Evaluation of the Effects of Solvents Used in the Fabrication of Microfluidic Devices on Cell Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of COP-Based Microfluidic Structures and Plates
2.2. Solvent-Bonding Process for COP Device Fabrication
2.3. VUV-Photobonding Process for COP Device Fabrication
2.4. Peeling Test
2.5. Leakage Test
2.6. ECM Coating of Microfluidic Channels
2.7. Evaluation of the Amount of Coated ECM in the COP Devices
2.8. Cell Culture
2.9. Cell Culture in the COP Devices
2.10. Apoptosis Assays
2.11. Microscopic Cell Imaging
2.12. Single Cell Profiling Based on Microscopic Images
2.13. Statistical Analysis
3. Results
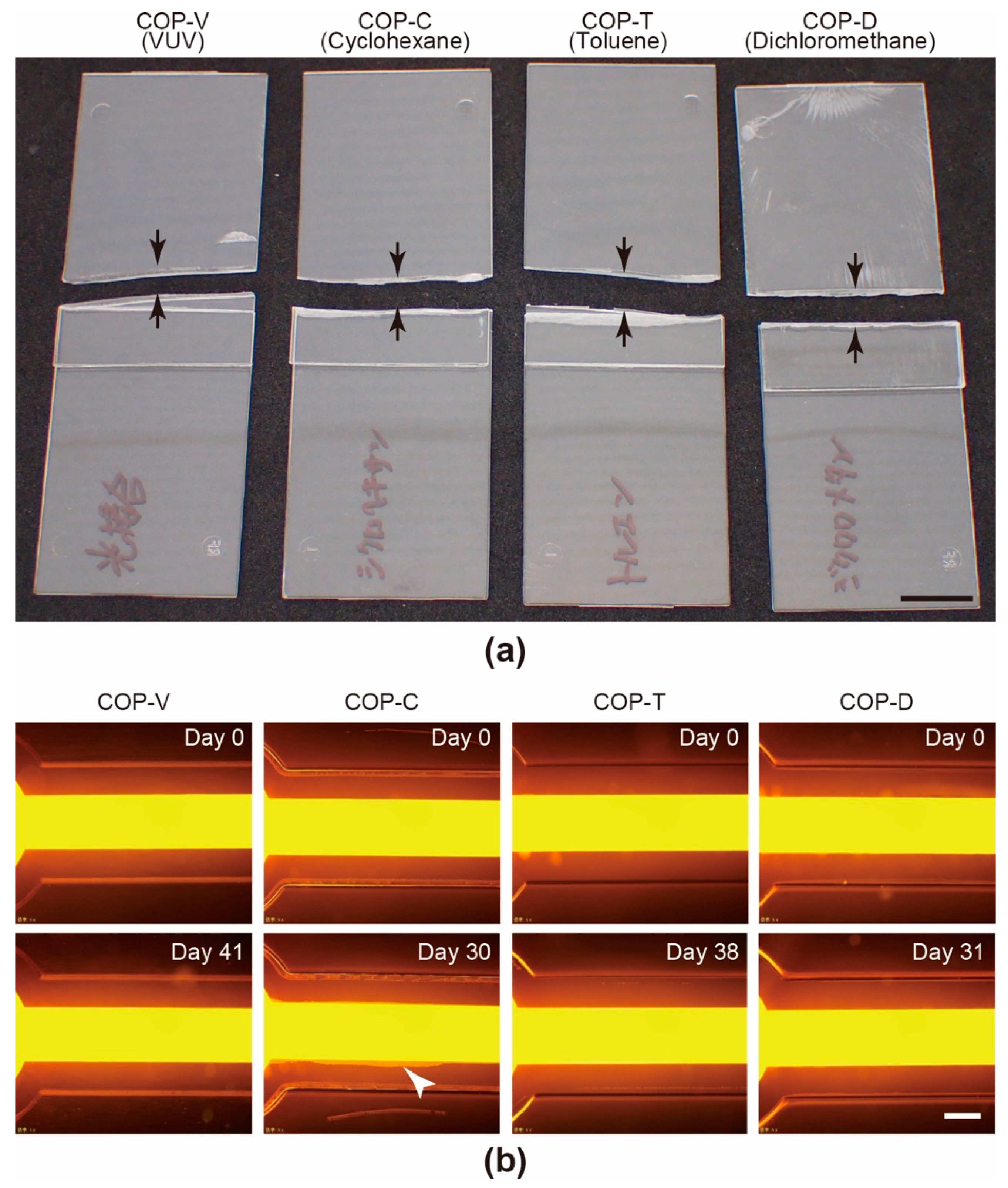
3.1. Fabrication of COP-Based Microfluidic Devices
3.2. Effect of Residual Solvent on the ECM Coating in COP-Based Microfluidic Devices
3.3. Effect of Solvent on SH-SY5Y Cells Cultured in COP Devices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baßler, K.; Günther, P.; Schulte-Schrepping, J.; Becker, M.; Biernat, P. Single Cell Methods: Sequencing and Proteomics; Proserpio, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1979, ISBN 978-1-4939-9239-3. [Google Scholar]
- Zilionis, R.; Nainys, J.; Veres, A.; Savova, V.; Zemmour, D.; Klein, A.M.; Mazutis, L. Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc. 2017, 12, 44–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Masterman-Smith, M.D.; Graham, N.A.; Jiao, J.; Mottahedeh, J.; Laks, D.R.; Ohashi, M.; DeJesus, J.; Kamei, K.; Lee, K.-B.; et al. A microfluidic platform for systems pathology: Multiparameter single-cell signaling measurements of clinical brain tumor specimens. Cancer Res. 2010, 70, 6128–6138. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cordero, J.L.; Maerkl, S.J. Microfluidic systems for cancer diagnostics. Curr. Opin. Biotechnol. 2020, 65, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Dauth, S.; Hassell, B.; Herland, A.; Jain, A.; Jang, K.-J.; Karalis, K.; Kim, H.J.; MacQueen, L.; Mahmoodian, R.; et al. Engineered In Vitro Disease Models. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 195–262. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Wang, Y.I.; Narasimhan Sriram, N.; Jackson, M.; Long, C.; Hickman, J.J.; Shuler, M.L. Recent Advances in Body-on-a-Chip Systems. Anal. Chem. 2019, 91, 330–351. [Google Scholar] [CrossRef]
- Soto-Gutierrez, A.; Gough, A.; Vernetti, L.A.; Taylor, D.L.; Monga, S.P. Pre-clinical and clinical investigations of metabolic zonation in liver diseases: The potential of microphysiology systems. Exp. Biol. Med. 2017, 242, 1605–1616. [Google Scholar] [CrossRef]
- Kamei, K.; Kato, Y.; Hirai, Y.; Ito, S.; Satoh, J.; Oka, A.; Tsuchiya, T.; Chen, Y.; Tabata, O. Integrated heart/cancer on a chip to reproduce the side effects of anti-cancer drugs in vitro. RSC Adv. 2017, 7, 36777–36786. [Google Scholar] [CrossRef]
- Hutson, M.S.; Alexander, P.G.; Allwardt, V.; Aronoff, D.M.; Bruner-Tran, K.L.; Cliffel, D.E.; Davidson, J.M.; Gough, A.; Markov, D.A.; McCawley, L.J.; et al. Organs-on-Chips as Bridges for Predictive Toxicology. Appl. In Vitro Toxicol. 2016, 2, 97–102. [Google Scholar] [CrossRef]
- van Meer, B.J.; de Vries, H.; Firth, K.S.A.; van Weerd, J.; Tertoolen, L.G.J.; Karperien, H.B.J.; Jonkheijm, P.; Denning, C.; IJzerman, A.P.; Mummery, C.L. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484. [Google Scholar] [CrossRef]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 2012, 12, 1224. [Google Scholar] [CrossRef]
- Carter, S.-S.D.; Atif, A.-R.; Kadekar, S.; Lanekoff, I.; Engqvist, H.; Varghese, O.P.; Tenje, M.; Mestres, G. PDMS leaching and its implications for on-chip studies focusing on bone regeneration applications. Organs Chip 2020, 2, 100004. [Google Scholar] [CrossRef]
- Campbell, S.B.; Wu, Q.; Yazbeck, J.; Liu, C.; Okhovatian, S.; Radisic, M. Beyond Polydimethylsiloxane: Alternative Materials for Fabrication of Organ-on-a-Chip Devices and Microphysiological Systems. ACS Biomater. Sci. Eng. 2020. [Google Scholar] [CrossRef]
- Auner, A.W.; Tasneem, K.M.; Markov, D.A.; McCawley, L.J.; Hutson, M.S. Chemical-PDMS binding kinetics and implications for bioavailability in microfluidic devices. Lab Chip 2019, 19, 864–874. [Google Scholar] [CrossRef]
- Tsao, C.-W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, J.; Zhou, T.; Song, L. Fabricating Microstructures on Glass for Microfluidic Chips by Glass Molding Process. Micromachines 2018, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Seker, E. Configurable microfluidic platform for investigating therapeutic delivery from biomedical device coatings. Lab Chip 2017, 17, 3331–3337. [Google Scholar] [CrossRef] [PubMed]
- Shoffner, M. Chip PCR. I. Surface passivation of microfabricated silicon-glass chips for PCR. Nucleic Acids Res. 1996, 24, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K.; Hirai, Y.; Yoshioka, M.; Makino, Y.; Yuan, Q.; Nakajima, M.; Chen, Y.; Tabata, O. Phenotypic and Transcriptional Modulation of Human Pluripotent Stem Cells Induced by Nano/Microfabrication Materials. Adv. Healthc. Mater. 2013, 2, 287–291. [Google Scholar] [CrossRef]
- Puza, S.; Gencturk, E.; Odabasi, I.E.; Iseri, E.; Mutlu, S.; Ulgen, K.O. Fabrication of cyclo olefin polymer microfluidic devices for trapping and culturing of yeast cells. Biomed. Microdevices 2017, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Xiaodong, W.; Fan, Y. Microfluidic chip made of COP (cyclo-olefin polymer) and comparion to PMMA (polymethylmethacrylate) microfluidic chip. J. Mater. Process. Technol. 2008, 208, 63–69. [Google Scholar] [CrossRef]
- Nunes, P.S.; Ohlsson, P.D.; Ordeig, O.; Kutter, J.P. Cyclic olefin polymers: Emerging materials for lab-on-a-chip applications. Microfluid. Nanofluidics 2010, 9, 145–161. [Google Scholar] [CrossRef]
- Sun, H.; Chan, C.-W.; Wang, Y.; Yao, X.; Mu, X.; Lu, X.; Zhou, J.; Cai, Z.; Ren, K. Reliable and reusable whole polypropylene plastic microfluidic devices for a rapid, low-cost antimicrobial susceptibility test. Lab Chip 2019, 19, 2915–2924. [Google Scholar] [CrossRef]
- Rötting, O.; Röpke, W.; Becker, H.; Gärtner, C. Polymer microfabrication technologies. Microsyst. Technol. 2002, 8, 32–36. [Google Scholar] [CrossRef]
- Tsao, C.-W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluidics 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Yamanaka, M.; Wen, X.; Imamura, S.; Sakai, R.; Terada, S.; Kamei, K. Cyclo olefin polymer-based solvent-free mass-productive microphysiological systems. Biomed. Mater. 2021, 16, 035009. [Google Scholar] [CrossRef] [PubMed]
- Bal-Price, A.; Hogberg, H.T.; Crofton, K.M.; Daneshian, M.; FitzGerald, R.E.; Fritsche, E.; Heinonen, T.; Bennekou, S.H.; Klima, S.; Piersma, A.H.; et al. Recommendation on test readiness criteria for new approach methods in toxicology: Exemplified for developmental neurotoxicity. ALTEX 2018, 35, 306–352. [Google Scholar] [CrossRef]
- Meulenberg, C.J.W.; de Groot, A.; Westerink, R.H.S.; Vijverberg, H.P.M. Organic solvent-induced changes in membrane geometry in human SH-SY5Y neuroblastoma cells—A common narcotic effect? Neurotoxicology 2016, 55, 74–82. [Google Scholar] [CrossRef] [PubMed]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef]
- García, M.T.; Gracia, I.; Duque, G.; de Lucas, A.; Rodríguez, J.F. Study of the solubility and stability of polystyrene wastes in a dissolution recycling process. Waste Manag. 2009, 29, 1814–1818. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.-W.; Liu, J.; DeVoe, D.L. Droplet formation from hydrodynamically coupled capillaries for parallel microfluidic contact spotting. J. Micromech. Microeng. 2008, 18, 025013. [Google Scholar] [CrossRef]
- Mair, D.A.; Rolandi, M.; Snauko, M.; Noroski, R.; Svec, F.; Fréchet, J.M.J. Room-Temperature Bonding for Plastic High-Pressure Microfluidic Chips. Anal. Chem. 2007, 79, 5097–5102. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.M.D.; Sadri, A.; Young, E.W.K. Liquid phase solvent bonding of plastic microfluidic devices assisted by retention grooves. Lab Chip 2015, 15, 3785–3792. [Google Scholar] [CrossRef] [PubMed]
- Jayadev, R.; Sherwood, D.R. Basement membranes. Curr. Biol. 2017, 27, R207–R211. [Google Scholar] [CrossRef]
- Kastana, P.; Zahra, F.T.; Ntenekou, D.; Katraki-Pavlou, S.; Beis, D.; Lionakis, M.S.; Mikelis, C.M.; Papadimitriou, E. Matrigel Plug Assay for In Vivo Evaluation of Angiogenesis. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1952, pp. 219–232. ISBN 9781493991334. [Google Scholar]
- Smith, J.; Ockleford, C.D. Laser scanning confocal examination and comparison of nidogen (entactin) with laminin in term human amniochorion. Placenta 1994, 15, 95–106. [Google Scholar] [CrossRef]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Microfluidic device fabrication mediated by surface chemical bonding. Analyst 2020, 145, 4096–4110. [Google Scholar] [CrossRef]
- Wade, A.; McKinney, A.; Phillips, J.J. Matrix regulators in neural stem cell functions. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2520–2525. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef]
- Kamei, K.; Mashimo, Y.; Yoshioka, M.; Tokunaga, Y.; Fockenberg, C.; Terada, S.; Koyama, Y.; Nakajima, M.; Shibata-Seki, T.; Liu, L.; et al. Microfluidic-Nanofiber Hybrid Array for Screening of Cellular Microenvironments. Small 2017, 13, 1603104. [Google Scholar] [CrossRef] [PubMed]
- Changede, R.; Cai, H.; Wind, S.J.; Sheetz, M.P. Integrin nanoclusters can bridge thin matrix fibres to form cell–matrix adhesions. Nat. Mater. 2019, 18, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Tachizaki, T.; Sakaguchi, R.; Terada, S.; Kamei, K.; Hirori, H. Driving Intracellular Metal Ions of Human Induced Pluripotent Stem Cells with Intense Terahertz Pulses. Opt. Lett. 2020, 45, 6078–6081. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lim, J.; Park, J.Y.; Lee, S.-H. Concise Review: Stem Cell Microenvironment on a Chip: Current Technologies for Tissue Engineering and Stem Cell Biology. Stem Cells Transl. Med. 2015, 4, 1352–1368. [Google Scholar] [CrossRef]
- Sauer, U.G.; Deferme, L.; Gribaldo, L.; Hackermüller, J.; Tralau, T.; van Ravenzwaay, B.; Yauk, C.; Poole, A.; Tong, W.; Gant, T.W. The challenge of the application of ’omics technologies in chemicals risk assessment: Background and outlook. Regul. Toxicol. Pharmacol. 2017, 91, S14–S26. [Google Scholar] [CrossRef]
- Trujillo-de Santiago, G.; Flores-Garza, B.G.; Tavares-Negrete, J.A.; Lara-Mayorga, I.M.; González-Gamboa, I.; Zhang, Y.S.; Rojas-Martínez, A.; Ortiz-López, R.; Álvarez, M.M. The Tumor-on-Chip: Recent Advances in the Development of Microfluidic Systems to Recapitulate the Physiology of Solid Tumors. Materials 2019, 12, 2945. [Google Scholar] [CrossRef]
- Aref, A.R.; Campisi, M.; Ivanova, E.; Portell, A.; Larios, D.; Piel, B.P.; Mathur, N.; Zhou, C.; Coakley, R.V.; Bartels, A.; et al. 3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade. Lab Chip 2018, 18, 3129–3143. [Google Scholar] [CrossRef]









| Solvent | Cyclohexane | Dichloromethane | Toluene |
|---|---|---|---|
| Temperature | 30 °C | 30 °C | 30 °C |
| Exposure time | 3 min | 1 min 30 s | 4 min 30 s |
| Hot press strength | 3 kN (1200 N/cm2) | 1 kN (400 N/cm2) | 1 kN (400 N/cm2) |
| Hot press time | 5 min | 5 min | 5 min |
| Hot press temp. | 90 °C | 90 °C | 90 °C |
| Fabrication Parameters | COP-V 1 | COP-C 2 | COP-D 2 | COP-T 2 |
|---|---|---|---|---|
| COP microfluidic structure | RC3 | RC2 | RC2 | RC2 |
| Channel height | 320 µm | 290 µm | 290 µm | 290 µm |
| Target height | 250 µm | 250 µm | 250 µm | 250 µm |
| Bonding process | Vacuum ultraviolet (VUV) photobonding | Solvent bonding (cyclohexane) | Solvent bonding (dichloromethane) | Solvent bonding (toluene) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Takahashi, S.; Hatakeyama, K.; Kamei, K.-i. Evaluation of the Effects of Solvents Used in the Fabrication of Microfluidic Devices on Cell Cultures. Micromachines 2021, 12, 550. https://doi.org/10.3390/mi12050550
Wen X, Takahashi S, Hatakeyama K, Kamei K-i. Evaluation of the Effects of Solvents Used in the Fabrication of Microfluidic Devices on Cell Cultures. Micromachines. 2021; 12(5):550. https://doi.org/10.3390/mi12050550
Chicago/Turabian StyleWen, Xiaopeng, Seiichiro Takahashi, Kenji Hatakeyama, and Ken-ichiro Kamei. 2021. "Evaluation of the Effects of Solvents Used in the Fabrication of Microfluidic Devices on Cell Cultures" Micromachines 12, no. 5: 550. https://doi.org/10.3390/mi12050550
APA StyleWen, X., Takahashi, S., Hatakeyama, K., & Kamei, K.-i. (2021). Evaluation of the Effects of Solvents Used in the Fabrication of Microfluidic Devices on Cell Cultures. Micromachines, 12(5), 550. https://doi.org/10.3390/mi12050550







