in cellulose-based helices isolated from Erodium awns. Soft Matter 2019, 15, 2838–2847. [Google Scholar] [CrossRef]
- Klak, C.; Reeves, G.; Hedderson, T. Unmatched tempo of evolution in Southern African semi-desert ice plants. Nature 2004, 427, 63–65. [Google Scholar] [CrossRef]
- Parolin, P. Ombrohydrochory: Rain-operated seed dispersal in plants–With special regard to jet-action dispersal in Aizoaceae. Flora 2006, 201, 511–518. [Google Scholar] [CrossRef]
- Elbaum, R.; Abraham, Y. Insights into the microstructures of hygroscopic movement in plant seed dispersal. Plant Sci. 2014, 223, 124–133. [Google Scholar] [CrossRef]
- Hedrich, R.; Neher, E. Venus flytrap: How an excitable, carnivorous plant works. Trends Plant Sci. 2018, 23, 220–234. [Google Scholar] [CrossRef]
- Volkov, A.G.; Vilfranc, C.L.; Murphy, V.A.; Mitchell, C.M.; Volkova, M.I.; O’Neal, L.; Markin, V.S. Electrotonic and action potentials in the Venus flytrap. J. Plant Physiol. 2013, 170, 838–846. [Google Scholar] [CrossRef]
- Scherzer, S.; Shabala, L.; Hedrich, B.; Fromm, J.; Bauer, H.; Munz, E.; Jakob, P.; Al-Rascheid, K.A.; Kreuzer, I.; Becker, D. Insect haptoelectrical stimulation of Venus flytrap triggers exocytosis in gland cells. Proc. Natl. Acad. Sci. USA. 2017, 114, 4822–4827. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Adesina, T.; Jovanov, E. Closing of Venus flytrap by electrical stimulation of motor cells. Plant Signal. Behav. 2007, 2, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, A.; Iwata, G.Z.; Scherzer, S.; Bougas, L.; Rolfs, K.; Jodko-Władzińska, A.; Voigt, J.; Hedrich, R.; Budker, D. Action potentials induce biomagnetic fields in carnivorous Venus flytrap plants. Sci. Rep. 2021, 11, 1438. [Google Scholar] [CrossRef] [PubMed]
- Sachse, R.; Westermeier, A.; Mylo, M.; Nadasdi, J.; Bischoff, M.; Speck, T.; Poppinga, S. Snapping mechanics of the Venus flytrap (Dionaea muscipula). Proc. Natl. Acad. Sci. USA 2020, 117, 16035–16042. [Google Scholar] [CrossRef]
- Adlassnig, W.; Lendl, T.; Peroutka, M.; Lang, I. Deadly glue—Adhesive traps of carnivorous plants. In Biological Adhesive Systems; Springer: Vienna, Austria, 2010; pp. 15–28. [Google Scholar]
- Krausko, M.; Perutka, Z.; Šebela, M.; Šamajová, O.; Šamaj, J.; Novák, O.; Pavlovič, A. The role of electrical and jasmonate signalling in the recognition of captured prey in the carnivorous sundew plant Drosera capensis. New Phytol. 2017, 213, 1818–1835. [Google Scholar] [CrossRef] [PubMed]
- Pavlovič, A.; Krausko, M.; Adamec, L. A carnivorous sundew plant prefers protein over chitin as a source of nitrogen from its traps. Plant Physiol. Biochem. 2016, 104, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Poppinga, S.; Hartmeyer, S.R.H.; Seidel, R.; Masselter, T.; Hartmeyer, I.; Speck, T. Catapulting tentacles in a sticky carnivorous plant. PLoS ONE 2012, 7, e45735. [Google Scholar] [CrossRef]
- Guo, Q.; Dai, E.; Han, X.; Xie, S.; Chao, E.; Chen, Z. Fast nastic motion of plants and bioinspired structures. J. R. Soc. Interface 2015, 12, 20150598. [Google Scholar] [CrossRef]
- Volkov, A.G.; Foster, J.C.; Baker, K.D.; Markin, V.S. Mechanical and electrical anisotropy in Mimosa pudica pulvini. Plant Signal. Behav. 2010, 5, 1211–1221. [Google Scholar] [CrossRef]
- Scorza, L.C.; Dornelas, M.C. Plants on the move: Towards common mechanisms governing mechanically-induced plant movements. Plant Signal. Behav. 2011, 6, 1979–1986. [Google Scholar] [CrossRef]
- Le, X.; Lu, W.; Zhang, J.; Chen, T. Recent progress in biomimetic anisotropic hydrogel actuators. Adv. Sci. 2019, 6, 1801584. [Google Scholar] [CrossRef] [PubMed]
- Kharlampieva, E.; Kozlovskaya, V.; Sukhishvili, S.A. Layer-by-layer hydrogen-bonded polymer films: From fundamentals to applications. Adv. Mater. 2009, 21, 3053–3065. [Google Scholar] [CrossRef]
- He, X.; Sun, Y.; Wu, J.; Wang, Y.; Chen, F.; Fan, P.; Zhong, M.; Xiao, S.; Zhang, D.; Yang, J. Dual-stimulus bilayer hydrogel actuators with rapid, reversible, bidirectional bending behaviors. J. Mater. Chem. C 2019, 7, 4970–4980. [Google Scholar] [CrossRef]
- Myung, D.; Waters, D.; Wiseman, M.; Duhamel, P.E.; Noolandi, J.; Ta, C.N.; Frank, C.W. Progress in the development of interpenetrating polymer network hydrogels. Polym. Adv. Technol. 2008, 19, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Kozhunova, E.Y.; Makhaeva, E.E.; Khokhlov, A.R. Collapse of thermosensitive polyelectrolyte semi-interpenetrating networks. Polymer 2012, 53, 2379–2384. [Google Scholar] [CrossRef]
- Tominaga, T.; Tirumala, V.R.; Lee, S.; Lin, E.K.; Gong, J.P.; Wu, W.-l. Thermodynamic interactions in double-network hydrogels. J. Phys. Chem. B 2008, 112, 3903–3909. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, X.; Gao, Y.; Serpe, M.J. Reversible bidirectional bending of hydrogel-based bilayer actuators. J. Mater. Chem. B 2017, 5, 2804–2812. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, P.; Le, X.; Lu, W.; Théato, P.; Ma, C.; Du, B.; Zhang, J.; Huang, Y.; Chen, T. Mimosa inspired bilayer hydrogel actuator functioning in multi-environments. J. Mater. Chem. C 2018, 6, 1320–1327. [Google Scholar] [CrossRef]
- Duan, J.; Liang, X.; Zhu, K.; Guo, J.; Zhang, L. Bilayer hydrogel actuators with tight interfacial adhesion fully constructed from natural polysaccharides. Soft Matter 2017, 13, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wu, R.; Li, H.; Ren, W.; Du, J.; Xu, S.; Wang, J. Electric field-induced gradient strength in nanocomposite hydrogel through gradient crosslinking of clay. J. Mater. Chem. B 2015, 3, 4426–4430. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, Y.; Wang, X.; An, W.; Xu, S.; Liao, W.; Wang, Y. Photothermal nanocomposite hydrogel actuator with electric-field-induced gradient and oriented structure. ACS Appl. Mater. Interfaces 2018, 10, 7688–7692. [Google Scholar] [CrossRef]
- Maeda, S.; Hara, Y.; Sakai, T.; Yoshida, R.; Hashimoto, S. Self-walking gel. Adv. Mater. 2007, 19, 3480–3484. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Liu, B.; Li, H.; Wu, Y.; Zhang, Y.; Lin, Z.; Wu, M.; Ruan, C.; et al. Direct 3D Printing of High Strength Biohybrid Gradient Hydrogel Scaffolds for Efficient Repair of Osteochondral Defect. Adv. Funct. Mater. 2018, 28, 1706644. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Gao, Q.; Niu, X.; Shao, L.; Zhou, L.; Lin, Z.; Sun, A.; Fu, J.; Chen, Z.; Hu, J.; Liu, Y. 3D printing of complex GelMA-based scaffolds with nanoclay. Biofabrication 2019, 11, 035006. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, D.; Xu, H.; Yang, Y.; An, W.; Yu, L.; Xiao, Z.; Xu, S. A Fast, Reversible, and Robust Gradient Nanocomposite Hydrogel Actuator with Water-Promoted Thermal Response. Macromol. Rapid. Commun. 2018, 39, 1700863. [Google Scholar] [CrossRef] [PubMed]
- Athas, J.C.; Nguyen, C.P.; Zarket, B.C.; Gargava, A.; Nie, Z.; Raghavan, S.R. Enzyme-Triggered Folding of Hydrogels: Toward a Mimic of the Venus Flytrap. ACS Appl. Mater. Interfaces 2016, 8, 19066–19074. [Google Scholar] [CrossRef]
- Wu, Z.L.; Moshe, M.; Greener, J.; Therien-Aubin, H.; Nie, Z.; Sharon, E.; Kumacheva, E. Three-dimensional shape transformations of hydrogel sheets induced by small-scale modulation of internal stresses. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Peng, X.; Liu, T.; Zhang, Q.; Shang, C.; Bai, Q.W.; Wang, H. Surface patterning of hydrogels for programmable and complex shape deformations by ion inkjet printing. Adv. Funct. Mater. 2017, 27, 1701962. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Chen, F.; Zhou, D.; Li, B.; Zhou, X.; Gan, T.; Handschuh-Wang, S.; Zhou, X. Softening and Shape Morphing of Stiff Tough Hydrogels by Localized Unlocking of the Trivalent Ionically Cross-Linked Centers. Macromol. Rapid. Commun. 2018, 39, 1800143. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, R.; Wu, J.; Song, J.; Bai, H.; Li, B.; Zhao, Q.; Xie, T. Ultrafast digital printing toward 4D shape changing materials. Adv. Funct. Mater. 2017, 29, 1605390. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Yuan, C.; Peng, X.; Wang, T.; Qi, H.J.; Dunn, M.L. Direct 4D printing via active composite materials. Sci. Adv. 2017, 3, e1602890. [Google Scholar] [CrossRef] [PubMed]

| Type | Reference | Hydrogel | Response Time | Tensile Strength (kPa) | Motion | Stimulus |
|---|---|---|---|---|---|---|
| Bilayer | [58] | pNIPAM, GO | 16–24 s | 83 | Bending | Temperature |
| [62] | pNIPAM, pDADMAC | Bending | Temperature, pH | |||
| [63] | pNIPAM, (AAc-co-AAm) | ~60 s | Bending | Temperature | ||
| [64] | CS, CMC | 240 s | 62 | Coiling | pH | |
| Gradient | [66] | pNIPAM, GO | 40 s | Bending | Photo | |
| [67] | NAGA, NAT, TRIS-acrylamide | 410 | Temperature | |||
| [71] | pNIPAM, Laponite | 20 s | 290 | Bending | Temperature | |
| Pattern | [72] | PEGDA, GelMA-co-PEGDMA | 20.3/1.8 | Folding, twisting | pH, enzyme | |
| [73] | pNIPAM, AMPS | 2 h | Twisting | Temperature | ||
| [76] | HEA, HEMA, PSPMA, LA, HD | Bending, folding | Temperature | |||
| [77] | TangoBLack+, VeroClear | Bending, folding | Temperature |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhou, Y.; Zhang, B.; Zhang, C.; Wang, J.; Wang, Z. Recent Progress on Plant-Inspired Soft Robotics with Hydrogel Building Blocks: Fabrication, Actuation and Application. Micromachines 2021, 12, 608. https://doi.org/10.3390/mi12060608
Xu Z, Zhou Y, Zhang B, Zhang C, Wang J, Wang Z. Recent Progress on Plant-Inspired Soft Robotics with Hydrogel Building Blocks: Fabrication, Actuation and Application. Micromachines. 2021; 12(6):608. https://doi.org/10.3390/mi12060608
Chicago/Turabian StyleXu, Zhenyu, Yongsen Zhou, Baoping Zhang, Chao Zhang, Jianfeng Wang, and Zuankai Wang. 2021. "Recent Progress on Plant-Inspired Soft Robotics with Hydrogel Building Blocks: Fabrication, Actuation and Application" Micromachines 12, no. 6: 608. https://doi.org/10.3390/mi12060608
APA StyleXu, Z., Zhou, Y., Zhang, B., Zhang, C., Wang, J., & Wang, Z. (2021). Recent Progress on Plant-Inspired Soft Robotics with Hydrogel Building Blocks: Fabrication, Actuation and Application. Micromachines, 12(6), 608. https://doi.org/10.3390/mi12060608








