First-Principles Studies for Electronic Structure and Optical Properties of Strontium Doped β-Ga2O3
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Properties
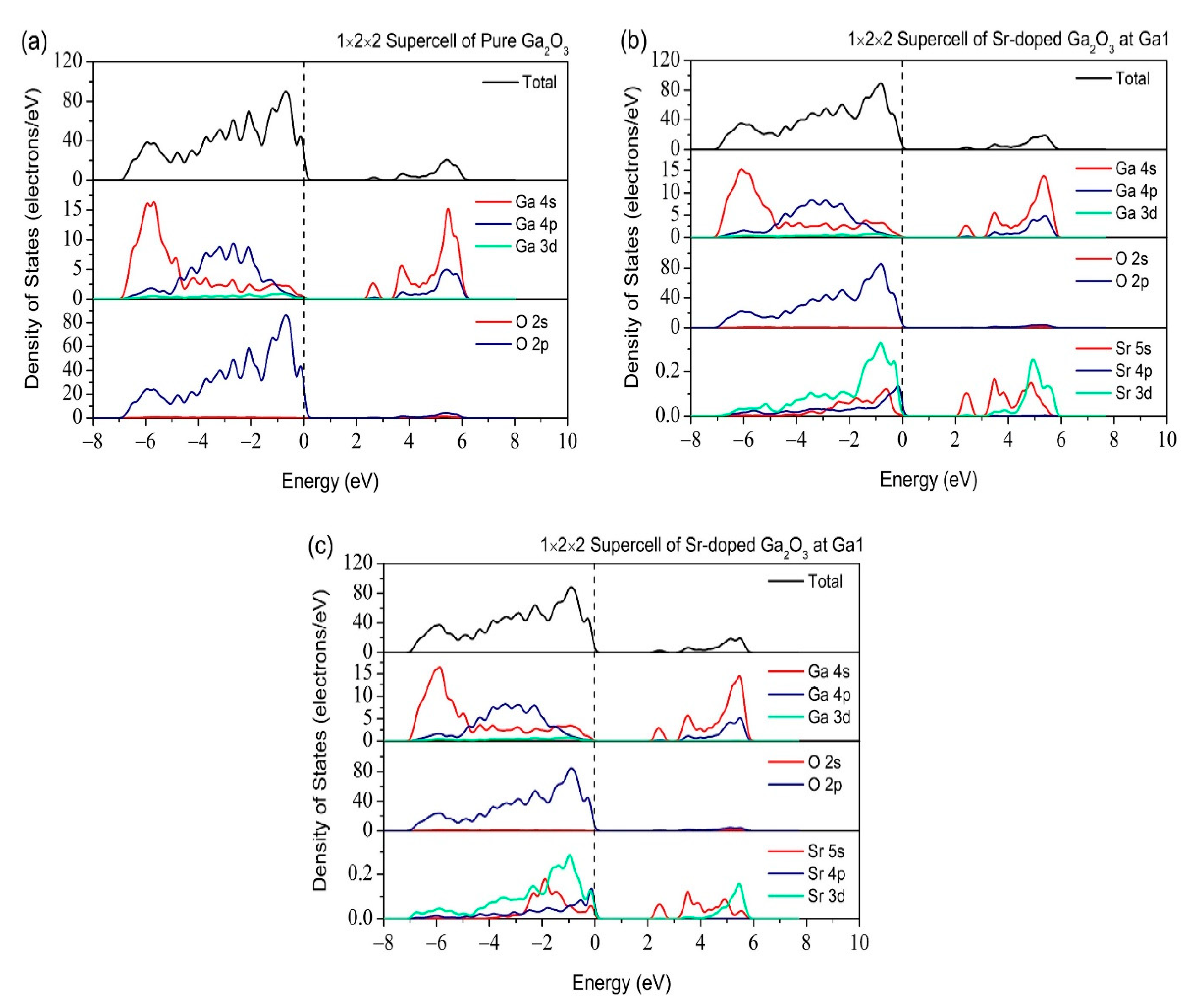
3.2. Properties of Electronic Structure
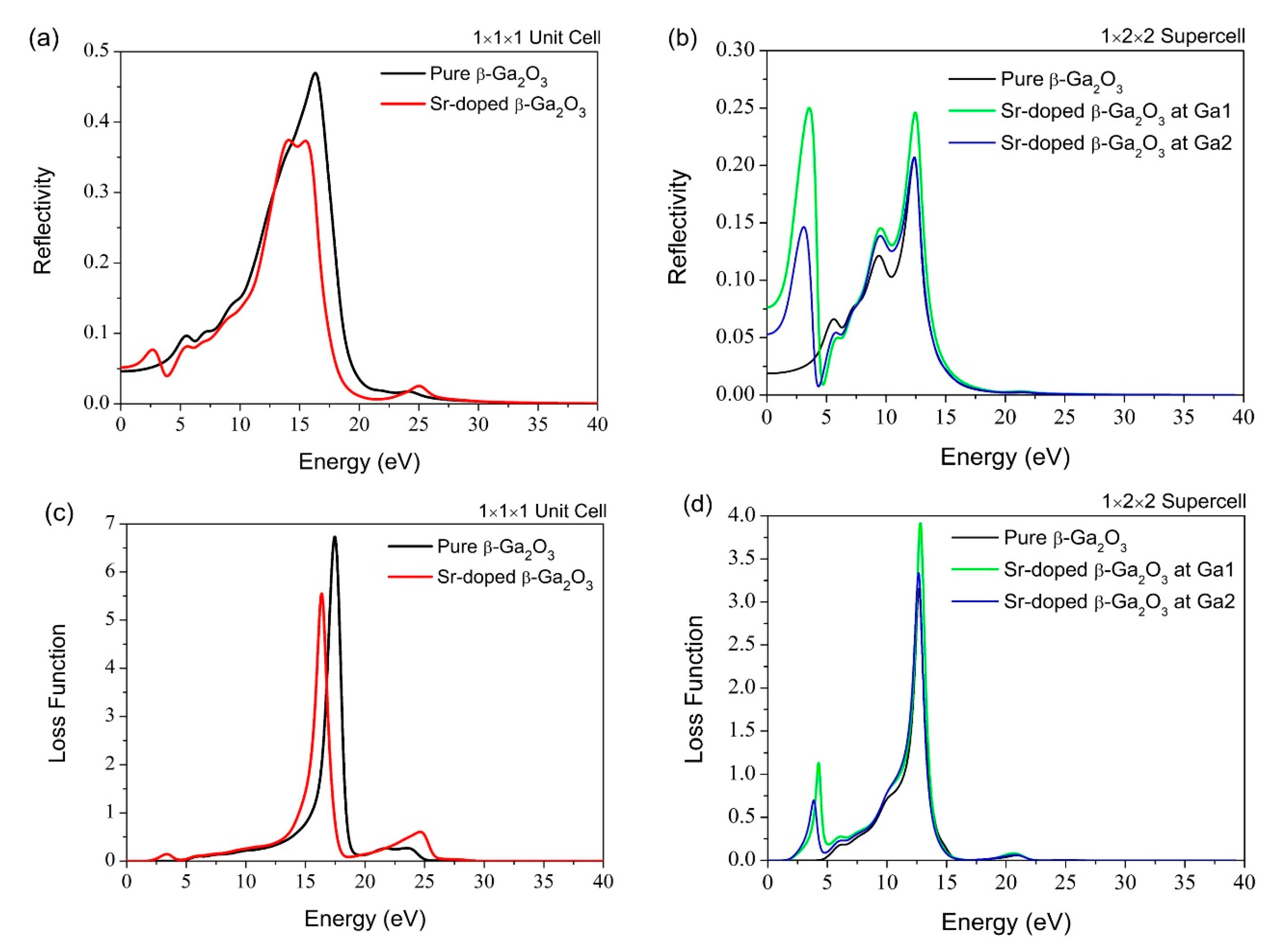
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roy, R.; Hill, V.G.; Osborn, E.F. Polymorphism of Ga2O3 and the system Ga2O3—H2O. J. Am. Chem. Soc. 1952, 74, 719–722. [Google Scholar] [CrossRef]
- Stepanov, S.I.; Nikolaev, V.I.; Bougrov, V.E.; Romanov, A.E. Gallium oxide: Properties and applications—A review. Rev. Adv. Mater. Sci. 2016, 44, 63–86. [Google Scholar]
- Bakhtatou, A.; Ersan, F. Effects of the number of layers on the vibrational, electronic and optical properties of alpha lead oxide. Phys. Chem. Chem. Phys. 2019, 21, 3868–3876. [Google Scholar] [CrossRef]
- Ersan, F.; Sarıkurt, S.; Sarikurt, S. Monitoring the electronic, thermal and optical properties of two-dimensional MoO2 under strain via vibrational spectroscopies: A first-principles investigation. Phys. Chem. Chem. Phys. 2019, 21, 19904–19914. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Edwards, T.E.J.; Della Ventura, N.M.; Casari, D.; Huszár, E.; Fu, L.; Zhou, L.; Maeder, X.; Schwiedrzik, J.J.; Utke, I.; et al. Synthesis of model Al-Al2O3 multilayer systems with monolayer oxide thickness control by circumventing native oxidation. Thin Solid Films 2020, 711, 138287. [Google Scholar] [CrossRef]
- Zywotko, D.R.; Zandi, O.; Faguet, J.; Abel, P.R.; George, S.M. ZrO2 monolayer as a removable etch stop layer for thermal Al2O3 atomic layer etching using hydrogen fluoride and trimethylaluminum. Chem. Mater. 2020. [Google Scholar] [CrossRef]
- Galazka, Z. β-Ga2O3 for wide-bandgap electronics and optoelectronics. Semicond. Sci. Technol. 2018, 33, 113001. [Google Scholar] [CrossRef]
- Ghadi, H.; McGlone, J.F.; Feng, Z.; Bhuiyan, A.F.M.A.U.; Zhao, H.; Arehart, A.R.; Ringel, S.A. Influence of growth temperature on defect states throughout the bandgap of MOCVD-grown β-Ga2O3. Appl. Phys. Lett. 2020, 117. [Google Scholar] [CrossRef]
- Lee, I.; Kumar, A.; Zeng, K.; Singisetti, U.; Yao, X. Modeling and Power Loss Evaluation of Ultra Wide Band Gap Ga2O3 Device for High Power Applications. In Proceedings of the 2017 IEEE Energy Conversion Congress and Exposition (ECCE 2017), Cincinnati, OH, USA, 1–5 October 2017; pp. 4377–4382. [Google Scholar] [CrossRef]
- Green, A.J.; Chabak, K.D.; Heller, E.; Mccandless, J.P.; Moser, N.A.; Fitch, R.C.; Walker, D.E.; Tetlak, S.E.; Crespo, A.; Leedy, K.D.; et al. Recent progress of β-Ga2O3 MOSFETs for power electronic applications. Appl. Phys. Lett. 2016, 109, 391–394. [Google Scholar]
- Pearton, S.J.; Yang, J.; Iv, P.H.C.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5. [Google Scholar] [CrossRef]
- Li, P.; Shi, H.; Chen, K.; Guo, D.; Cui, W.; Zhi, Y.; Wang, S.; Wu, Z.; Chen, Z.; Tang, W. Construction of GaN/Ga2O3 p–n junction for an extremely high responsivity self-powered UV photodetector. J. Mater. Chem. C 2017, 5, 10562–10570. [Google Scholar] [CrossRef]
- Jinno, R.; Uchida, T.; Kaneko, K.; Fujita, S. Control of crystal structure of Ga2O3 on sapphire substrate by introduction of α-(AlxGa1−x)2O3 buffer layer. Phys. Status Solidi Basic Res. 2018, 255, 3–7. [Google Scholar] [CrossRef]
- Wheeler, V.D.; Nepal, N.; Boris, D.R.; Qadri, S.B.; Nyakiti, L.O.; Lang, A.; Koehler, A.; Foster, G.; Walton, S.G.; Eddy, C.R.; et al. Phase control of crystalline Ga2O3 films by plasma-enhanced atomic layer deposition. Chem. Mater. 2020, 32, 1140–1152. [Google Scholar] [CrossRef]
- Lorenz, M.; Woods, J.; Gambino, R. Gambino, Some electrical properties of the semiconductor β-Ga2O3. J. Phys. Chem. Solids 1967, 28, 403–404. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, M.; Chen, C.; Zhu, Z.; Zheng, X.; Chen, Z.; Guo, Y.; Liu, C.; Yan, Y.; Fang, G. Efficient and stable nonfullerene-graded heterojunction inverted perovskite solar cells with inorganic Ga2O3 tunneling protective nanolayer. Adv. Funct. Mater. 2018, 28, 1–11. [Google Scholar] [CrossRef]
- Wurdack, M.; Yun, T.; Estrecho, E.; Syed, N.; Bhattacharyya, S.; Pieczarka, M.; Zavabeti, A.; Chen, S.; Haas, B.; Müller, J.; et al. Ultrathin Ga2O3 glass: A large-scale passivation and protection material for monolayer WS 2. Adv. Mater. 2020, 1–6. [Google Scholar] [CrossRef]
- Choi, Y.H.; Baik, K.H.; Kim, S.; Kim, J. Photoelectrochemical etching of ultra-wide bandgap β-Ga2O3 semiconductor in phosphoric acid and its optoelectronic device application. Appl. Surf. Sci. 2021, 539, 148130. [Google Scholar] [CrossRef]
- Afzal, A. β-Ga2O3 nanowires and thin films for metal oxide semiconductor gas sensors: Sensing mechanisms and performance enhancement strategies. J. Mater. 2019, 5, 542–557. [Google Scholar] [CrossRef]
- Ayhan, M.E.; Shinde, M.; Todankar, B.; Desai, P.; Ranade, A.K.; Tanemura, M.; Kalita, G. Ultraviolet radiation-induced photovoltaic action in γ-CuI/β-Ga2O3 heterojunction. Mater. Lett. 2020, 262, 127074. [Google Scholar] [CrossRef]
- Negi, G.; Goswami-Giri, A.S. Potentiometric graphite coated electrode based on ditertbutyl-cyclohexano18crown6 for detection of strontium (II) ions. Anal. Chem. Lett. 2018, 8, 25–34. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Nervi, C.; Chierotti, M.R.; Rubini, K.; Gobetto, R.; Bigi, A. Strontium and zinc substitution in β-tricalcium phosphate: An X-ray diffraction, solid state NMR and ATR-FTIR study. J. Funct. Biomater. 2019, 10, 20. [Google Scholar] [CrossRef]
- Sasa, K.; Honda, M.; Hosoya, S.; Takahashi, T.; Takano, K.; Ochiai, Y.; Sakaguchi, A.; Kurita, S.; Satou, Y.; Sueki, K. A sensitive method for Sr-90 analysis by accelerator mass spectrometry. J. Nucl. Sci. Technol. 2021, 58, 72–79. [Google Scholar] [CrossRef]
- Bikit, K.; Knezevic, J.; Mrdja, D.; Todorovic, N.; Kuzmanovic, P.; Forkapic, S.; Nikolov, J.; Bikit, I. Application of 90Sr for industrial purposes and dose assessment. Radiat. Phys. Chem. 2021, 179, 109260. [Google Scholar] [CrossRef]
- Turkington, G.; Gamage, K.A.A.; Graham, J. Detection of Strontium-90, a Review and the Potential for Direct In Situ Detection. In Proceedings of the 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Sydney, Australia, 10–17 November 2018. [Google Scholar] [CrossRef]
- Semenishchev, V.S.; Voronina, A.V. Isotopes of strontium: Properties and applications. Handb. Environ. Chem. 2020, 88, 25–42. [Google Scholar] [CrossRef]
- Jin, Y.; Lei, Z.; Tong, X.; Zhilin, Z.; Shuo, L.; Wenjie, T. Research Progress on Recycling Technology of Lead-containing Glass in Waste Cathode Ray Tubes. In Proceedings of the IOP Conference Series: Earth Environmental Science, Bucharest, Romania, 16–18 October 2020; Volume 446, p. 5. [Google Scholar] [CrossRef]
- Cabrera, M.; Pérez, P.; Rosales, J.; Agrela, F. Feasible use of cathode ray tube glass (CRT) and recycled aggregates as unbound and cement-treated granular materials for road sub-bases. Materials 2020, 13, 748. [Google Scholar] [CrossRef]
- Iasir, A.R.M.; Hammond, K.D. Pseudopotential for plane-wave density functional theory studies of metallic uranium. Comput. Mater. Sci. 2020, 171, 109221. [Google Scholar] [CrossRef]
- Torres, E.; Kaloni, T. Projector augmented-wave pseudopotentials for uranium-based compounds. Comput. Mater. Sci. 2020, 171, 109237. [Google Scholar] [CrossRef]
- Mondal, A.; Mohamed, M.; Ping, L.; Taib, M.M.; Samat, M.; Haniff, M.M.; Bahru, R. First-principles studies for electronic structure and optical properties of p-type calcium doped α-Ga2O3. Materials 2021, 14, 604. [Google Scholar] [CrossRef]
- Guo, R.; Su, J.; Yuan, H.; Zhang, P.; Lin, Z.; Zhang, J.; Chang, J.; Hao, Y. Surface functionalization modulates the structural and optoelectronic properties of two-dimensional Ga2O3. Mater. Today Phys. 2020, 12. [Google Scholar] [CrossRef]
- Cohen, A.J.; Mori-Sánchez, P.; Yang, W. Challenges for density functional theory. Chem. Rev. 2012, 112, 289–320. [Google Scholar] [CrossRef]
- Roehrens, D.; Brendt, J.; Samuelis, D.; Martin, M. On the ammonolysis of Ga2O3: An XRD, neutron diffraction and XAS investigation of the oxygen-rich part of the system Ga2O3–GaN. J. Solid State Chem. 2010, 183, 532–541. [Google Scholar] [CrossRef][Green Version]
- Qian, Y.; Guo, D.; Chu, X.; Shi, H.; Zhu, W.; Wang, K.; Huang, X.; Wang, H.; Wang, S.; Li, P.; et al. Mg-doped p-type β-Ga2O3 thin film for solar-blind ultraviolet photodetector. Mater. Lett. 2017, 209, 558–561. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, J.; Yang, Y.; Pan, D.; Xing, Y.; Shi, X.; Xia, X.; Liang, H. Catalytic growth and characterization of single crystalline Zn doped p-type β-Ga2O3 nanowires. J. Alloy. Compd. 2016, 687, 964–968. [Google Scholar] [CrossRef]
- Mykhaylyk, V.B.; Kraus, H.; Kapustianyk, V.; Rudko, M. Low temperature scintillation properties of Ga2O3. Appl. Phys. Lett. 2019, 115, 081103. [Google Scholar] [CrossRef]
- Liao, C.-H.; Li, K.-H.; Torres-Castanedo, C.G.; Zhang, G.; Li, X. Wide range tunable bandgap and composition β-phase (AlGa)2O3 thin film by thermal annealing. Appl. Phys. Lett. 2021, 118, 032103. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, F.; Liang, X.; Gong, H.; Liu, Q.; Li, L.; Qin, X.; Huang, X.; Huang, C. Electronic transport properties in metal doped beta-Ga2O3: A first principles study. Phys. B Condens. Matter 2019, 562, 124–130. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, L.; Tang, X.; Hu, J.; Sun, J.; Zhang, Y.; Zhang, Y.; Jia, R. Progress of ultra-wide bandgap Ga2O3 semiconductor materials in power MOSFETs. IEEE Trans. Power Electron. 2020, 35, 5157–5179. [Google Scholar] [CrossRef]
- Mohamed, M.; Unger, I.; Janowitz, C.; Manzke, R.; Galazka, Z.; Uecker, R.; Fornari, R. The surface band structure of β-Ga2O3. J. Phys. Conf. Ser. 2011, 286. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Zhao, G.; Xie, W. First-principles study on electronic structure and optical properties of Sn-doped β-Ga2O3. Phys. B Condens. Matter 2010, 405, 3899–3903. [Google Scholar] [CrossRef]
- Yan, J.; Qu, C.; Jinliang, Y.; Chong, Q. Electronic structure and optical properties of F-dopedβ-Ga2O3 from first principles cal culations. J. Semicond. 2016, 37. [Google Scholar] [CrossRef]
- Vincenzo, F. Dielectric scaling of the self-energy scissor operator in semiconductors and insulators. Phys. Rev. B 1995, 51, 196–198. [Google Scholar]
- Godby, R.W.; Schlüter, M.; Sham, L.J. Self-energy operators and exchange-correlation potentials in semiconductors. Phys. Rev. B 1988, 37, 10159–10175. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Xia, C.; Pei, G.; Deng, Q.; Yang, Z.; Xu, W.; Shi, H.; Wu, F.; Wu, Y.; et al. Growth and spectral characterization of β-Ga2O3 single crystals. J. Phys. Chem. Solids 2006, 67, 2448–2451. [Google Scholar] [CrossRef]
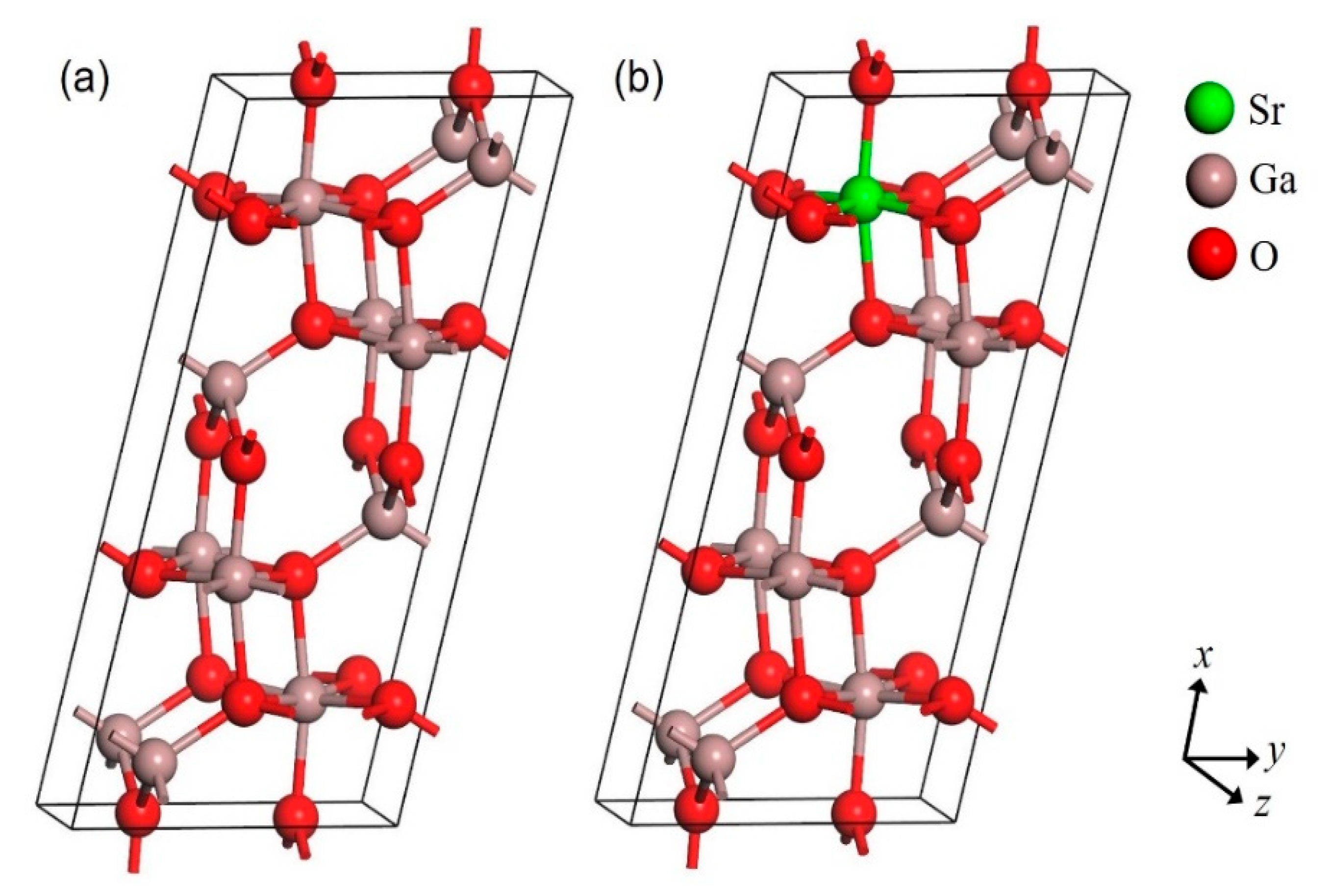
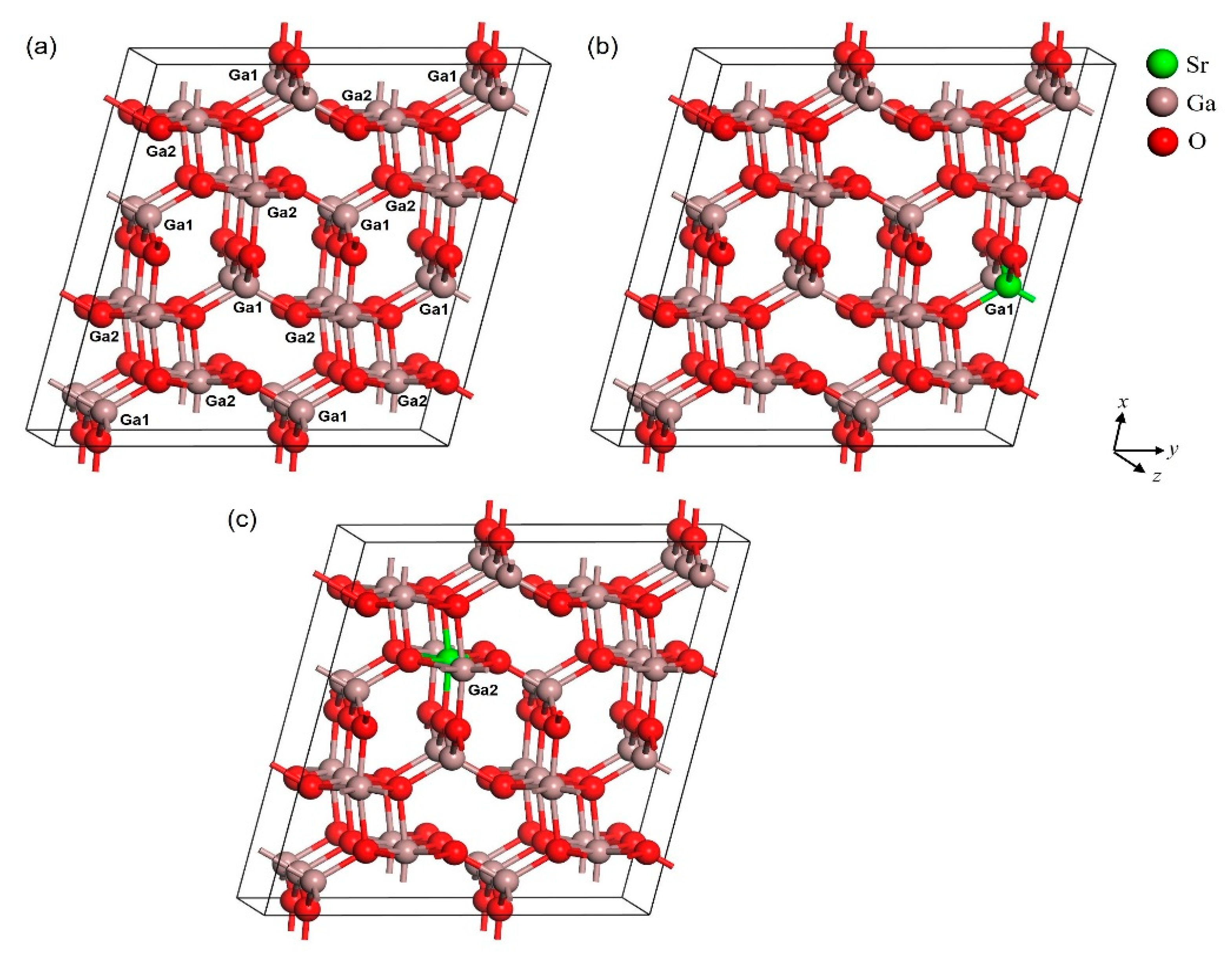
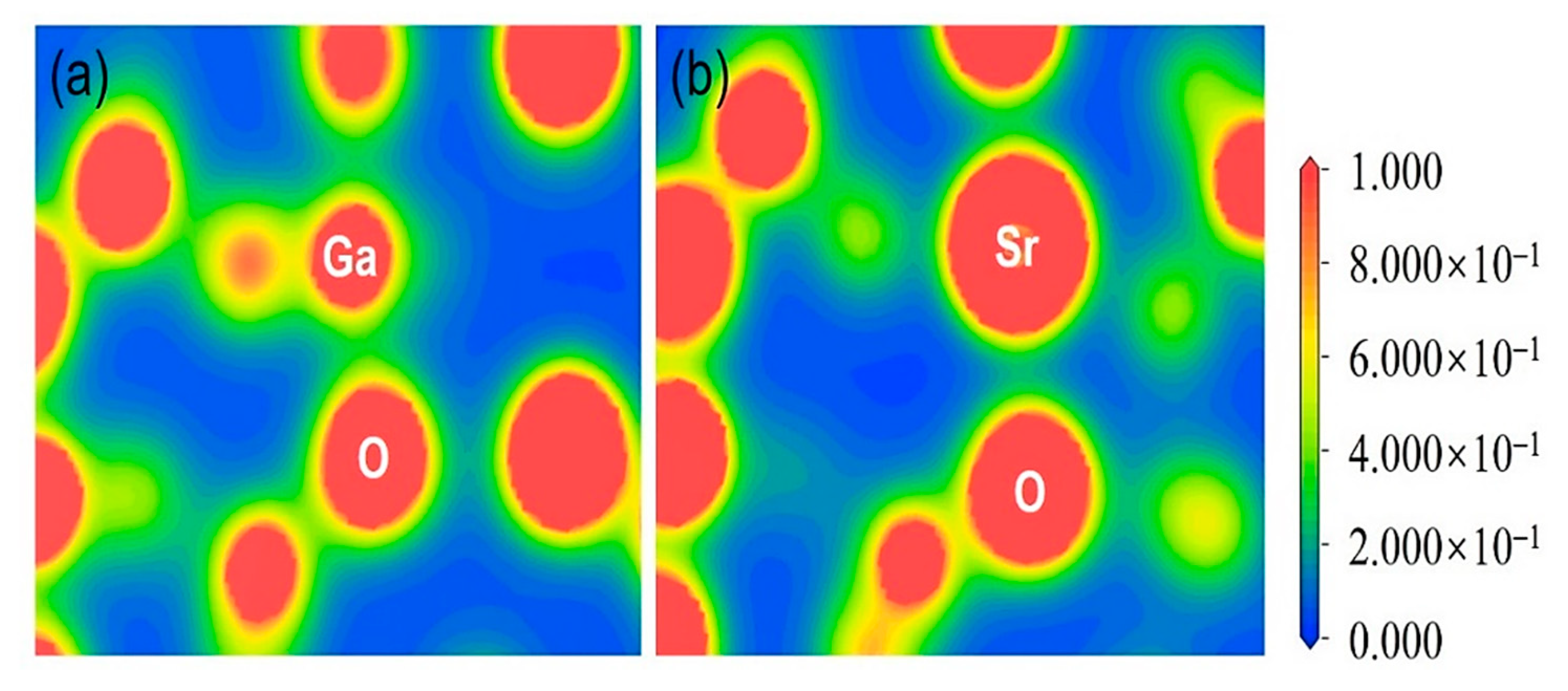
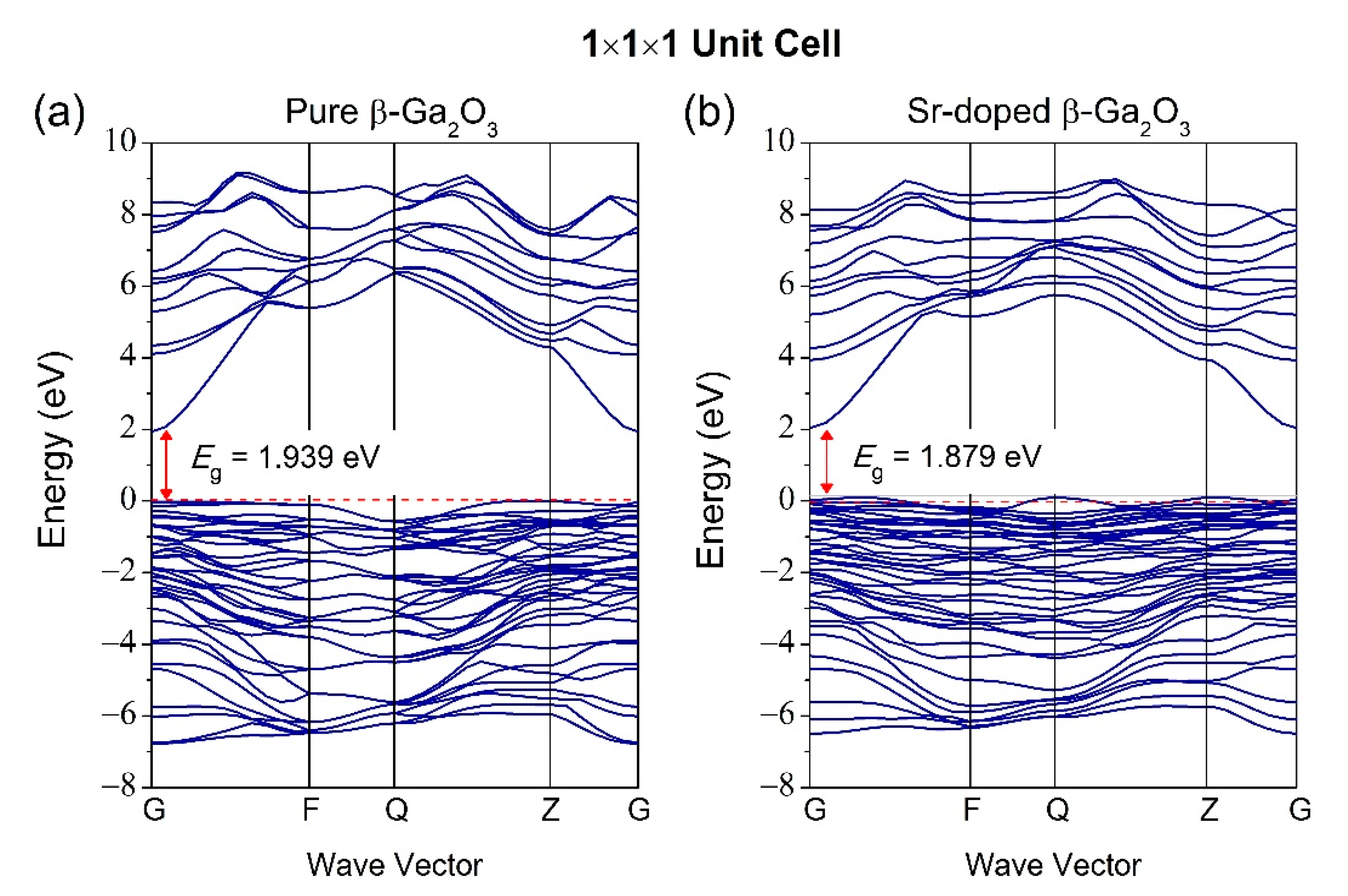
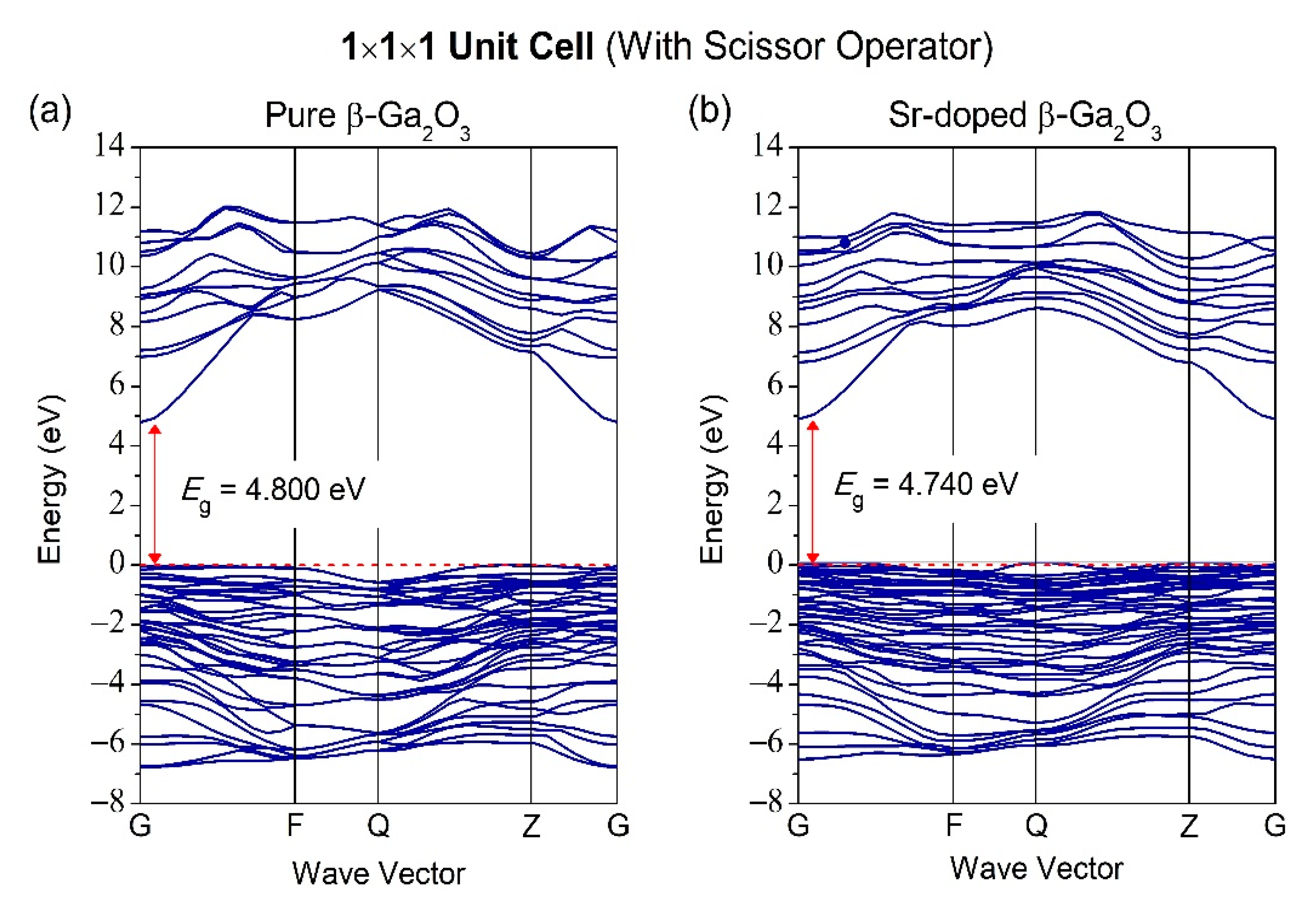
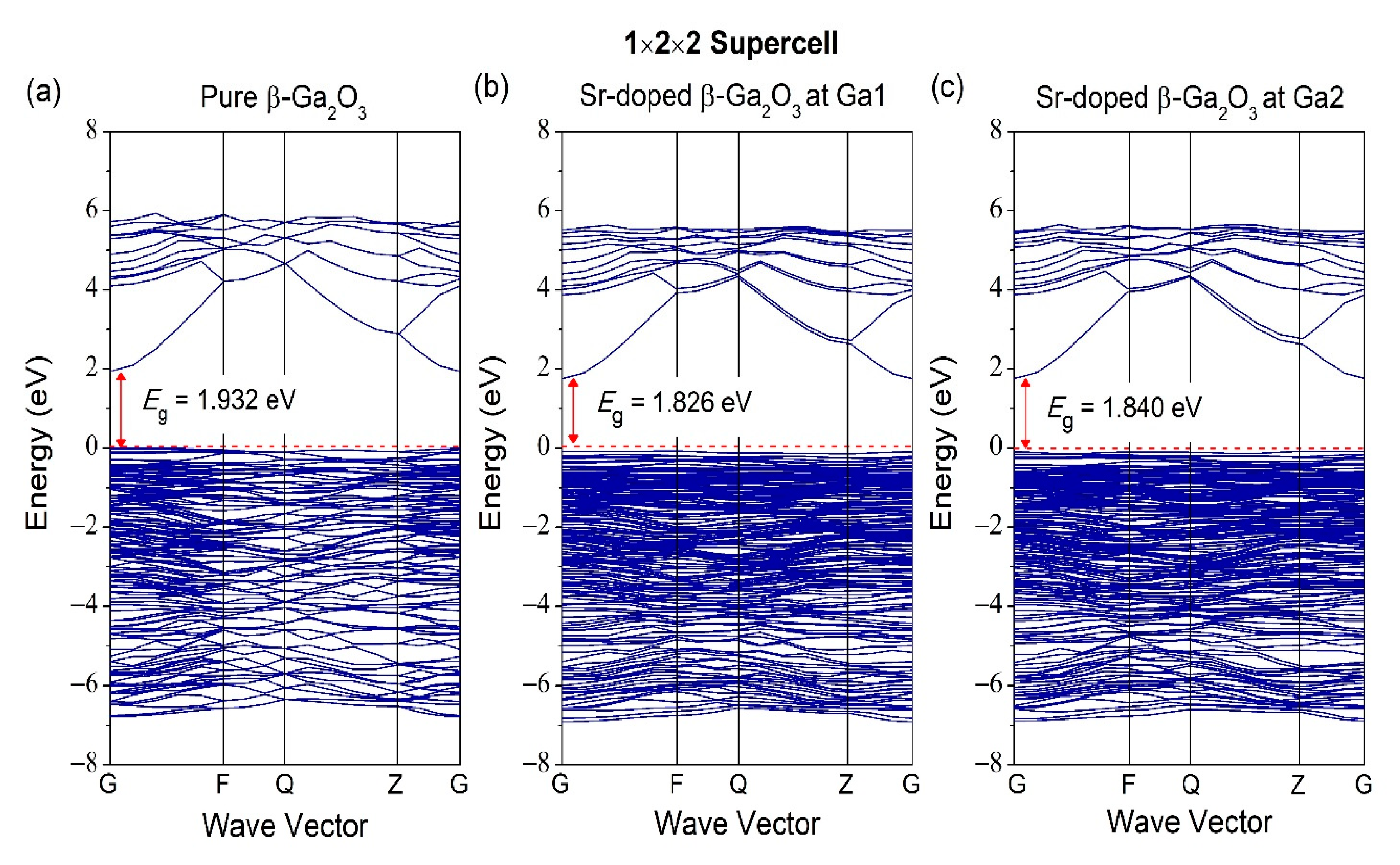
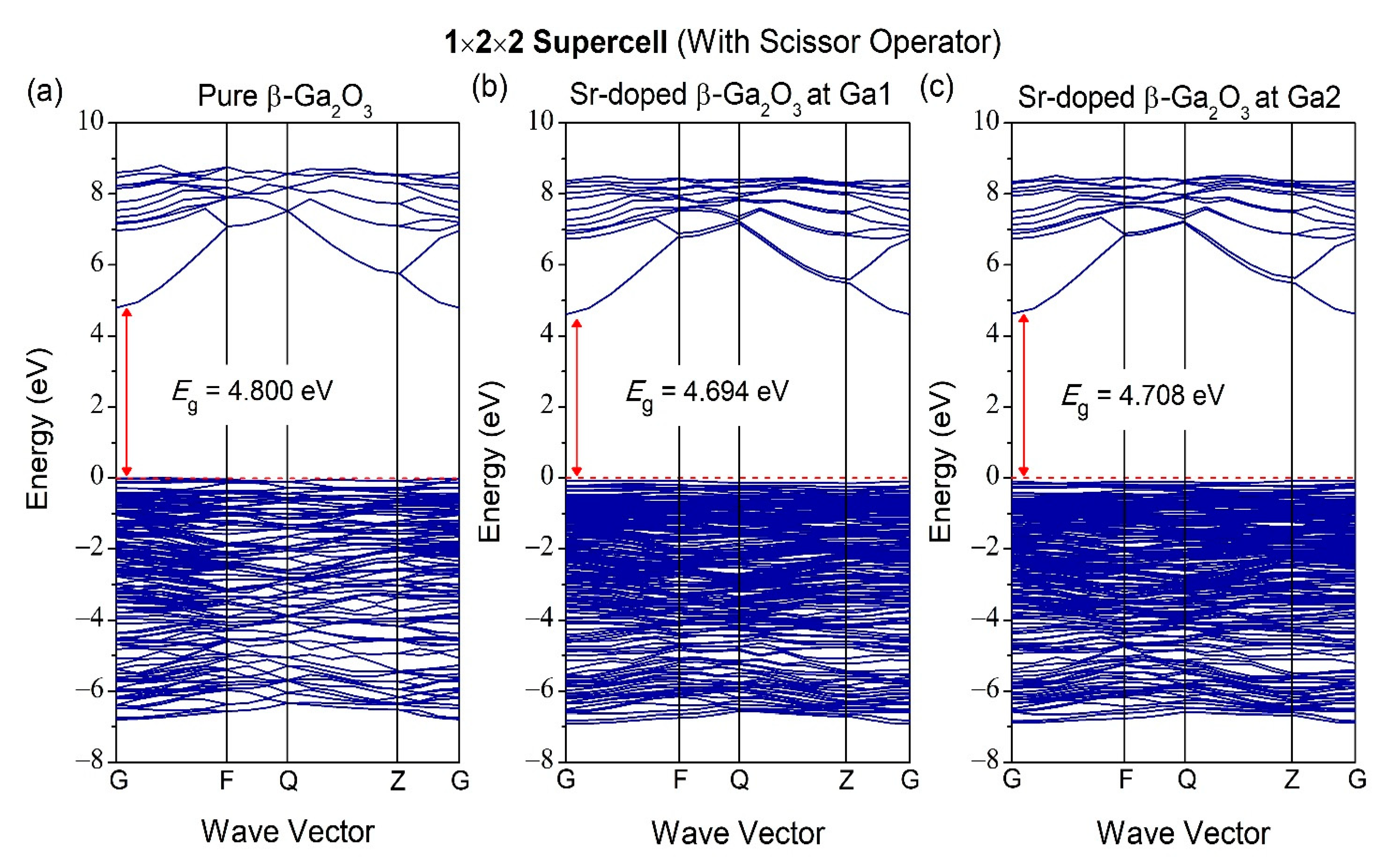



| Structures | Parameters | GGA-PBE | Experiment [37] |
|---|---|---|---|
| β-Ga2O3 | a (Å) | 12.494 (+2.29%) | 12.214 |
| b (Å) | 3.096 (+1.94%) | 3.037 | |
| c (Å) | 5.898 (+1.72%) | 5.798 | |
| Cell Volume (Å3) | 221.647 (+6.14%) | 208.835 | |
| Cell Angle (°) | α = 90° β = 103.705° γ = 90° | α = 90° β = 103.830° γ = 90° | |
| Sr-doped β-Ga2O3 | a (Å) | 12.506 (+0.10%) | - |
| b (Å) | 3.158 (+2.00%) | - | |
| c (Å) | 5.794 (–1.76%) | - | |
| Cell Volume (Å3) | 222.317 (+0.30%) | - | |
| Cell Angle (°) | α = 90° | - | |
| β = 103.701° | - | ||
| γ = 90° | - |
| Parameters | Pure β-Ga2O3 | Sr-Doped β-Ga2O3 at Ga1 | Sr-Doped β-Ga2O3 at Ga2 |
|---|---|---|---|
| a (Å) | 12.497 | 12.545 (+0.38%) | 12.599 (+0.82%) |
| b (Å) | 6.187 | 6.218 (+0.50%) | 6.233 (+0.74%) |
| c (Å) | 11.806 | 11.937 (+1.11%) | 11.857 (+0.43%) |
| Cell Volume (Å3) | 886.770 | 903.865 | 904.761 |
| Cell Angle (°) | α = 90° β = 103.723° γ = 90° | α = 90° β = 103.903° γ = 90° | α = 90° β = 103.667° γ = 90° |
| Bond Length | 1 × 1 × 1 Unit Cell | |
|---|---|---|
| Pure β-Ga2O3 | Sr-Doped β-Ga2O3 | |
| Ga-O (Å) | 1.964 | 1.965 |
| O-O (Å) | 2.861 | 2.864 |
| Sr-O (Å) | - | 2.426 |
| Bond Length | 1 × 2 × 2 Supercell | ||
|---|---|---|---|
| Pure β-Ga2O3 | Sr-Doped β-Ga2O3 at Ga1 | Sr-Doped β-Ga2O3 at Ga2 | |
| Ga-O (Å) | 1.971 | 1.979 | 1.973 |
| O-O (Å) | 2.882 | 2.873 | 2.876 |
| Sr-O (Å) | - | 2.229 | 2.366 |
| Bonding Characteristics | Ionic | Weak Ionic | Weak Covalent | Covalent |
|---|---|---|---|---|
| Bond Population | 0–0.32 | 0.32–0.50 | 0.50–0.75 | 0.75–1.0 |
| Pure Ga2O3 | Sr-Doped β-Ga2O3 | |||
|---|---|---|---|---|
| without SO | with SO | without SO | with SO | |
| Absorption edge | 400–700 nm | 100–300 nm | 400–700 nm | 100–300 nm |
| Dielectric constant | 3.05 | 2.39 | 5.37 | 2.53 |
| Refractive index | 1.75 | 1.55 | 2.33 | 1.59 |
| Reflectivity peak | 13.3 eV | 16.3 eV | 11.1 eV | 15.5 eV |
| Loss function peak | 14.3 eV | 17.4 eV | 13.3 eV | 16.3 eV |
| Pure Ga2O3 | Sr-Doped β-Ga2O3 at Ga1 | Sr-Doped β-Ga2O3 at Ga2 | ||||
|---|---|---|---|---|---|---|
| without SO | with SO | without SO | with SO | without SO | with SO | |
| Absorption edge | 400–700 nm | 100–300 nm | 400–700 nm | 100–300 nm | 400–700 nm | 100–300 nm |
| Dielectric constant | 2.18 | 1.74 | 14.7 | 3.11 | 9.63 | 2.55 |
| Refractive index | 1.48 | 1.32 | 3.88 | 1.76 | 3.13 | 1.60 |
| Reflectivity peak | 9.46 eV | 12.3 eV | 9.46 eV | 3.54 eV | 9.33 eV | 12.3 eV |
| Loss function peak | 9.69 eV | 12.7 eV | 9.69 eV | 12.8 eV | 9.57 eV | 12.5 eV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kean Ping, L.; Mohamed, M.A.; Kumar Mondal, A.; Mohamad Taib, M.F.; Samat, M.H.; Berhanuddin, D.D.; Menon, P.S.; Bahru, R. First-Principles Studies for Electronic Structure and Optical Properties of Strontium Doped β-Ga2O3. Micromachines 2021, 12, 348. https://doi.org/10.3390/mi12040348
Kean Ping L, Mohamed MA, Kumar Mondal A, Mohamad Taib MF, Samat MH, Berhanuddin DD, Menon PS, Bahru R. First-Principles Studies for Electronic Structure and Optical Properties of Strontium Doped β-Ga2O3. Micromachines. 2021; 12(4):348. https://doi.org/10.3390/mi12040348
Chicago/Turabian StyleKean Ping, Loh, Mohd Ambri Mohamed, Abhay Kumar Mondal, Mohamad Fariz Mohamad Taib, Mohd Hazrie Samat, Dilla Duryha Berhanuddin, P. Susthitha Menon, and Raihana Bahru. 2021. "First-Principles Studies for Electronic Structure and Optical Properties of Strontium Doped β-Ga2O3" Micromachines 12, no. 4: 348. https://doi.org/10.3390/mi12040348
APA StyleKean Ping, L., Mohamed, M. A., Kumar Mondal, A., Mohamad Taib, M. F., Samat, M. H., Berhanuddin, D. D., Menon, P. S., & Bahru, R. (2021). First-Principles Studies for Electronic Structure and Optical Properties of Strontium Doped β-Ga2O3. Micromachines, 12(4), 348. https://doi.org/10.3390/mi12040348







