The Application of Nanomaterials for the Electrochemical Detection of Antibiotics: A Review
Abstract
1. Introduction
2. Antibiotics Electrochemical Detection Methods
2.1. Antibiotics Electrochemical Detection Strategies
2.1.1. Biosensors Based on Molecular Imprinted Polymers (MIPs)
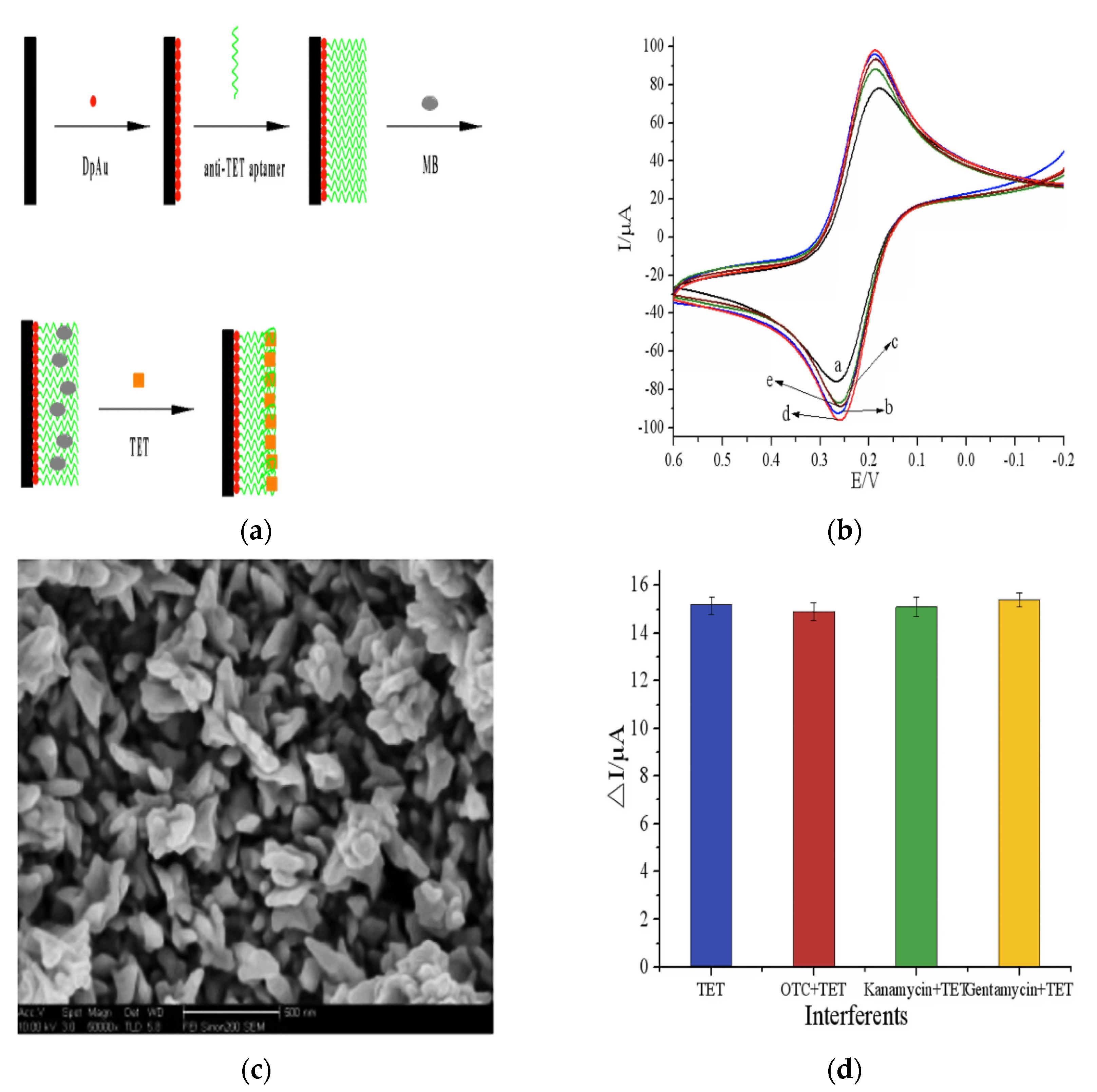
2.1.2. Biosensors Based on Aptamers (Apts)
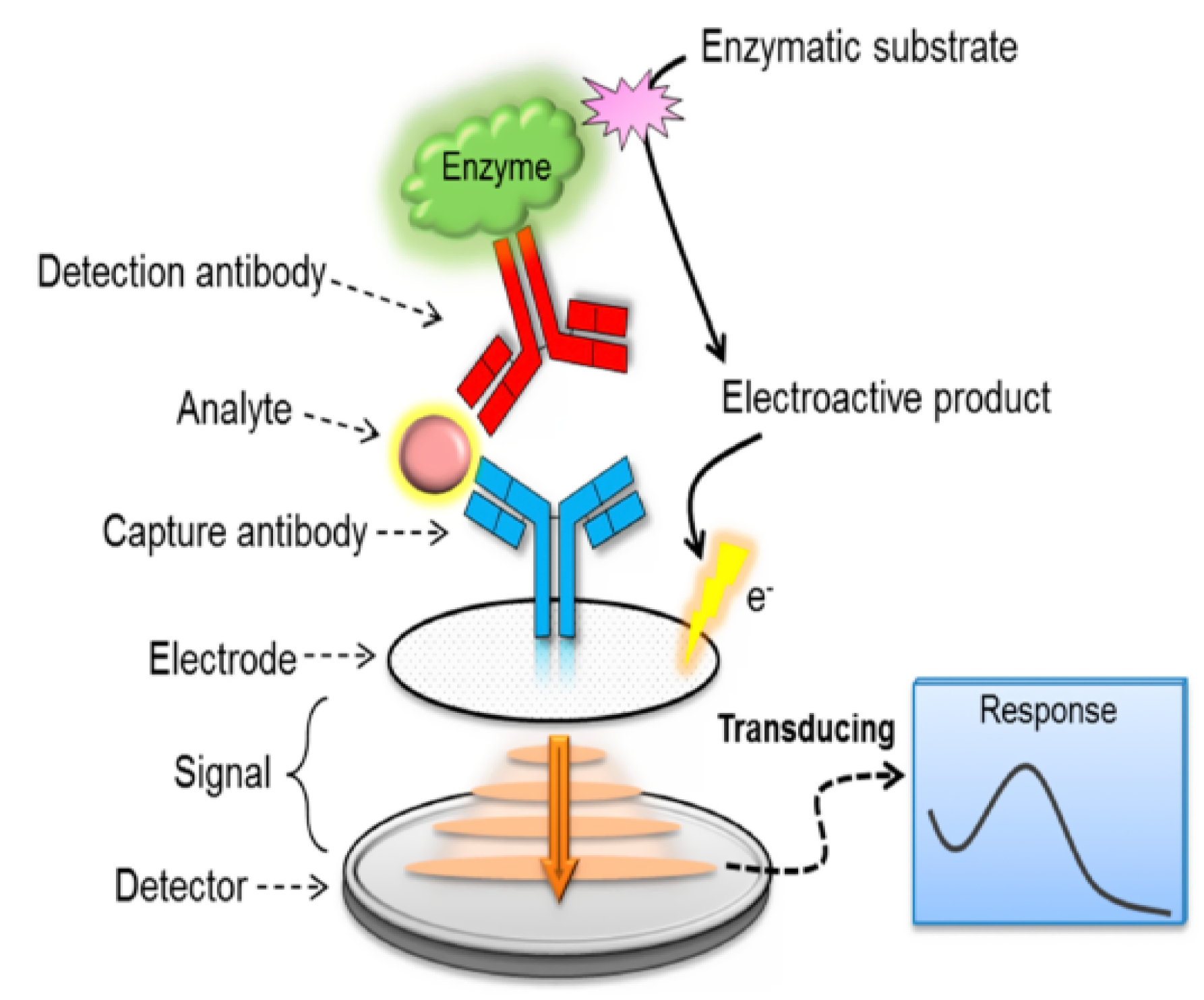
2.1.3. Biosensors Based on Immuno-Complex
2.1.4. Enzyme/Receptor-Mediated Biosensors
2.2. Antibiotics Electrochemical Sensing Using Nanomaterials
2.2.1. Quantum Dots
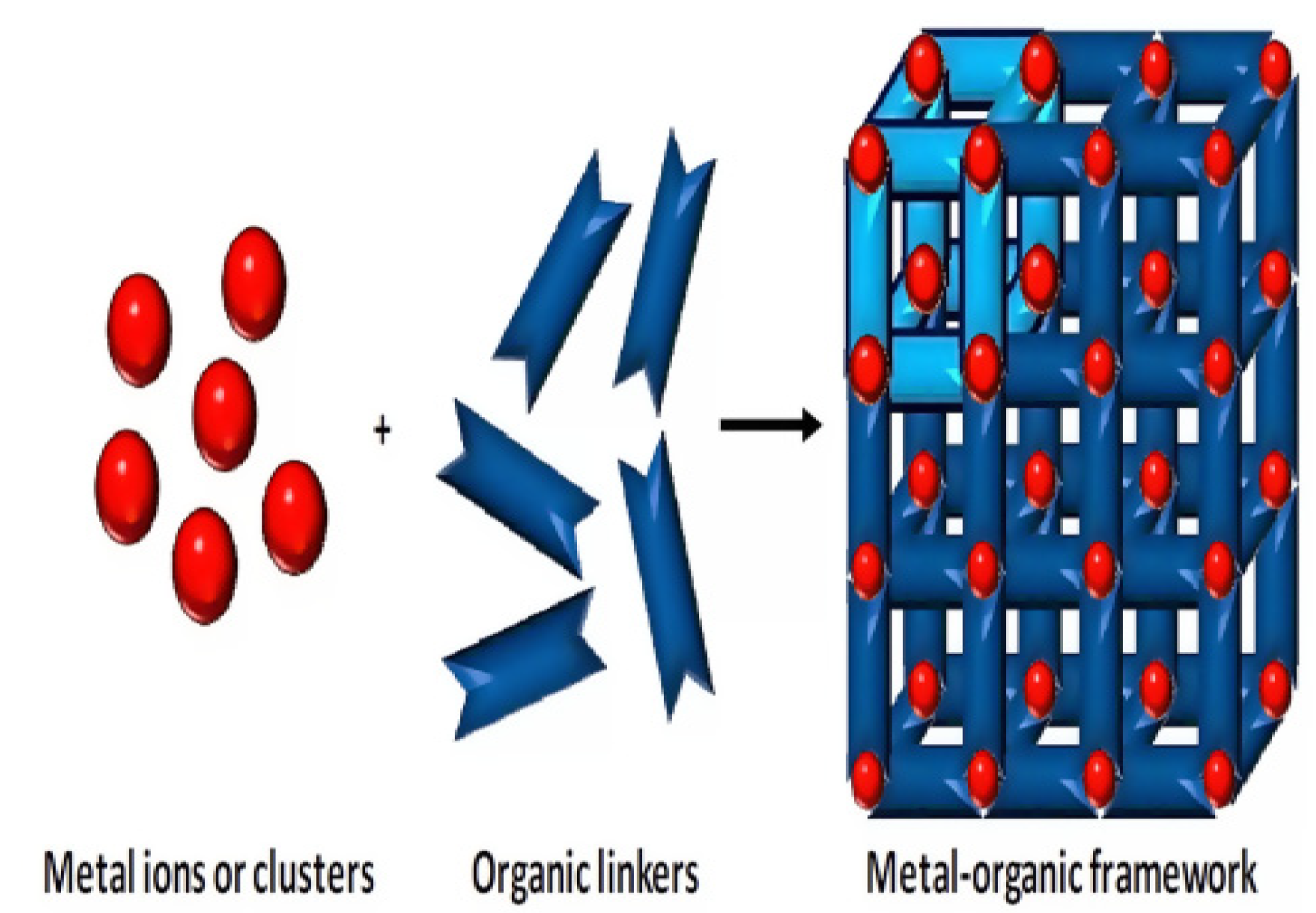
2.2.2. Metal-Organic Frameworks
2.2.3. Metal Nanoparticles
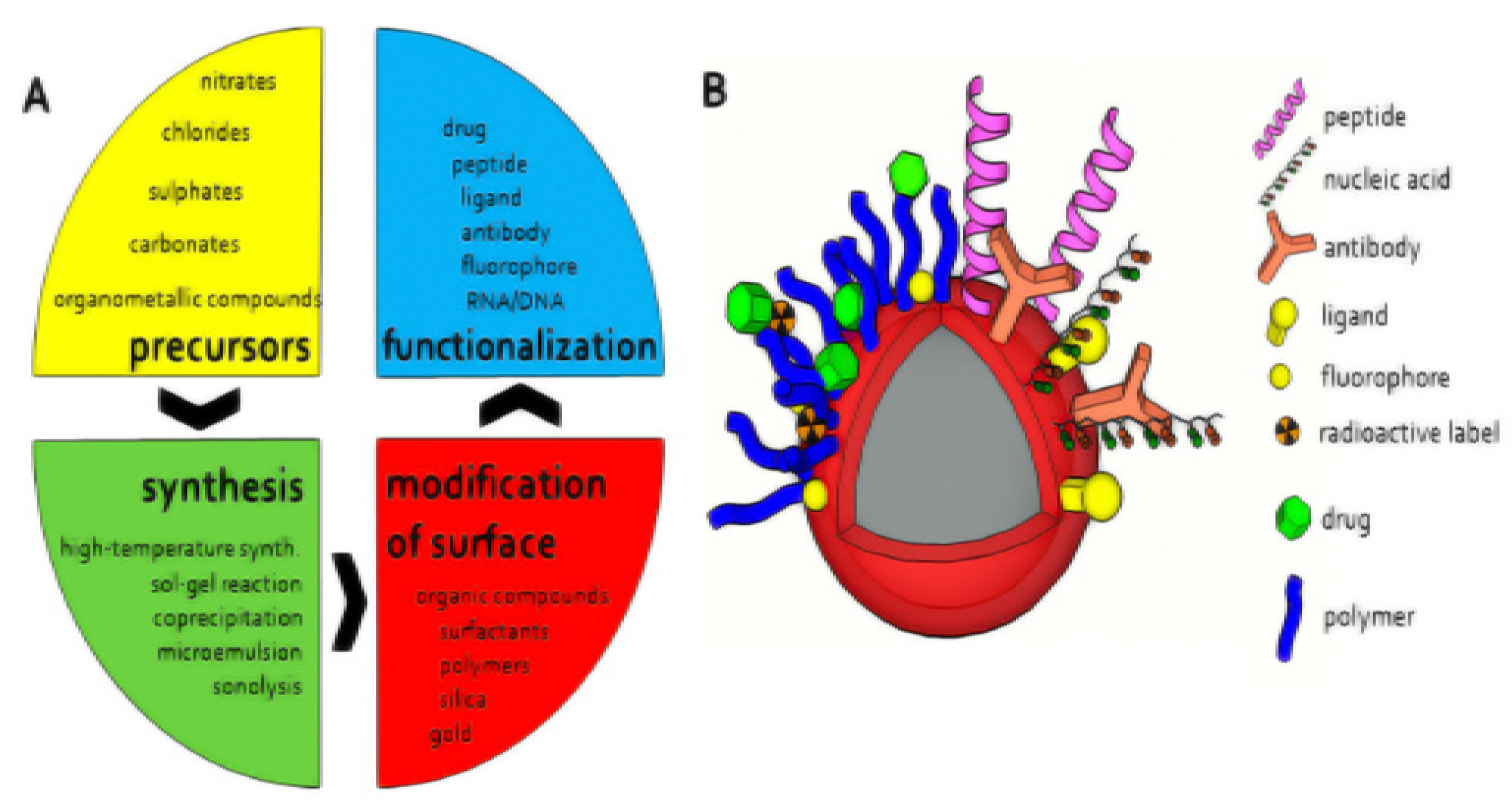
2.2.4. Magnetic Nanomaterials
2.2.5. Carbon Nanomaterials
Graphene-Based Nanomaterials
Carbon Nanotubes
Other Carbon Nanomaterials
3. Evaluation of the Potential of Different Nanomaterials-Based Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kiambi, S.; Mwanza, R.; Sirma, A.; Czerniak, C.; Kimani, T.; Kabali, E.; Dorado-Garcia, A.; Eckford, S.; Price, C.; Gikonyo, S. Understanding Antimicrobial use Contexts in the Poultry Sector: Challenges for Small-Scale Layer Farms in Kenya. Antibiotics 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.C.; da Silva Rocha, C.; Tavares, D.S.; de Morais Calado, S.L.; Gomes, M.P. Veterinary antibiotics and plant physiology: An overview. Sci. Total Environ. 2021, 767, 144902. [Google Scholar] [CrossRef] [PubMed]
- Cetuk, H.; Anishkin, A.; Scott, A.J.; Rempe, S.B.; Ernst, R.K.; Sukharev, S. Partitioning of Seven Different Classes of Antibiotics into LPS Monolayers Supports Three Different Permeation Mechanisms through the Outer Bacterial Membrane. Langmuir 2021, 37, 1372–1385. [Google Scholar] [CrossRef]
- Aghdam, E.M.; Hejazi, M.S.; Barzegar, A. Riboswitches: From living biosensors to novel targets of antibiotics. Gene 2016, 592, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Tadić, Đ.; Hernandez, M.J.B.; Cerqueira, F.; Matamoros, V.; Piña, B.; Bayona, J.M. Occurrence and human health risk assessment of antibiotics and their metabolites in vegetables grown in field-scale agricultural systems. J. Hazard. Mater. 2021, 401, 123424. [Google Scholar] [CrossRef]
- Kim, H.Y.; Chang, R.Y.K.; Morales, S.; Chan, H.-K. Bacteriophage-Delivering Hydrogels: Current Progress in Combating Antibiotic Resistant Bacterial Infection. Antibiotics 2021, 10, 130. [Google Scholar] [CrossRef]
- Denooz, R.; Charlier, C. Simultaneous determination of five β-lactam antibiotics (cefepim, ceftazidim, cefuroxim, meropenem and piperacillin) in human plasma by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B 2008, 864, 161–167. [Google Scholar] [CrossRef]
- Preu, M.; Guyot, D.; Petz, M. Development of a gas chromatography—Mass spectrometry method for the analysis of aminoglycoside antibiotics using experimental design for the optimisation of the derivatisation reactions. J. Chromatogr. A 1998, 818, 95–108. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Detection of Pyoluteorin by thin layer chromatography. In Plant-Microbe Interactions; Springer: Cham, Switzerland, 2021; pp. 175–176. [Google Scholar]
- Bueno, D.; Istamboulie, G.; Muñoz, R.; Marty, J.L. Determination of mycotoxins in food: A review of bioanalytical to analytical methods. Appl. Spectrosc. Rev. 2015, 50, 728–774. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Liang, L. Recent development of antibiotic detection in food and environment: The combination of sensors and nanomaterials. Microchimica Acta 2021, 188, 1–22. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Alsaiari, N.S.; Tahoon, M.A.; Rebah, F.B. The application of nanomaterials as electrode modifiers for the electrochemical detection of ascorbic acid. Int. J. Electrochem. Sci. 2020, 15, 3327–3346. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Tahoon, M.A.; Alsaiari, N.S.; Shabbir, M.; Rebah, F.B. Application of Functionalized Nanomaterials as Effective Adsorbents for the Removal of Heavy Metals from Wastewater: A Review. Curr. Anal. Chem. 2021, 17, 4–19. [Google Scholar] [CrossRef]
- Amari, A.; Alalwan, B.; Siddeeg, S.M.; Tahoon, M.A.; Alsaiari, N.S.; Rebah, F.B. Biomolecules Behavior on a Surface of Boron Doped/un-doped Graphene Nanosheets. Int. J. Electrochem. Sci. 2020, 15, 11427–11436. [Google Scholar] [CrossRef]
- Tahoon, M.A.; Siddeeg, S.M.; Alsaiari, N.S.; Mnif, W.; Rebah, F.B. Effective heavy metals removal from water using nanomaterials: A review. Processes 2020, 8, 645. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Amari, A.; Tahoon, M.A.; Alsaiari, N.S.; Rebah, F.B. Removal of meloxicam, piroxicam and Cd+ 2 by Fe3O4/SiO2/glycidyl methacrylate-S-SH nanocomposite loaded with laccase. Alex. Eng. J. 2020, 59, 905–914. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Tahoon, M.A.; Rebah, F.B. Simultaneous Removal of Calconcarboxylic Acid, NH4+ and PO43− from Pharmaceutical Effluent Using Iron Oxide-Biochar Nanocomposite Loaded with Pseudomonas putida. Processes 2019, 7, 800. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Tahoon, M.A.; Mnif, W.; Rebah, F.B. Iron oxide/chitosan magnetic nanocomposite immobilized manganese peroxidase for decolorization of textile wastewater. Processes 2020, 8, 5. [Google Scholar] [CrossRef]
- Amari, A.; Al Mesfer, M.K.; Alsaiari, N.S.; Danish, M.; Alshahrani, A.M.; Tahoon, M.A.; Rebah, F.B. Electrochemical and Optical Properties of Tellurium Dioxide (TeO2) Nanoparticles. Int. J. Electrochem. Sci. 2021, 16, 210235. [Google Scholar] [CrossRef]
- Das, S.; Ngashangva, L.; Goswami, P. Carbon Dots: An Emerging Smart Material for Analytical Applications. Micromachines 2021, 12, 84. [Google Scholar] [CrossRef]
- Qian, L.; Thiruppathi, A.R.; Elmahdy, R.; van der Zalm, J.; Chen, A. Graphene-oxide-based electrochemical sensors for the sensitive detection of pharmaceutical drug naproxen. Sensors 2020, 20, 1252. [Google Scholar] [CrossRef]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-Based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, J.; Wang, C.; Tian, Y.; Zhou, N. Simultaneous electrochemical detection of multiple antibiotic residues in milk based on aptamers and quantum dots. Anal. Methods 2016, 8, 1981–1988. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Barek, J. How to Improve the Performance of Electrochemical Sensors via Minimization of Electrode Passivation. Chemosensors 2021, 9, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Canoura, J.; Alkhamis, O.; Xiao, Y. Immobilization Strategies for Enhancing Sensitivity of Electrochemical Aptamer-Based Sensors. ACS Appl. Mater. Interfaces 2021, 9491–9499. [Google Scholar] [CrossRef]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular imprinted polymers for separation science: A review of reviews. J. Sep. Sci. 2013, 36, 609–628. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhang, Y.; Wei, G. Highly sensitive molecularly imprinted electrochemical sensor based on the double amplification by an inorganic prussian blue catalytic polymer and the enzymatic effect of glucose oxidase. Anal. Chem. 2012, 84, 1888–1893. [Google Scholar] [CrossRef]
- Jafari, S.; Dehghani, M.; Nasirizadeh, N.; Baghersad, M.H.; Azimzadeh, M. Label-Free electrochemical detection of Cloxacillin antibiotic in milk samples based on molecularly imprinted polymer and graphene oxide-gold nanocomposite. Measurement 2019, 145, 22–29. [Google Scholar] [CrossRef]
- Kumar, S.; Karfa, P.; Majhi, K.C.; Madhuri, R. Photocatalytic, fluorescent BiPO4@ Graphene oxide based magnetic molecularly imprinted polymer for detection, removal and degradation of ciprofloxacin. Mater. Sci. Eng. C 2020, 111, 110777. [Google Scholar] [CrossRef]
- Bi, H.; Wu, Y.; Wang, Y.; Liu, G.; Ning, G.; Xu, Z. A molecularly imprinted polymer combined with dual functional Au@ Fe3O4 nanocomposites for sensitive detection of kanamycin. J. Electroanal. Chem. 2020, 870, 114216. [Google Scholar] [CrossRef]
- Yuphintharakun, N.; Nurerk, P.; Chullasat, K.; Kanatharana, P.; Davis, F.; Sooksawat, D.; Bunkoed, O. A nanocomposite optosensor containing carboxylic functionalized multiwall carbon nanotubes and quantum dots incorporated into a molecularly imprinted polymer for highly selective and sensitive detection of ciprofloxacin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Nakamura, Y. Aptamers: A review of their chemical properties and modifications for therapeutic application. Molecules 2019, 24, 4229. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Kumar, S.; Ortega, G.A.; Srinivasan, S.; Rajabzadeh, A.R. Target specific aptamer-induced self-assembly of fluorescent graphene quantum dots on palladium nanoparticles for sensitive detection of tetracycline in raw milk. Food Chem. 2021, 346, 128893. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Sun, X.; Guo, Y. Multiplex electrochemical aptasensor for detecting multiple antibiotics residues based on carbon fiber and mesoporous carbon-gold nanoparticles. Sens. Actuators B Chem. 2018, 265, 217–226. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xu, Q.; Cao, Y.; Chen, Y. An electrochemical aptasensor for multiplex antibiotics detection based on metal ions doped nanoscale MOFs as signal tracers and RecJf exonuclease-assisted targets recycling amplification. Talanta 2016, 161, 867–874. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhang, H.; Yan, Z.; Li, T.; Chen, Y.; Xu, Q.; Jiang, Q. Electrochemical simultaneous assay of chloramphenicol and PCB72 using magnetic and aptamer-modified quantum dot-encoded dendritic nanotracers for signal amplification. Microchim. Acta 2016, 183, 1099–1106. [Google Scholar] [CrossRef]
- Huang, S.; Gan, N.; Li, T.; Zhou, Y.; Cao, Y.; Dong, Y. Electrochemical aptasensor for multi-antibiotics detection based on endonuclease and exonuclease assisted dual recycling amplification strategy. Talanta 2018, 179, 28–36. [Google Scholar] [CrossRef]
- Pan, C.; Wei, H.; Han, Z.; Wu, F.; Mao, L. Enzymatic electrochemical biosensors for in situ neurochemical measurement. Curr. Opin. Electrochem. 2020, 19, 162–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Q.; Xu, C.; Li, H.; Wu, D.; Cai, Y.; Mao, K.; Cui, Z.; Du, B. Label-Free electrochemical immunosensor for sensitive detection of kanamycin. Sens. Actuators B Chem. 2011, 155, 618–625. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, S.; Hu, Y.; Li, Z.; Luo, F.; He, Z. Electrochemical immunosensor based on the chitosan-magnetic nanoparticles for detection of tetracycline. Food Anal. Methods 2016, 9, 2972–2978. [Google Scholar] [CrossRef]
- Giroud, F.; Gorgy, K.; Gondran, C.; Cosnier, S.; Pinacho, D.G.; Marco, M.-P.; Sánchez-Baeza, F.J. Impedimetric Immunosensor Based on a Polypyrrole− Antibiotic Model Film for the Label-Free Picomolar Detection of Ciprofloxacin. Anal. Chem. 2009, 81, 8405–8409. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, F.; Wang, H.; Gao, L.; Wang, Z. A sensor based on Au nanoparticles/carbon nitride/graphene composites for the detection of chloramphenicol and ciprofloxacin. ECS J. Solid State Sci. Technol. 2018, 7, M201. [Google Scholar] [CrossRef]
- Wu, H.; Fan, S.; Zhang, W.; Chen, H.; Peng, L.; Jin, X.; Ma, J.; Zhang, H. Amperometric immunosensor based on covalent immobilization of new methylene blue and penicillin polyclonal antibody for determination of penicillin G in milk. Anal. Methods 2014, 6, 497–502. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Chen, G.; Tang, D. Biotin-avidin-conjugated metal sulfide nanoclusters for simultaneous electrochemical immunoassay of tetracycline and chloramphenicol. Microchim. Acta 2014, 181, 257–262. [Google Scholar] [CrossRef]
- Zacco, E.; Adrián, J.; Galve, R.; Marco, M.-P.; Alegret, S.; Pividori, M.I. Electrochemical magneto immunosensing of antibiotic residues in milk. Biosens. Bioelectron. 2007, 22, 2184–2191. [Google Scholar] [CrossRef]
- Cho, I.-H.; Lee, J.; Kim, J.; Kang, M.-S.; Paik, J.K.; Ku, S.; Cho, H.-M.; Irudayaraj, J.; Kim, D.-H. Current technologies of electrochemical immunosensors: Perspective on signal amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Kling, A.; Chatelle, C.; Armbrecht, L.; Qelibari, E.; Kieninger, J.; Dincer, C.; Weber, W.; Urban, G. Multianalyte antibiotic detection on an electrochemical microfluidic platform. Anal. Chem. 2016, 88, 10036–10043. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Callera, W.F.; Sotomayor, M.D.; Bueno, P.R. Penicillinase-Based amperometric biosensor for penicillin G. Electrochem. Commun. 2014, 38, 131–133. [Google Scholar] [CrossRef]
- Faridah, S.; Hazana, R.; Gayah, A.; Norzaili, Z.; Azima, A.; Nur Azura, M.; Zamri, I. Electrochemical sensors for detection of tetracycline antibiotics. Malays. Soc. Anim. Prod. 2012, 15, 67–80. [Google Scholar]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Mostafa, M.; El Nady, J.; Ebrahim, S.M.; Elshaer, A. Synthesis, structural, and optical properties of Mn2+ doped ZnS quantum dots for biosensor application. Opt. Mater. 2021, 112, 110732. [Google Scholar] [CrossRef]
- Hwang, J.; Le, A.D.D.; Trinh, C.T.; Le, Q.T.; Lee, K.-G.; Kim, J. Green synthesis of reduced-graphene oxide quantum dots and application for colorimetric biosensor. Sens. Actuators A Phys. 2021, 318, 112495. [Google Scholar] [CrossRef]
- Vijian, D.; Chinni, S.V.; Yin, L.S.; Lertanantawong, B.; Surareungchai, W. Non-Protein coding RNA-based genosensor with quantum dots as electrochemical labels for attomolar detection of multiple pathogens. Biosens. Bioelectron. 2016, 77, 805–811. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef]
- Shan, J.; Li, R.; Yan, K.; Zhu, Y.; Zhang, J. In situ anodic stripping of Cd (II) from CdS quantum dots for electrochemical sensing of ciprofloxacin. Sens. Actuators B Chem. 2016, 237, 75–80. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Bao, T.; Hu, T.-X.; Wen, W.; Zhang, X.-H.; Wang, S.-F. Voltammetric determination of levofloxacin using a glassy carbon electrode modified with poly (o-aminophenol) and graphene quantum dots. Microchim. Acta 2017, 184, 127–135. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef]
- Carrasco, S. Metal-Organic frameworks for the development of biosensors: A current overview. Biosensors 2018, 8, 92. [Google Scholar] [CrossRef]
- Bougrini, M.; Florea, A.; Cristea, C.; Sandulescu, R.; Vocanson, F.; Errachid, A.; Bouchikhi, B.; El Bari, N.; Jaffrezic-Renault, N. Development of a novel sensitive molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework for tetracycline detection in honey. Food Control 2016, 59, 424–429. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Li, Y.; Chen, J.; Li, X.; Zhang, Y.; Zhang, Y. Novel nanostructured MIL-101 (Cr)/XC-72 modified electrode sensor: A highly sensitive and selective determination of chloramphenicol. Sens. Actuators B Chem. 2017, 247, 756–764. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xu, Q.; Cao, Y.; Chen, Y. A novel aptamer-metal ions-nanoscale MOF based electrochemical biocodes for multiple antibiotics detection and signal amplification. Sens. Actuators B Chem. 2017, 242, 1201–1209. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Li, T.; Wang, Y.; Xu, Q.; Chen, Y. An electrochemical aptasensor for multiplex antibiotics detection using Y-shaped DNA-based metal ions encoded probes with NMOF substrate and CSRP target-triggered amplification strategy. Anal. Chim. Acta 2017, 968, 30–39. [Google Scholar] [CrossRef]
- Zhou, N.; Ma, Y.; Hu, B.; He, L.; Wang, S.; Zhang, Z.; Lu, S. Construction of Ce-MOF@ COF hybrid nanostructure: Label-Free aptasensor for the ultrasensitive detection of oxytetracycline residues in aqueous solution environments. Biosens. Bioelectron. 2019, 127, 92–100. [Google Scholar] [CrossRef]
- Munonde, T.S.; Nomngongo, P.N. Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples. Sensors 2021, 21, 131. [Google Scholar] [CrossRef]
- Chandra, P.; Noh, H.-B.; Won, M.-S.; Shim, Y.-B. Detection of daunomycin using phosphatidylserine and aptamer co-immobilized on Au nanoparticles deposited conducting polymer. Biosens. Bioelectron. 2011, 26, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chandra, P.; Song, K.-M.; Ban, C.; Shim, Y.-B. Label-Free detection of kanamycin based on the aptamer-functionalized conducting polymer/gold nanocomposite. Biosens. Bioelectron. 2012, 36, 29–34. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.; Luo, J.; Tian, Y.; Zhou, N. Direct electrochemical detection of kanamycin based on peroxidase-like activity of gold nanoparticles. Anal. Chim. Acta 2016, 936, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, L.; Yu, F.; Ye, B.-C.; Li, Y. Molecularly imprinted polymer functionalized nanoporous Au-Ag alloy microrod: Novel supportless electrochemical platform for ultrasensitive and selective sensing of metronidazole. Electrochim. Acta 2016, 208, 10–16. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Sun, X. A label-free electrochemical aptasensor based on electrodeposited gold nanoparticles and methylene blue for tetracycline detection. Int. J. Electrochem. Sci. 2015, 10, 3668–3679. [Google Scholar]
- Bagheri Hashkavayi, A.; Bakhsh Raoof, J.; Ojani, R.; Hamidi Asl, E. Label-Free electrochemical aptasensor for determination of chloramphenicol based on gold nanocubes-modified screen-printed gold electrode. Electroanalysis 2015, 27, 1449–1456. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.B.; Azimi, R.; Ojani, R. Label-Free and sensitive aptasensor based on dendritic gold nanostructures on functionalized SBA-15 for determination of chloramphenicol. Anal. Bioanal. Chem. 2016, 408, 2557–2565. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.B. Design an aptasensor based on structure-switching aptamer on dendritic gold nanostructures/Fe3O4@ SiO2/DABCO modified screen printed electrode for highly selective detection of epirubicin. Biosens. Bioelectron. 2017, 91, 650–657. [Google Scholar] [CrossRef] [PubMed]
- del Torno-de Román, L.; Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Tyrosinase based biosensor for the electrochemical determination of sulfamethoxazole. Sens. Actuators B Chem. 2016, 227, 48–53. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, G.; Liu, S.; Yu, A. Ultrasensitive electrochemical aptasensing of kanamycin antibiotic by enzymatic signal amplification with a horseradish peroxidase-functionalized gold nanoprobe. Sens. Actuators B Chem. 2018, 273, 1762–1767. [Google Scholar] [CrossRef]
- Afkhami, A.; Soltani-Felehgari, F.; Madrakian, T. Gold nanoparticles modified carbon paste electrode as an efficient electrochemical sensor for rapid and sensitive determination of cefixime in urine and pharmaceutical samples. Electrochim. Acta 2013, 103, 125–133. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, H.; Quan, X. Gold modified microelectrode for direct tetracycline detection. Front. Environ. Sci. Eng. 2012, 6, 313–319. [Google Scholar] [CrossRef]
- Beytur, M.; Kardaş, F.; Akyıldırım, O.; Özkan, A.; Bankoğlu, B.; Yüksek, H.; Yola, M.L.; Atar, N. A highly selective and sensitive voltammetric sensor with molecularly imprinted polymer based silver@ gold nanoparticles/ionic liquid modified glassy carbon electrode for determination of ceftizoxime. J. Mol. Liq. 2018, 251, 212–217. [Google Scholar] [CrossRef]
- Jakubec, P.; Urbanová, V.; Medříková, Z.; Zbořil, R. Advanced sensing of antibiotics with magnetic gold nanocomposite: Electrochemical detection of chloramphenicol. Chem. Eur. J. 2016, 22, 14279–14284. [Google Scholar] [CrossRef]
- Kushikawa, R.T.; Silva, M.R.; Angelo, A.C.; Teixeira, M.F. Construction of an electrochemical sensing platform based on platinum nanoparticles supported on carbon for tetracycline determination. Sens. Actuators B Chem. 2016, 228, 207–213. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Hosseini-Nassab, N.; Kamalzadeh, Z. Fabrication of an electrochemical sensor based on the electrodeposition of Pt nanoparticles on multiwalled carbon nanotubes film for voltammetric determination of ceftriaxone in the presence of lidocaine, assisted by factorial-based response-surface methodology. J. Solid State Electrochem. 2014, 18, 77–88. [Google Scholar] [CrossRef]
- Campanile, R.; Scardapane, E.; Forente, A.; Granata, C.; Germano, R.; Di Girolamo, R.; Minopli, A.; Velotta, R.; Della Ventura, B.; Iannotti, V. Core-Shell magnetic nanoparticles for highly sensitive magnetoelastic immunosensor. Nanomaterials 2020, 10, 1526. [Google Scholar] [CrossRef] [PubMed]
- Netto, C.G.; Toma, H.E.; Andrade, L.H. Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J. Mol. Catal. B Enzym. 2013, 85, 71–92. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Justino, C.I.; Rocha-Santos, T.A.; Cardoso, S.; Duarte, A.C. Strategies for enhancing the analytical performance of nanomaterial-based sensors. TrAC Trends Anal. Chem. 2013, 47, 27–36. [Google Scholar] [CrossRef]
- Yu, S.; Wei, Q.; Du, B.; Wu, D.; Li, H.; Yan, L.; Ma, H.; Zhang, Y. Label-Free immunosensor for the detection of kanamycin using Ag@ Fe3O4 nanoparticles and thionine mixed graphene sheet. Biosens. Bioelectron. 2013, 48, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Hou, W.; Jiao, Y.; Guo, Y.; Sun, X.; Zhao, J.; Wang, X. Ultra-Sensitive aptasensor based on il and Fe3O4 nanoparticles for tetracycline detection. Int. J. Electrochem. Sci. 2017, 12, 7426–7434. [Google Scholar] [CrossRef]
- Jahanbani, S.; Benvidi, A. Comparison of two fabricated aptasensors based on modified carbon paste/oleic acid and magnetic bar carbon paste/Fe3O4@ oleic acid nanoparticle electrodes for tetracycline detection. Biosens. Bioelectron. 2016, 85, 553–562. [Google Scholar] [CrossRef]
- Yin, Y.; Qin, X.; Wang, Q.; Yin, Y. A novel electrochemical aptasensor for sensitive detection of streptomycin based on gold nanoparticle-functionalized magnetic multi-walled carbon nanotubes and nanoporous PtTi alloy. RSC Adv. 2016, 6, 39401–39408. [Google Scholar] [CrossRef]
- Yan, Z.; Gan, N.; Li, T.; Cao, Y.; Chen, Y. A sensitive electrochemical aptasensor for multiplex antibiotics detection based on high-capacity magnetic hollow porous nanotracers coupling exonuclease-assisted cascade target recycling. Biosens. Bioelectron. 2016, 78, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tang, D.; Zhang, B.; Que, X.; Yang, H.; Chen, G. Au (III)-promoted magnetic molecularly imprinted polymer nanospheres for electrochemical determination of streptomycin residues in food. Biosens. Bioelectron. 2013, 41, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Gálvez, A.; Ait-Lahcen, A.; Mercante, L.A.; Morales-Narváez, E.; Amine, A.; Merkoçi, A. Molecularly imprinted polymer-decorated magnetite nanoparticles for selective sulfonamide detection. Anal. Chem. 2016, 88, 3578–3584. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Zhang, Z.; Yang, Z.; Zeng, J.; Jiang, Y. Imprinted electrochemical sensor based on magnetic multi-walled carbon nanotube for sensitive determination of kanamycin. J. Electroanal. Chem. 2015, 755, 7–14. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Allafchian, A.R. Multiwall carbon nanotubes decorated with NiFe2O4 magnetic nanoparticles, a new catalyst for voltammetric determination of cefixime. Colloids Surf. B Biointerfaces 2013, 102, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Asadpour-Zeynali, K.; Mollarasouli, F. Novel electrochemical biosensor based on PVP capped CoFe2O4@ CdSe core-shell nanoparticles modified electrode for ultra-trace level determination of rifampicin by square wave adsorptive stripping voltammetry. Biosens. Bioelectron. 2017, 92, 509–516. [Google Scholar] [CrossRef]
- Dehdashtian, S.; Gholivand, M.B.; Shamsipur, M. Construction of a sensitive and selective sensor for morphine using chitosan coated Fe3O4 magnetic nanoparticle as a modifier. Mater. Sci. Eng. C 2016, 58, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, D.; Ma, R.; Zhang, X.; Rao, J.; Yin, Y.; Wang, X.; Yi, F. Flexible temperature sensors based on carbon nanomaterials. J. Mater. Chem. B 2021, 1941–1964. [Google Scholar] [CrossRef] [PubMed]
- Alwarappan, S.; Erdem, A.; Liu, C.; Li, C.-Z. Probing the electrochemical properties of graphene nanosheets for biosensing applications. J. Phys. Chem. C 2009, 113, 8853–8857. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, S. Applications of carbon nanotubes and graphene for electrochemical sensing of environmental pollutants. J. Nanosci. Nanotechnol. 2016, 16, 7852–7872. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A.; Aydar, S.; Ozyurt, D.; Demirata, B. Determination of tetracycline on the surface of a high-performance graphene modified screen-printed carbon electrode in milk and honey samples. Curr. Nanosci. 2016, 12, 527–533. [Google Scholar] [CrossRef]
- Borowiec, J.; Wang, R.; Zhu, L.; Zhang, J. Synthesis of nitrogen-doped graphene nanosheets decorated with gold nanoparticles as an improved sensor for electrochemical determination of chloramphenicol. Electrochim. Acta 2013, 99, 138–144. [Google Scholar] [CrossRef]
- Qin, X.; Yin, Y.; Yu, H.; Guo, W.; Pei, M. A novel signal amplification strategy of an electrochemical aptasensor for kanamycin, based on thionine functionalized graphene and hierarchical nanoporous PtCu. Biosens. Bioelectron. 2016, 77, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, L.; Huang, L.; Han, Z.; Wang, J.; Pan, H. A low detection limit penicillin biosensor based on single graphene nanosheets preadsorbed with hematein/ionic liquids/penicillinase. Mater. Sci. Eng. C 2014, 39, 92–99. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, L.; Zhang, J. Electrochemical sensor for levofloxacin based on molecularly imprinted polypyrrole-graphene-gold nanoparticles modified electrode. Sens. Actuators B Chem. 2014, 192, 642–647. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.-C.; Zhang, J.-W. A highly selective electrochemical sensor for chloramphenicol based on three-dimensional reduced graphene oxide architectures. Talanta 2016, 161, 567–573. [Google Scholar] [CrossRef]
- Yadav, M.; Ganesan, V.; Gupta, R.; Yadav, D.K.; Sonkar, P.K. Cobalt oxide nanocrystals anchored on graphene sheets for electrochemical determination of chloramphenicol. Microchem. J. 2019, 146, 881–887. [Google Scholar] [CrossRef]
- Sebastian, N.; Yu, W.-C.; Balram, D. Electrochemical detection of an antibiotic drug chloramphenicol based on a graphene oxide/hierarchical zinc oxide nanocomposite. Inorg. Chem. Front. 2019, 6, 82–93. [Google Scholar] [CrossRef]
- Wang, K.-P.; Zhang, Y.-C.; Zhang, X.; Shen, L. Green preparation of chlorine-doped graphene and its application in electrochemical sensor for chloramphenicol detection. SN Appl. Sci. 2019, 1, 157. [Google Scholar] [CrossRef]
- Wen, Y.; Liao, X.; Deng, C.; Liu, G.; Yan, Q.; Li, L.; Wang, X. Imprinted voltammetric streptomycin sensor based on a glassy carbon electrode modified with electropolymerized poly (pyrrole-3-carboxy acid) and electrochemically reduced graphene oxide. Microchim. Acta 2017, 184, 935–941. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, G.; Zhang, M. Electrochemical sensor based on electro-polymerization of β-cyclodextrin and reduced-graphene oxide on glassy carbon electrode for determination of gatifloxacin. Sens. Actuators B Chem. 2016, 228, 59–65. [Google Scholar] [CrossRef]
- Xi, X.; Ming, L. A voltammetric sensor based on electrochemically reduced graphene modified electrode for sensitive determination of midecamycin. Anal. Methods 2012, 4, 3013–3018. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Roostaee, M. Modification of carbon paste electrode with NiO/graphene oxide nanocomposite and ionic liquids for fabrication of high sensitive voltammetric sensor on sulfamethoxazole analysis. J. Mol. Liq. 2016, 220, 329–333. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Arduini, F. Nanomaterials and Cross-Cutting Technologies for Fostering Smart Electrochemical Biosensors in the Detection of Chemical Warfare Agents. Appl. Sci. 2021, 11, 720. [Google Scholar] [CrossRef]
- Moraes, F.C.; Silva, T.A.; Cesarino, I.; Lanza, M.R.; Machado, S.A. Antibiotic detection in urine using electrochemical sensors based on vertically aligned carbon nanotubes. Electroanalysis 2013, 25, 2092–2099. [Google Scholar] [CrossRef]
- Zhou, L.; Li, D.-J.; Gai, L.; Wang, J.-P.; Li, Y.-B. Electrochemical aptasensor for the detection of tetracycline with multi-walled carbon nanotubes amplification. Sens. Actuators B Chem. 2012, 162, 201–208. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, G.; Sun, X.; Wang, X. Electrochemical aptasensor based on multiwalled carbon nanotubes and graphene for tetracycline detection. IEEE Sens. J. 2014, 15, 1951–1958. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y. Electrochemical behavior of adriamycin at an electrode modified with silver nanoparticles and multi-walled carbon nanotubes, and its application. Microchim. Acta 2010, 169, 161–165. [Google Scholar] [CrossRef]
- Hajian, R.; Mehrayin, Z.; Mohagheghian, M.; Zafari, M.; Hosseini, P.; Shams, N. Fabrication of an electrochemical sensor based on carbon nanotubes modified with gold nanoparticles for determination of valrubicin as a chemotherapy drug: Valrubicin-DNA interaction. Mater. Sci. Eng. C 2015, 49, 769–775. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, H.; Quan, X.; Chen, S. Electrochemical determination of tetracycline using molecularly imprinted polymer modified carbon nanotube-gold nanoparticles electrode. Electroanalysis 2011, 23, 1863–1869. [Google Scholar] [CrossRef]
- Lian, W.; Liu, S.; Yu, J.; Li, J.; Cui, M.; Xu, W.; Huang, J. Electrochemical sensor using neomycin-imprinted film as recognition element based on chitosan-silver nanoparticles/graphene-multiwalled carbon nanotubes composites modified electrode. Biosens. Bioelectron. 2013, 44, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Rastgar, S. Construction of an electrochemical sensor based on the electrodeposition of Au-Pt nanoparticles mixtures on multi-walled carbon nanotubes film for voltammetric determination of cefotaxime. Analyst 2012, 137, 2706–2715. [Google Scholar] [CrossRef]
- Munawar, A.; Tahir, M.A.; Shaheen, A.; Lieberzeit, P.A.; Khan, W.S.; Bajwa, S.Z. Investigating nanohybrid material based on 3D CNTs@ Cu nanoparticle composite and imprinted polymer for highly selective detection of chloramphenicol. J. Hazard. Mater. 2018, 342, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Cesarino, I.; Cesarino, V.; Lanza, M.R. Carbon nanotubes modified with antimony nanoparticles in a paraffin composite electrode: Simultaneous determination of sulfamethoxazole and trimethoprim. Sens. Actuators B Chem. 2013, 188, 1293–1299. [Google Scholar] [CrossRef]
- Kor, K.; Zarei, K. Electrochemical determination of chloramphenicol on glassy carbon electrode modified with multi-walled carbon nanotube–cetyltrimethylammonium bromide-poly (diphenylamine). J. Electroanal. Chem. 2014, 733, 39–46. [Google Scholar] [CrossRef]
- Gomaa, E.A.; Negm, A.; Tahoon, M.A.K. Study of redox behavior of Cu (II) and interaction of Cu (II) with lysine in the aqueous medium using cyclic voltammetry. Eur. J. Chem. 2016, 7, 341–346. [Google Scholar] [CrossRef][Green Version]
- Yalikun, N.; Mamat, X.; Li, Y.; Hu, X.; Wågberg, T.; Dong, Y.; Hu, G. Synthesis of an iron-nitrogen co-doped ordered mesoporous carbon-silicon nanocomposite as an enhanced electrochemical sensor for sensitive and selective determination of chloramphenicol. Colloids Surf. B Biointerfaces 2018, 172, 98–104. [Google Scholar] [CrossRef]
- Gan, T.; Lv, Z.; Liu, N.; Shi, Z.; Sun, J.; Liu, Y. Electrochemical Detection Method for Chlorotetracycline based on Enhancement of Yolk-Shell Structured Carbon Sphere@ MnO2. J. Electrochem. Soc. 2015, 162, H200. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Z.; Fu, J.; Zhao, W.; Guo, Y.; Sun, X.; Zhang, H. Ratiometric electrochemical aptasensor based on ferrocene and carbon nanofibers for highly specific detection of tetracycline residues. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deroco, P.B.; Rocha-Filho, R.C.; Fatibello-Filho, O. A new and simple method for the simultaneous determination of amoxicillin and nimesulide using carbon black within a dihexadecylphosphate film as electrochemical sensor. Talanta 2018, 179, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, B.; Yang, C.; Zhang, Z.; He, L.; Fang, S.; Qu, X.; Zhang, Q. Electrochemical biosensing based on protein-directed carbon nanospheres embedded with SnOx and TiO2 nanocrystals for sensitive detection of tobramycin. Biosens. Bioelectron. 2018, 99, 176–185. [Google Scholar] [CrossRef]
- Song, Y.; Duan, F.; Zhang, S.; Tian, J.-Y.; Zhang, Z.; Wang, Z.-W.; Liu, C.-S.; Xu, W.-M.; Du, M. Iron oxide@ mesoporous carbon architectures derived from an Fe (II)-based metal organic framework for highly sensitive oxytetracycline determination. J. Mater. Chem. A 2017, 5, 19378–19389. [Google Scholar] [CrossRef]
- Simioni, N.B.; Silva, T.A.; Oliveira, G.G.; Fatibello-Filho, O. A nanodiamond-based electrochemical sensor for the determination of pyrazinamide antibiotic. Sens. Actuators B Chem. 2017, 250, 315–323. [Google Scholar] [CrossRef]
- Yin, J.; Guo, W.; Qin, X.; Zhao, J.; Pei, M.; Ding, F. A sensitive electrochemical aptasensor for highly specific detection of streptomycin based on the porous carbon nanorods and multifunctional graphene nanocomposites for signal amplification. Sens. Actuators B Chem. 2017, 241, 151–159. [Google Scholar] [CrossRef]
- Son, J.; Buck, E.C.; Riechers, S.L.; Yu, X.Y. Stamping Nanoparticles onto the Electrode for Rapid Electrochemical Analysis in Microfluidics. Micromachines 2021, 12, 60. [Google Scholar] [CrossRef]
- Gomaa, E.A.; Tahoon, M.A. Ion association and solvation behavior of copper sulfate in binary aqueous–methanol mixtures at different temperatures. J. Mol. Liq. 2016, 214, 19–23. [Google Scholar] [CrossRef]
- Gomaa, E.A.; Tahoon, M.A.; Negm, A. Aqueous micro-solvation of Li+ ions: Thermodynamics and energetic studies of Li+-(H2O) n (n = 1–6) structures. J. Mol. Liq. 2017, 241, 595–602. [Google Scholar] [CrossRef]
- Gomaa, E.A.; Negm, A.; Tahoon, M.A. Conductometric and volumetric study of copper sulphate in aqueous ethanol solutions at different temperatures. J. Taibah Univ. Sci. 2017, 11, 741–748. [Google Scholar] [CrossRef]
- Gomaa, E.A.; Tahoon, M.A.; Shokr, A. Ionic association and solvation study of CoSO4 in aqueous-organic solvents at different temperatures. Chem. Data Collect. 2016, 3, 58–67. [Google Scholar] [CrossRef]
- Tahoon, M.; Gomaa, E.; Suleiman, M. Aqueous Micro-hydration of Na+ (H–O) n = 1–7 Clusters: DFT Study. Open Chem. 2019, 17, 260–269. [Google Scholar] [CrossRef]
- Ben Rebah, F.; Siddeeg, S.M.; Tahoon, M.A. Thermodynamic Parameters and Solvation Behavior of 1-Ethyle-3-methylimidazolium Tetrafluoroborate and 1-Butyl-3-methylimidazolium Tetrafluoroborate in N, N-Dimethylformamide and Acetonitrile at Different Temperature. Egypt. J. Chem. 2019, 62, 393–404. [Google Scholar] [CrossRef]


| Method | Principle | Limit of Detection | Applications |
|---|---|---|---|
| Electrochemical impedance spectroscopy | Small-amplitude sinusoidal AC excitation signal is applied to measure the resistive properties | 10−12 M | Study of antigen-antibodies reaction, corrosion, and electron transfer kinetics |
| Chronoamperometry | The stepped potential is applied and the current measured | 10−5 M | Measure electrode process mechanism, working electrode surface area, and analytes diffusion coefficient |
| Stripping technique | Worked electrode carries the pre-concentrated analyte then analyte stripped by application of scan potential from the electrode | 10−9 M | Detection of trace elements |
| Square wave voltammetry | Current is determined as a consequence of square wave potential superposed on staircase waveform | 10−8 M | Detection of trace elements, the study of catalytic homogeneous chemical reactions, and electrode kinetics |
| Differential Pulse voltammetry | Current is determined as a function of applied voltage superposed as regular voltage pulses superposed on the potential linear sweep or stair steps | 10−7 M | Detection of trace elements |
| Linear Sweep Voltammetry | Voltage is applied then the current measured on the working electrode surface | 10−5 M | Determination of analytes concentrations, unknown reactions, and irreversible reactions |
| Cyclic Voltammetry | Voltage is applied then the current measured on the working electrode surface | 10−5 M | Assessment of reaction products, trace reaction intermediates, and study redox reactions |
| Electrode | Interface | Transduction Method | Antibiotics Detected | Limit of Detection (nM) | Selectivity | Real Samples | Ref. |
|---|---|---|---|---|---|---|---|
| Au | QD–cDNA2/cDNA1/ Cap-DNA | SWASV | Tetracycline, chloramphenicol, and streptomycin | 20, 5, and 10, respectively | - | Milk | [23] |
| GCE | Dendritic probe encoded with magnetic aptamer QDs | SWV | chloramphenicol | 0.001 | Oxytetracycline and kanamycin | Fish | [37] |
| GCE | PoAP/GQD | LSV | Levofloxacin | 10 | Norfloxacin, lomefloxacin, enrofloxacin, and ciprofloxacin. | Milk | [57] |
| GCE | CdS QDs | DPASV | Ciprofloxacin | 23 | Gentamycin, erythromycin, kanamycin, chloramphenicol, and ofloxacin, | Human urine | [56] |
| Au | Ce-MOF@COF | EIS | Oxytetracycline | 0.000036 | Kanamycin, streptomycin sulfate, doxycyclinehyclate, bleomycin, and ampicillin | Urine, water, and milk | [66] |
| GCE | Y-DNA-NMOF | SWV | Oxytetracycline, and chloramphenicol | 0.000049, and 0.000034, respectively | gentamicin sulfate, tetracycline, doxycycline, and kanamycin | Milk | [64] |
| GCE | NMOF Probe labeled with magnetic aptamer | SWV | Kanamycin and oxytetracycline | 0.00016, and 0.00019, respectively | Gentamicin sulfate, doxycycline, streptomycin, chloramphenicol, and Chlortetracycline | Milk | [36] |
| GCE | Aptamer-metal ions NMOF Biocodes | SWV | Chloramphenicol and kanamycin | 0.00020, and 0.00017, respectively | metal ions (K, Ca, Mg), oxytetracycline, and chlortetracycline | Milk | [63] |
| GCE | MIL-101(Cr)/XC-72 | DPV | Chloramphenicol | 1.6 | Amikacin, gentamicin, neomycin, rutin, quercetin, penicillin, kanamycin, kitasamycin, tetracycline, and chlortetracycline | Milk, eye drop, and honey | [62] |
| Au | MMOF-MIP | LSV | Tetracycline | 0.00000023 | Doxycycline | Honey | [61] |
| GCE | CoFe2O4@CdSe capped with PVP | SWV | Rifampicin | 0.00000005 | Glucose, L-threonine, uric acid, pyrazinamide, and isoniazid | Pharmaceutical drug and serum | [79] |
| GCE | NiFe2O4-MWCNTs | CV | Cefixime | 19 | Ascorbic acid, glucose, tartaric acid, CO3−2, SO4−2, NH4+, and Ca+2 | Plasma, urine, and tablets | [78] |
| CE | MMIP/CE | DPV and CV | Kanamycin | 0.03 | Erythromycin, streptomycin, and gentamycin | Milk and animal food derivatives | [77] |
| SPCE | MIP decorated Fe3O4 MNPs | EIS | Sulfamethoxazole | 0.002 | Sulfacetamide and sulfadiazine | Seawater | [76] |
| GCE | Aptamer/NP-PtTi/ Au@MWCNTs–Fe3O4 | DPV | Streptomycin | 0.02 | Streptomycin, neomycin sulfate, kanamycin sulfate, and terramycin | Milk | [73] |
| MBCPE | Fe3O4 NPs@OA/antiTET | EIS | Tetracycline | 0.000004 | Doxycycline and oxytetracycline | Serum, honey, milk, and drugs | [72] |
| SPE | Fe3O4/IL | CV | Tetracycline | 1.00 | - | Milk | [71] |
| Au | Ab-MNPs-chitosan | DPV | Tetracycline | 0.08 | Chloramphenicol, penicillin, gentamycin, and erythromycin | Milk | [41] |
| GCE | TH-GS/GA/ Ag@Fe3O4-Ab | SWV | Kanamycin | 0.04 | Neomycin, gentamicin, vitamin C, and glucose | Animal foods | [70] |
| GCE | Pt NPs/C | DPV | Tetracycline | 4281 | - | Human urine | [97] |
| GCE | Pt Nps/MWCNT | LSV | Ceftriaxone | 9.02 | Lidocaine | Human serum | [98] |
| GCE | MIP/Ag@Au Nps/Ils | DPV | Ceftizoxime | 0.003 | Dopamine and ascorbic acid | Pharmaceuticals | [95] |
| GCE | Fe3O4-CMC@Au | SWV | Chloramphenicol | 67.00 | Ca+2, glucose, xanthine, cysteine, uric acid, and ascorbic acid | Urine | [96] |
| CPE | GNPs/MWCPE | SWV | Cefixime | 4.00 | Caffeine, glucose, oxalic acid, uric acid, citric acid, and ascorbic acid, | Tablets and human urine | [93] |
| ME | gold colloids | CV | Tetracycline | 200 | - | - | [94] |
| SPCE | Tyr-AuNPs | Amperometric | Sulfamethoxazole | 22 × 103 | - | Water | [91] |
| Graphite SPE | HEM/Apt/AuNPs/ SBA-15@ DABCO | DPV | Chloramphenicol | 5.00 | Florfenicol, amoxicillin, cephalexin, and cefixime | Blood serum | [89] |
| Interdigitated Au SPE | Aptamer/AuNCs-Cys | SWV | Chloramphenicol | 5.00 | Florfenicol, amoxicillin, cephalexin, cefixime, and chloramphenicol | Human serum | [89] |
| GCE | MB/Anti-TET/AuNps | CV | Tetracycline | 0.005 | Gentamycin sulfate, kanamycin monosulfate, and oxytetracycline hydrochloride | Milk | [87] |
| MIP/NPAMR | MIP/NPAMR | CV | Metronidazole | 0.00003 | Dimetridazole, 4 -nitroimidazole, and 1,2 dimethylimidazole | Tablets and fish tissues | [86] |
| SPE | Aptamer/poly-DPB/ AuNPs | LSV | Kanamycin | 9.5 | Sulfadimethoxine, tetracycline, ampicillin, streptomycin, and neomycin | Milk | [83] |
| GCE | AuNPs/poly TTBA/ PS/aptamer/AuNPs | DPV | Daunomycin | 0.053 | Adriamycin, anthraquinone, neomycin, chloramphenicol, kanamycin, and tetracycline | Human urine | [82] |
| GCE | SGN-hematein/ILs/ penicillinase | DPV | Penicillin | 0.0002 | Levofloxacin hydrochloride, streptomycin sulfate, and Kanamycin sulfate, | Milk | [44,105] |
| GCE | Au/N-G | EIS and LSV | Chloramphenicol | 591.0 | Chlortetracycline, Oxytetracycline, and Metronidazole | Eye drop | [103] |
| GCE | Graphene | LSV | Midecamycin | 101.0 | Isovalerylspiramycin, acetylspiramycin, josamycin, and Kitasamycin | Urine and serum samples | [113] |
| GCE | Au/C3N4/GN | SWV | Ciprofloxacin and Chloramphenicol | 421.0 and 28.0 | - | Milk | [43] |
| GCE | Anti-Kan/WGS/PBCTS/NPG | SWV | Kanamycin | 0.014 | Neamine, neomycin, and gentamicin | Pork meat | [40] |
| GCE | β-cyclodextrin/rGO | DPV | Gatifloxacin | 21.0 | Norfloxacin, ofloxacin, ciprofloxacin, and moxifloxacin | Pharmaceuticals, and human urine | [112] |
| GCE | PoAP/GQD | DPV | Levofloxacin | 11.0 | Norfloxacin, lomefloxacin, enrofloxacin, and ciprofloxacin | Milk | [57] |
| GCE | Cl-RGO | DPV | Chloramphenicol | 1000 | Tetracycline, Erythromycin, penicillin G, and cysteine | Eye drops, water, calf plasma, and milk | [110] |
| GCE | CO3O4@rGO | Chronoamperometry and DPV | Chloramphenicol | 551.0 | glutathione, cysteine, and uric acid | Honey and milk | [108] |
| GCE | GO/ZnO | DPV | Chloramphenicol | 11.0 | 4-amino phenol, 4-nitro phenol, 4-nitroaniline, 4-nitrobenzene, Cl-, and Ca+2 | Eye drops, milk, and honey | [109] |
| GCE | PPy3C/ERGO | DPV | Streptomycin | 0.6 | Gentamycin, kanamycin, amikacin, neomycin, and dihydrostreptomycin | Honey and porcine kidney | [110] |
| GCE | 3D RGO | DPV | Chloramphenicol | 151.0 | uric acid, cysteine, taurine, and glutathione | Milk and eye drops | [107] |
| GCE | MIP/G-AuNPs | DPV | Levofloxacin | 531.0 | Norfloxacin, prulifloxacin, oxytetracycline, and chlortetracycline | Levofloxacin capsule | [106] |
| GCE | Aptamer/HNP–PtCu/ GR-TH/GCE | DPV | Kanamycin | 0.0009 | Human chorionic gonadotropin, tyrosine, dopamine, TSH hormone. | Chicken liver and pork meat | [104] |
| GCE | Au-Pt Nps/MWCNT | LSV | Cefotaxime | 1.0 | Glucose, dopamine, and ascorbic acid | Plasma | [124] |
| Au | ssDNA/SWCNT | SWV | Levofloxacin | 75.0 | - | Urine | [117] |
| GCE | 3DCNTs@ Cu NPs@MIP | CV | Chloramphenicol | 10 × 103 | Florfenicol, clindamycin, Dansyl chloride, and thiamphenicol | Milk | [125] |
| Au | MIPs/GR-MWCNTs/ CS-SNP | Amperometry, and CV | Neomycin | 7.65 | Erythromycin, kanamycin, streptomycin, and gentamycin | Honey and milk | [123] |
| GCE | MWCNT-GNPs/MIP | CV | Tetracycline | 91.0 | Chloramphenicol, nafcillin, and oxytetracycline | - | [122] |
| Au | ssDNA/AuNPs/en/ MWCNTs | CV | Valrubicin | 19.0 | K+, paracetamol, Na+, glucose, urea, azithromycin, ascorbic acid, and Caffeine | Blood and human urine | [121] |
| GCE | MWCNT-CTAB-PDPA | Stripping DPV | Chloramphenicol | 3.0 | Streptomycin, ceftazidime, cefotaxime, and ceftizoxime | Honey and milk | [127] |
| Paraffin | MWCNT-Sb Nps | DPV | Trimethoprim, and Sulfamethoxazole | 32.0 and 25.0 | Carbaryl, and 17β Estradiol | Natural H2O | [126] |
| GCE | AgNPs/MWCNTsCOOH | DPV | Adriamycin | 1.80 | - | Ct-DNA | [120] |
| GCE | Anti-TET/GA/(CS-PBGR)2/MWCNTs-CS | DPV | Tetracycline | 0.006 | Gentamycin sulfate, kanamycinmonosulfate, and oxytetracycline | Milk | [119] |
| GCE | Anti-TET/MWCNTs | DPV | Tetracycline | 6.0 | Doxycycline hydrochloride and oxytetracycline | Milk | [118] |
| GCE | Si–Fe/NOMC/GCE | DPV | Chloramphenicol | 31.0 | Florfenicol, benzylpenicillin potassium, chlortetracycline hydrochloride, gentamicin sulfate, and thiamphenicol | Eye drop | [129] |
| GCE | CS @MnO2 | DPV | Chlortetracycline | 261.0 | Rifampicin, Oxytetracycline, and chloramphenicol | Fish, shrimp, and milk | [130] |
| GCE | CB-DHP | SWV | Amoxicillin | 121.0 | Humic acid, vermicompost, albumin, glucose, K+, and Na+ | Water and urine | [132] |
| SPE | CdS-KAP+ PbS-STP/ cKAP+ cSTP/OMCAuNPs/CNF | DPV | Streptomycin and kanamycin | 0.05 and 0.09 | Oxytetracycline, tobramycin, neomycin, and gentamycin | Milk | [35] |
| GCE | Aptamer/ SnOx@TiO2@mC | EIS | Tobramycin | 0.02 | Doxycycline, oxytetracycline, and Kanamycin | Human serum and urine | [133] |
| GCE | Aptamer/Fe3O4@mC | EIS | Oxytetracycline | 0.00006 | Chlortetracycline, doxycycline, and tetracycline | Milk | [134] |
| GCE | Nanodiamonds | SWV | Pyrazinamide | 221.0 | - | Human serum and urine | [135] |
| GCE | STR Aptamer/GRFe3O4-AuNPs/PCNR | DPV | Streptomycin | 0.05 | Glucose, methionine, ascorbic acid, and penicillin | Milk | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaiari, N.S.; Katubi, K.M.M.; Alzahrani, F.M.; Siddeeg, S.M.; Tahoon, M.A. The Application of Nanomaterials for the Electrochemical Detection of Antibiotics: A Review. Micromachines 2021, 12, 308. https://doi.org/10.3390/mi12030308
Alsaiari NS, Katubi KMM, Alzahrani FM, Siddeeg SM, Tahoon MA. The Application of Nanomaterials for the Electrochemical Detection of Antibiotics: A Review. Micromachines. 2021; 12(3):308. https://doi.org/10.3390/mi12030308
Chicago/Turabian StyleAlsaiari, Norah Salem, Khadijah Mohammedsaleh M Katubi, Fatimah Mohammed Alzahrani, Saifeldin M. Siddeeg, and Mohamed A. Tahoon. 2021. "The Application of Nanomaterials for the Electrochemical Detection of Antibiotics: A Review" Micromachines 12, no. 3: 308. https://doi.org/10.3390/mi12030308
APA StyleAlsaiari, N. S., Katubi, K. M. M., Alzahrani, F. M., Siddeeg, S. M., & Tahoon, M. A. (2021). The Application of Nanomaterials for the Electrochemical Detection of Antibiotics: A Review. Micromachines, 12(3), 308. https://doi.org/10.3390/mi12030308







