Rapid Fabrication of Superhydrophobic Virtual Walls for Microfluidic Gas Extraction and Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Hydrophobized Silica Gel
2.3. Preparation of Artificial Saliva
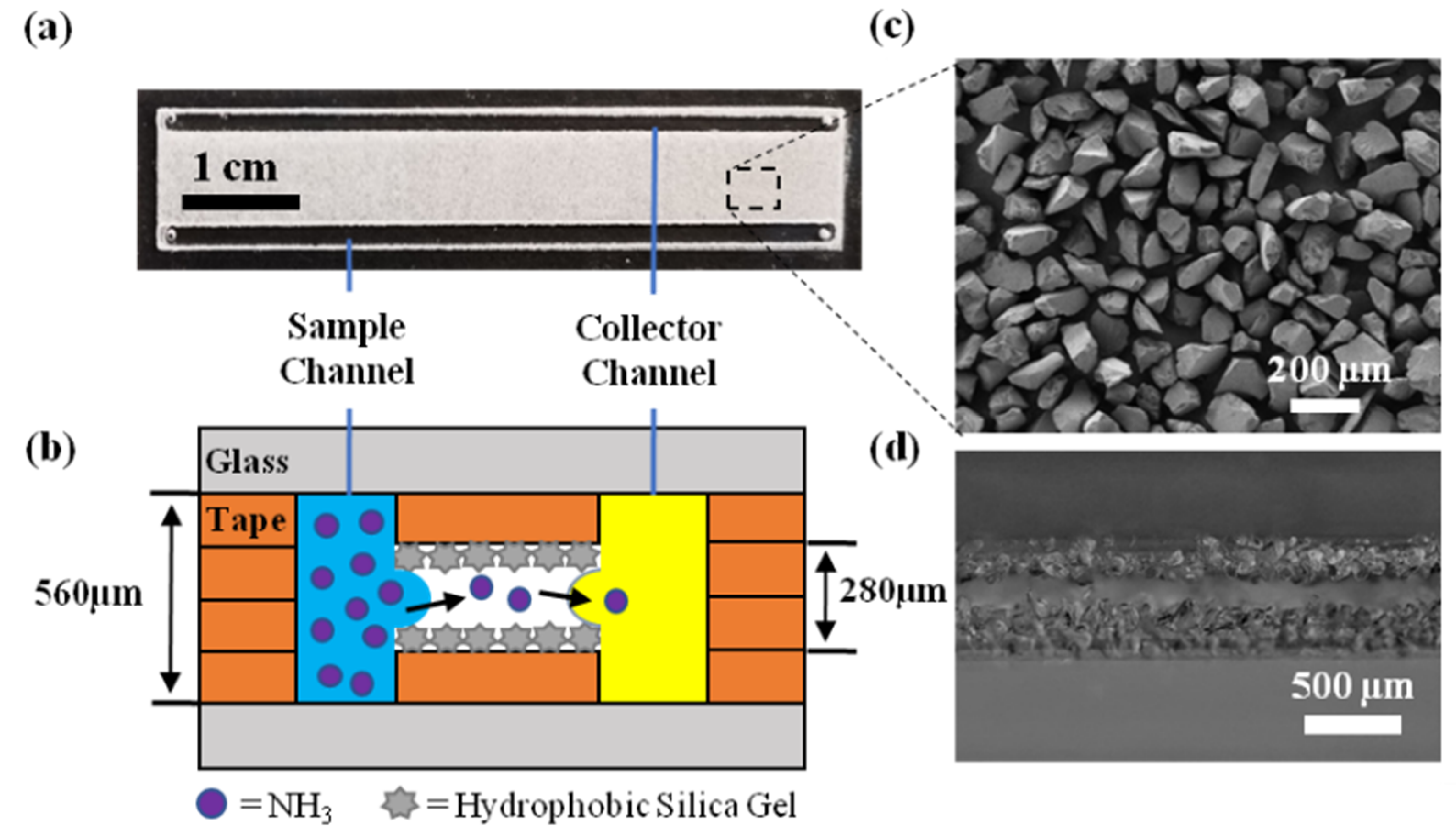
2.4. Chip Design
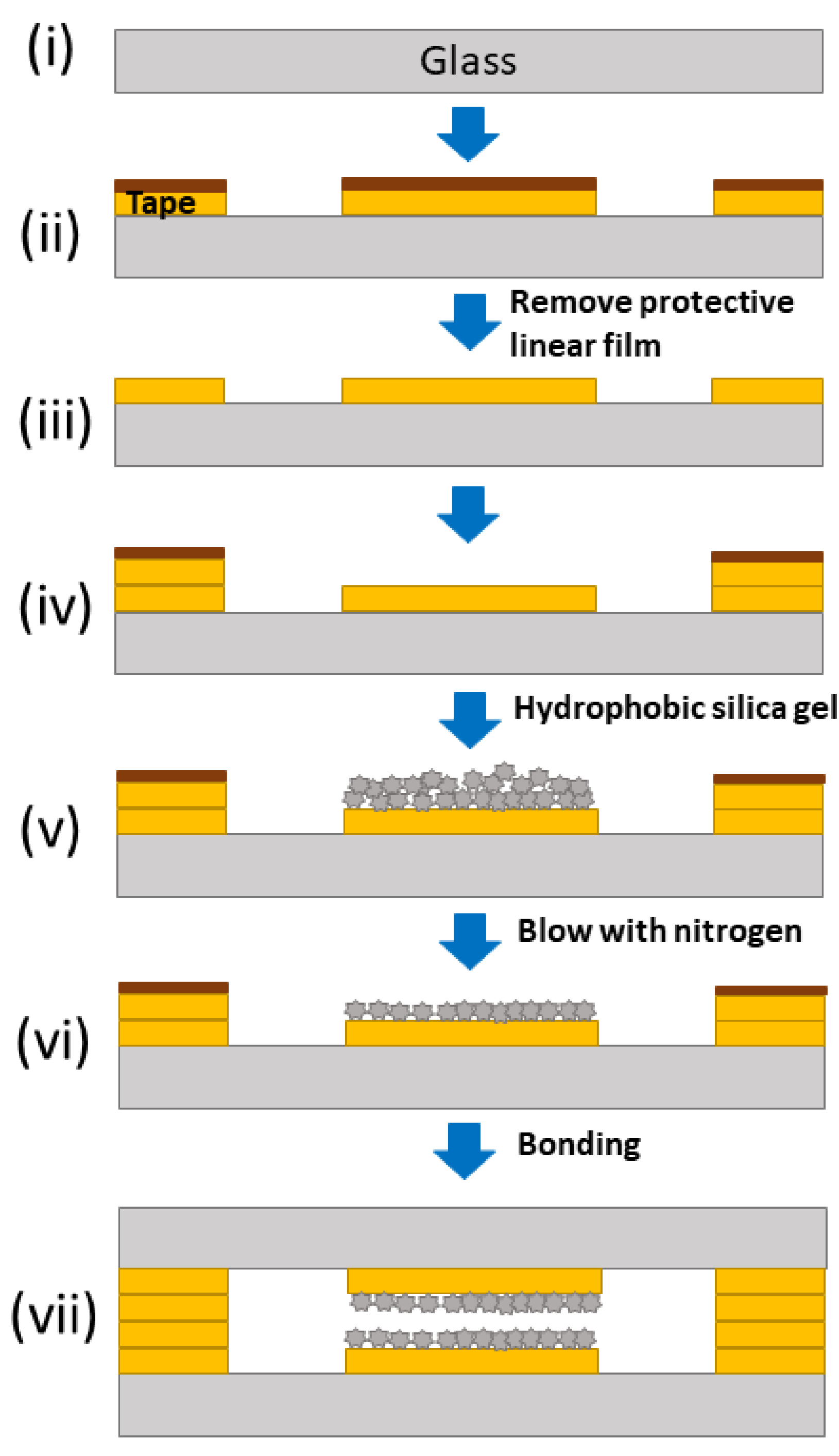
2.5. Chip Fabrication
2.6. Operating Procedure
2.7. Surface Characterization
3. Results and Discussion
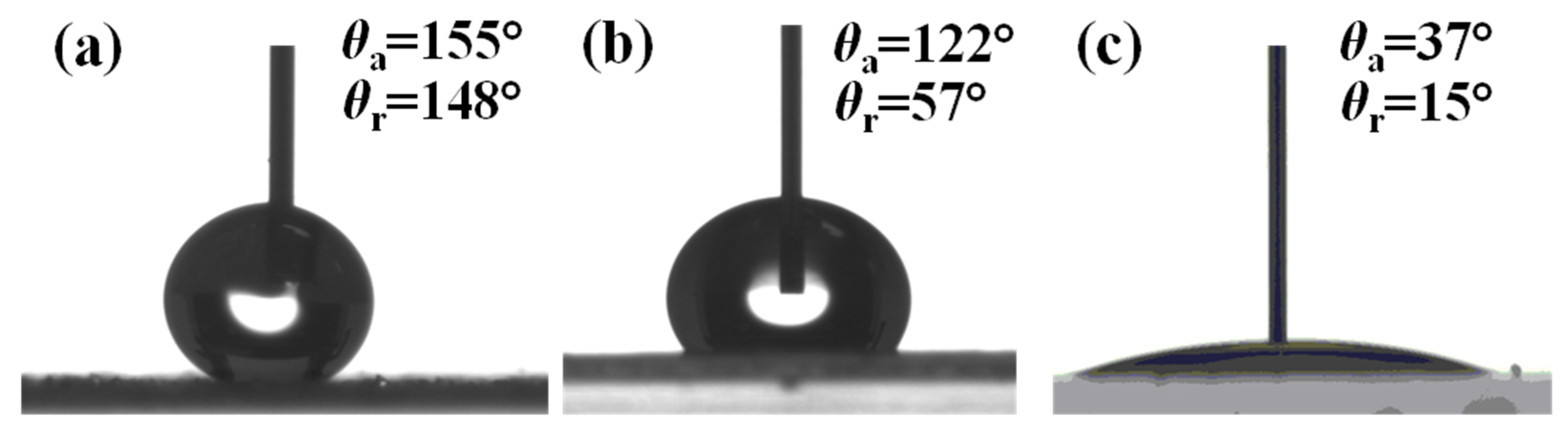
3.1. Surface Characterization
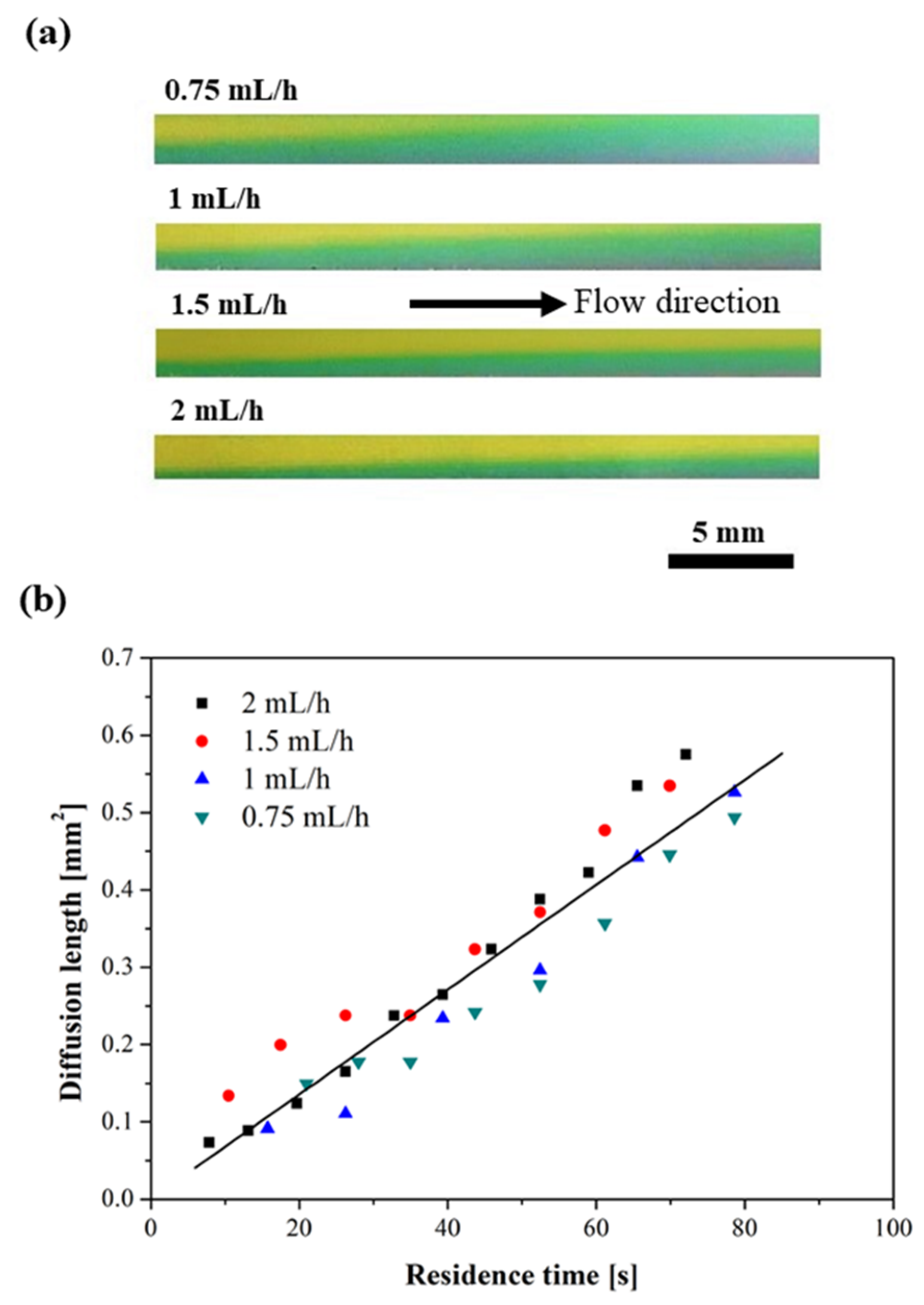
3.2. Flow Stability Consideration
3.3. Extraction of Ammonia
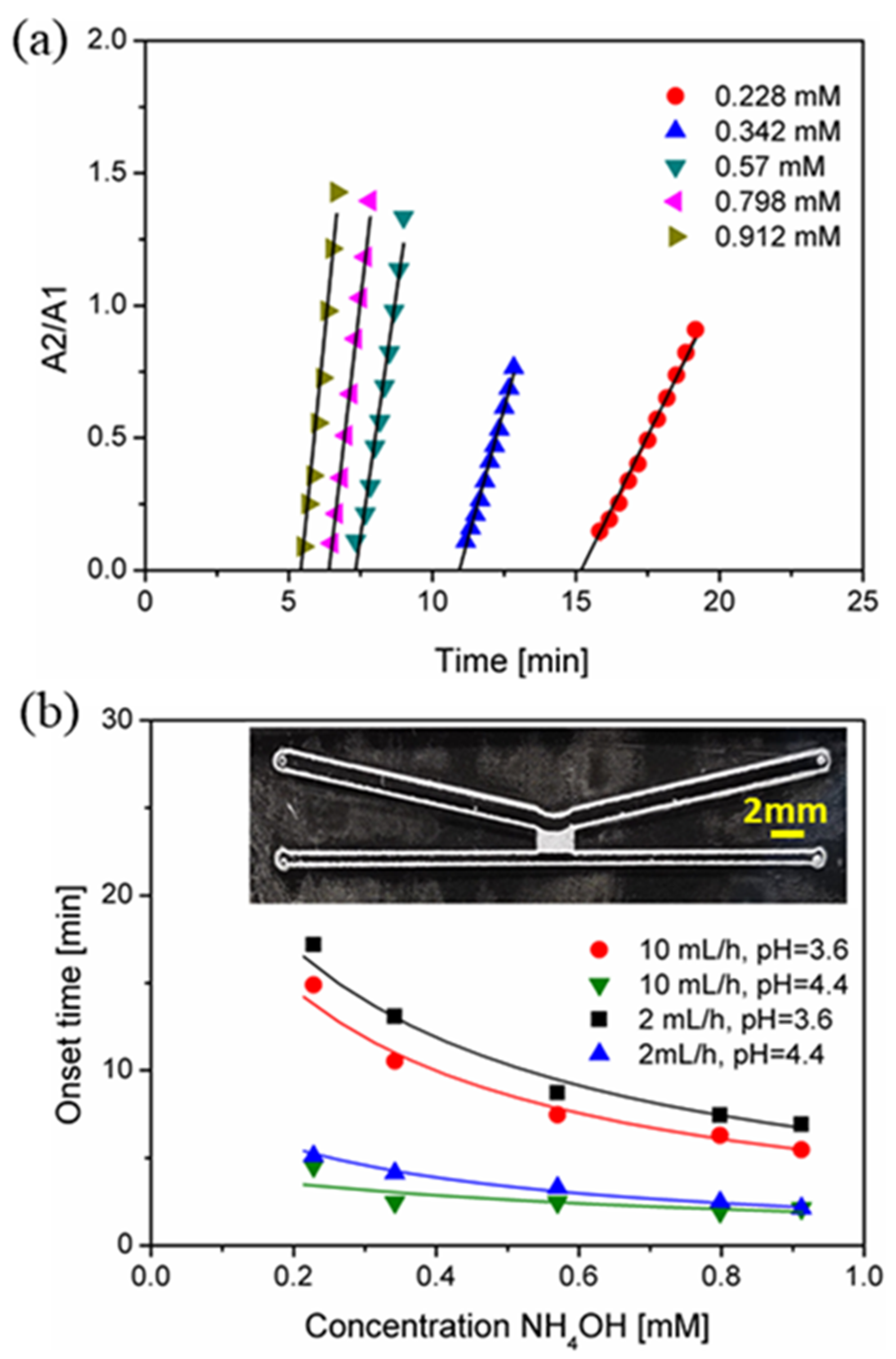
3.4. Concentration-Dependent Sensing on the Chip
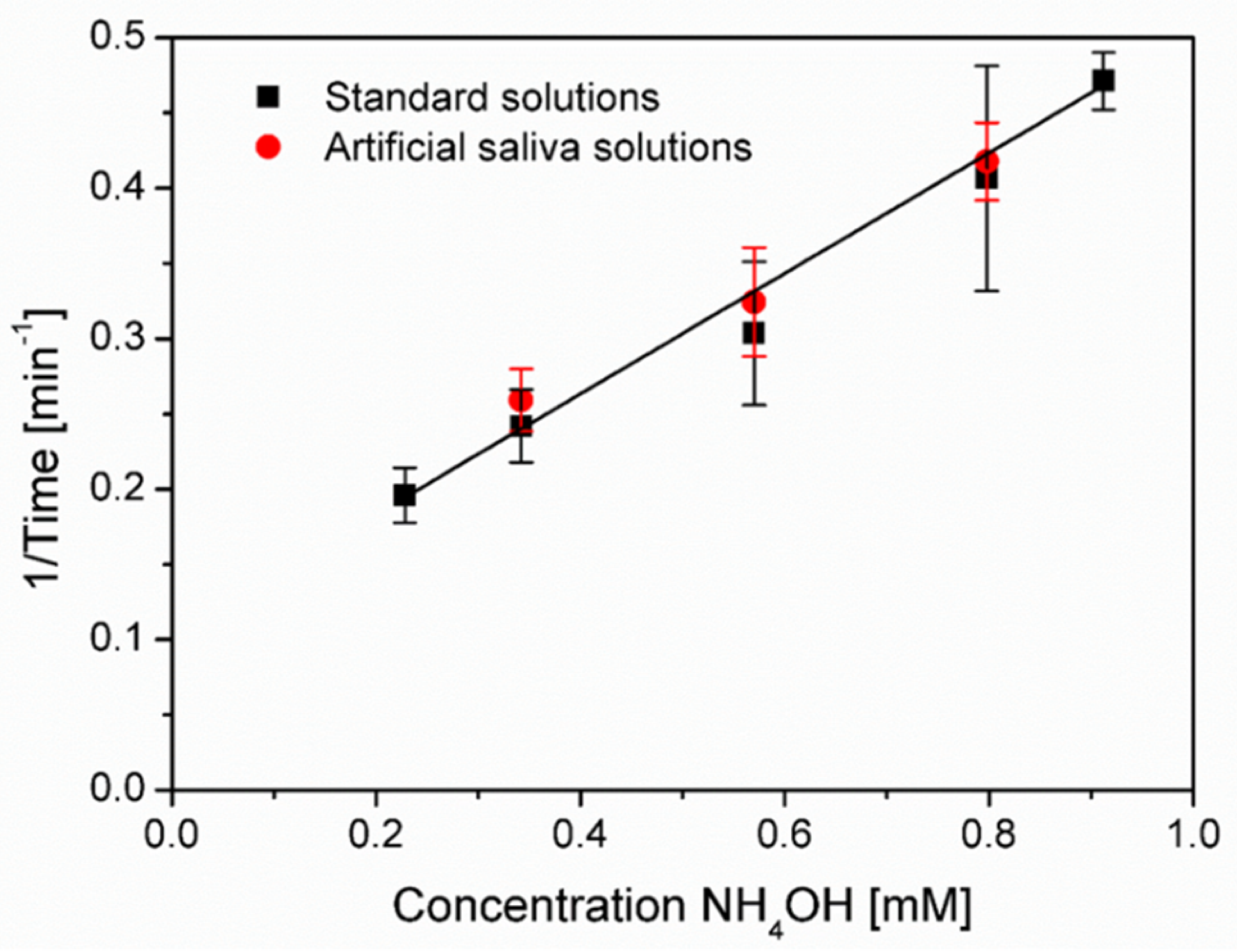
3.5. Ammonia Sensing in Artificial Saliva
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ho, C.K.; Itamura, M.T.; Kelley, M.J.; Hughes, R.C. Review of Chemical Sensors for In-Situ Monitoring of Volatile Contaminants; Sandia Report: Albuquerque, MN, USA, 2001. [Google Scholar]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; von den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Gardner, J.W.; Shin, H.W.; Hines, E.L.; Dow, C.S. An electronic nose system for monitoring the quality of potable water. Sens. Actuators B Chem. 2000, 69, 336–341. [Google Scholar] [CrossRef]
- Dewettinck, T.; van Hege, K.; Verstraete, W. The electronic nose as a rapid sensor for volatile compounds in treated domestic wastewater. Water Res. 2001, 35, 2475–2483. [Google Scholar] [CrossRef]
- Si, P.; Mortensen, J.; Komolov, A.; Denborg, J.; Møller, P.J. Polymer coated quartz crystal microbalance sensors for detection of volatile organic compounds in gas mixtures. Anal. Chim. Acta 2007, 597, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Kim, J.; Miyazaki, Y.; Shiratori, S. Electrospun nanofibrous membranes coated quartz crystal microbalance as gas sensor for NH3 detection. Sens. Actuators B Chem. 2004, 101, 373–380. [Google Scholar] [CrossRef]
- Zhao, B.; Moore, J.S.; Beebe, D.J. Surface-Directed Liquid Flow Inside Microchannels. Science 2001, 291, 1023. [Google Scholar] [CrossRef]
- Phansi, P.; Sumantakul, S.; Wongpakdee, T.; Fukana, N.; Ratanawimarnwong, N.; Sitanurak, J.; Nacapricha, D. Membraneless Gas-Separation Microfluidic Paper-Based Analytical Devices for Direct Quantitation of Volatile and Nonvolatile Compounds. Anal. Chem. 2016, 88, 8749–8756. [Google Scholar] [CrossRef]
- Choengchan, N.; Mantim, T.; Wilairat, P.; Dasgupta, P.K.; Motomizu, S.; Nacapricha, D. A membraneless gas diffusion unit: Design and its application to determination of ethanol in liquors by spectrophotometric flow injection. Anal. Chim. Acta 2006, 579, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Mornane, P.; van den Haak, J.; Cardwell, T.J.; Cattrall, R.W.; Dasgupta, P.K.; Kolev, S.D. Thin layer distillation for matrix isolation in flow analysis. Talanta 2007, 72, 741–746. [Google Scholar] [CrossRef]
- Ratanawimarnwong, N.; Pluangklang, T.; Chysiri, T.; Nacapricha, D. New membraneless vaporization unit coupled with flow systems for analysis of ethanol. Anal. Chim. Acta 2013, 796, 61–67. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Estela, J.M.; Segundo, M.A.; Cerdà, V. A membraneless gas-diffusion unit–multisyringe flow injection spectrophotometric method for ammonium determination in untreated environmental samples. Talanta 2011, 84, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.K.; Souza, P.A.F.; Kamogawa, M.Y.; Reis, B.F.; Rocha, F.R.P. A new strategy for membraneless gas-liquid separation in flow analysis: Determination of dissolved inorganic carbon in natural waters. Microchem. J. 2019, 145, 1218–1223. [Google Scholar] [CrossRef]
- Handique, K.; Gogoi, B.; Burke, D.; Mastrangelo, C.; Burns, M. Microfluidic flow control using selective hydrophobic patterning: SPIE. In Proceedings of the Proceedings Volume 3224, Micromachined Devices and Components III, Austin, TX, USA, 5 September 1997. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Fu, J.-Z.; Gao, Q.; Qiu, J.-J. Developments of 3D Printing Microfluidics and Applications in Chemistry and Biology: A Review. Electroanalysis 2016, 28, 1658–1678. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.-J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef]
- Ni, Y.; Ji, R.; Long, K.; Bu, T.; Chen, K.; Zhuang, S. A review of 3D-printed sensors. Appl. Spectrosc. Rev. 2017, 52, 623–652. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed Microfluidic Devices: Fabrication, Advantages and Limitations-a Mini Review. Anal. Methods. 2016, 8, 6005–6012. [Google Scholar] [CrossRef]
- Lisowski, P.; Zarzycki, P.K. Microfluidic Paper-Based Analytical Devices (μPADs) and Micro Total Analysis Systems (μTAS): Development, Applications and Future Trends. Chromatographia 2013, 76, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Morbioli, G.G.; Mazzu-Nascimento, T.; Stockton, A.M.; Carrilho, E. Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μPADs)-A review. Anal. Chim. Acta 2017, 970, 1–22. [Google Scholar] [CrossRef]
- Webster, M.; Kumar, V.S. Lab on a Stamp: Paper-Based Diagnostic Tools. Clin. Chem. 2012, 58, 956–958. [Google Scholar] [CrossRef][Green Version]
- Charmet, J.; Rodrigues, R.; Yildirim, E.; Challa, P.K.; Roberts, B.; Dallmann, R.; Whulanza, Y. Low-Cost Microfabrication Tool Box. Micromachines 2020, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Patko, D.; Mártonfalvi, Z.; Kovacs, B.; Vonderviszt, F.; Kellermayer, M.; Horvath, R. Microfluidic channels laser-cut in thin double-sided tapes: Cost-effective biocompatible fluidics in minutes from design to final integration with optical biochips. Sens. Actuators B Chem. 2014, 196, 352–356. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, T.; Wang, L.; Liu, Y.; Chen, G.; Zhou, H.; Zhang, M. Tape-Assisted Photolithographic-Free Microfluidic Chip Cell Patterning for Tumor Metastasis Study. Anal. Chem. 2018, 90, 777–784. [Google Scholar] [CrossRef]
- Nath, P.; Fung, D.; Kunde, Y.A.; Zeytun, A.; Branch, B.; Goddard, G. Rapid prototyping of robust and versatile microfluidic components using adhesive transfer tapes. Lab Chip 2010, 10, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Neuville, A.; Renaud, L.; Luu, T.T.; Minde, M.W.; Jettestuen, E.; Vinningland, J.L.; Hiorth, A.; Dysthe, D.K. Xurography for microfluidics on a reactive solid. Lab Chip 2017, 17, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, J.I.; Betancourt, H.A.; García-López, E.; Rodriguez, C.A.; Siller, H.R. Rapid Fabrication of Disposable Micromixing Arrays Using Xurography and Laser Ablation. Micromachines 2017, 8, 144. [Google Scholar] [CrossRef]
- Hizawa, T.; Takano, A.; Parthiban, P.; Doyle, P.S.; Hashimoto, E.I.M. Rapid prototyping of fluoropolymer microchannels by xurography for improved solvent resistance. Biomicrofluidics 2018, 12, 064105. [Google Scholar] [CrossRef]
- Bartholomeusz, D.A.; Boutte, R.W.; Andrade, J.D. Xurography: Rapid prototyping of microstructures using a cutting plotter. J Microelectromech Syst. 2005, 14, 1364–1374. [Google Scholar] [CrossRef]
- Renaud, L.; Selloum, D.; Tingry, S. Xurography for 2D and multi-level glucose/O2 microfluidic biofuel cell. Microfluid Nanofluidics 2015, 18, 1407–1416. [Google Scholar] [CrossRef]
- Cosson, S.; Aeberli, L.G.; Brandenberg, N.; Lutolf, M.P. Ultra-rapid prototyping of flexible, multi-layered microfluidic devices via razor writing. Lab Chip 2015, 15, 72–76. [Google Scholar] [CrossRef]
- Gal, J.-Y.; Fovet, Y.; Adib-Yadzi, M. About a synthetic saliva for in vitro studies. Talanta 2001, 53, 1103–1115. [Google Scholar] [CrossRef]
- Kwon, O.-J.; Tang, S.; Myung, S.-W.; Lu, N.; Choi, H.-S. Surface characteristics of polypropylene film treated by an atmospheric pressure plasma. Surf. Coat. Technol. 2005, 192, 1–10. [Google Scholar] [CrossRef]
- Tao, G.; Gong, A.; Lu, J.; Sue, H.-J.; Bergbreiter, D.E. Surface Functionalized Polypropylene: Synthesis, Characterization, and Adhesion Properties. Macromolecules 2001, 34, 7672–7679. [Google Scholar] [CrossRef]
- Priest, C.; Albrecht, T.W.J.; Sedev, R.; Ralston, J. Asymmetric Wetting Hysteresis on Hydrophobic Microstructured Surfaces. Langmuir 2009, 25, 5655–5660. [Google Scholar] [CrossRef]
- Aligwe, P.A.; Sirkar, K.K.; Canlas, C.J. Hollow fiber gas membrane-based removal and recovery of ammonia from water in three different scales and types of modules. Sep. Purif. Technol. 2019, 224, 580–590. [Google Scholar] [CrossRef]
- Rankin, D.W.H. CRC handbook of chemistry and physics, 89th edition, edited by David R. Lide. Crystallogr. Rev. 2009, 15, 223–224. [Google Scholar] [CrossRef]
- Elmas, S.; Pospisilova, A.; Sekulska, A.A.; Vasilev, V.; Nann, T.; Thornton, S.; Priest, C. Photometric Sensing of Active Chlorine, Total Chlorine, and pH on a Microfluidic Chip for Online Swimming Pool Monitoring. Sensors 2020, 20, 3099. [Google Scholar] [CrossRef]
- Zilberman, Y.; Sonkusale, S.R. Microfluidic optoelectronic sensor for salivary diagnostics of stomach cancer. Biosens. Bioelectron. 2015, 67, 465–471. [Google Scholar] [CrossRef]
| Flow Rate, mL/h | Liquid Pressure, [Pa] | Resistant Pressure, [Pa] | ||
|---|---|---|---|---|
| Capillary Pressure | ||||
| 0.75 | 19 | 196 | 215 | 470 |
| 1 | 26 | 196 | 221 | 470 |
| 1.5 | 38 | 196 | 234 | 470 |
| 2 | 51 | 196 | 247 | 470 |
| 10 | 256 | 196 | 452 | 470 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, W.; Yang, D.; Priest, C. Rapid Fabrication of Superhydrophobic Virtual Walls for Microfluidic Gas Extraction and Sensing. Micromachines 2021, 12, 514. https://doi.org/10.3390/mi12050514
Raj W, Yang D, Priest C. Rapid Fabrication of Superhydrophobic Virtual Walls for Microfluidic Gas Extraction and Sensing. Micromachines. 2021; 12(5):514. https://doi.org/10.3390/mi12050514
Chicago/Turabian StyleRaj, Wojciech, Daisy Yang, and Craig Priest. 2021. "Rapid Fabrication of Superhydrophobic Virtual Walls for Microfluidic Gas Extraction and Sensing" Micromachines 12, no. 5: 514. https://doi.org/10.3390/mi12050514
APA StyleRaj, W., Yang, D., & Priest, C. (2021). Rapid Fabrication of Superhydrophobic Virtual Walls for Microfluidic Gas Extraction and Sensing. Micromachines, 12(5), 514. https://doi.org/10.3390/mi12050514







