Can 3D Printing Bring Droplet Microfluidics to Every Lab?—A Systematic Review
Abstract
1. Introduction
- Technology, referring to reviews on 3DP technology as it relates to microfluidics, challenges, limitations and comparisons of options, among other factors;
- Applications, be they biological or chemical, as well as the droplet microfluidics applications of 3DP microfluidic chips;
- Future potential, namely novel technology options that 3DP technology can offer (e.g., cloud-based manufacturing, digital design automation, as well as the potential of constructing integrated 3DP microfluidic systems (system integration));
- Other, which are papers that did not fit in the above categories.
2. 3D-Printed Droplet Microfluidics
2.1. 3D Printing Techniques
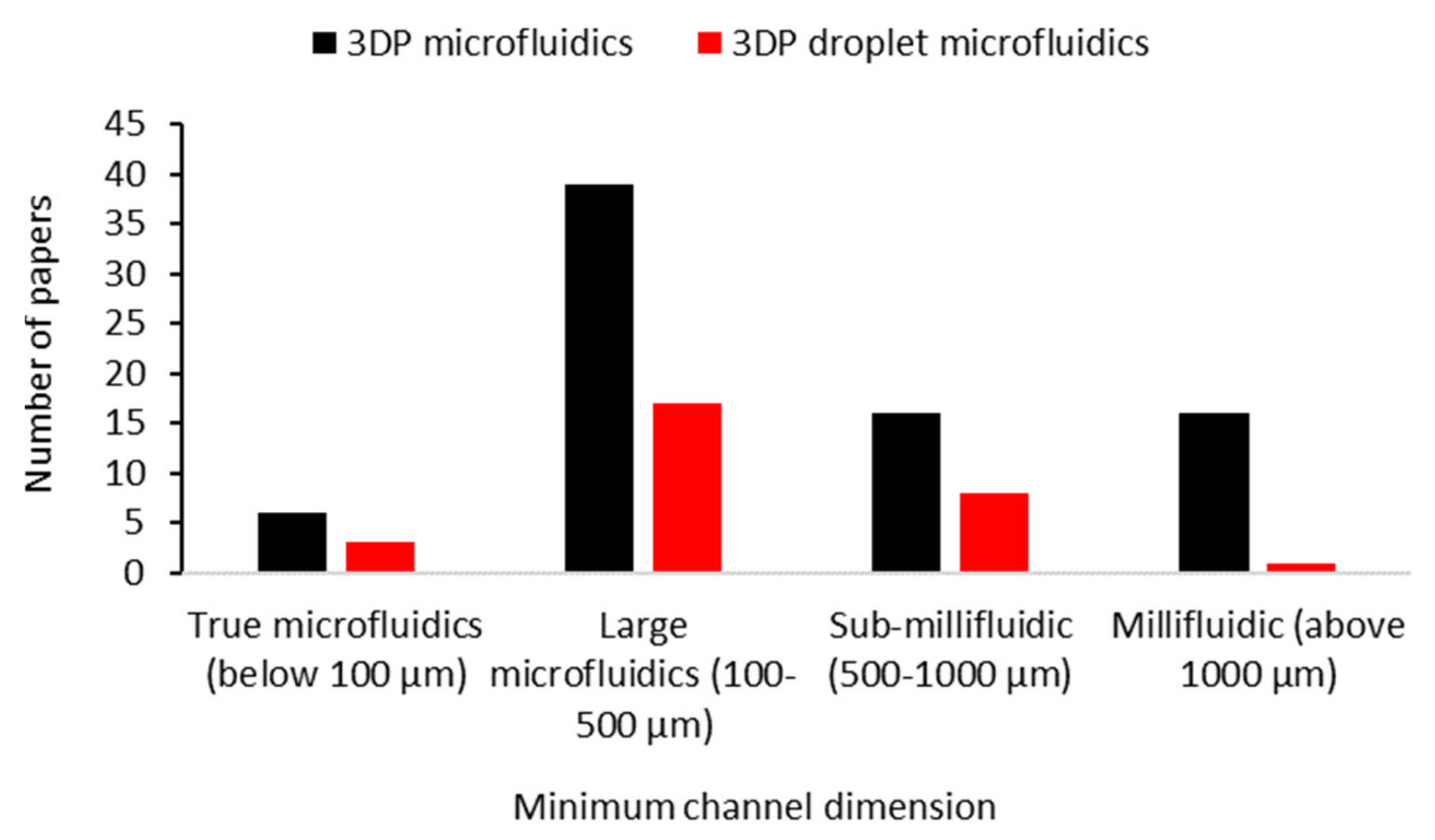
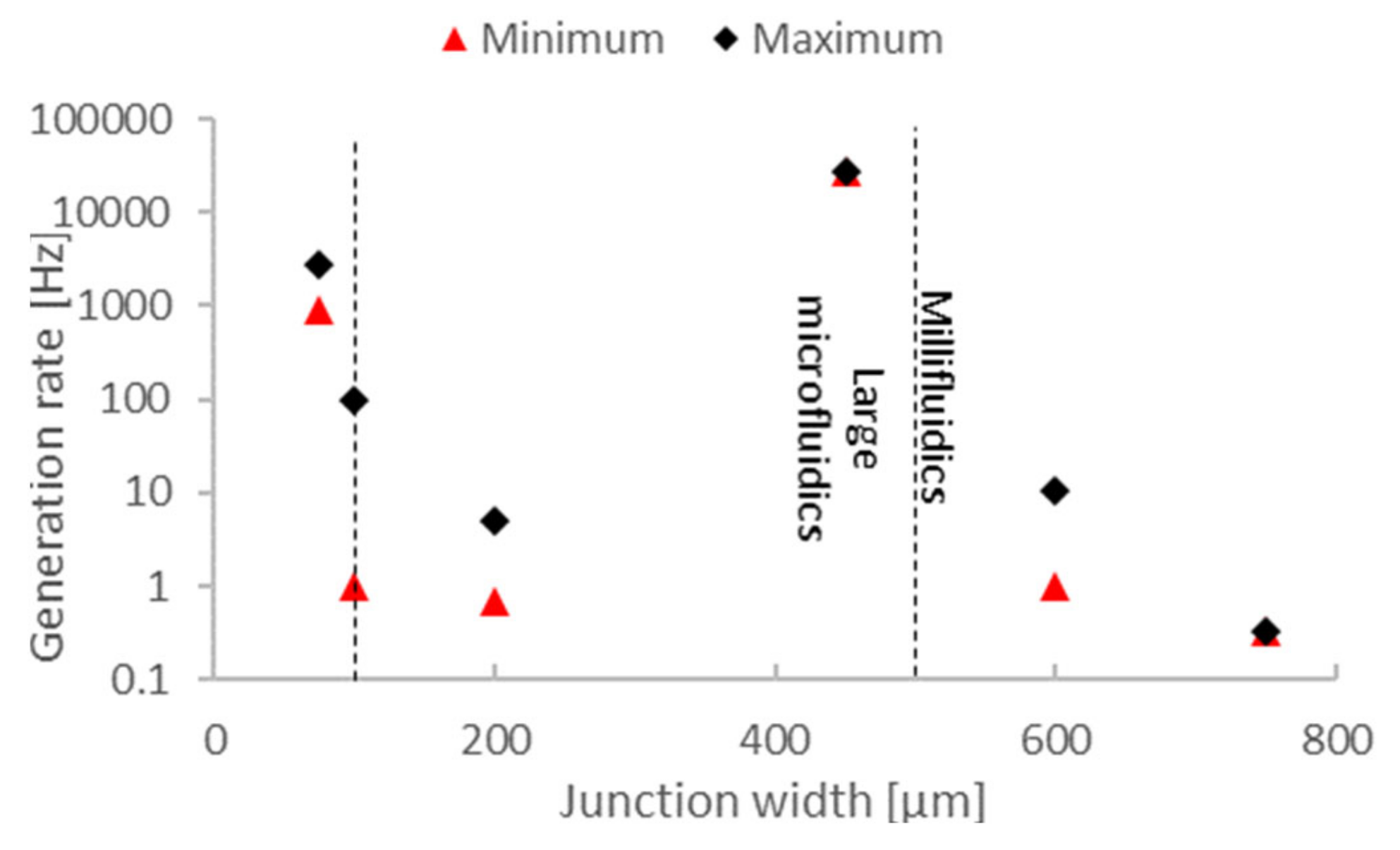
2.2. Droplet Generation Performance of 3D-Printed Droplet Microfluidics
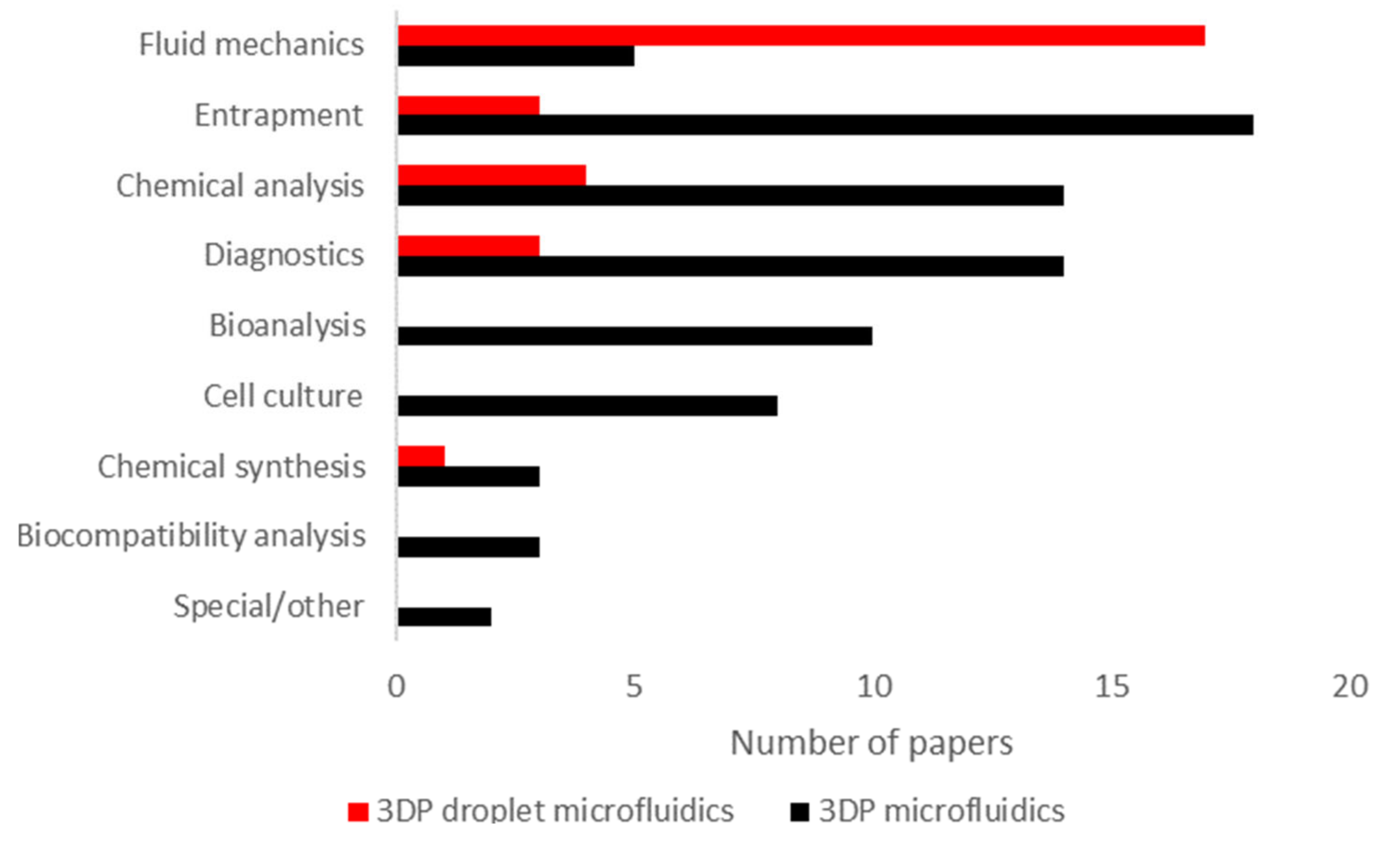
2.3. Applications of 3D-Printed Droplet Microfluidics
- Entrapment [39,91,113], or immobilizing microparticles (e.g., cells and proteins). Commonly, this involves using a lattice or micropillar array, microwells or similar structures. The category can also include other means of immobilization (e.g., immobilization with chemical or electromagnetic means);
- Bioanalysis, covering the implementation of bioanalytical techniques such as gel electrophoresis or viscometry in an integrated format. This also includes the biomonitoring of liquid samples (e.g., impedimetric flow cells);
- Cell culture, as in cell culturing applications. In this category, the focus is on sustaining cells, not necessarily monitoring their activity or response to stimuli;
- Chemical synthesis [68], such as pharmaceuticals. This category also includes nanoparticle synthesis;
- Biocompatibility analysis, analyzing the biocompatibility of 3DP microfluidics;
- Special or other, which includes works that do not fall into any other category (e.g., implementing fluidic logic circuits).
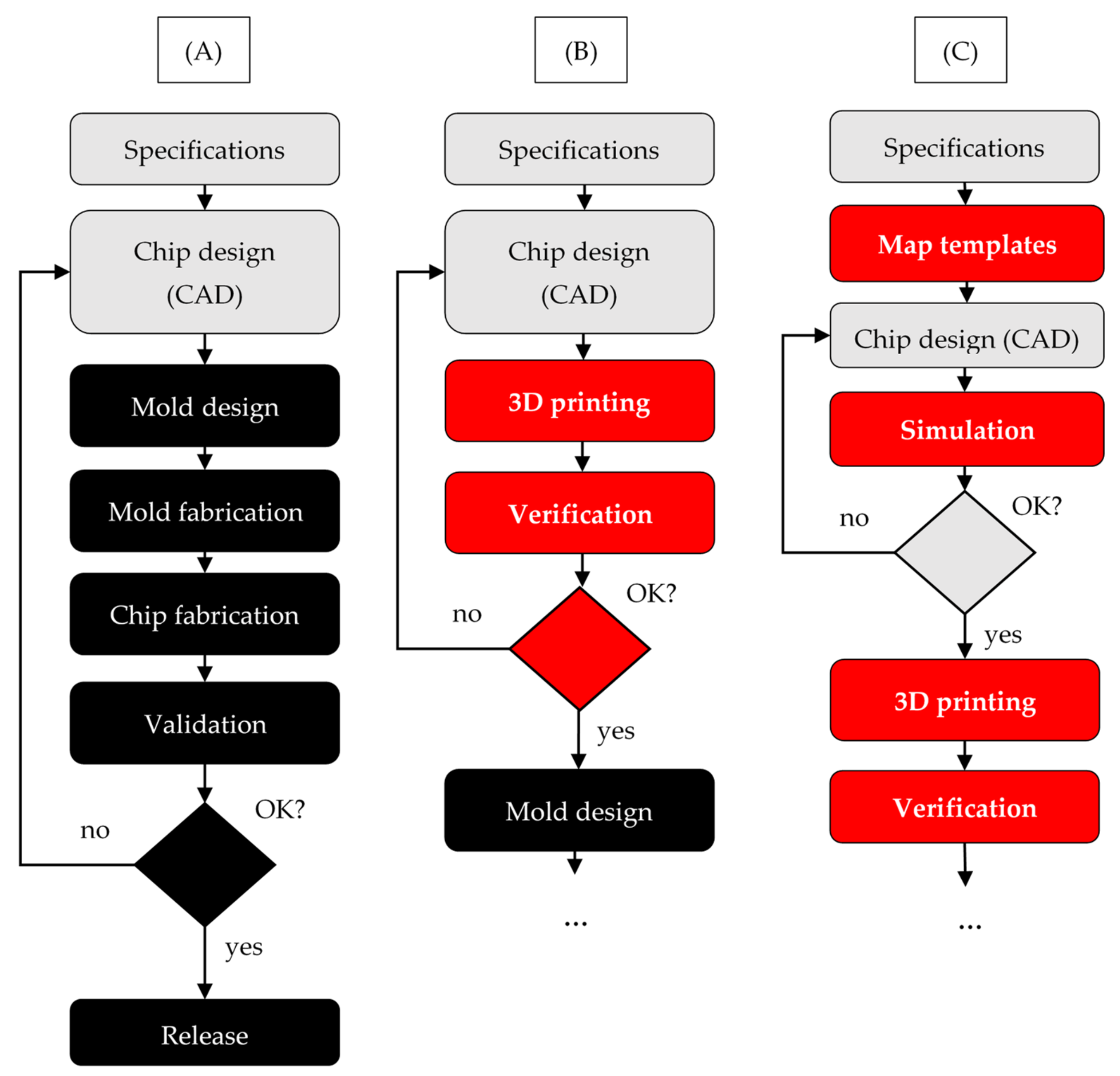
3. Democratizing 3DP Droplet Microfluidics
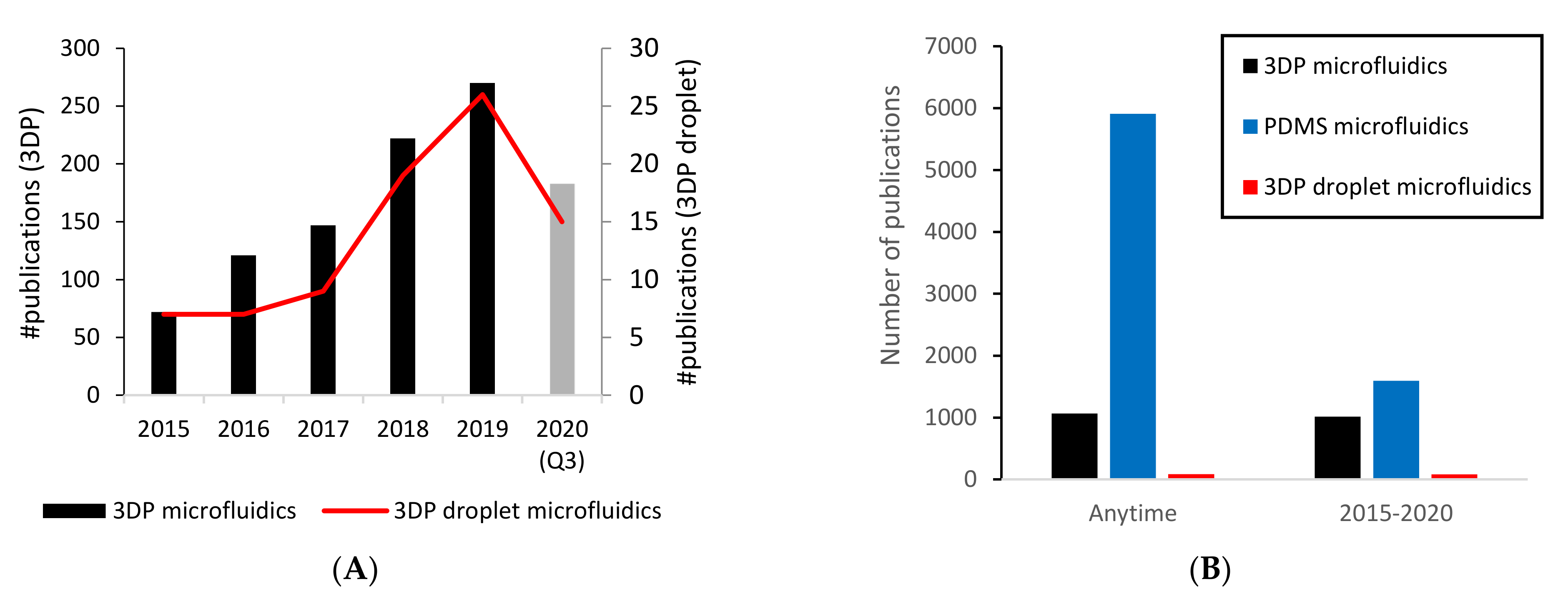
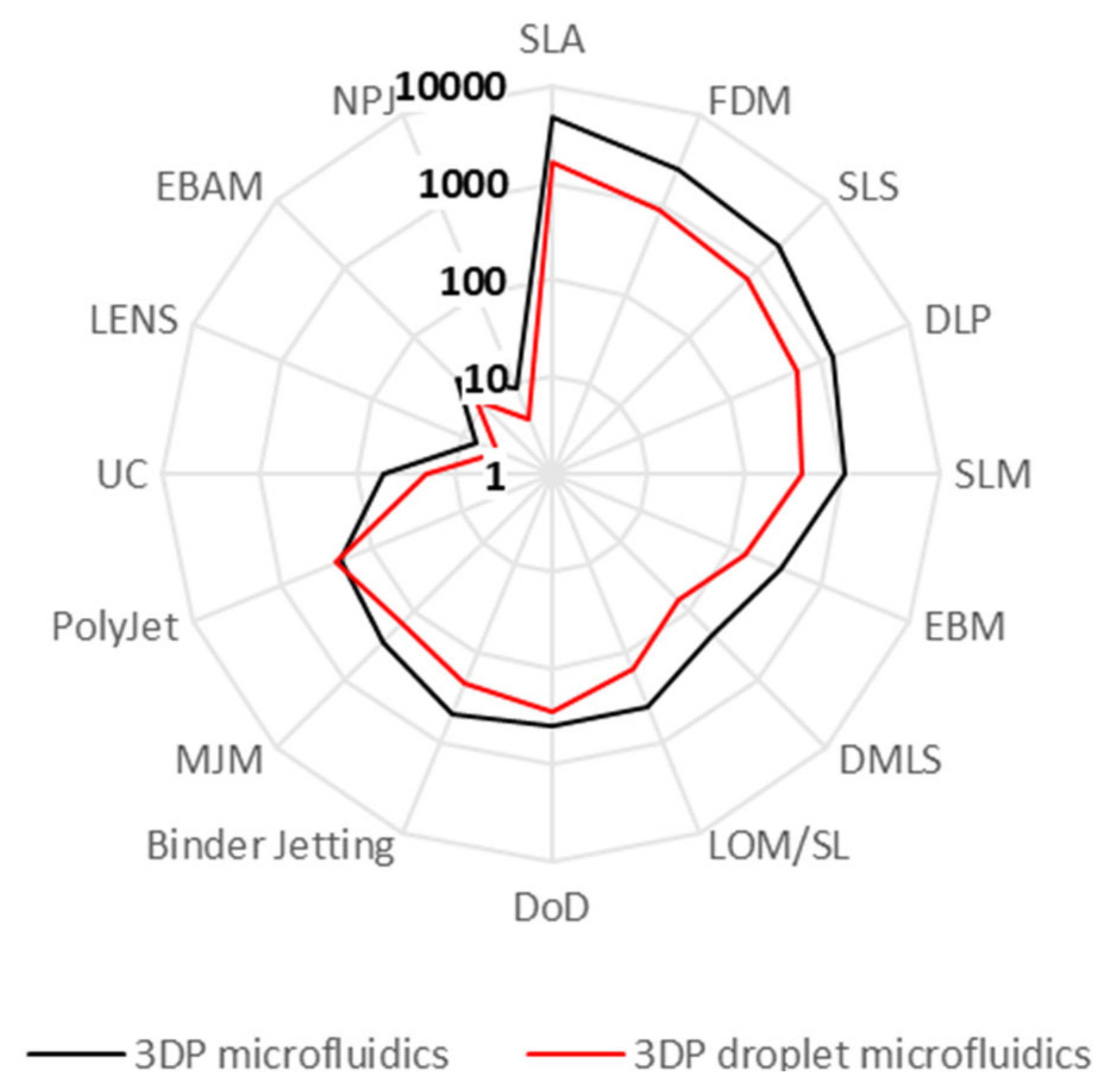
3.1. Increasing Availability in Mainstream Laboratories
3.2. Digital Design Automation and Future Potential
- Reduced logistics. Since only 3D printing material needs to be supplied and stocked, logistics costs, overhead and environmental impacts are significantly reduced. Furthermore, new designs can be transferred digitally and do not require transporting physical masters, as would be the case for PDMS, or molds, as would be the case for injection molding;
- Design flexibility. There are no dedicated molds or masters, and therefore design changes can be quickly implemented, regardless of the number of printers involved or the number of parts printed;
- Automation. Printers can run autonomously and can be monitored remotely. In the case of SLA printers, washing and post-curing stations are also offered that can automatically finish fabricating the part. The operator only needs to move parts between machines and remove the supports at the end.
4. Discussion
5. Conclusions
- SLA and FDM are the best candidates for the democratization of 3DP microfluidics. Both offer the same high level of automation and flexibility; however, both have their unique technical challenges. Among other issues, SLA parts primarily have issues with biocompatibility, and FDM has issues with surface quality, those being being watertightness and printing overhangs;
- Open access hardware platforms with digital design automation, together with online design repositories and verification of designs via simulation, could greatly speed up microfluidic chip design as well as reduce the associated costs.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.A.; Popma, A.; Wong, W.; Nguyen, T.; Pan, Y.; Xu, J. 3D printing: An emerging tool for novel microfluidics and lab-on-a-chip applications. Microfluid. Nanofluid. 2016, 20, 1–18. [Google Scholar] [CrossRef]
- Padash, M.; Enz, C.; Carrara, S. Microfluidics by additive manufacturing for wearable biosensors: A review. Sensors 2020, 20, 4236. [Google Scholar] [CrossRef]
- Gross, B.; Lockwood, S.Y.; Spence, D.M. Recent advances in analytical chemistry by 3D printing. Anal. Chem. 2017, 89, 57–70. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Y.; Fu, J.Z.; Gao, Q.; Qiu, J.J. Developments of 3D Printing Microfluidics and Applications in Chemistry and Biology: A Review. Electroanalysis 2016, 28, 1658–1678. [Google Scholar] [CrossRef]
- Cocovi-Solberg, D.J.; Worsfold, P.J.; Miró, M. Opportunities for 3D printed millifluidic platforms incorporating on-line sample handling and separation. TrAC Trends Anal. Chem. 2018, 108, 13–22. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef]
- Nielsen, A.V.; Beauchamp, M.J.; Nordin, G.P.; Woolley, A.T. 3D Printed Microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef] [PubMed]
- Pranzo, D.; Larizza, P.; Filippini, D.; Percoco, G. Extrusion-based 3D printing of microfluidic devices for chemical and biomedical applications: A topical review. Micromachines 2018, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.J.; Nordin, G.P.; Woolley, A.T. Moving from millifluidic to truly microfluidic sub-100-μm cross-section 3D printed devices. Anal. Bioanal. Chem. 2017, 409, 4311–4319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Three-dimensional-printing for microfluidics or the other way around? Int. J. Bioprint. 2019, 5, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Wu, S.Y.; Yang, C.; Restaino, M.; Lin, L. 3D printed microfluidics and microelectronics. Microelectron. Eng. 2018, 189, 52–68. [Google Scholar] [CrossRef]
- Carve, M.; Wlodkowic, D. 3D-printed chips: Compatibility of additive manufacturing photopolymeric substrata with biological applications. Micromachines 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Bhattacharjee, N.; Folch, A. Digital Manufacturing for Microfluidics. Annu. Rev. Biomed. Eng. 2019. [Google Scholar] [CrossRef] [PubMed]
- Santana, H.S.; Palma, M.S.A.; Lopes, M.G.M.; Souza, J.; Lima, G.A.S.; Taranto, O.P.; Silva, J.L. Microfluidic Devices and 3D Printing for Synthesis and Screening of Drugs and Tissue Engineering. Ind. Eng. Chem. Res. 2020, 59, 3794–3810. [Google Scholar] [CrossRef]
- Priyadarshini, B.M.; Dikshit, V.; Zhang, Y. 3D-printed bioreactors for in vitro modeling and analysis. Int. J. Bioprint. 2020, 6, 267. [Google Scholar]
- Manzanares Palenzuela, C.L.; Pumera, M. (Bio)Analytical chemistry enabled by 3D printing: Sensors and biosensors. TrAC Trends Anal. Chem. 2018, 103, 110–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, S.; Yu, J. Chemical and biochemical analysis on lab-on-a-chip devices fabricated using three-dimensional printing. TrAC Trends Anal. Chem. 2016, 85, 166–180. [Google Scholar] [CrossRef]
- Chan, H.N.; Tan, M.J.A.; Wu, H. Point-of-care testing: Applications of 3D printing. Lab Chip 2017, 17, 2713–2739. [Google Scholar] [CrossRef]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-L.; Lee, P.-Y.; Yang, C.-L.; Huang, W.-Y.; Lin, Y.-S. Recent Advances in Applications of Droplet Microfluidics. Micromachines 2015, 6, 1249–1271. [Google Scholar] [CrossRef]
- Kaminski, T.S.; Scheler, O.; Garstecki, P. Droplet microfluidics for microbiology: Techniques, applications and challenges. Lab Chip 2016, 16, 2168–2187. [Google Scholar] [CrossRef] [PubMed]
- Gwak, H.; Kim, J.; Kashefi-Kheyrabadi, L.; Kwak, B.; Hyun, K.-A.; Jung, H.-I. Progress in Circulating Tumor Cell Research Using Microfluidic Devices. Micromachines 2018, 9, 353. [Google Scholar] [CrossRef]
- Mashaghi, S.; Abbaspourrad, A.; Weitz, D.A.; van Oijen, A.M. Droplet microfluidics: A tool for biology, chemistry and nanotechnology. TrAC Trends Anal. Chem. 2016, 82, 118–125. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef] [PubMed]
- Dixit, C.K.; Kadimisetty, K.; Rusling, J. 3D-printed miniaturized fluidic tools in chemistry and biology. TrAC Trends Anal. Chem. 2018, 106, 37–52. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef]
- Zhang, J.M.; Ji, Q.; Duan, H. Three-dimensional printed devices in droplet microfluidics. Micromachines 2019, 10, 754. [Google Scholar] [CrossRef]
- Weisgrab, G.; Ovsianikov, A.; Costa, P.F. Functional 3D Printing for Microfluidic Chips. Adv. Mater. Technol. 2019, 4, 1900275. [Google Scholar] [CrossRef]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Yew, M.; Koh, K.; Ren, Y. Advances in Droplet Microfluidics with Off-the-Shelf Devices and Other Novel Designs. In Advances in Microfluidic Technologies for Energy and Environmental Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Bishop, G.W.; Satterwhite-Warden, J.E.; Kadimisetty, K.; Rusling, J.F. 3D-printed bioanalytical devices. Nanotechnology 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, M.; Kadimisetty, K.; Bhalerao, K.S.; Chen, T.; Rusling, J.F. 3D-Printed Immunosensor Arrays for Cancer Diagnostics. Sensors 2020, 20, 4514. [Google Scholar] [CrossRef] [PubMed]
- Arivarasi, A.; Kumar, A. Classification of challenges in 3D printing for combined electrochemical and microfluidic applications: A review. Rapid Prototyp. J. 2019, 25, 1328–1346. [Google Scholar]
- Gong, H.; Bickham, B.P.; Woolley, A.T.; Nordin, G.P. Custom 3D printer and resin for 18 μm × 20 μm microfluidic flow channels. Lab Chip 2017, 17, 2899–2909. [Google Scholar] [CrossRef]
- Kamperman, T.; Teixeira, L.M.; Salehi, S.S.; Kerckhofs, G.; Guyot, Y.; Geven, M.; Geris, L.; Grijpma, D.; Blanquer, S.; Leijten, J. Engineering 3D parallelized microfluidic droplet generators with equal flow profiles by computational fluid dynamics and stereolithographic printing. Lab Chip 2020, 20, 490–495. [Google Scholar] [CrossRef]
- Shallan, A.I.; Smejkal, P.; Corban, M.; Guijt, R.M.; Breadmore, M.C. Cost-effective three-dimensional printing of visibly transparent microchips within minutes. Anal. Chem. 2014, 86, 3124–3130. [Google Scholar] [CrossRef]
- Nelson, M.D.; Ramkumar, N.; Gale, B.K. Flexible, transparent, sub-100 μm microfluidic channels with fused deposition modeling 3D-printed thermoplastic polyurethane. J. Micromech. Microeng. 2019, 29. [Google Scholar] [CrossRef]
- Li, F.; MacDonald, N.P.; Guijt, R.M.; Breadmore, M.C. Multimaterial 3D Printed Fluidic Device for Measuring Pharmaceuticals in Biological Fluids. Anal. Chem. 2019, 91, 1758–1763. [Google Scholar] [CrossRef] [PubMed]
- Castiaux, A.D.; Pinger, C.W.; Hayter, E.A.; Bunn, M.E.; Martin, R.S.; Spence, D.M. PolyJet 3D-Printed Enclosed Microfluidic Channels without Photocurable Supports. Anal. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, G.; Han, Q.; Cheng, Y. Formation of magnetic ionic liquid-water Janus droplet in assembled 3D-printed microchannel. Chem. Eng. J. 2021, 406. [Google Scholar] [CrossRef]
- Song, R.; Abbasi, M.S.; Lee, J. Fabrication of 3D printed modular microfluidic system for generating and manipulating complex emulsion droplets. Microfluid. Nanofluid. 2019, 23. [Google Scholar] [CrossRef]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Venkatesh, S.; Avetisyan, A.; Ekman, K.F.; Raulinaitis, A.; Tsai, A.; Wienkers, A.; Korner, K.; et al. 3D printed microfluidic circuitry via multijet-based additive manufacturing. Lab Chip 2016, 16, 668–678. [Google Scholar] [CrossRef]
- Cai, S.; Shi, H.; Li, G.; Xue, Q.; Zhao, L.; Wang, F.; Hu, B. 3D-printed concentration-controlled microfluidic chip with diffusion mixing pattern for the synthesis of alginate drug delivery microgels. Nanomaterials 2019, 9, 1451. [Google Scholar] [CrossRef]
- Adamski, K.; Kubicki, W.; Walczak, R. 3D Printed Electrophoretic Lab-on-chip for DNA Separation. Procedia Eng. 2016, 168, 1454–1457. [Google Scholar] [CrossRef]
- Kadimisetty, K.; Song, J.; Doto, A.M.; Hwang, Y.; Peng, J.; Mauk, M.G.; Bushman, F.D.; Gross, R.; Jarvis, J.N.; Liu, C. Fully 3D printed integrated reactor array for point-of-care molecular diagnostics. Biosens. Bioelectron. 2018, 109, 156–163. [Google Scholar] [CrossRef]
- Knowlton, S.; Yu, C.H.; Ersoy, F.; Emadi, S.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic chips with patterned, cell-laden hydrogel constructs. Biofabrication 2016, 8. [Google Scholar] [CrossRef]
- Macdonald, N.P.; Cabot, J.M.; Smejkal, P.; Guijt, R.M.; Paull, B.; Breadmore, M.C. Comparing Microfluidic Performance of Three-Dimensional (3D) Printing Platforms. Anal. Chem. 2017, 89, 3858–3866. [Google Scholar] [CrossRef]
- Kadimisetty, K.; Spak, A.P.; Bhalerao, K.S.; Sharafeldin, M.; Mosa, I.M.; Lee, N.H.; Rusling, J.F. Automated 4-sample protein immunoassays using 3D-printed microfluidics. Anal. Methods 2018, 10, 4000–4006. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.N.; Shu, Y.; Xiong, B.; Chen, Y.Y.; Chen, Y.Y.; Tian, Q.; Michael, S.A.; Shen, B.; Wu, H. Simple, Cost-Effective 3D Printed Microfluidic Components for Disposable, Point-of-Care Colorimetric Analysis. ACS Sens. 2016, 1, 227–234. [Google Scholar] [CrossRef]
- Zhu, F.; Macdonald, N.P.; Skommer, J.; Wlodkowic, D. Biological implications of lab-on-a-chip devices fabricated using multi-jet modelling and stereolithography processes. In Bio-MEMS and Medical Microdevices II; SPIE: Bellingham, WA, USA, 2015; Volume 9518, p. 951808. [Google Scholar]
- Zhang, L.; Deraney, R.N.; Tripathi, A. Adsorption and isolation of nucleic acids on cellulose magnetic beads using a three-dimensional printed microfluidic chip. Biomicrofluidics 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N.P.; Zhu, F.; Hall, C.J.; Reboud, J.; Crosier, P.S.; Patton, E.E.; Wlodkowic, D.; Cooper, J.M. Assessment of biocompatibility of 3D printed photopolymers using zebrafish embryo toxicity assays. Lab Chip 2016, 16, 291–297. [Google Scholar] [CrossRef]
- Zhu, F.; Skommer, J.; MacDonald, N.P.; Friedrich, T.; Kaslin, J.; Wlodkowic, D. Three-dimensional printed millifluidic devices for zebrafish embryo tests. Biomicrofluidics 2015, 9. [Google Scholar] [CrossRef]
- Zhu, F.; Skommer, J.; Friedrich, T.; Kaslin, J.; Wlodkowic, D. 3D printed polymers toxicity profiling: A caution for biodevice applications. In Proceedings of the Micro/Nano Materials, Devices, and Systems. Microtechnologies, Barcelona, Spain, 4–6 May 2015; p. 96680Z. [Google Scholar]
- Bishop, G.W.; Satterwhite-Warden, J.E.; Bist, I.; Chen, E.; Rusling, J.F. Electrochemiluminescence at Bare and DNA-Coated Graphite Electrodes in 3D-Printed Fluidic Devices. ACS Sens. 2016, 1, 197–202. [Google Scholar] [CrossRef]
- Piironen, K.; Haapala, M.; Talman, V.; Järvinen, P.; Sikanen, T. Cell adhesion and proliferation on common 3D printing materials used in stereolithography of microfluidic devices. Lab Chip 2020, 20, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.J.Y.; Islam, A.; Dasgupta, R.; Iyer, N.G.; Leo, H.L.; Toh, Y.C. A 3D printed microfluidic perfusion device for multicellular spheroid cultures. Biofabrication 2017, 9. [Google Scholar] [CrossRef]
- Zhang, J.M.; Ji, Q.; Liu, Y.; Huang, J.; Duan, H. An integrated micro-millifluidic processing system. Lab Chip 2018, 18, 3393–3404. [Google Scholar] [CrossRef]
- Zhu, F.; Macdonald, N.P.; Cooper, J.M.; Wlodkowic, D. Additive manufacturing of lab-on-a-chip devices: Promises and challenges. In Proceedings of the Micro/Nano Materials, Devices, and Systems, Melbourne, Australia, 8–11 December 2013; p. 892344. [Google Scholar]
- Takenaga, S.; Schneider, B.; Erbay, E.; Biselli, M.; Schnitzler, T.; Schöning, M.J.; Wagner, T. Fabrication of biocompatible lab-on-chip devices for biomedical applications by means of a 3D-printing process. Phys. Status Solidi Appl. Mater. Sci. 2015, 212, 1347–1352. [Google Scholar] [CrossRef]
- Patrick, W.G.; Nielsen, A.A.K.; Keating, S.J.; Levy, T.J.; Wang, C.W.; Rivera, J.J.; Mondragón-Palomino, O.; Carr, P.A.; Voigt, C.A.; Oxman, N.; et al. DNA assembly in 3D printed fluidics. PLoS ONE 2015, 10, e0143636. [Google Scholar] [CrossRef]
- Brandhoff, L.; van den Driesche, S.; Lucklum, F.; Vellekoop, M.J. Creation of hydrophilic microfluidic devices for biomedical application through stereolithography. In Bio-MEMS and Medical Microdevices II; SPIE: Bellingham, WA, USA, 2015; Volume 9518, p. 95180D. [Google Scholar]
- Li, F.; Macdonald, N.P.; Guijt, R.M.; Breadmore, M.C. Using Printing Orientation for Tuning Fluidic Behavior in Microfluidic Chips Made by Fused Deposition Modeling 3D Printing. Anal. Chem. 2017, 89, 12805–12811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Li, E.Q.; Aguirre-Pablo, A.A.; Thoroddsen, S.T. A simple and low-cost fully 3D-printed non-planar emulsion generator. RSC Adv. 2016, 6, 2793–2799. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, J.M.; Liu, Y.; Li, X.; Lv, P.; Jin, D.; Duan, H. A Modular Microfluidic Device via Multimaterial 3D Printing for Emulsion Generation. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, A.L.; Borenstein, J.T.; Velasquez-Garcia, L.F. Monolithic, 3D-Printed microfluidic platform for recapitulation of dynamic tumor microenvironments. J. Microelectromech. Syst. 2018, 27, 1009–1022. [Google Scholar] [CrossRef]
- Anycubic Anycubic Photon Zero 3D Resin Printer—ANYCUBIC 3D Printing. Available online: https://www.anycubic.com/products/anycubic-photon-zero (accessed on 31 January 2021).
- Chohan, J.S.; Singh, R. Pre and post processing techniques to improve surface characteristics of FDM parts: A state of art review and future applications. Rapid Prototyp. J. 2017, 23, 495–513. [Google Scholar] [CrossRef]
- Erkal, J.L.; Selimovic, A.; Gross, B.C.; Lockwood, S.Y.; Walton, E.L.; McNamara, S.; Martin, R.S.; Spence, D.M. 3D printed microfluidic devices with integrated versatile and reusable electrodes. Lab Chip 2014, 14, 2023–2032. [Google Scholar] [CrossRef]
- Anderson, K.B.; Lockwood, S.Y.; Martin, R.S.; Spence, D.M. A 3D printed fluidic device that enables integrated features. Anal. Chem. 2013, 85, 5622–5626. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Anderson, K.B.; Meisel, J.E.; McNitt, M.I.; Spence, D.M. Polymer Coatings in 3D-Printed Fluidic Device Channels for Improved Cellular Adherence Prior to Electrical Lysis. Anal. Chem. 2015, 87, 6335–6341. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Lockwood, S.Y.; Spence, D.M. 3D-printed fluidic devices enable quantitative evaluation of blood components in modified storage solutions for use in transfusion medicine. Analyst 2014, 139, 3219–3226. [Google Scholar] [CrossRef]
- Munshi, A.S.; Martin, R.S. Microchip-based electrochemical detection using a 3-D printed wall-jet electrode device. Analyst 2016, 141, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.A. A Multi-Channel 3D-Printed Bioreactor for Evaluation of Growth and Production in the Microalga Dunaliella sp. Available online: http://digitalcommons.library.umaine.edu/etd/2560 (accessed on 19 March 2021).
- Jans, A.; Lölsberg, J.; Omidinia-Anarkoli, A.; Viermann, R.; Möller, M.; De Laporte, L.; Wessling, M.; Kuehne, A.J.C. High-throughput production of micrometer sized double emulsions and microgel capsules in parallelized 3D printed microfluidic devices. Polymers 2019, 11, 1887. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Dolomite Single Emulsion Systems 2018. Available online: https://www.dolomite-microfluidics.com/microfluidic-systems/single-emulsions/ (accessed on 19 March 2021).
- Fiedler, B.L.; Van Buskirk, S.; Carter, K.P.; Qin, Y.; Carpenter, M.C.; Palmer, A.E.; Jimenez, R. Droplet Microfluidic Flow Cytometer For Sorting On Transient Cellular Responses Of Genetically-Encoded Sensors. Anal. Chem. 2017, 89, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Holzner, G.; Choo, J.; DeMello, A. High-throughput microfluidic imaging flow cytometry. Curr. Opin. Biotechnol. 2019, 55, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Au, A.K.; Lee, W.; Folch, A. Mail-order microfluidics: Evaluation of stereolithography for the production of microfluidic devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef]
- Kuo, A.P.; Bhattacharjee, N.; Lee, Y.S.; Castro, K.; Kim, Y.T.; Folch, A. High-Precision Stereolithography of Biomicrofluidic Devices. Adv. Mater. Technol. 2019, 4. [Google Scholar] [CrossRef]
- Su, C.K.; Peng, P.J.; Sun, Y.C. Fully 3D-Printed Preconcentrator for Selective Extraction of Trace Elements in Seawater. Anal. Chem. 2015, 87, 6945–6950. [Google Scholar] [CrossRef] [PubMed]
- Warr, C.; Valdoz, J.C.; Bickham, B.P.; Knight, C.J.; Franks, N.A.; Chartrand, N.; Van Ry, P.M.; Christensen, K.A.; Nordin, G.P.; Cook, A.D. Biocompatible PEGDA Resin for 3D Printing. ACS Appl. Bio Mater. 2020, 3, 2239–2244. [Google Scholar] [CrossRef]
- Costa, P.F.; Albers, H.J.; Linssen, J.E.A.; Middelkamp, H.H.T.; Van Der Hout, L.; Passier, R.; Van Den Berg, A.; Malda, J.; Van Der Meer, A.D. Mimicking arterial thrombosis in a 3D-printed microfluidic: In vitro vascular model based on computed tomography angiography data. Lab Chip 2017, 17, 2785–2792. [Google Scholar] [CrossRef]
- Beckwith, A.L.; Velásquez-García, L.F.; Borenstein, J.T. Microfluidic Model for Evaluation of Immune Checkpoint Inhibitors in Human Tumors. Adv. Healthc. Mater. 2019, 8. [Google Scholar] [CrossRef]
- Santangelo, M.F.; Libertino, S.; Turner, A.P.F.; Filippini, D.; Mak, W.C. Integrating printed microfluidics with silicon photomultipliers for miniaturised and highly sensitive ATP bioluminescence detection. Biosens. Bioelectron. 2018, 99, 464–470. [Google Scholar] [CrossRef]
- Zhang, J.M.; Aguirre-Pablo, A.A.; Li, E.Q.; Buttner, U.; Thoroddsen, S.T. Droplet generation in cross-flow for cost-effective 3D-printed “plug-and-play” microfluidic devices. RSC Adv. 2016, 6, 81120–81129. [Google Scholar] [CrossRef]
- Morimoto, Y.; Kiyosawa, M.; Takeuchi, S. Three-dimensional printed microfluidic modules for design changeable coaxial microfluidic devices. Sens. Actuators B Chem. 2018, 274, 491–500. [Google Scholar] [CrossRef]
- Al Mughairy, B.; Al-Lawati, H.A.J.; Suliman, F.E.O. Investigating the impact of metal ions and 3D printed droplet microfluidics chip geometry on the luminol-potassium periodate chemiluminescence system for estimating total phenolic content in olive oil. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, W.; Xu, F.; Lu, W.; Hu, L.; Zhou, J.; Zhang, C.; Jiang, Z. Microfluidic droplet formation in co-flow devices fabricated by micro 3D printing. J. Food Eng. 2021, 290. [Google Scholar] [CrossRef]
- Riche, C.T.; Roberts, E.J.; Gupta, M.; Brutchey, R.L.; Malmstadt, N. Flow invariant droplet formation for stable parallel microreactors. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Lee, W.; Kwon, D.; Choi, W.; Jung, G.Y.; Au, A.K.; Folch, A.; Jeon, S. 3D-Printed Microfluidic Device for the Detection of Pathogenic Bacteria Using Size-based Separation in Helical Channel with Trapezoid Cross-Section. Sci. Rep. 2015, 5, 7717. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, T.; Harding, M.J.; Harris, R.A.; Friel, R.J.; Christie, S.D.R. Customisable 3D printed microfluidics for integrated analysis and optimisation. Lab Chip 2016, 16, 3362–3373. [Google Scholar] [CrossRef]
- Oh, S.; Kim, B.; Lee, J.K.; Choi, S. 3D-printed capillary circuits for rapid, low-cost, portable analysis of blood viscosity. Sens. Actuators B Chem. 2018, 259, 106–113. [Google Scholar] [CrossRef]
- Valentin, T.M.; Leggett, S.E.; Chen, P.Y.; Sodhi, J.K.; Stephens, L.H.; McClintock, H.D.; Sim, J.Y.; Wong, I.Y. Stereolithographic printing of ionically-crosslinked alginate hydrogels for degradable biomaterials and microfluidics. Lab Chip 2017, 17, 3474–3488. [Google Scholar] [CrossRef]
- Lee, W.; Kwon, D.; Chung, B.; Jung, G.Y.; Au, A.; Folch, A.; Jeon, S. Ultrarapid detection of pathogenic bacteria using a 3D immunomagnetic flow assay. Anal. Chem. 2014, 86, 6683–6688. [Google Scholar] [CrossRef]
- Kise, D.P.; Reddish, M.J.; Brian Dyer, R. Sandwich-format 3D printed microfluidic mixers: A flexible platform for multi-probe analysis. J. Micromech. Microeng. 2015, 25. [Google Scholar] [CrossRef]
- Romanov, V.; Samuel, R.; Chaharlang, M.; Jafek, A.R.; Frost, A.; Gale, B.K. FDM 3D Printing of High-Pressure, Heat-Resistant, Transparent Microfluidic Devices. Anal. Chem. 2018, 90, 10450–10456. [Google Scholar] [CrossRef]
- Bressan, L.P.; Adamo, C.B.; Quero, R.F.; De Jesus, D.P.; Da Silva, J.A.F. A simple procedure to produce FDM-based 3D-printed microfluidic devices with an integrated PMMA optical window. Anal. Methods 2019, 11, 1014–1020. [Google Scholar] [CrossRef]
- Salentijn, G.I.J.; Oomen, P.E.; Grajewski, M.; Verpoorte, E. Fused Deposition Modeling 3D Printing for (Bio)analytical Device Fabrication: Procedures, Materials, and Applications. Anal. Chem. 2017, 89, 7053–7061. [Google Scholar] [CrossRef] [PubMed]
- Malinauskas, M.; Rekštyte, S.; Lukoševičius, L.; Butkus, S.; Balčiunas, E.; Pečiukaityte, M.; Baltriukiene, D.; Bukelskiene, V.; Butkevičius, A.; Kucevičius, P.; et al. 3D microporous scaffolds manufactured via combination of fused filament fabrication and direct laser writing ablation. Micromachines 2014, 5, 839–858. [Google Scholar] [CrossRef]
- Bishop, G.W.; Satterwhite, J.E.; Bhakta, S.; Kadimisetty, K.; Gillette, K.M.; Chen, E.; Rusling, J.F. 3D-Printed fluidic devices for nanoparticle preparation and flow-injection amperometry using integrated prussian blue nanoparticle-modified electrodes. Anal. Chem. 2015, 87, 5437–5443. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Baxani, D.K.; Jamieson, W.D.; Xu, W.; Rocha, V.G.; Barrow, D.A.; Castell, O.K. Formation of Polarized, Functional Artificial Cells from Compartmentalized Droplet Networks and Nanomaterials, Using One-Step, Dual-Material 3D-Printed Microfluidics. Adv. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Femmer, T.; Jans, A.; Eswein, R.; Anwar, N.; Moeller, M.; Wessling, M.; Kuehne, A.J.C. High-Throughput Generation of Emulsions and Microgels in Parallelized Microfluidic Drop-Makers Prepared by Rapid Prototyping. ACS Appl. Mater. Interfaces 2015, 7, 12635–12638. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.C.; Figueredo, F.; Ribeiro, L.E.B.; Cortón, E.; Coltro, W.K.T. Label-free counting of Escherichia coli cells in nanoliter droplets using 3D printed microfluidic devices with integrated contactless conductivity detection. Anal. Chim. Acta 2019, 1071, 36–43. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhao, L.; Tang, C.; Shi, H.; Wang, F.; Hu, B. Droplet-based PCR in a 3D-printed microfluidic chip for miRNA-21 detection. Anal. Methods 2019, 11, 3386–3393. [Google Scholar] [CrossRef]
- Männel, M.J.; Selzer, L.; Bernhardt, R.; Thiele, J. Optimizing Process Parameters in Commercial Micro-Stereolithography for Forming Emulsions and Polymer Microparticles in Nonplanar Microfluidic Devices. Adv. Mater. Technol. 2019, 4. [Google Scholar] [CrossRef]
- Ye, B.; Xu, H.; Bao, B.; Xuan, J.; Zhang, L. 3D-printed air-blast microfluidic nozzles for preparing calcium alginate microparticles. RSC Adv. 2017, 7, 48826–48834. [Google Scholar] [CrossRef]
- Morgan, A.J.L.; San Jose, L.H.; Jamieson, W.D.; Wymant, J.M.; Song, B.; Stephens, P.; Barrow, D.A.; Castell, O.K. Simple and versatile 3D printed microfluidics using fused filament fabrication. PLoS ONE 2016, 11, e0152023. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.C.; Chagas, C.L.S.; Ribeiro, L.E.B.; Coltro, W.K.T. 3D printing of microfluidic devices with embedded sensing electrodes for generating and measuring the size of microdroplets based on contactless conductivity detection. Sens. Actuators B Chem. 2017, 251, 427–432. [Google Scholar] [CrossRef]
- Donvito, L.; Galluccio, L.; Lombardo, A.; Morabito, G.; Nicolosi, A.; Reno, M. Experimental validation of a simple, low-cost, T-junction droplet generator fabricated through 3D printing. J. Micromech. Microeng. 2015, 25. [Google Scholar] [CrossRef]
- Van Nguyen, H.; Nguyen, H.Q.; Nguyen, V.D.; Seo, T.S. A 3D printed screw-and-nut based droplet generator with facile and precise droplet size controllability. Sens. Actuators B Chem. 2019, 296. [Google Scholar] [CrossRef]
- Zhou, Z.; Kong, T.; Mkaouar, H.; Salama, K.N.; Zhang, J.M. A hybrid modular microfluidic device for emulsion generation. Sens. Actuators A Phys. 2018, 280, 422–428. [Google Scholar] [CrossRef]
- Duong, L.H.; Chen, P.C. Simple and low-cost production of hybrid 3D-printed microfluidic devices. Biomicrofluidics 2019, 13. [Google Scholar] [CrossRef]
- Lee, J.M.; Zhang, M.; Yeong, W.Y. Characterization and evaluation of 3D printed microfluidic chip for cell processing. Microfluid. Nanofluid. 2016, 20, 1–15. [Google Scholar] [CrossRef]
- Urrios, A.; Parra-Cabrera, C.; Bhattacharjee, N.; Gonzalez-Suarez, A.M.; Rigat-Brugarolas, L.G.; Nallapatti, U.; Samitier, J.; Deforest, C.A.; Posas, F.; Garcia-Cordero, J.L.; et al. 3D-printing of transparent bio-microfluidic devices in PEG-DA. Lab Chip 2016, 16, 2287–2294. [Google Scholar] [CrossRef]
- Krejcova, L.; Nejdl, L.; Rodrigo, M.A.M.; Zurek, M.; Matousek, M.; Hynek, D.; Zitka, O.; Kopel, P.; Adam, V.; Kizek, R. 3D printed chip for electrochemical detection of influenza virus labeled with CdS quantum dots. Biosens. Bioelectron. 2014, 54, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Vlachova, J.; Tmejova, K.; Kopel, P.; Korabik, M.; Zitka, J.; Hynek, D.; Kynicky, J.; Adam, V.; Kizek, R. A 3D microfluidic chip for electrochemical detection of hydrolysed nucleic bases by a modified glassy carbon electrode. Sensors 2015, 15, 2438–2452. [Google Scholar] [CrossRef]
- Chudobova, D.; Cihalova, K.; Skalickova, S.; Zitka, J.; Rodrigo, M.A.M.; Milosavljevic, V.; Hynek, D.; Kopel, P.; Vesely, R.; Adam, V.; et al. 3D-printed chip for detection of methicillin-resistant Staphylococcus aureus labeled with gold nanoparticles. Electrophoresis 2015, 36, 457–466. [Google Scholar] [CrossRef]
- Anciaux, S.K.; Geiger, M.; Bowser, M.T. 3D Printed Micro Free-Flow Electrophoresis Device. Anal. Chem. 2016, 88, 7675–7682. [Google Scholar] [CrossRef]
- Parker, E.K.; Nielsen, A.V.; Beauchamp, M.J.; Almughamsi, H.M.; Nielsen, J.B.; Sonker, M.; Gong, H.; Nordin, G.P.; Woolley, A.T. 3D printed microfluidic devices with immunoaffinity monoliths for extraction of preterm birth biomarkers. Anal. Bioanal. Chem. 2019, 411, 5405–5413. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.J.; Nielsen, A.V.; Gong, H.; Nordin, G.P.; Woolley, A.T. 3D Printed Microfluidic Devices for Microchip Electrophoresis of Preterm Birth Biomarkers. Anal. Chem. 2019, 91, 7418–7425. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, É.M.; Murer, R.C.; Santos, J.M.; Carvalho, R.M.; Eberlin, M.N.; Augusto, F.; Poppi, R.J.; Gobbi, A.L.; Hantao, L.W. Simple, Expendable, 3D-Printed Microfluidic Systems for Sample Preparation of Petroleum. Anal. Chem. 2017, 89, 3460–3467. [Google Scholar] [CrossRef]
- Lim, C.; Lee, Y.; Kulinsky, L. Fabrication of a malaria-Ab ELISA bioassay platform with utilization of syringe-based and 3D printed assay automation. Micromachines 2018, 9, 502. [Google Scholar] [CrossRef]
- Kadimisetty, K.; Mosa, I.M.; Malla, S.; Satterwhite-Warden, J.E.; Kuhns, T.M.; Faria, R.C.; Lee, N.H.; Rusling, J.F. 3D-printed supercapacitor-powered electrochemiluminescent protein immunoarray. Biosens. Bioelectron. 2016, 77, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, X.; Xie, X.; Bahnemann, J.; Lin, X.; Wu, X.; Wang, S.; Hoffmann, M.R. Propidium monoazide pretreatment on a 3D-printed microfluidic device for efficient PCR determination of “live: Versus dead” microbial cells. Environ. Sci. Water Res. Technol. 2018, 4, 956–963. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- PrusaResearch Original Prusa MINI—Prusa3d.com—Open-Source 3D Printers by Josef Prusa. Available online: https://www.prusa3d.com/original-prusa-mini/ (accessed on 7 January 2021).
- Anycubic Anycubic Photon Series LCD-Based SLA Resin 3D Printers—ANYCUBIC 3D Printing. Available online: https://www.anycubic.com/collections/anycubic-photon-3d-printers (accessed on 7 January 2021).
- PrusaResearch PrusaSlicer—Prusa3D—3D Printers from Josef Průša. Available online: https://www.prusa3d.com/prusaslicer/ (accessed on 3 February 2021).
- Thingiverse Thingiverse—Digital Designs for Physical Objects. Available online: https://www.thingiverse.com/ (accessed on 3 February 2021).
- MIT Metafluidics—Open Repository for Fluidic Systems. Available online: https://metafluidics.org/ (accessed on 31 January 2021).
- Haag, S.; Anderl, R. Digital twin—Proof of concept. Manuf. Lett. 2018, 15, 64–66. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X. Fluid and Cell Behaviors Along a 3D Printed Alginate/Gelatin/Fibrin Channel. Biotechnol. Bioeng 2015, 112, 1683–1695. [Google Scholar] [CrossRef]
- Spigarelli, L.; Bertana, V.; Marchisio, D.; Scaltrito, L.; Ferrero, S.; Cocuzza, M.; Marasso, S.L.; Canavese, G.; Pirri, C.F. A passive two-way microfluidic device for low volume blood-plasma separation. Microelectron. Eng. 2019, 209, 28–34. [Google Scholar] [CrossRef]
- Park, Y.J.; Yu, T.; Yim, S.J.; You, D.; Kim, D.P. A 3D-printed flow distributor with uniform flow rate control for multi-stacked microfluidic systems. Lab Chip 2018, 18, 1250–1258. [Google Scholar] [CrossRef]
- Hosseini, F.; Rahimi, M. Computational fluid dynamics and experimental investigations on liquid–liquid mass transfer in T-type microchannels with different mixing channel barrier shapes. Sep. Sci. Technol. 2020, 55, 3502–3516. [Google Scholar] [CrossRef]
- Adamopoulou, T.; Nawada, S.; Deridder, S.; Wouters, B.; Desmet, G.; Schoenmakers, P.J. Experimental and numerical study of band-broadening effects associated with analyte transfer in microfluidic devices for spatial two-dimensional liquid chromatography created by additive manufacturing. J. Chromatogr. A 2019, 1598, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Pardy, T.; Rang, T.; Tulp, I. Thermal analysis of a disposable, instrument-free DNA amplification lab-on-a-chip platform. Sensors 2018, 18, 1812. [Google Scholar] [CrossRef]
- Pardy, T.; Tulp, I.; Kremer, C.; Rang, T.; Stewart, R. Integrated self-regulating resistive heating for isothermal nucleic acid amplification tests (NAAT) in Lab-on-a-Chip (LoC) devices. PLoS ONE 2017, 12, e0189968. [Google Scholar] [CrossRef] [PubMed]
- Philippin, N.; Frey, A.; Michatz, M.; Kuehne, I. 3D-Printed Microfluidic Chip System for Dielectrophoretic Manipulation of Colloids. In Proceedings of the COSMOL Conference, Lausanne, Switzerland, 22 October 2018. [Google Scholar]
- Santana, H.S.; Lameu da Silva, J., Jr.; Taranto, O.P. (Eds.) Process Analysis, Design, and Intensification in Microfluidics and Chemical Engineering; Advances in Chemical and Materials Engineering; IGI Global: Hershey, PA, USA, 2019; ISBN 9781522571384. [Google Scholar]
- Nguyen, N.-T.T.; Wereley, S.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics, 3rd ed.; Artech House Publishers: Norwood, MA, USA, 2019. [Google Scholar]
- Choi, S.H.; Kim, D.S.; Kwon, T.H. Microinjection molded disposable microfluidic lab-on-a-chip for efficient detection of agglutination. Microsyst. Technol. 2009, 15, 309–316. [Google Scholar] [CrossRef]
- Attia, U.M.; Marson, S.; Alcock, J.R. Micro-injection moulding of polymer microfluidic devices. Microfluid. Nanofluid. 2009, 7, 1–28. [Google Scholar] [CrossRef]
- Anycubic Anycubic Plant-Based UV Eco-Resin—ANYCUBIC 3D Printing. Available online: https://www.anycubic.com/products/anycubic-plant-based-uv-resin (accessed on 15 February 2021).


| Feature | Topic | Covered in Detail (***) | Covered (**) | Mentioned (*) |
|---|---|---|---|---|
| Technology | 3D printing techniques (e.g., SLA, FDM), materials, chip printing | [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,29] | [20,21,22,30,31,32,33,34,35,36] | [18,19] |
| Comparison of commercial 3D printers (table) | - | [1,11] | - | |
| Printing functional elements (actuators and sensors) | [32] | - | - | |
| 3D-printed PDMS casts | - | [9,11] | - | |
| Integration with 3D printed electronics | - | [15] | [3,4] | |
| Technical challenges and limitations | [37] | [1,6,17,30,31] | [21] | |
| Applications | Biological or chemical analysis and synthesis applications | [6,18,19,20,21,22,36] | [5,7,11,30,35] | - |
| Cell cultures and organ-on-chip applications | [18,19] | - | [6,21,30] | |
| Bioprinting | - | [5,8,18] | [2,6] | |
| Biological and chemical compatibility | [16] | [2,14,17] | [1,4,11,19,31] | |
| Droplet microfluidics | [31] | - | [14] | |
| Future Potential | Design automation tools (e.g., CAD + CFD or FEM) | - | - | - |
| Digital or cloud-based manufacturing (Internet prefab libraries, modular systems) | - | [2,3,14,22,32] | [17] | |
| Repeatability and reproducibility of geometry or dimensions, tolerances System integration | - - | - [22] | - [31] | |
| Other | Statistical analysis | - | - | [8,14] |
| Technology Group | Technique | Channel Dimension (Typical) (µm) | Channel Dimension (Best) (µm) | Printing Time (Best) (h) | Chip Cost (EUR) |
|---|---|---|---|---|---|
| Vat Polymerization | SLA (stereolithography), including LCD (liquid crystal display) and laser-based DLP (digital light projection) | 100–1000 200–500 | 18 [38] 150 [39] | <0.5 [40] <0.5 [39] | 0.5–1 |
| Material Extrusion | FDM (fused deposition modeling) | 200–800 | 40 [41] | <0.5 [42] | <0.5 |
| Material Jetting | PJM (PolyJet modeling) | 500–1500 | 54 [43] | 0.5 [44] | >1 |
| MJM (MultiJet modeling) | 100–500 | 100 [45] | 4 [46] | ||
| MJP (MultiJet printing) | 200–500 | 200 [47] | 3 [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyimah, N.; Scheler, O.; Rang, T.; Pardy, T. Can 3D Printing Bring Droplet Microfluidics to Every Lab?—A Systematic Review. Micromachines 2021, 12, 339. https://doi.org/10.3390/mi12030339
Gyimah N, Scheler O, Rang T, Pardy T. Can 3D Printing Bring Droplet Microfluidics to Every Lab?—A Systematic Review. Micromachines. 2021; 12(3):339. https://doi.org/10.3390/mi12030339
Chicago/Turabian StyleGyimah, Nafisat, Ott Scheler, Toomas Rang, and Tamas Pardy. 2021. "Can 3D Printing Bring Droplet Microfluidics to Every Lab?—A Systematic Review" Micromachines 12, no. 3: 339. https://doi.org/10.3390/mi12030339
APA StyleGyimah, N., Scheler, O., Rang, T., & Pardy, T. (2021). Can 3D Printing Bring Droplet Microfluidics to Every Lab?—A Systematic Review. Micromachines, 12(3), 339. https://doi.org/10.3390/mi12030339








