Biofabrication in Congenital Cardiac Surgery: A Plea from the Operating Theatre, Promise from Science
Abstract
1. Introduction
2. Objectives
- To summarize clinical scene and needs for biomaterials in congenital and pediatric cardiac surgery.
- To present the state of biofabrication relevant to congenital and pediatric cardiac surgery.
- To highlight avenues of translational research and collaboration.
3. Scope of the Problem
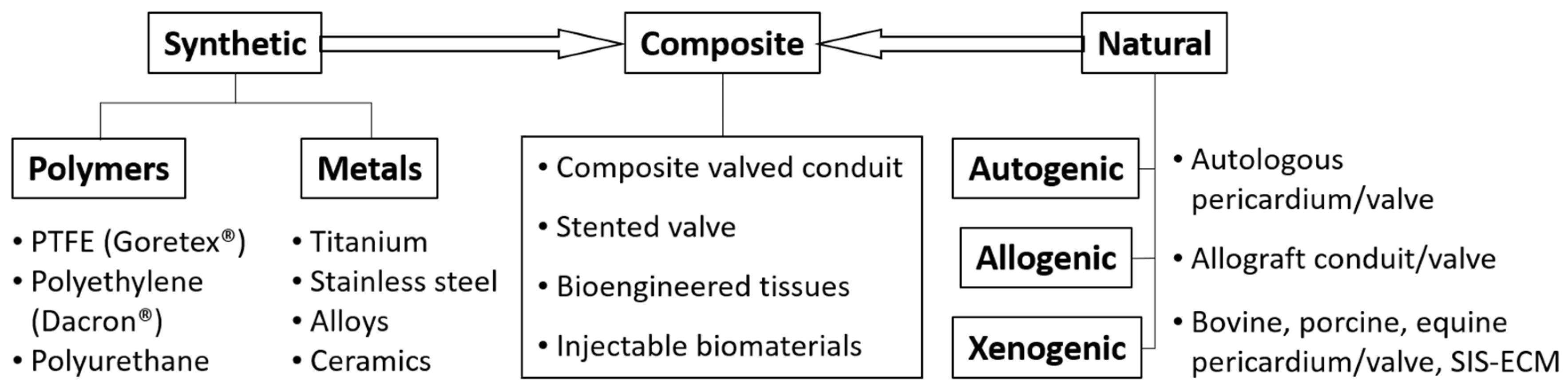
4. Biomaterials Clinically Utilized in Congenital Cardiac Surgery at Present
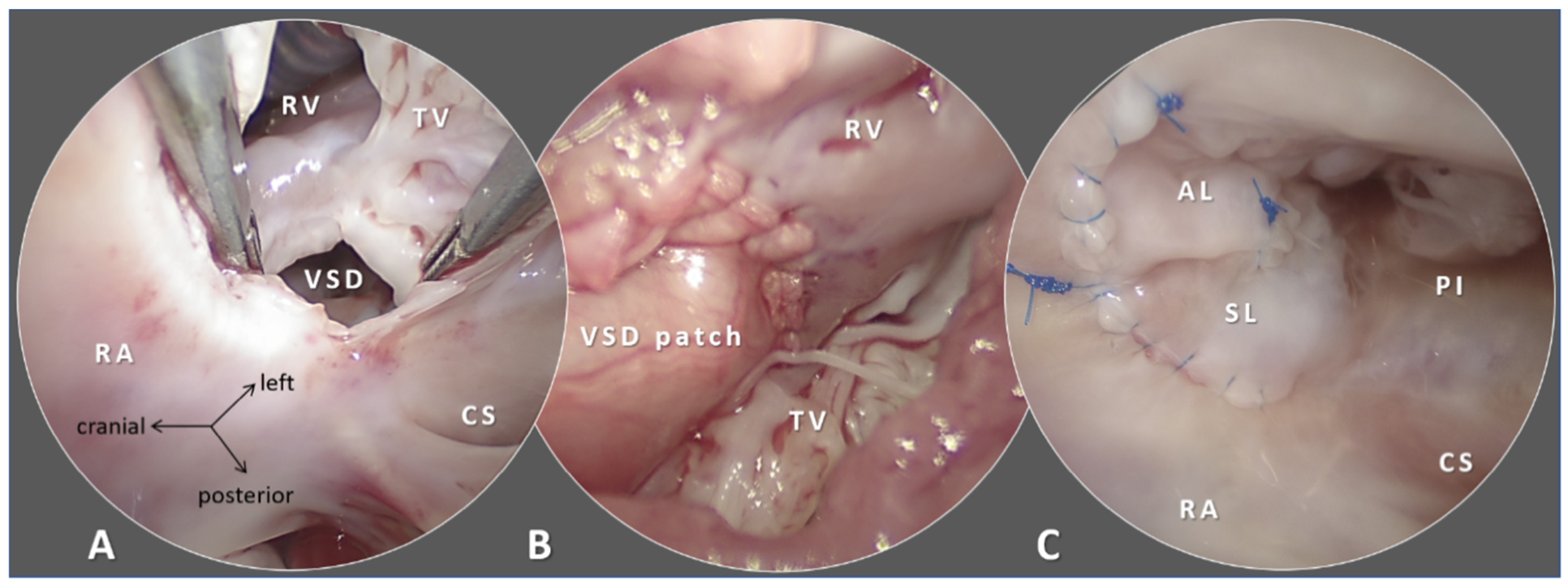
4.1. Patches: Predominantly Non-Cylindrical Reconstruction
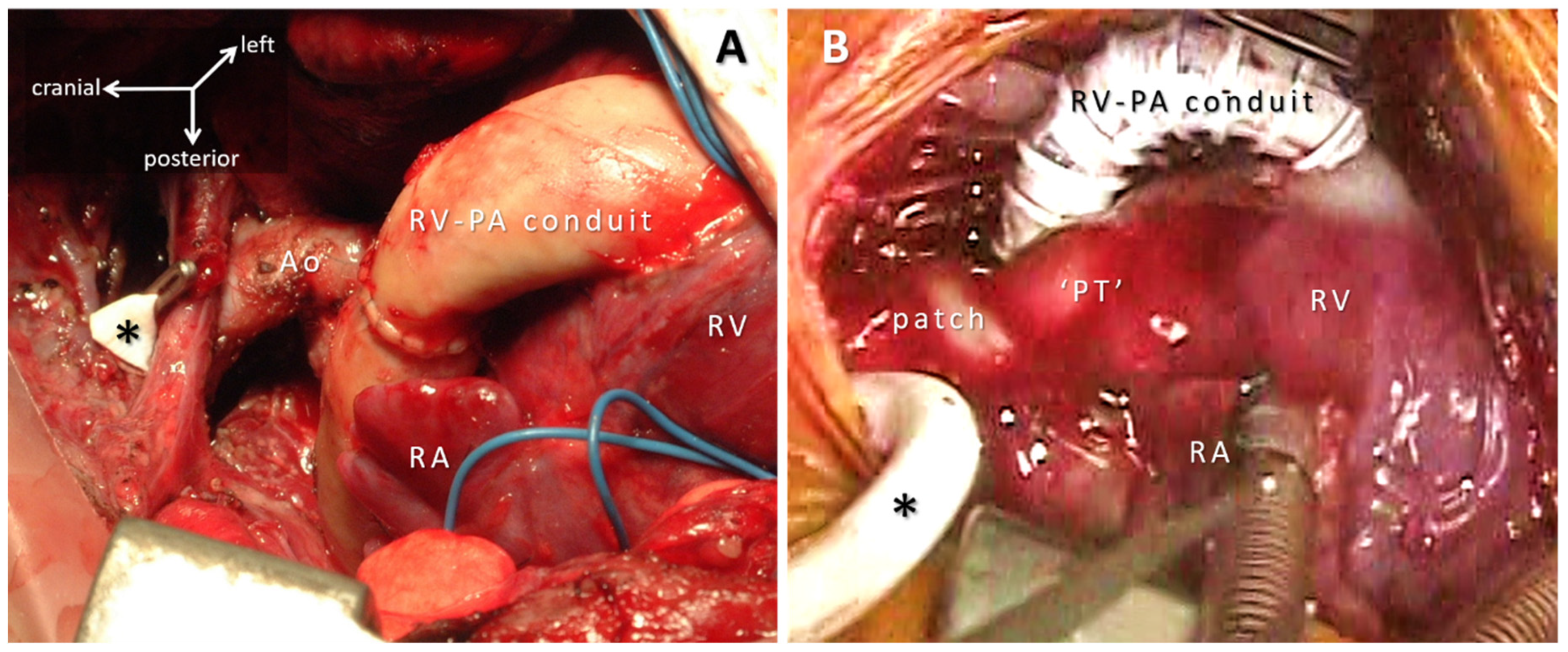
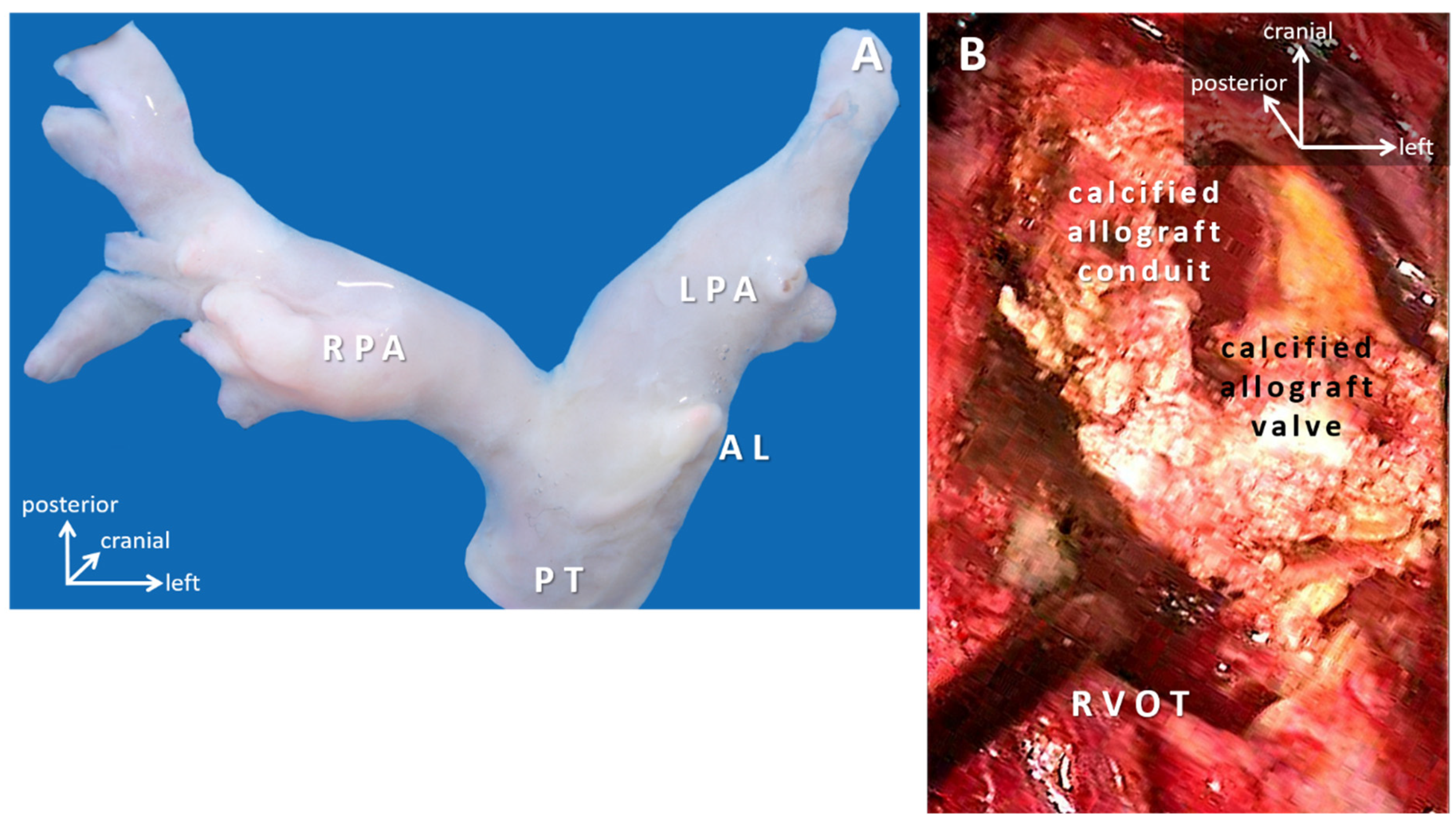
4.2. Conduits, Valved-Conduits: Cylindrical Reconstructions
4.3. Valve Replacement and Valve Reconstruction in Patients with Somatic Growth
4.4. Biodegradable Devices: Disappear Once Their Job Is Done
5. Biofabrication for Congenital Cardiac Surgery
5.1. Acellular versus Cellular Constructs
5.2. Tissue Engineering and Bioprinting
5.2.1. Replicating Biomimicry
5.2.2. Cells
5.2.3. Materials
5.2.4. The Role of the Basement Membrane (BM) and Growth Potential
- There are several points to consider before this approach could be successfully used for cardiac tissue replacements:
- While the first and most-important step in the embryonic development of cardiac tissues namely the epithelial-to-mesenchymal transition (EMT) is understood, the complete developmental process of cardiac tissues such as valves is not fully understood yet [88]. Efforts to appreciate the missing links in the developmental process will go a long way in replicating the organogenesis using stem cells in vitro or in vivo.
- An ideal ratio of CMs:ECs:CFs for optimal myocardial viability from various stem cell sources has to be determined. A similar ratio should be established for the cardiac valves and great arteries.
- Perfect biomimicry of the cardiac BM by novel biofabrication strategies such as bioprinting should be explored.
- Sources of stem cells that are ethically non-controversial and easily isolated such as UCSCs and their differentiation into CMs and other associated cell lineages require further studying.
- An ideal bioink for bioprinting cardiac tissues, that would have the right type and concentration of cells, growth factors, and polymers/hydrogels that would promote the native developmental biology signals and cues needs to be formulated.
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ASD | atrial septal defect |
| AVSD | atrioventricular septal defect |
| BM | basement membrane |
| BMSC | bone marrow-derived stem cell |
| CFs | cardiac fibroblasts |
| CHD | congenital heart disease |
| CMs | cardiomyocytes |
| ECs | endothelial cells |
| ePTFE | expanded polytetrafluoroethylene |
| HLA | human leukocyte antigen |
| iPSCs | induced pluripotent stem cells |
| LV | left ventricular |
| MAPCA | major aortopulmonary collateral artery |
| MPA | main pulmonary artery |
| MC | monocusp ventricular outflow patch |
| PDA | patent arterial duct |
| PV | pulmonary valve |
| RV | right ventricle |
| RVOT | right ventricular outflow tract |
| SIS-ECM | small intestine submucosa extracellular matrix |
| TAP | transannular patch |
| TGA | transposition of the great arteries |
| TOF | tetralogy of Fallot |
| UCSC | umbilical cord and placental stem cell |
| VSD | ventricular septal defect |
References
- Congenital Heart Disease Statistics 2006, British Heart Foundation. Available online: www.heartstats.org (accessed on 20 March 2008).
- Hoffman, J.I. The global burden of congenital heart disease: Review article. Cardiovasc. J. Afr. 2013, 24, 141–145. [Google Scholar] [CrossRef]
- Zimmerman, M.S.; Smith, A.G.C.; Sable, C.A.; Echko, M.M.; Wilner, L.B.; Olsen, H.E.; Atalay, H.T.; Awasthi, A.; Bhutta, Z.A.; Boucher, J.L.; et al. Global, regional, and national burden of congenital heart disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc. Health 2020, 4, 185–200. [Google Scholar] [CrossRef]
- Daebritz, S.H. Update in Adult Congenital Cardiac Surgery. Pediatr. Cardiol. 2007, 96–104. [Google Scholar] [CrossRef]
- Webb, G.D. Challenges in the care of adult patients with congenital heart defects. Heart 2003, 89, 465–469. [Google Scholar] [CrossRef]
- Wren, C.; O’Sullivan, J.J. Survival with congenital heart disease and need for follow-up in adult life. Heart 2001, 85, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Tynan, M.J.; Becker, A.E.; Macartney, F.J.; Jimenez, M.Q.; Shinebourne, E.A.; Anderson, R.H. Nomenclature and classification of congenital heart disease. Heart 1979, 41, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, A.R.; Mayer, J.E.; Jonas, R.A.; Lock, J.E.; Wessel, D.L.; Hickey, P.R. The neonate with critical congenital heart disease: Repair—A surgical challenge. J. Thorac. Cardiovasc. Surg. 1989, 98 Pt 2, 869–875. [Google Scholar] [CrossRef]
- Fontan, F.; Baudet, E. Surgical repair of tricuspid atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef]
- Norwood, W.I.; Kirklin, J.K.; Sanders, S.P. Hypoplastic left heart syndrome: Experience with palliative surgery. Am. J. Cardiol. 1980, 45, 87–91. [Google Scholar] [CrossRef]
- Zimmermann, W.-H.; Cesnjevar, R. Cardiac Tissue Engineering: Implications for Pediatric Heart Surgery. Pediatr. Cardiol. 2009, 30, 716–723. [Google Scholar] [CrossRef]
- Delius, R.E.; Rademecker, M.A.; De Leval, M.R.; Elliott, M.J.; Stark, J. Is a high-risk biventricular repair always preferable to conversion to a single ventricle repair? J. Thorac. Cardiovasc. Surg. 1996, 112, 1561–1569. [Google Scholar] [CrossRef]
- Ohuchi, H. Adult patients with Fontan circulation: What we know and how to manage adults with Fontan circulation? J. Cardiol. 2016, 68, 181–189. [Google Scholar] [CrossRef] [PubMed]
- D’Udekem, Y.; Iyengar, A.J.; Cochrane, A.D.; Grigg, L.; Ramsay, J.M.; Wheaton, G.R.; Penny, D.J.; Brizard, C.P. The Fontan Procedure: Contemporary Techniques Have Improved Long-Term Outcomes. Circulation 2007, 116, 157. [Google Scholar] [CrossRef]
- The Society of Thoracic Surgeons Congenital Heart Surgery Data Summary, All Patients STS Period Ending 12/31/2018. Available online: https://www.sts.org/sites/default/files/Congenital-STSExecSummary_AllPatients.pdf (accessed on 15 December 2020).
- Coulson, J.D.; Seddon, M.R.; Readdy, W.F. Advancing Safety in Pediatric Cardiology—Approaches Developed in Aviation. Congenit. Cardiol. Today 2008, 6, 1–10. Available online: https://www.congenitalcardiologytoday.com/index_files/CCT-MAR08-NA.pdf (accessed on 1 December 2020).
- Sutton, I. Process. Risk and Reliability Management, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Jacobs, J.P.; Mayer, J.E.J.; Mavroudis, C.; O’Brien, S.M.; Austin, E.H., 3rd; Pasquali, S.K.; Hill, K.D.; Overman, D.M.; St Louis, J.D.; Karamlou, T.; et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2017 Update on Outcomes and Quality. Ann. Thorac. Surg. 2017, 103, 699–709. [Google Scholar] [CrossRef]
- Chi-Square Calculator, Social Science Statistics. Available online: https://www.socscistatistics.com/tutorials/chisquare/default.aspx (accessed on 10 December 2020).
- Galantowicz, M. Encouraging Six-Month Results with Gore Novel Biosynthetic Tissue Valve. Available online: https://www.prnewswire.com/news-releases/w-l-gore--associates-announces-encouraging-six-month-results-with-its-novel-biosynthetic-tissue-valve-301180050.html (accessed on 15 December 2020).
- Jonas, R.A. Choosing the right biomaterial. In Comprehensive Surgical Management of Congenital Heart Disease, 2nd ed.; Jonas, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 247–266. [Google Scholar]
- Rastelli, G.C. A new approach to “anatomic” repair of transposition of the great arteries. Mayo Clin. Proc. 1969, 44, 1–12. [Google Scholar]
- Us, M.H.; Sungun, M.; Sanioglu, S.; Pocan, S.; Cebeci, B.S.; Ogus, T.; Ucak, A.; Guler, A. A Retrospective Comparison of Bovine Pericardium and Polytetrafluoroethylene Patch for Closure of Ventricular Septal Defects. J. Int. Med. Res. 2004, 32, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Maglione, J.; Bergersen, L.; Lock, J.E.; McElhinney, D.B. Ultra-High-Pressure Balloon Angioplasty for Treatment of Resistant Stenoses within or Adjacent to Previously Implanted Pulmonary Arterial Stents. Circ. Cardiovasc. Interv. 2009, 2, 52–58. [Google Scholar] [CrossRef]
- Schoof, P.H.; Hazekamp, M.G.; Van Ulzen, K.; Bartelings, M.M.; Bruyn, J.A.; Helbing, W.; Huysmans, H.A. Autologous pericardium for ventricular septal defect closure. J. Heart Valve Dis. 1998, 7, 407–409. [Google Scholar]
- Lam, M.T.; Wu, J.C. Biomaterial applications in cardiovascular tissue repair and regeneration. Expert Rev. Cardiovasc. Ther. 2012, 10, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Ferrans, V.J.; Jones, M.; Boyce, S.W.; Kawanami, O.; Roberts, W.C. Histologic and ultrastructural features of normal human parietal pericardium. Am. J. Cardiol. 1980, 46, 744–753. [Google Scholar] [CrossRef]
- Vitanova, K.; Cleuziou, J.; Von Ohain, J.P.; Burri, M.; Eicken, A.; Lange, R. Recoarctation After Norwood I Procedure for Hypoplastic Left Heart Syndrome: Impact of Patch Material. Ann. Thorac. Surg. 2017, 103, 617–621. [Google Scholar] [CrossRef]
- Herrmann, J.L.; Lewis, M.J.; Fuller, S.; Mascio, C.E. Aneurysm formation after the Norwood procedure: Case report and review of the literature. J. Thorac. Cardiovasc. Surg. 2014, 147, e55–e56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seybold-Epting, W.; Chiariello, L.; Hallman, G.L.; Cooley, D.A. Aneurysm of Pericardial Right Ventricular Outflow Tract Patches. Ann. Thorac. Surg. 1977, 24, 237–240. [Google Scholar] [CrossRef]
- Burkhart, H.M.; Moody, S.A.; Ensing, G.J.; Brown, J.W. Ventricular septal aneurysm after atrioventricular septal repair with pericardium. Ann. Thorac. Surg. 1996, 61, 1838–1839. [Google Scholar] [CrossRef]
- Kawashima, Y.; Nakano, S.; Kato, M.; Danno, M.; Sato, K. Fate of pericardium utilized for the closure of ventricular septal defect. Postoperative ventricular septal aneurysm. J. Thorac. Cardiovasc. Surg. 1974, 68, 209–218. [Google Scholar] [CrossRef]
- Bennink, G.B.; Hitchcock, F.J.; Molenschot, M.; Hutter, P.; Sreeram, N. Aneurysmal pericardial patch producing right ventricular inflow obstruction. Ann. Thorac. Surg. 2001, 71, 1346–1347. [Google Scholar] [CrossRef]
- Chanda, J. Prevention of calcification of heart valve bioprostheses: An experimental study in rat. Ann Thorac Surg. 1995, 60 (Suppl. S2), S339–S342. [Google Scholar] [CrossRef]
- Sinha, P.; Zurakowski, D.; Kumar, T.S.; He, D.; Rossi, C.; Jonas, R.A. Effects of glutaraldehyde concentration, pretreatment time, and type of tissue (porcine versus bovine) on postimplantation calcification. J. Thorac. Cardiovasc. Surg. 2012, 143, 224–227. [Google Scholar] [CrossRef]
- Vesely, I.; Mako, W.J. Comparison of the compressive buckling of porcine aortic valve cusps and bovine pericardium. J. Heart Valve Dis. 1998, 7, 34–39. [Google Scholar]
- Liao, K.; Frater, R.W.; LaPietra, A.; Ciuffo, G.; Ilardi, C.F.; Seifter, E. Time-dependent effect of glutaraldehyde on the tendency to calcify of both autografts and xenografts. Ann. Thorac. Surg. 1995, 60 (Suppl. S2), S343–S347. [Google Scholar] [CrossRef]
- Maizato, M.J.; Pires, M.D.; Canzian, M.; Higa, O.Z.; Pitombo, R.N.; Leirner, A.A. Histological Evaluation of Biocompatibility of Lyophilized Bovine Pericardium Implanted Subcutaneously in Rats. Artif. Organs 2008, 32, 268–271. [Google Scholar] [CrossRef]
- Huanglee, L.L.H.; Cheung, D.T.; Nimni, M.E. Biochemical-changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived cross-links. J. Biomed. Mater. Res. 1990, 24, 1185–1201. [Google Scholar] [CrossRef]
- Zouhair, S.; Sasso, E.D.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Neethling, W.M.; Strange, G.; Firth, L.; Smit, F.E. Evaluation of a tissue-engineered bovine pericardial patch in paediatric patients with congenital cardiac anomalies: Initial experience with the ADAPT-treated CardioCel® patch. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, M.; Cudak, E.; Mrówczyński, W.; Nałęcz, T.K.; Westerski, P.; Wojtalik, M. Application of the CardioCel bovine pericardial patch—A preliminary report. Pol. J. Cardio-Thorac. Surg. 2016, 3, 210–212. [Google Scholar] [CrossRef]
- Ashcraft, T.M.; Jones, K.; Border, W.L.; Eghtesady, P.; Pearl, J.M.; Khoury, P.R.; Manning, P.B. Factors Affecting Long-Term Risk of Aortic Arch Recoarctation After the Norwood Procedure. Ann. Thorac. Surg. 2008, 85, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.R. Invited letter concerning: Accelerated degeneration of aortic allografts in infants and young children. J. Thorac. Cardiovasc. Surg. 1994, 107, 1162–1164. [Google Scholar] [CrossRef]
- Chang, Q.; Jing, H.; Sun, M.; Xu, P. Exploring the role of short-course cyclosporin a therapy in preventing homograft valve calcification after transplantation. Cell. Immunol. 2014, 287, 36–45. [Google Scholar] [CrossRef]
- Hooper, D.K.; Hawkins, J.A.; Fuller, T.C.; Profaizer, T.; Shaddy, R.E. Panel-reactive antibodies late after allograft implantation in children. Ann. Thorac. Surg. 2005, 79, 641–644. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Quintessenza, J.A.; Boucek, R.J.; Morell, V.O.; Botero, L.M.; Badhwar, V.; Van Gelder, H.M.; Asante-Korang, A.; McCormack, J.; Daicoff, G.R. Pediatric Cardiac Transplantation in Children with High Panel Reactive Antibody. Ann. Thorac. Surg. 2004, 78, 1703–1709. [Google Scholar] [CrossRef]
- Mahle, W.T.; Hu, C.; Trachtenberg, F.; Menteer, J.; Kindel, S.J.; Dipchand, A.I.; Richmond, M.E.; Daly, K.P.; Henderson, H.T.; Lin, K.Y.; et al. Heart failure after the Norwood procedure: An analysis of the Single Ventricle Reconstruction Trial. J. Heart Lung Transplant. 2018, 37, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.; Ruzmetov, M.; Huynh, D.; Rodefeld, M.D.; Turrentine, M.W.; Fiore, A.C. Rastelli Operation for Transposition of the Great Arteries with Ventricular Septal Defect and Pulmonary Stenosis. Ann. Thorac. Surg. 2011, 91, 188–194. [Google Scholar] [CrossRef] [PubMed]
- De Level, M.R.; McKay, R.; Jones, M.; Stark, J.; Macartney, F.J. Modified Blalock-Taussig shunt. Use of subclavian artery orifice as flow regulator in prosthetic systemic-pulmonary artery shunts. J. Thorac. Cardiovasc. Surg. 1981, 81, 112–119. [Google Scholar]
- Sano, S.; Ishino, K.; Kawada, M.; Yoshizumi, K.; Takeuchi, M.; Ohtsuki, S.-I. Experience over five years using a shunt placed between the right ventricle and the pulmonary arteries during initial reconstruction of hypoplasia of the left heart. Cardiol. Young 2004, 14 (Suppl. S3), 90–95. [Google Scholar] [CrossRef][Green Version]
- Marcelletti, C.; Corno, A.; Giannico, S.; Marino, B. Inferior vena cava-pulmonary artery extracardiac conduit. A new form of right heart bypass. J. Thorac. Cardiovasc. Surg. 1990, 100, 228–232. [Google Scholar] [CrossRef]
- Dasi, L.P.; Pekkan, K.; Katajima, H.D.; Yoganathan, A.P. Functional analysis of Fontan energy dissipation. J. Biomech. 2008, 41, 2246–2252. [Google Scholar] [CrossRef]
- Corno, A.F.; Owen, M.J.; Cangiani, A.; Hall, E.J.C.; Rona, A. Physiological Fontan Procedure. Front. Pediatr. 2019, 7. [Google Scholar] [CrossRef]
- Ross, D. Homograft Replacement of the Aortic Valve. Lancet 1962, 280, 487. [Google Scholar] [CrossRef]
- Yankah, A.C.; Alexi-Meskhishvili, V.; Weng, Y.; Berger, F.; Lange, P.; Hetzer, R. Performance of aortic and pulmonary homografts in the right ventricular outflow tract in children. J. Heart Valve Dis. 1995, 4, 392–395. [Google Scholar]
- Mayer, J.E. Uses of homograft conduits for right ventricle to pulmonary artery connections in the neonatal period. Semin. Thorac. Cardiovasc. Surg. 1995, 7, 130–132. [Google Scholar] [PubMed]
- Mitchell, R.N.; Jonas, R.A.; Schoen, F.J. Structure-function correlations in cryopreserved allograft cardiac valves. Ann. Thorac. Surg. 1995, 60, S108–S113. [Google Scholar] [CrossRef]
- Rajani, B.; Mee, R.B.; Ratliff, N.B. Evidence for rejection of homograft cardiac valves in infants. J. Thorac. Cardiovasc. Surg. 1998, 115, 111–117. [Google Scholar] [CrossRef]
- Baskett, R.J.; Ross, D.B.; Nanton, M.A.; Murphy, D.A. Factors in the early failure of cryopreserved homograft pulmonary valves in children: Preserved immunogenicity? J. Thorac. Cardiovasc. Surg. 1996, 112, 1170–1179. [Google Scholar] [CrossRef]
- Forbess, J.M. Conduit selection for right ventricular outflow tract reconstruction: Contemporary options and outcomes. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2004, 7, 115–124. [Google Scholar] [CrossRef]
- Göber, V.; Berdat, P.; Pavlovic, M.; Pfammatter, J.-P.; Carrel, T.P. Adverse Mid-Term Outcome Following RVOT Reconstruction Using the Contegra Valved Bovine Jugular Vein. Ann. Thorac. Surg. 2005, 79, 625–631. [Google Scholar] [CrossRef]
- Brown, J.W.; Ruzmetov, M.; Rodefeld, M.D.; Vijay, P.; Darragh, R.K. Valved Bovine Jugular Vein Conduits for Right Ventricular Outflow Tract Reconstruction in Children: An Attractive Alternative to Pulmonary Homograft. Ann. Thorac. Surg. 2006, 82, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Rastan, A.J.; Walther, T.; Daehnert, I.; Hambsch, J.; Mohr, F.W.; Janousek, J.; Kostelka, M. Bovine Jugular Vein Conduit for Right Ventricular Outflow Tract Reconstruction: Evaluation of Risk Factors for Mid-Term Outcome. Ann. Thorac. Surg. 2006, 82, 1308–1315. [Google Scholar] [CrossRef]
- Pearl, J.M.; Cooper, D.S.; Bove, K.E.; Manning, P.B. Early failure of the shelhigh pulmonary valve conduit in infants. Ann. Thorac. Surg. 2002, 74, 542–549. [Google Scholar] [CrossRef]
- Yuan, S.-M.; Mishaly, D.; Shinfeld, A.; Raanani, E. Right ventricular outflow tract reconstruction: Valved conduit of choice and clinical outcomes. J. Cardiovasc. Med. 2008, 9, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Falk, V.; Baumgartner, H.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardio-Thorac. Surg. 2017, 52, 616–664. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef]
- Siddiqui, R.F.; Abraham, J.R.; Butany, J. Bioprosthetic heart valves: Modes of failure. Histopatholology 2009, 55, 135–144. [Google Scholar] [CrossRef]
- Durko, A.P.; Head, S.J.; Pibarot, P.; Atluri, P.; Bapat, V.; Cameron, D.E.; Casselman, F.P.A.; Chen, E.P.; Dahle, G.; Ebels, T.; et al. Characteristics of surgical prosthetic heart valves and problems around labelling: A document from the European Association for Cardio-Thoracic Surgery (EACTS)—The Society of Thoracic Surgeons (STS)—American Association for Thoracic Surgery (AATS) Valve Labelling Task Force. Eur. J. Cardio-Thorac. Surg. 2019, 55, 1025–1036. [Google Scholar] [CrossRef]
- Tasca, G.; Vismara, R.; Trinca, F.; Riva, B.; Gamba, A.; Lobiati, E. Opening/closing pattern of Trifecta and Freestyle valves versus native aortic valve: Are stentless valves more physiologic than a stented valve? J. Card. Surg. 2017, 32, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Leonardo da Vinci. Moment of closure of the aortic valve. Clark 19082r (detail), cca. 1513. In Leonardo Da Vinci on the Human Anatomy; Leonardo’s Anatomic Drawings and Notes; O’Malley, C.D., Saunders, C.M., Eds.; Gramercy: New York, NY, USA, 2003; p. 78. [Google Scholar]
- Pasquali, S.K.; Cohen, M.S.; Shera, D.; Wernovsky, G.; Spray, T.L.; Marino, B.S. The Relationship Between Neo-Aortic Root Dilation, Insufficiency, and Reintervention Following the Ross Procedure in Infants, Children, and Young Adults. J. Am. Coll. Cardiol. 2007, 49, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Bové, T.; Bradt, N.; Martens, T.; De Wolf, D.; François, K.; de Beco, G.; Sluysmans, T.; Rubay, J.; Poncelet, A. The Pulmonary Autograft After the Ross Operation: Results of 25-Year Follow-Up in a Pediatric Cohort. Ann. Thorac. Surg. 2021, 111, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Durko, A.P.; Yacoub, M.H.; Kluin, J. Tissue Engineered Materials in Cardiovascular Surgery: The Surgeon’s Perspective. Front. Cardiovasc. Med. 2020, 7. [Google Scholar] [CrossRef]
- Odim, J.; Laks, H.; Allada, V.; Child, J.; Wilson, S.; Gjertson, D. Results of Aortic Valve-Sparing and Restoration with Autologous Pericardial Leaflet Extensions in Congenital Heart Disease. Ann. Thorac. Surg. 2005, 80, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Pattar, S.S.; Hassanabad, A.F.; Fedak, P.W.M. Acellular Extracellular Matrix Bioscaffolds for Cardiac Repair and Regeneration. Front. Cell Dev. Biol. 2019, 7, 63. [Google Scholar] [CrossRef]
- Al Halees, Z.; Al Shahid, M.; Al Sanei, A.; Sallehuddin, A.; Duran, C. Up to 16 years follow-up of aortic valve reconstruction with pericardium: A stentless readily available cheap valve? Eur. J. Cardio-Thorac. Surg. 2005, 28, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Bacha, E.A.; Satou, G.M.; Moran, A.M.; Zurakowski, D.; Marx, G.R.; Keane, J.F.; Jonas, R.A. Valve-sparing operation for balloon-induced aortic regurgitation in congenital aortic stenosis. J. Thorac. Cardiovasc. Surg. 2001, 122, 162–168. [Google Scholar] [CrossRef][Green Version]
- Ozaki, S.; Kawase, I.; Yamashita, H.; Uchida, S.; Nozawa, Y.; Takatoh, M.; Hagiwara, S. A total of 404 cases of aortic valve reconstruction with glutaraldehyde-treated autologous pericardium. J. Thorac. Cardiovasc. Surg. 2014, 147, 301–306. [Google Scholar] [CrossRef]
- D’Udekem, Y. Aortic valve repair in children. Ann. Cardiothorac. Surg. 2013, 2, 100–104. [Google Scholar] [CrossRef]
- Yamagishi, M.; Kurosawa, H. Outflow reconstruction of tetralogy of Fallot using a Gore-Tex valve. Ann. Thorac. Surg. 1993, 56, 1414–1416. [Google Scholar] [CrossRef]
- Kaza, A.K.; Lim, H.-G.; DiBardino, D.J.; Bautista-Hernandez, V.; Robinson, J.; Allan, C.; Laussen, P.; Fynn-Thompson, F.; Bacha, E.; Del Nido, P.J.; et al. Long-term results of right ventricular outflow tract reconstruction in neonatal cardiac surgery: Options and outcomes. J. Thorac. Cardiovasc. Surg. 2009, 138, 911–916. [Google Scholar] [CrossRef]
- Samadi, M.; Khoshfetrat, M.; Keykha, A.; Javadi, S.H. The effects of monocusp valve implantation and transannular patch angioplasty on pulmonary regurgitation and right ventricular failure after total correction of tetralogy of fallot. Biomed. Res. Ther. 2020, 7, 3799–3806. [Google Scholar] [CrossRef]
- Brown, J.W.; Ruzmetov, M.; Vijay, P.; Rodefeld, M.D.; Turrentine, M.W. Right ventricular outflow tract reconstruction with a polytetrafluoroethylene monocusp valve: A twelve-year experience. J. Thorac. Cardiovasc. Surg. 2007, 133, 1336–1343. [Google Scholar] [CrossRef]
- Hoerstrup, S.P.; Sodian, R.; Daebritz, S.; Wang, J.; Bacha, E.A.; Martin, D.P.; Moran, A.M.; Guleserian, K.J.; Sperling, J.S.; Kaushal, S.; et al. Functional Living Trileaflet Heart Valves Grown In Vitro. Circulation 2000, 102 (Suppl. S3), 44–49. [Google Scholar] [CrossRef]
- Chester, A.H.; Grande-Allen, K.J. Which Biological Properties of Heart Valves Are Relevant to Tissue Engineering? Front. Cardiovasc. Med. 2020, 7, 63. [Google Scholar] [CrossRef]
- Sewell-Loftin, M.K.; Chun, Y.W.; Khademhosseini, A.; Merryman, W.D. EMT-Inducing Biomaterials for Heart Valve Engineering: Taking Cues from Developmental Biology. J. Cardiovasc. Transl. Res. 2011, 4, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Garcia, A.; Oliver-De-La-Cruz, J.; Mirabet, V.; Gandía, C.; Villagrasa, A.; Sodupe, E.; Escobedo-Lucea, C. Heart valve tissue engineering: How far is the bedside from the bench? Expert Rev. Mol. Med. 2015, 17, e16. [Google Scholar] [CrossRef]
- Hibino, N.; McGillicuddy, E.; Matsumura, G.; Ichihara, Y.; Naito, Y.; Breuer, C.; Shinoka, T. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 2010, 139, 431–436. [Google Scholar] [CrossRef]
- Shoji, T.; Shinoka, T. Tissue engineered vascular grafts for pediatric cardiac surgery. Transl. Pediatr. 2018, 7, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.; Geddes, L.; Obermiller, J. Extracellular Matrix for Myocardial Repair. Heart Surg. Forum 2003, 6, 20. [Google Scholar] [CrossRef]
- Crapo, P.M.; Wang, Y. Small intestinal submucosa gel as a potential scaffolding material for cardiac tissue engineering. Acta Biomater. 2010, 6, 2091–2096. [Google Scholar] [CrossRef]
- Tottey, S.; Johnson, S.A.; Crapo, P.M.; Reing, J.E.; Zhang, L.; Jiang, H.; Medberry, C.J.; Reines, B.; Badylak, S.F. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials 2011, 32, 128–136. [Google Scholar] [CrossRef]
- Huang, Y.; Wong, Y.S.; Ng, H.C.A.; Boey, F.Y.C.; Venkatraman, S. Translation in cardiovascular stents and occluders: From biostable to fully degradable. Bioeng. Transl. Med. 2017, 2, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, S.; Luo, Z.; Butera, G.; Cao, Q.; Zhang, F.; Lu, M.; Wang, S.; Wang, W.; Pan, X. First-in-Human Experience with a Novel Fully Bioabsorbable Occluder for Ventricular Septal Defect. JACC Cardiovasc. Interv. 2020, 13, 1139–1141. [Google Scholar] [CrossRef]
- Mirensky, T.L.; Breuer, C.K. The Development of Tissue-Engineered Grafts for Reconstructive Cardiothoracic Surgical Applications. Pediatr. Res. 2008, 63, 559–568. [Google Scholar] [CrossRef]
- VijayaVenkataRaman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- VijayaVenkataRaman, S. A Perspective on Bioprinting Ethics. Artif. Organs 2016, 40, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.C.; Kundu, J.; Subia, B. Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications. In Tissue Engineering; Intech: Rijeka, Croatia, 2010. [Google Scholar] [CrossRef]
- Teoh, J.H.; Thamizhchelvan, A.M.; Davoodi, P.; Ramasamy, S.; VijayaVenkataRaman, S.; Yang, Q.; DiColandrea, T.; Zhao, H.; Fuh, J.Y.; Liou, Y.-C.; et al. Investigation of the application of a Taylor-Couette bioreactor in the post-processing of bioprinted human dermal tissue. Biochem. Eng. J. 2019, 151, 107317. [Google Scholar] [CrossRef]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.L.Y.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, e1800672. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, M.; Chapman, D.; Babyn, P.; Chen, X.; Kelly, M.E. UV-Assisted 3D Bioprinting of Nanoreinforced Hybrid Cardiac Patch for Myocardial Tissue Engineering. Tissue Eng. Part C Methods 2018, 24, 74–88. [Google Scholar] [CrossRef]
- Ong, C.S.; Fukunishi, T.; Zhang, H.; Huang, C.Y.; Nashed, A.; Blazeski, A.; DiSilvestre, D.; Vricella, L.; Conte, J.; Tung, L.; et al. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, S.J.; Cheng, H.-J.; Yoo, J.J.; Atala, A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Lerman, A. Bioprinting a cardiac valve. Biotechnol. Adv. 2015, 33, 1503–1521. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2012, 101, 1255–1264. [Google Scholar] [CrossRef]
- Duan, B.; Kapetanovic, E.; Hockaday, L.; Butcher, J. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014, 10, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Hoch, E.; Tovar, G.E.; Borchers, K. Bioprinting of artificial blood vessels: Current approaches towards a demanding goal. Eur. J. Cardio-Thorac. Surg. 2014, 46, 767–778. [Google Scholar] [CrossRef]
- Zhou, X.; Nowicki, M.; Sun, H.; Hann, S.Y.; Cui, H.; Esworthy, T.; Lee, J.D.; Plesniak, M.; Zhang, L.G. 3D Bioprinting-Tunable Small-Diameter Blood Vessels with Biomimetic Biphasic Cell Layers. ACS Appl. Mater. Interfaces 2020, 12, 45904–45915. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.Y.; Lee, K.-X.A.; Kuo, C.-N.; Shen, Y.-F. Bioprinting of artificial blood vessels. Int. J. Bioprint. 2018, 4. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
- Capulli, A.K.; Emmert, M.Y.; Pasqualini, F.S.; Kehl, D.; Caliskan, E.; Lind, J.U.; Sheehy, S.P.; Park, S.J.; Ahn, S.; Weber, B.; et al. JetValve: Rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement. Biomaterials 2017, 133, 229–241. [Google Scholar] [CrossRef]
- Weber, B.; Dijkman, P.E.; Scherman, J.; Sanders, B.B.; Emmert, M.Y.; Grünenfelder, J.; Verbeek, R.; Bracher, M.; Black, M.; Franz, T.; et al. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials 2013, 34, 7269–7280. [Google Scholar] [CrossRef]
- Driessen-Mol, A.; Emmert, M.Y.; Dijkman, P.E.; Frese, L.; Sanders, B.; Weber, B.; Cesarovic, N.; Sidler, M.; Leenders, J.; Jenni, R.; et al. Transcatheter Implantation of Homologous “Off-the-Shelf” Tissue-Engineered Heart Valves with Self-Repair Capacity. J. Am. Coll. Cardiol. 2014, 63, 1320–1329. [Google Scholar] [CrossRef]
- Dijkman, P.E.; Driessen-Mol, A.A.; Frese, L.; Hoerstrup, S.P.; Baaijens, F.P. Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 2012, 33, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Hobson, C.M.; Amoroso, N.J.; Amini, R.; Ungchusri, E.; Hong, Y.; D’Amore, A.; Sacks, M.S.; Wagner, W.R. Fabrication of elastomeric scaffolds with curvilinear fibrous structures for heart valve leaflet engineering. J. Biomed. Mater. Res. Part A 2015, 103, 3101–3106. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Zhang, M.; Bartosek, T.; Murry, C.E. Survival, Integration, and Differentiation of Cardiomyocyte Grafts: A study in normal and injured rat hearts. Circulation 1999, 100, 193–202. [Google Scholar] [CrossRef]
- Roche, C.D.; Brereton, R.J.L.; Ashton, A.W.; Jackson, C.; Gentile, C. Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery. Eur. J. Cardio-Thorac. Surg. 2020, 58, 500–510. [Google Scholar] [CrossRef]
- Soman, S.S.; VijayaVenkataRaman, S. Applications of 3D Bioprinted-Induced Pluripotent Stem Cells in Healthcare. Int. J. Bioprint. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Alatyyat, S.M.; Alasmari, H.M.; Aleid, O.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Umbilical cord stem cells: Background, processing and applications. Tissue Cell 2020, 65, 101351. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, H.E.; Smith, F.O. Cord Blood Hematopoietic Cell Transplantation. In Thomas’ Hematopoietic Cell Transplantation: Stem Cell Transplantation; Blackwell Publishing: Hoboken, NJ, USA, 2009; pp. 559–576. [Google Scholar] [CrossRef]
- Hunt, C.J. Cryopreservation of Human Stem Cells for Clinical Application: A Review. Transfus. Med. Hemotherapy 2011, 38, 107–123. [Google Scholar] [CrossRef]
- Weiss, M.L.; Troyer, D.L. Stem cells in the umbilical cord. Stem Cell Rev. Rep. 2006, 2, 155–162. [Google Scholar] [CrossRef]
- Kim, S.-W.; Han, H.; Chae, G.-T.; Lee, S.-H.; Bo, S.; Yoon, J.-H.; Lee, Y.-S.; Lee, K.-S.; Park, H.-K.; Kang, K.-S. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem Cells 2006, 24, 1620–1626. [Google Scholar] [CrossRef]
- Ichim, T.E.; Solano, F.; Brenes, R.; Glenn, E.; Chang, J.; Chan, K.; Riordan, N.H. Placental mesenchymal and cord blood stem cell therapy for dilated cardiomyopathy. Reprod. Biomed. Online 2008, 16, 898–905. [Google Scholar] [CrossRef]
- Roura, S.; Pujal, J.M.; Gálvez-Montón, C.; Bayes-Genis, A. Impact of Umbilical Cord Blood-Derived Mesenchymal Stem Cells on Cardiovascular Research. BioMed Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Mummery, C.L.; Zhang, J.; Ng, E.S.; Elliott, D.A.; Elefanty, A.G.; Kamp, T.J. Differentiation of Human Embryonic Stem Cells and Induced Pluripotent Stem Cells to Cardiomyocytes. Circ. Res. 2012, 111, 344–358. [Google Scholar] [CrossRef]
- Csöbönyeiová, M.; Polák, Š.; Danišovič, L. Perspectives of induced pluripotent stem cells for cardiovascular system regeneration. Exp. Biol. Med. 2015, 240, 549–556. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef]
- Sawkins, M.J.; Saldin, L.T.; Badylak, S.F.; White, L.J. ECM Hydrogels for Regenerative Medicine. In Extracellular Matrix for Tissue Engineering and Biomaterials; Berardi, A., Ed.; Humana Press: Cham, Switzerland, 2018; pp. 27–58. [Google Scholar] [CrossRef]
- Waters, R.; Alam, P.; Pacelli, S.; Chakravarti, A.R.; Ahmed, R.P.; Paul, A. Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater. 2018, 69, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C. Filling the Gaps between the In Vivo and In Vitro Microenvironment: Engineering of Spheroids for Stem Cell Technology. Curr. Stem Cell Res. Ther. 2016, 11, 652–665. [Google Scholar] [CrossRef]
- Polonchuk, L.; Chabria, M.; Badi, L.; Hoflack, J.-C.; Figtree, G.; Davies, M.J.; Gentile, C. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Campbell, M.; Chabria, M.; Figtree, G.A.; Polonchuk, L.; Gentile, C. Stem Cell-Derived Cardiac Spheroids as 3D In Vitro Models of the Human Heart Microenvironment. In Stem Cell Niche; Humana: New York, NY, USA, 2018; Volume 2002, pp. 51–59. [Google Scholar] [CrossRef]
- Mawad, D.; Mansfield, C.; Lauto, A.; Perbellini, F.; Nelson, G.W.; Tonkin, J.; Bello, S.O.; Carrad, D.J.; Micolich, A.P.; Mahat, M.M.; et al. A conducting polymer with enhanced electronic stability applied in cardiac models. Sci. Adv. 2016, 2, e1601007. [Google Scholar] [CrossRef] [PubMed]
- VijayaVenkataRaman, S.; Kannan, S.; Cao, T.; Fuh, J.Y.H.; Sriram, G.; Lu, W.F. 3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2019, 7, 266. [Google Scholar] [CrossRef]
- VijayaVenkataRaman, S.; Vialli, N.; Fuh, J.Y.H.; Lu, W.F. Conductive Collagen/PPy-b-PCL hydrogel for bioprinting of neural tissue constructs. Int. J. Bioprint. 2019, 5, 229. [Google Scholar] [CrossRef]
- Qazi, T.H.; Rai, R.; Dippold, D.; Roether, J.E.; Schubert, D.W.; Rosellini, E.; Barbani, N.; Boccaccini, A.R. Development and characterization of novel electrically conductive PANI–PGS composites for cardiac tissue engineering applications. Acta Biomater. 2014, 10, 2434–2445. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Hu, T.; Guo, B.; Ma, P.X. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. 2017, 59, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, F.F.; Contard, A.; Sabri, J.; Samuel, L. Rappaport, Fibronectin and basement membrane in cardiovascular organogenesis and disease pathogenesis. Cardiovasc. Res. 1996, 32, 433–442. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Amenta, P.S.; Patton, B.L. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004, 22, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Borg, T.K.; Liu, H.; Gao, B.Z. Interactive relationship between basement-membrane development and sarcomerogenesis in single cardiomyocytes. Exp. Cell Res. 2015, 330, 222–232. [Google Scholar] [CrossRef]
- Yang, H.; Borg, T.K.; Wang, Z.; Ma, Z.; Gao, B.Z. Role of the basement membrane in regulation of cardiac electrical properties. Ann. Biomed. Eng. 2014, 42, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Cunniffe, G.M.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering. Adv. Healthc. Mater. 2016, 5, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Yano, K.; Hata, Y.; Nishibayashi, A.; Hosokawa, K. DIEP Flap Breast Reconstruction Using 3-dimensional Surface Imaging and a Printed Mold. Plast. Reconstr. Surg. Glob. Open 2015, 3, e316. [Google Scholar] [CrossRef]
- Cohen, A.; Laviv, A.; Berman, P.; Nashef, R.; Abu-Tair, J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 661–666. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Prathep, S.; Sharma, D.; Fujita, Y.; Armstead, W.; Vavilala, M.S. Cardiac dysfunction following brain death after severe pediatric traumatic brain injury: A preliminary study of 32 children. Int. J. Crit. Illn. Inj. Sci. 2015, 5, 103–107. [Google Scholar] [CrossRef]
- Cooper, D.K.C. A Brief History of Cross-Species Organ Transplantation. Bayl. Univ. Med. Cent. Proc. 2012, 25, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Forgach, G.; Newman, S.A. Biological Physics of the Developing Embryo; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar] [CrossRef]

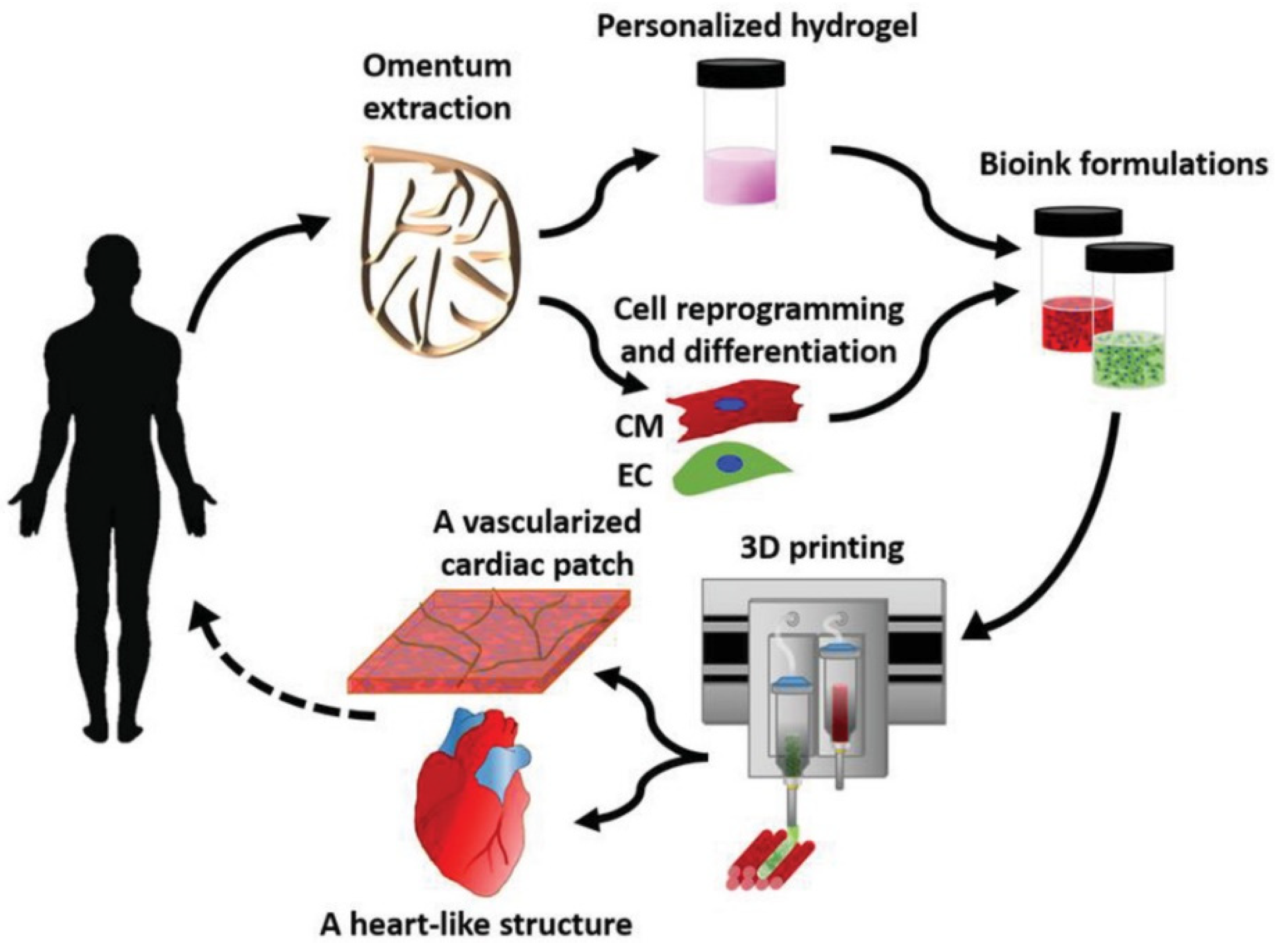
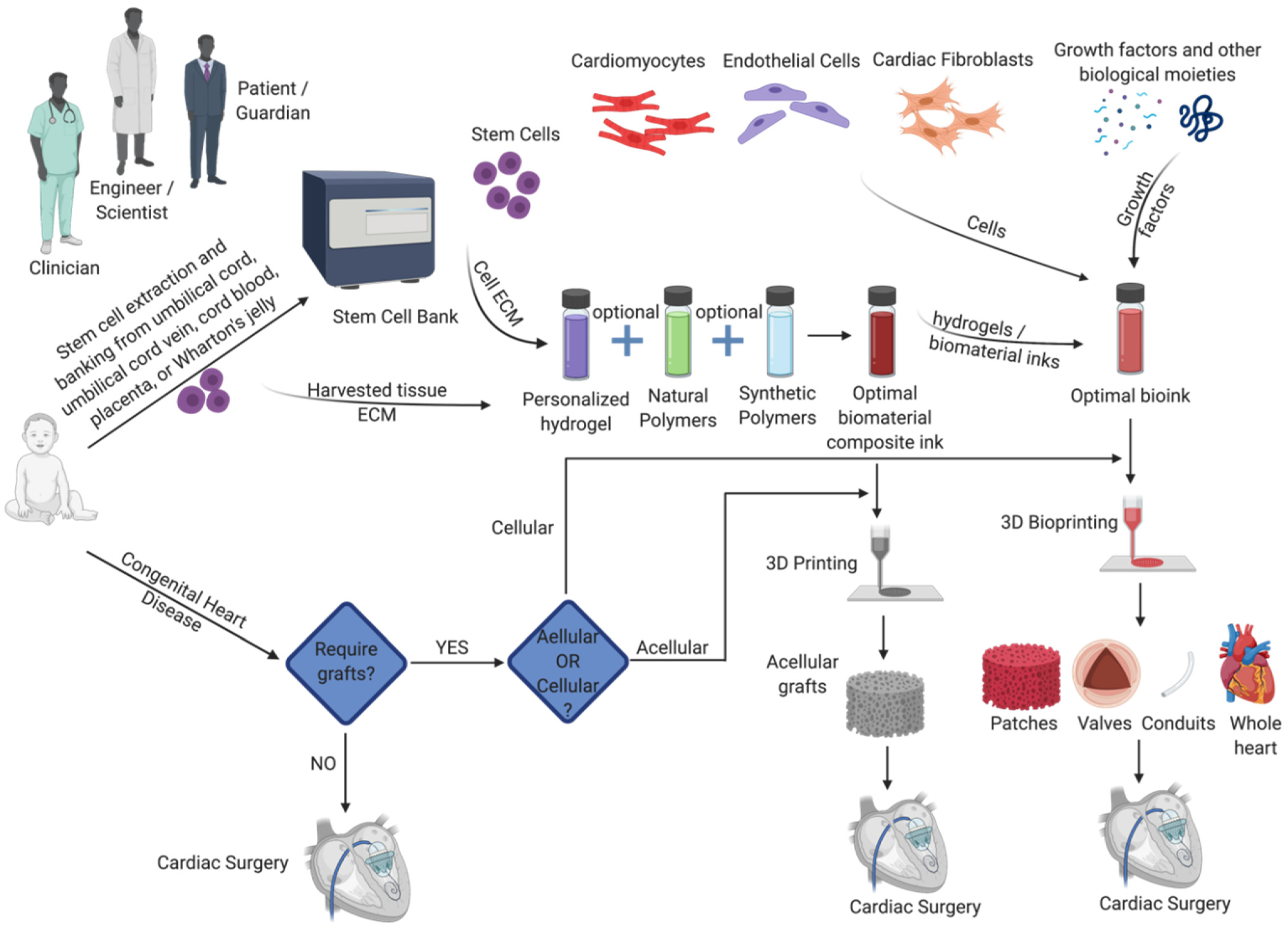
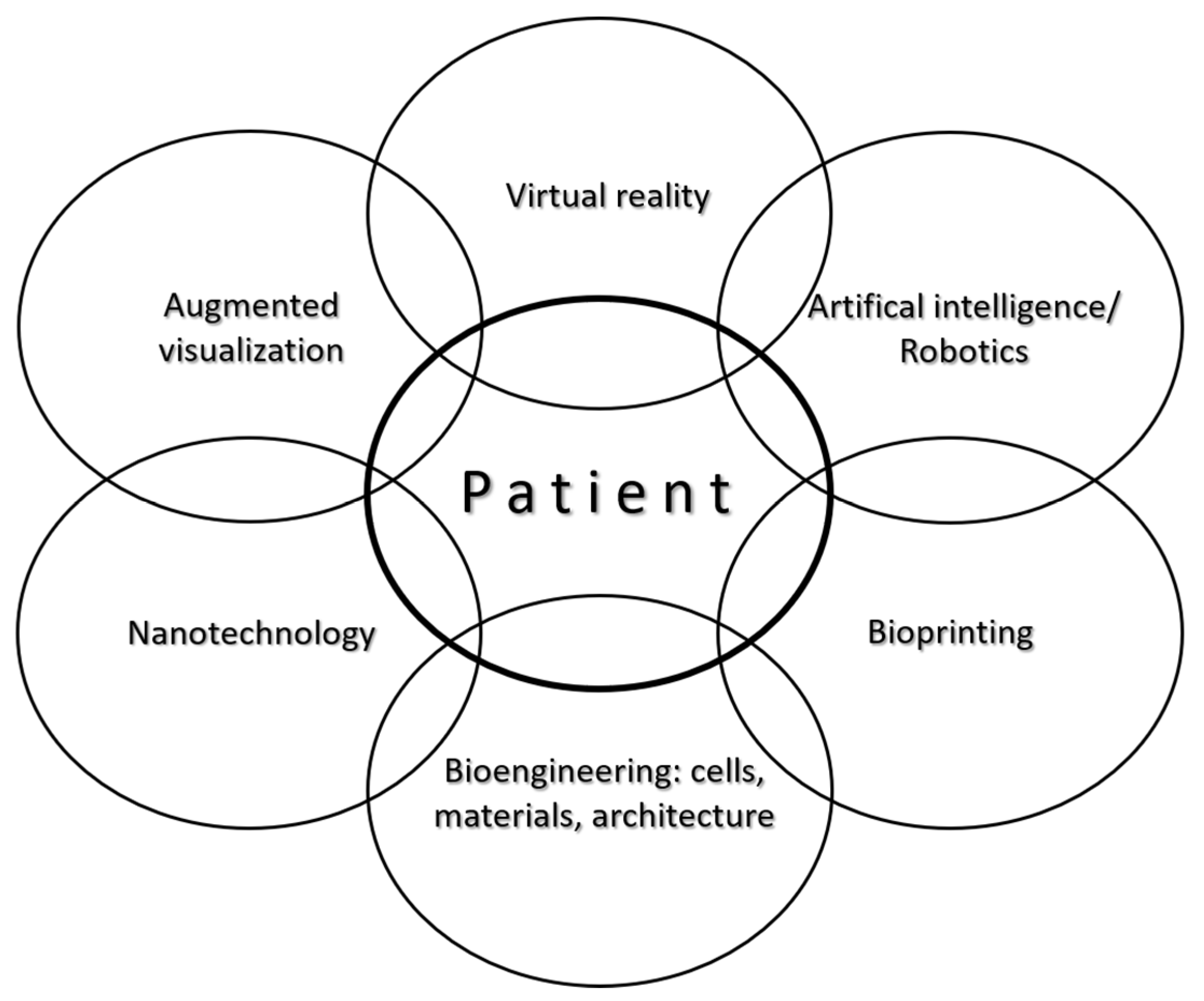
| Type of Construct | Clinical Scenarios | Specific Aspects and Anticipated Advantages |
|---|---|---|
| Acellular | Defect closure: ASD, VSD, etc. Intracardiac baffles: atrial separation (Mustard); intraventricular rerouting double outlet right ventricle (DORV); extracardiac: aortic arch repair | Structural robustness; flexibility; adaptability |
| Cellular | Regeneration of cardiac segments and structures: myocardium (univentricular heart), valves (pulmonary-, aortic atresia), vessels (pulmonary artery reconstruction) | Tissue integration, regeneration and growth |
| Composite | Conduits and valves to bridge gap (pulmonary atresia), augment hypoplastic and replace missing segments | Tissue integration, regeneration and growth |
| Naturally Derived Polymers | Synthetic Polymers | |
|---|---|---|
| Examples | Gelatin, chitosan, fibrin, alginate | Polylactic acid (PLA), polyglycolic acid (PGA), their copolymer: PLGA; polydioxanone (PDO, PDS) |
| Advantages | General availability, excellent biocompatibility; autologous (fibrin); improved cell adhesion and cell invasion | Easily mass produced and sterilized; ability to regulate the microstructure, degradation rate, and mechanical properties |
| Disadvantages | Poor mechanical strength and rapid degradation rate (gelatin, fibrin); poor cell adhesion (chitosan); possibility of transmission of pathogens | Poor cell adhesion and cell invasion; decreased remodeling and growth; inflammatory response |
| Bioengineered Tissues/Organs | Xenotransplantation | Allotransplantation | |
|---|---|---|---|
| Availability, supply: present | Unresolved | Unresolved | Limited |
| Availability, supply: ideal-case scenario | Unlimited | Unlimited | Limited |
| Difficulty in manufacturing | High | High | n/a |
| Organ dysfunction due to donor brain death | n/a | n/a | Possible |
| Pathophysiological barriers | Numerous: “create life” | Numerous: “outwit evolution” | Moderate: “outwit immunology” |
| Biomechanical properties | Poor; improving | Adequate | Adequate |
| Growth possibility, endurance | Unresolved | Unresolved | Adequate with limitations |
| Immunology | n/a | Highly problematic | Problematic |
| Transmission of pathogens, sterility | Unlikely | Less possible | Possible |
| Cultural/ethical considerations, public opinion | Accepted | Mixed | Culturally variable |
| Regulatory considerations, infrastructure | Significant | Significant | Moderate |
| Costs | Moderate | High | Moderate-high |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiraly, L.; Vijayavenkataraman, S. Biofabrication in Congenital Cardiac Surgery: A Plea from the Operating Theatre, Promise from Science. Micromachines 2021, 12, 332. https://doi.org/10.3390/mi12030332
Kiraly L, Vijayavenkataraman S. Biofabrication in Congenital Cardiac Surgery: A Plea from the Operating Theatre, Promise from Science. Micromachines. 2021; 12(3):332. https://doi.org/10.3390/mi12030332
Chicago/Turabian StyleKiraly, Laszlo, and Sanjairaj Vijayavenkataraman. 2021. "Biofabrication in Congenital Cardiac Surgery: A Plea from the Operating Theatre, Promise from Science" Micromachines 12, no. 3: 332. https://doi.org/10.3390/mi12030332
APA StyleKiraly, L., & Vijayavenkataraman, S. (2021). Biofabrication in Congenital Cardiac Surgery: A Plea from the Operating Theatre, Promise from Science. Micromachines, 12(3), 332. https://doi.org/10.3390/mi12030332







