Characterization and Separation of Live and Dead Yeast Cells Using CMOS-Based DEP Microfluidics
Abstract
1. Introduction
2. Materials and Methods
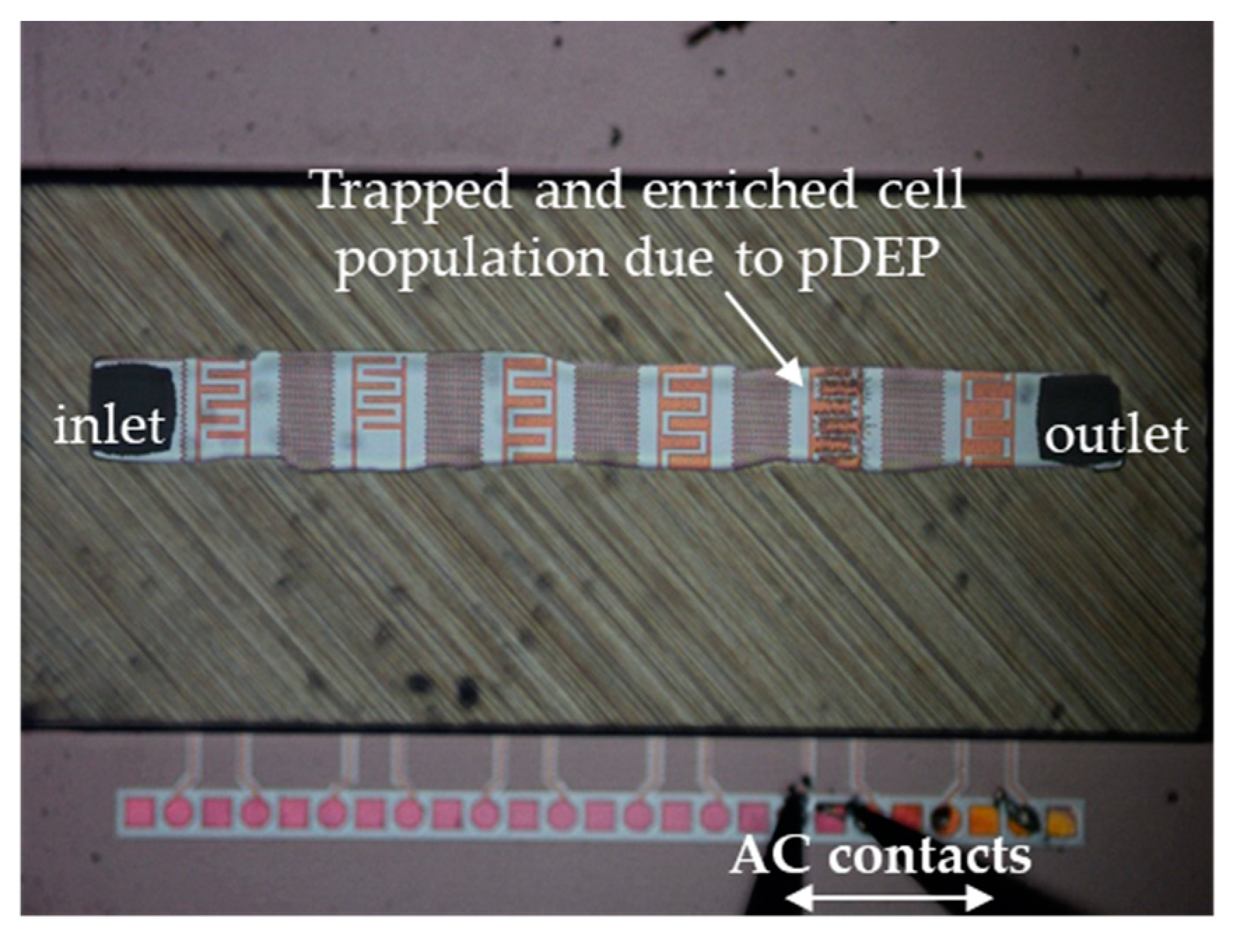
2.1. Microfluidic Device Design and DEP Operation
2.2. Sample Preparation
3. Theoretical Background
3.1. Dielectrophoresis Theory
3.2. Numerical Determinations of Clausius-Mossotti Factor
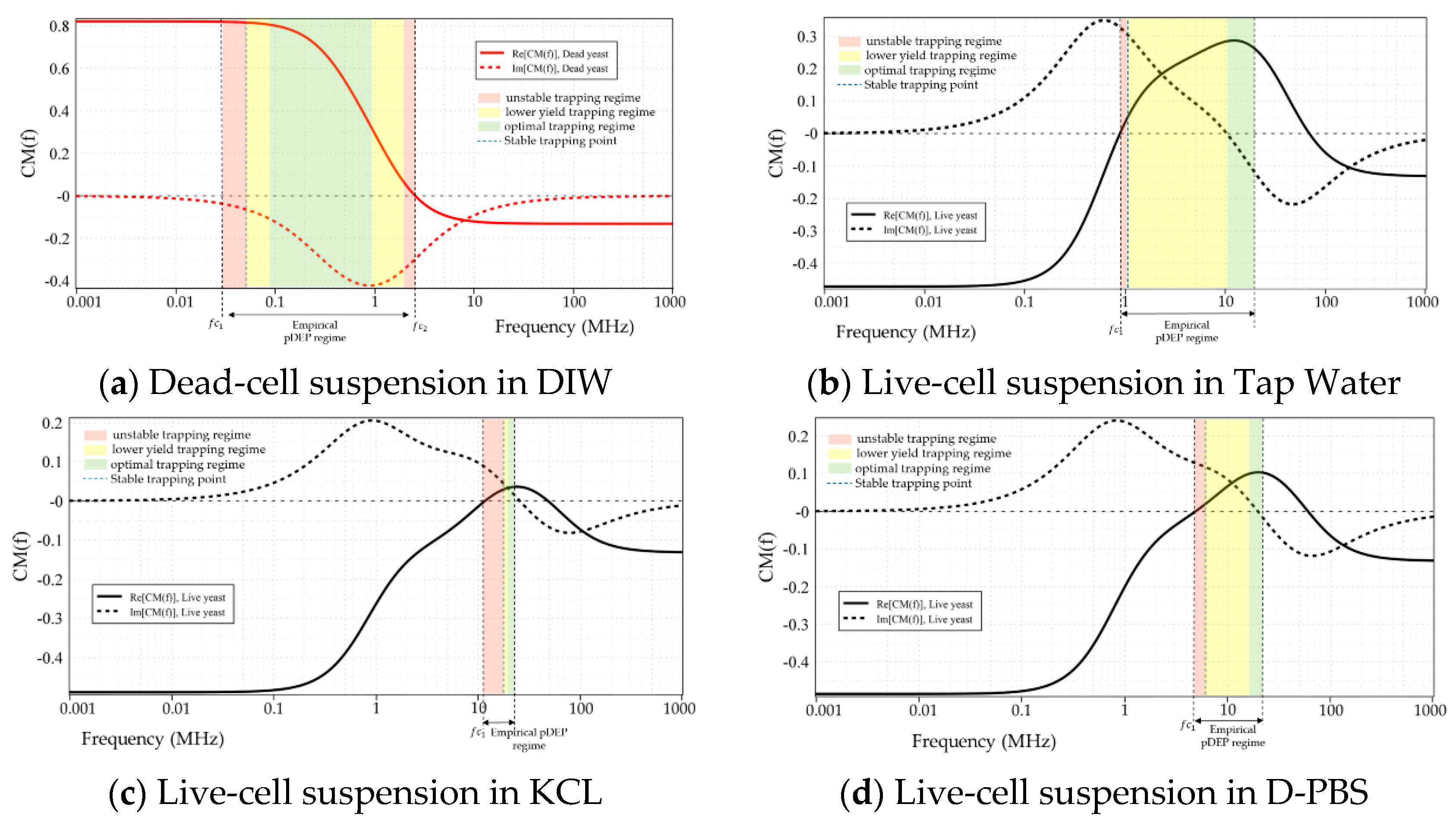
4. Results and Discussion
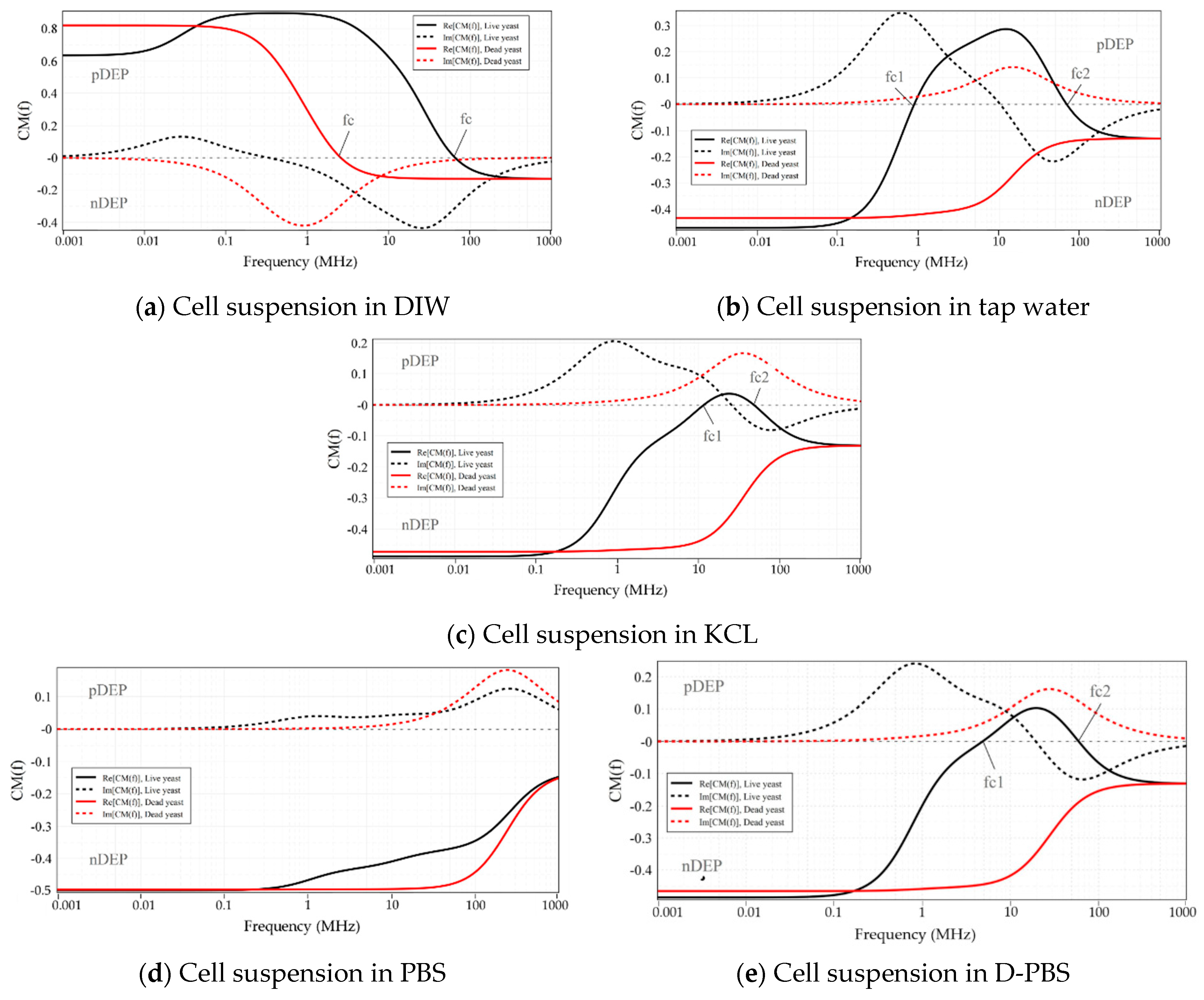
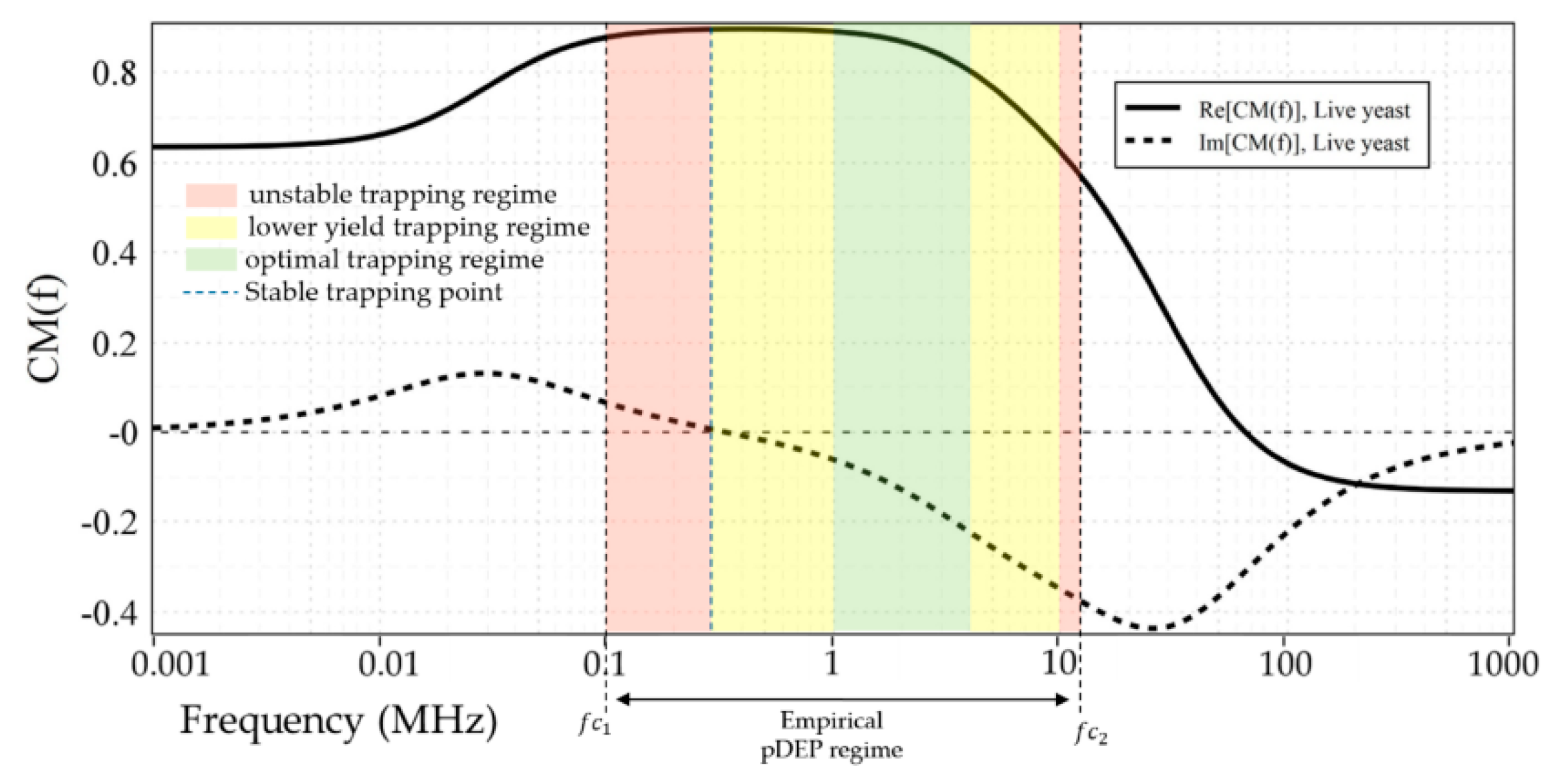
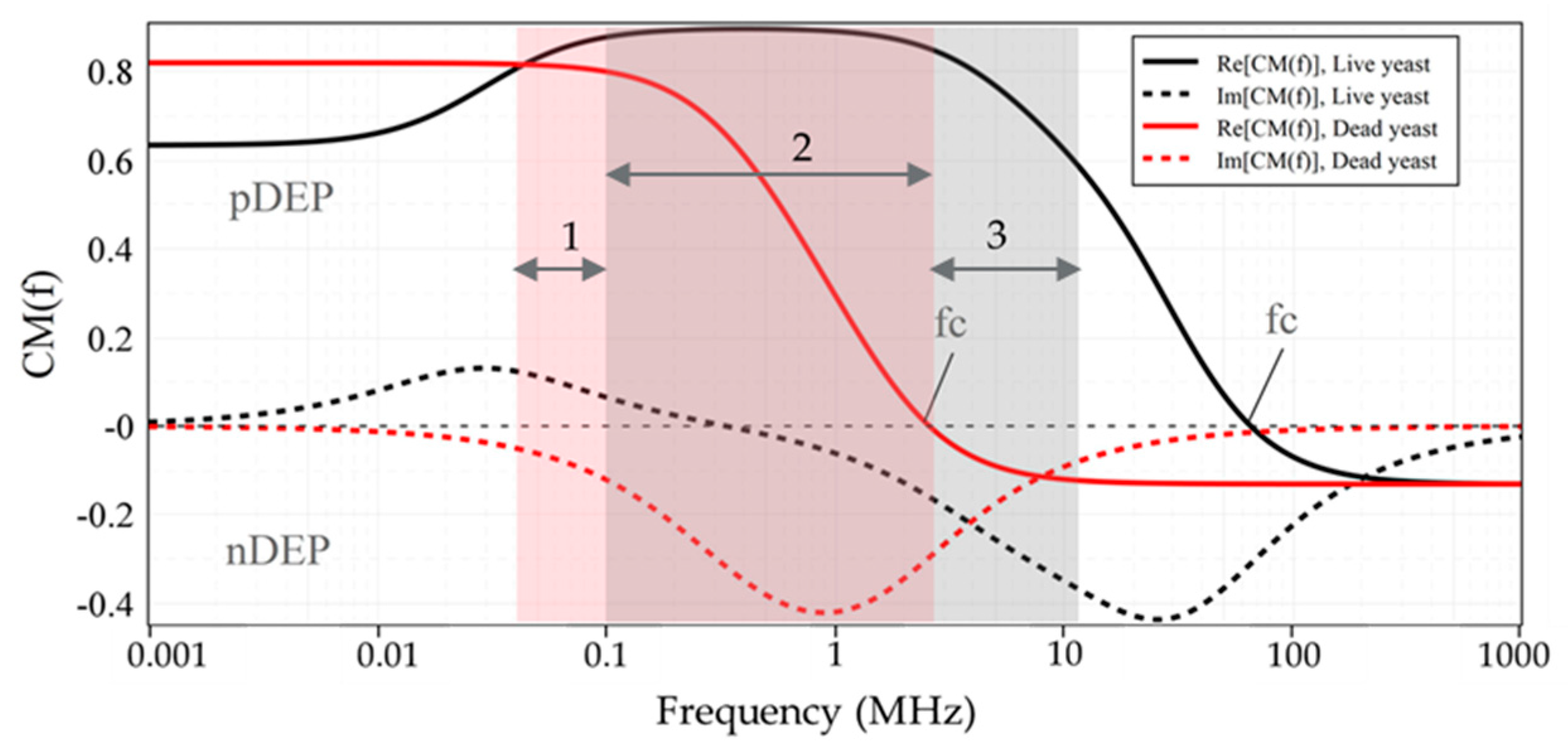
4.1. Characterization of Live and Dead Yeast Cells
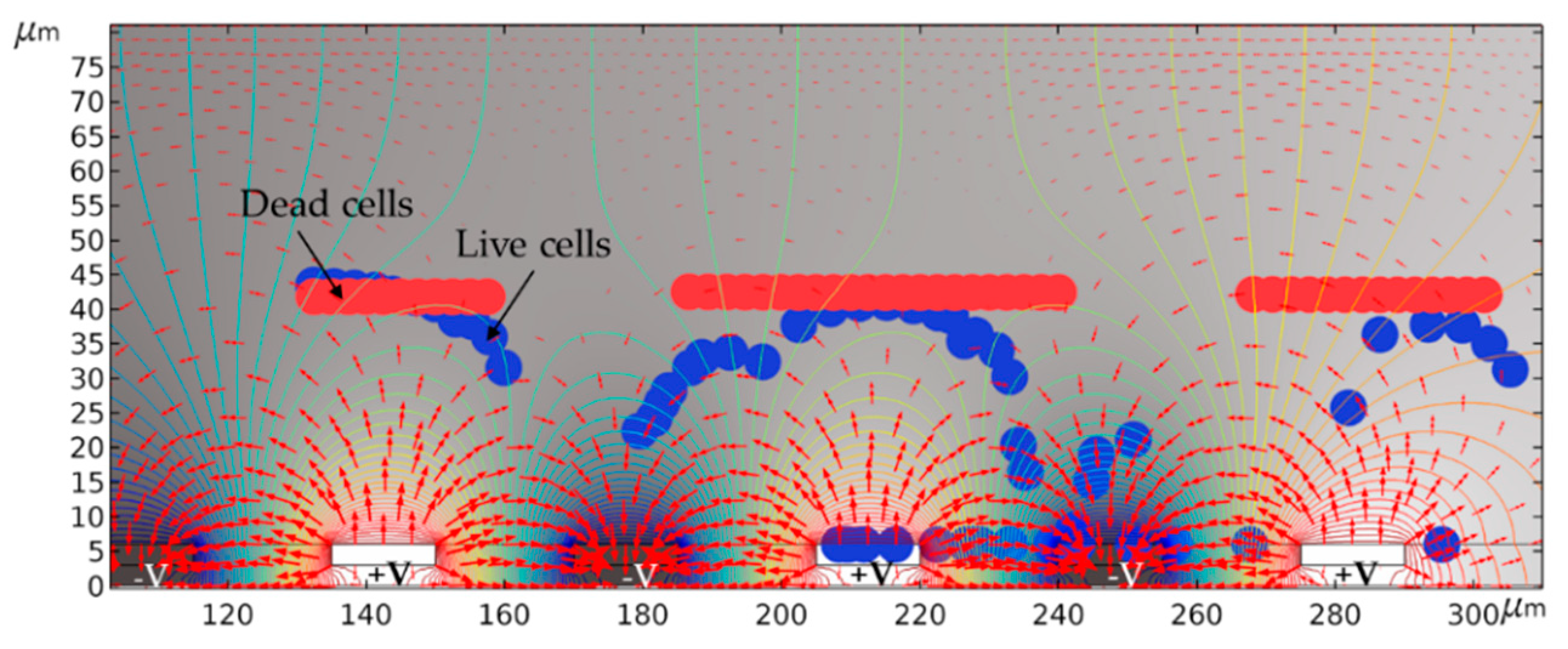
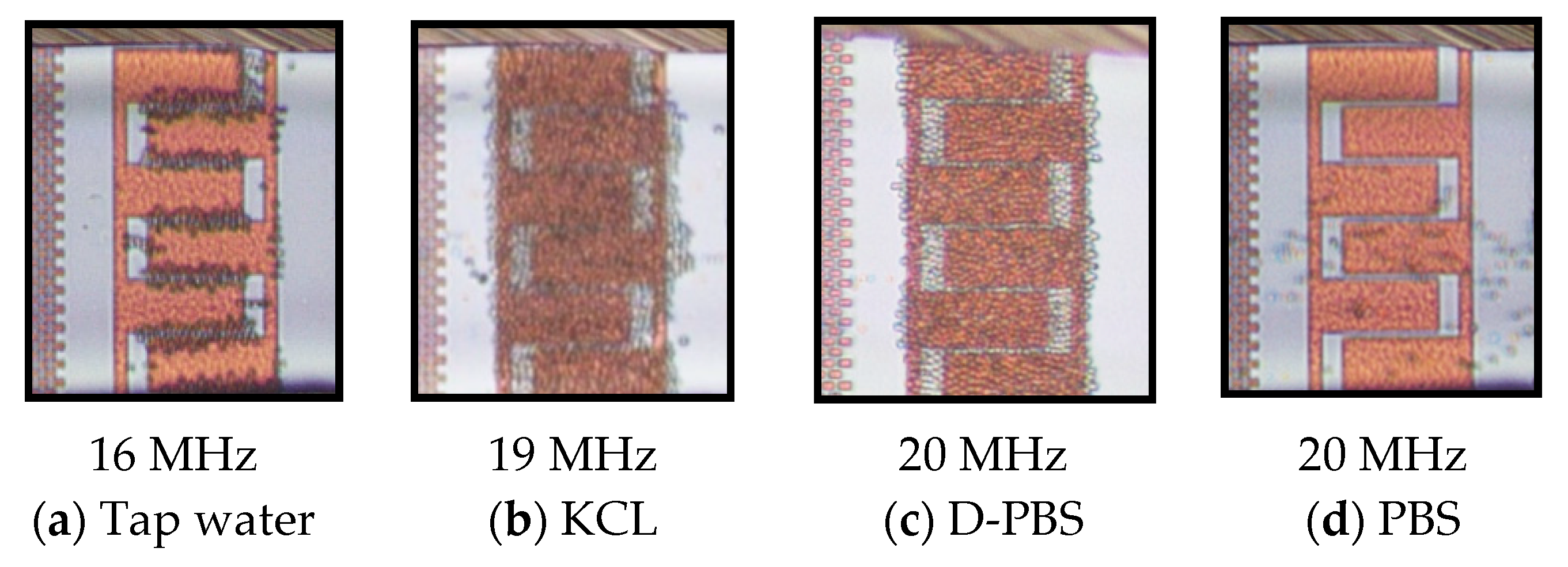
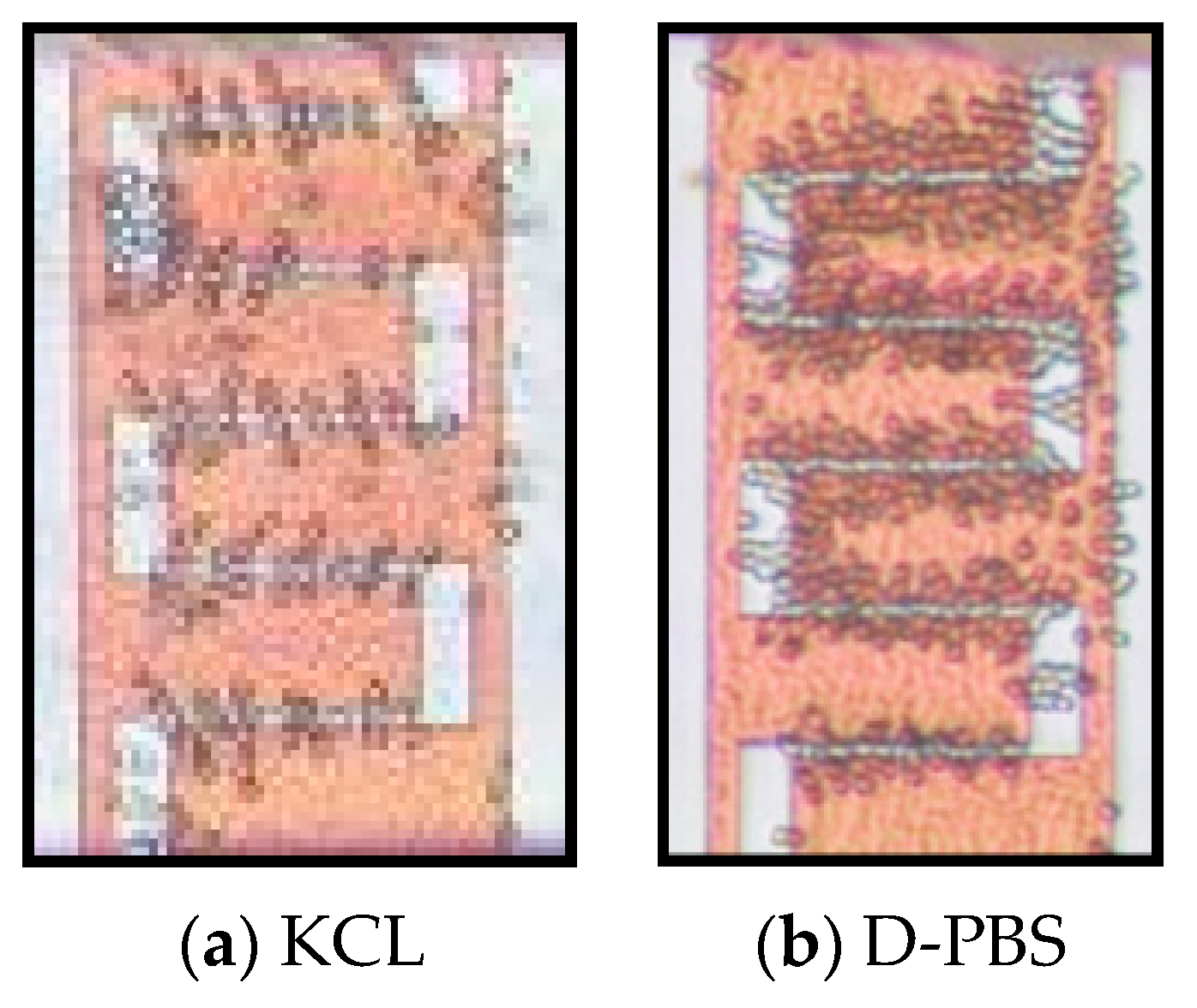
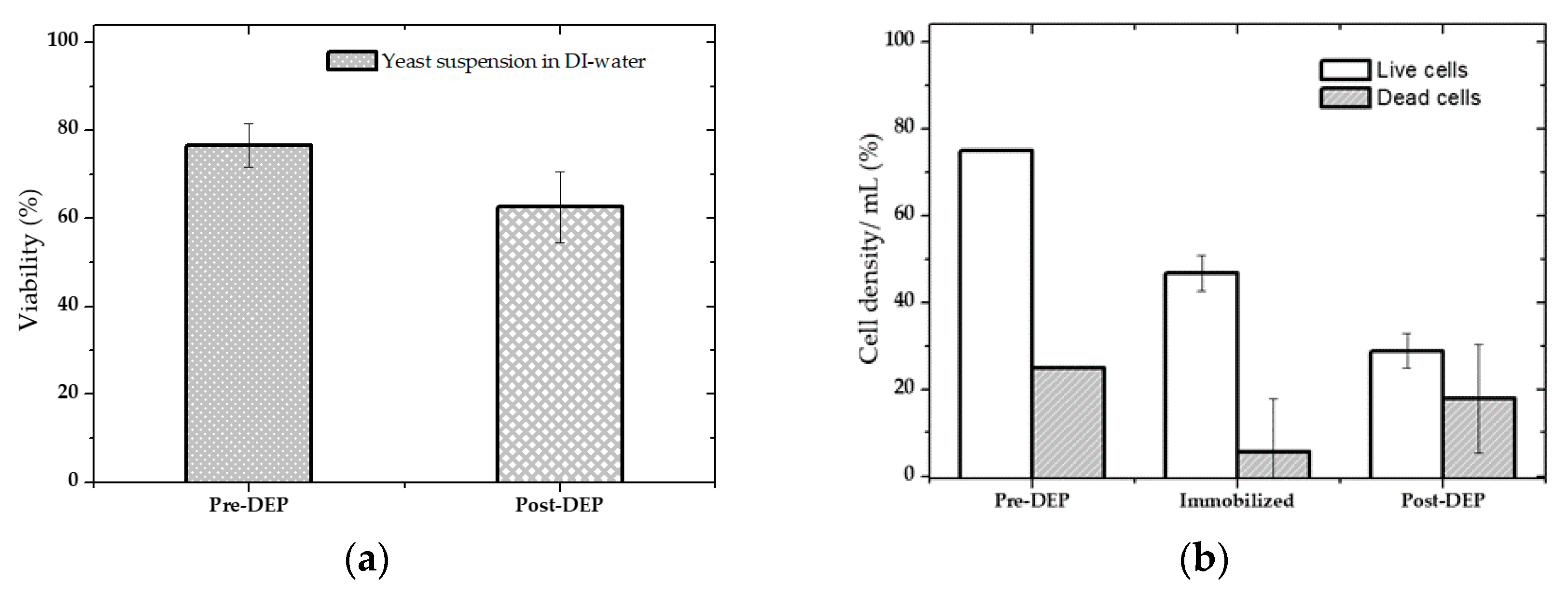
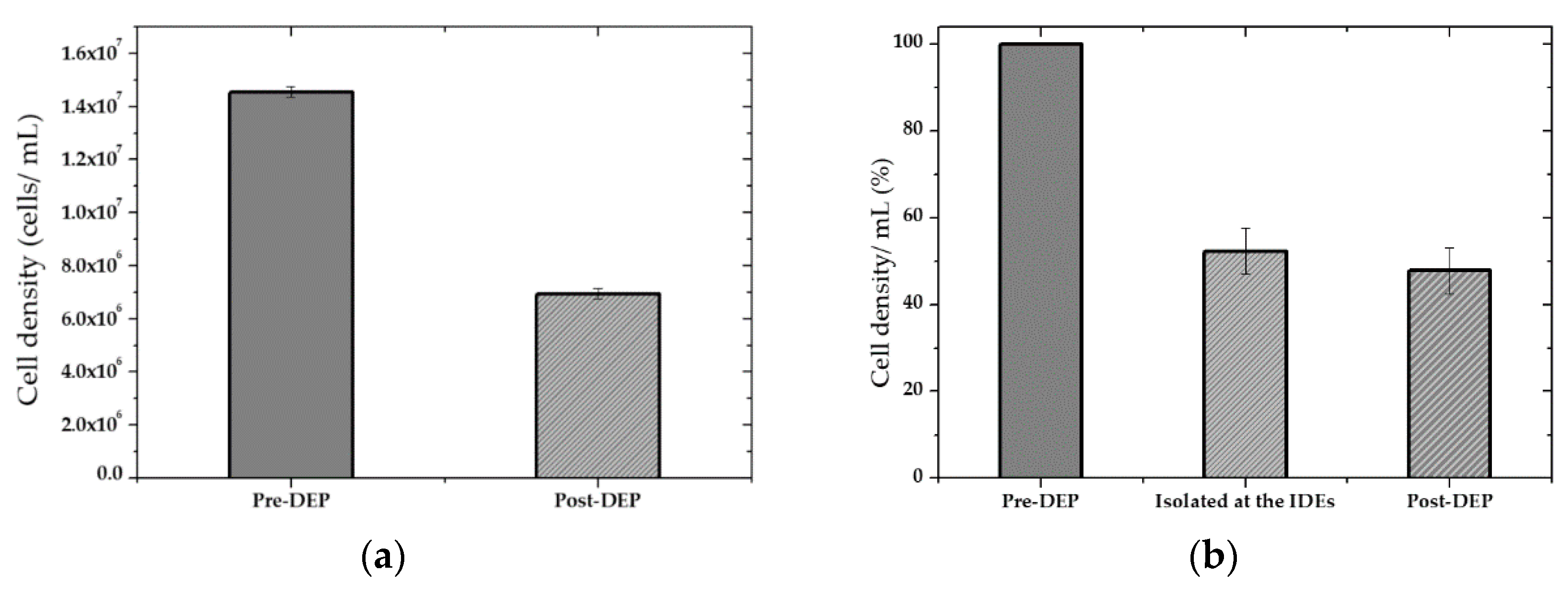
4.2. Cell Separation from a Cell Mixture
4.3. Isolation Efficiency Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demircan, Y.; Yilmaz, G.; Külah, H.; Demirci, U.; Khademhosseini, A.; Langer, R.; Blander, J. Electrophoresis and Dielectrophoresis for Lab-on-a-Chip (LOC) Analyses. In Microfluidic Technologies for Human Health; World Scientific: Singapore, 2013; pp. 341–375. [Google Scholar]
- Adekanmbi, E.O.; Srivastava, S.K. Dielectrophoretic applications for disease diagnostics using lab-on-a-chip platforms. Lab. Chip 2016, 16, 2148–2167. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, Y.; Akin, D.; Bashir, R. Characterization and modeling of a microfluidic dielectrophoresis filter for biological species. J. Microelectromechanical Syst. 2005, 14, 103–112. [Google Scholar] [CrossRef]
- Kersaudy-Kerhoas, M.; Dhariwal, R.; Desmulliez, M.P.Y. Recent advances in microparticle continuous separation. IET Nanobiotechnology 2008, 2, 1–13. [Google Scholar] [CrossRef]
- Tsutsui, H.; Ho, C.-M. Cell separation by non-inertial force fields in microfluidic systems. Mech. Res. Commun. 2009, 36, 92–103. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Bow, H.; Hou, H.W.; Tan, S.J.; Han, J.; Lim, C.T. Microfluidics for cell separation. Med. Biol. Eng. Comput. 2010, 48, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, K.S.; Jung, J.H.; Chang, C.B.; Sung, H.J. Optical mobility of blood cells for label-free cell separation applications. Appl. Phys. Lett. 2013, 102, 141911. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, T.; Xu, R.; Gao, W.; Zhao, H.; Shapter, J.G.; Wang, K.; Shen, Y.; Huang, P.; Gao, G.; et al. Large-scale immuno-magnetic cell sorting of T cells based on a self-designed high-throughput system for potential clinical application. Nanoscale 2017, 9, 13592–13599. [Google Scholar] [CrossRef]
- Quinn, M.M.; Jalalian, L.; Ribeiro, S.; Ona, K.; Demirci, U.; Cedars, M.I.; Rosen, M.P. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum. Reprod. 2018, 33, 1388–1393. [Google Scholar] [CrossRef]
- Bishop, D.P.; Blanes, L.; Wilson, A.B.; Wilbanks, T.; Killeen, K.; Grimm, R.; Wenzel, R.; Major, D.; Macka, M.; Clarke, D.; et al. Microfluidic high performance liquid chromatography-chip hyphenation to inductively coupled plasma–mass spectrometry. J. Chromatogr. A 2017, 1497, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Shi, Z.; Tang, W.; Huang, D.; Zhang, X.; Ni, Z. Improved understanding of particle migration modes in spiral inertial microfluidic devices. RSC Adv. 2015, 5, 77264–77273. [Google Scholar] [CrossRef]
- Gascoyne, P.R.C.; Shim, S. Isolation of Circulating Tumor Cells by Dielectrophoresis. Cancers 2014, 6, 545–579. [Google Scholar] [CrossRef]
- Gagnon, Z.R. Cellular dielectrophoresis: Applications to the characterization, manipulation, separation and patterning of cells. Electrophoresis 2011, 32, 2466–2487. [Google Scholar] [CrossRef]
- David, R.; Groebner, M.; Franz, W.-M. Magnetic Cell Sorting Purification of Differentiated Embryonic Stem Cells Stably Expressing Truncated Human CD4 as Surface Marker. Stem Cells 2005, 23, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Said, T.M.; Grunewald, S.; Paasch, U.; Glander, H.-J.; Baumann, T.; Kriegel, C.; Li, L.; Agarwal, A. Advantage of combining magnetic cell separation with sperm preparation techniques. Reprod. Biomed. Online 2005, 10, 740–746. [Google Scholar] [CrossRef]
- Werner, M.; Merenda, F.; Piguet, J.; Salathé, R.-P.; Vogel, H. Microfluidic array cytometer based on refractive optical tweezers for parallel trapping, imaging and sorting of individual cells. Lab. Chip 2011, 11, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B.; Radbruch, A.; Kümmel, T.; Wickenhauser, C.; Korb, H.; Hansmann, M.; Thiele, J.; Fischer, R. Magnetic activated cell sorting (MACS)-a new immunomagnetic method for megakaryocytic cell isolation: Comparison of different separation techniques. Eur. J. Haematol. 1994, 52, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Mach, A.J.; Di Carlo, D. Continuous scalable blood filtration device using inertial microfluidics. Biotechnol. Bioeng. 2010, 107, 302–311. [Google Scholar] [CrossRef]
- Khoshmanesh, K.; Zhang, C.; Tovar-Lopez, F.J.; Nahavandi, S.; Baratchi, S.; Kalantar-Zadeh, K.; Mitchell, A. Dielectrophoretic manipulation and separation of microparticles using curved microelectrodes. Electrophoresis 2009, 30, 3707–3717. [Google Scholar] [CrossRef]
- Pommer, M.S.; Zhang, Y.; Keerthi, N.; Chen, D.; Thomson, J.A.; Meinhart, C.D.; Soh, H.T. Dielectrophoretic separation of platelets from diluted whole blood in microfluidic channels. Electrophoresis 2008, 29, 1213–1218. [Google Scholar] [CrossRef]
- Pesch, G.R.; Du, F. A review of dielectrophoretic separation and classification of non-biological particles. Electrophoresis 2021, 42, 134–152. [Google Scholar] [CrossRef]
- Martinez-Duarte, R. Microfabrication technologies in dielectrophoresis applications-A review. Electrophoresis 2012, 33, 3110–3132. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.-F.; Chang, H.-C.; Hou, D.; Chang, H.-C. An integrated dielectrophoretic chip for continuous bioparticle filtering, focusing, sorting, trapping, and detecting. Biomicrofluidics 2007, 1, 021503. [Google Scholar] [CrossRef]
- Patel, S.; Showers, D.; Vedantam, P.; Tzeng, T.-R.; Qian, S.; Xuan, X. Microfluidic separation of live and dead yeast cells using reservoir-based dielectrophoresis. Biomicrofluidics 2012, 6, 034102. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, N.; Mernier, G.; Tornay, R.; Renaud, P. Separation of platelets from other blood cells in continuous-flow by dielectrophoresis field-flow-fractionation. Biomicrofluidics 2011, 5, 034122–341228. [Google Scholar] [CrossRef]
- Yildizhan, Y.; Erdem, N.; Islam, M.; Martinez-Duarte, R.; Elitas, M. Dielectrophoretic Separation of Live and Dead Monocytes Using 3D Carbon-Electrodes. Sensors 2017, 17, 2691. [Google Scholar] [CrossRef]
- Yafouz, B.; Kadri, N.A.; Ibrahim, F. Dielectrophoretic Manipulation and Separation of Microparticles Using Microarray Dot Electrodes. Sensors 2014, 14, 6356–6369. [Google Scholar] [CrossRef]
- Becker, F.F.; Wang, X.B.; Huang, Y.; Pethig, R.; Vykoukal, J.; Gascoyne, P.R. Separation of human breast cancer cells from blood by differential dielectric affinity. Proc. Natl. Acad. Sci. USA 1995, 92, 860–864. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, H.; Neuzil, P. DEP-on-a-Chip: Dielectrophoresis Applied to Microfluidic Platforms. Micromachines 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Gümüş, A.; Nassar, J.M.; Hussain, M.M. Personalized Healthcare: CMOS Enabled Microfluidic Systems for Healthcare Based Applications (Adv. Mater. 16/2018). Adv. Mater. 2018, 30, 1–26. [Google Scholar] [CrossRef]
- Nerguizian, V.; Stiharu, I.; Al-Azzam, N.; Diab, B.Y.; Alazzam, A. The effect of dielectrophoresis on living cells: Crossover frequencies and deregulation in gene expression. Analyst 2019, 144, 3853–3860. [Google Scholar] [CrossRef]
- Martínez-López, J.I.; Moncada-Hernández, H.; Baylon-Cardiel, J.L.; Martínez-Chapa, S.O.; Rito-Palomares, M.; Lapizco-Encinas, B.H. Characterization of electrokinetic mobility of microparticles in order to improve dielectrophoretic concentration. Anal. Bioanal. Chem. 2009, 394, 293–302. [Google Scholar] [CrossRef]
- Keeble, L.; Moser, N.; Rodriguez-Manzano, J.; Georgiou, P. ISFET-Based Sensing and Electric Field Actuation of DNA for On-Chip Detection: A Review. IEEE Sensors J. 2020, 20, 11044–11065. [Google Scholar] [CrossRef]
- Pohl, H.A. The Motion and Precipitation of Suspensoids in Divergent Electric Fields. J. Appl. Phys. 1951, 22, 869–871. [Google Scholar] [CrossRef]
- Tuukkanen, S.; Toppari, J.J.; Kuzyk, A.; Hirviniemi, L.; Hytönen, V.P.; Ihalainen, T.; Törmä, P. Carbon Nanotubes as Electrodes for Dielectrophoresis of DNA. Nano Lett. 2006, 6, 1339–1343. [Google Scholar] [CrossRef]
- Polevaya, Y.; Ermolina, I.; Schlesinger, M.; Ginzburg, B.-Z.; Feldman, Y. Time domain dielectric spectroscopy study of human cells. Biochim. et Biophys. Acta (BBA) Biomembr. 1999, 1419, 257–271. [Google Scholar] [CrossRef]
- Buyong, M.R.; Kayani, A.A.; Hamzah, A.A.; Majlis, B.Y. Dielectrophoresis Manipulation: Versatile Lateral and Vertical Mechanisms. Biosens. 2019, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Yang, L. A Review of Multifunctions of Dielectrophoresis in Biosensors and Biochips for Bacteria Detection. Anal. Lett. 2012, 45, 187–201. [Google Scholar] [CrossRef]
- Yang, L.; Guiseppi-Elie, A. Impedimetric Biosensors for Nano- and Microfluidics. In Encyclopedia of Microfluidics and Nanofluidics; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1364–1380. [Google Scholar]
- Yang, L.; Banada, P.P.; Chatni, M.R.; Lim, K.S.; Bhunia, A.K.; Ladisch, M.; Bashir, R. A multifunctional micro-fluidic system for dielectrophoretic concentration coupled with immuno-capture of low numbers of Listeria monocytogenes. Lab. Chip 2006, 6, 896–905. [Google Scholar] [CrossRef]
- Yang, L. Dielectrophoresis assisted immuno-capture and detection of foodborne pathogenic bacteria in biochips. Talanta 2009, 80, 551–558. [Google Scholar] [CrossRef]
- Miltenyi, S.; Müller, W.; Weichel, W.; Radbruch, A. High gradient magnetic cell separation with MACS. Cytometry 1990, 11, 231–238. [Google Scholar] [CrossRef]
- Gascoyne, P.R.C.; Wang, X.-B.; Huang, Y.; Becker, F.F. Dielectrophoretic separation of cancer cells from blood. IEEE Trans. Ind. Appl. 1997, 33, 670–678. [Google Scholar] [CrossRef]
- Zhu, K.; Kaprelyants, A.S.; Salina, E.G.; Markx, G.H. Separation by dielectrophoresis of dormant and nondormant bacterial cells ofMycobacterium smegmatis. Biomicrofluidics 2010, 4, 022809. [Google Scholar] [CrossRef]
- Choi, W.; Kim, J.-S.; Lee, H.; Lee, K.-K.; Koo, D.-B.; Park, J.-K. Dielectrophoretic oocyte selection chip for in vitro fertilization. Biomed. Microdevices 2007, 10, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Sabuncu, A.C.; Liu, J.A.; Beebe, S.J.; Beskok, A. Dielectrophoretic separation of mouse melanoma clones. Biomicrofluidics 2010, 4, 021101. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R.; Huang, Y.; Wang, X.-B.; Burt, J.P.H. Positive and negative dielectrophoretic collection of colloidal particles using interdigitated castellated microelectrodes. J. Phys. D Appl. Phys. 1992, 25, 881–888. [Google Scholar] [CrossRef]
- Demierre, N.; Braschler, T.; Linderholm, P.; Seger, U.; Van Lintel, H.; Renaud, P. Characterization and optimization of liquid electrodes for lateral dielectrophoresis. Lab. Chip 2007, 7, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-H.; Hsiung, S.-K.; Chen, C.-Y.; Tsai, M.-L.; Lee, G.-B. Automatic microfluidic platform for cell separation and nucleus collection. Biomed. Microdevices 2007, 9, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, S.; Noh, H.-S.; Hesketh, P.J.; Gottfried, D.S. Rapid, low cost microfabrication technologies toward realization of devices for dielectrophoretic manipulation of particles and nanowires. Sens. Actuators B Chem. 2006, 114, 392–401. [Google Scholar] [CrossRef]
- Zhang, C.; Khoshmanesh, K.; Tovar-Lopez, F.J.; Mitchell, A.; Wlodarski, W.; Klantar-Zadeh, K. Dielectrophoretic separation of carbon nanotubes and polystyrene microparticles. Microfluid. Nanofluidics 2009, 7, 633–645. [Google Scholar] [CrossRef]
- Suehiro, J. Fabrication and characterization of nanomaterial-based sensors using dielectrophoresis. Biomicrofluidics 2010, 4, 022804. [Google Scholar] [CrossRef]
- Li, Y.; Dalton, C.; Crabtree, H.J.; Nilsson, G.; Kaler, K.V.I.S. Continuous dielectrophoretic cell separation microfluidic device. Lab. Chip 2007, 7, 239–248. [Google Scholar] [CrossRef]
- Asbury, C.L.; Diercks, A.H.; van den Engh, G. Trapping of DNA by dielectrophoresis. Electrophoresis 2002, 23, 2658–2666. [Google Scholar] [CrossRef]
- Gadish, N.; Voldman, J. High-Throughput Positive-Dielectrophoretic Bioparticle Microconcentrator. Anal. Chem. 2006, 78, 7870–7876. [Google Scholar] [CrossRef]
- Flanagan, L.A.; Lu, J.; Wang, L.; Marchenko, S.A.; Jeon, N.L.; Lee, A.P.; Monuki, E.S. Unique Dielectric Properties Distinguish Stem Cells and Their Differentiated Progeny. Stem Cells 2008, 26, 656–665. [Google Scholar] [CrossRef]
- Ning, Y.; Ma, X.; Multari, C.R.; Luo, X.; Gholizadeh, V.; Palego, C.; Cheng, X.; Hwang, J.C.M. Improved broadband electrical detection of individual biological cells. In Proceedings of the 2015 IEEE MTT-S International Microwave Symposium, Phoenix, AZ, USA, 17–22 May 2015; pp. 1–3. [Google Scholar]
- Thomas, R.S.; Morgan, H.; Green, N.G. Negative DEP traps for single cell immobilisation. Lab. Chip 2009, 9, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Miled, M.A.; Massicotte, G.; Sawan, M. Dielectrophoresis-Based Integrated Lab-on-Chip for Nano and Micro-Particles Manipulation and Capacitive Detection. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kabiri, S.; Sonkusale, S. Dielectrophoretic lab-on-CMOS platform for trapping and manipulation of cells. Biomed. Microdevices 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Manczak, R.; Kaynak, C.B.; Kaynak, M.; Palego, C.; Lalloue, F.; Pothier, A.; Saada, S.; Provent, T.; Dalmay, C.; Bessette, B.; et al. UHF-Dielectrophoresis Crossover Frequency as a New Marker for Discrimination of Glioblastoma Undifferentiated Cells. IEEE J. Electromagn. RF Microw. Med. Biol. 2019, 3, 191–198. [Google Scholar] [CrossRef]
- Guha, S.; Schumann, U.; Jamal, F.I.; Wagner, D.; Meliani, C.; Schmidt, B.; Wenger, C.; Wessel, J.; Detert, M. Integrated high-frequency sensors in catheters for minimally invasive plaque characterization. In Proceedings of the 20th European Microelectronics and Packaging Conference and Exhibition: Enabling Technologies for a Better Life and Future, EMPC, Friedrichshafen, Germany, 14–16 September 2015; pp. 1–6. [Google Scholar]
- Guha, S.; Schmalz, K.; Meliani Ch Wenger, C. KW CMOS MEMS based Microfluidic System for Cytometry at 5GHz. In Proceedings of the MFHS, Microfluidic handling System, Entschede, The Netherlands, 10–12 October 2012. [Google Scholar]
- Guha, S.; Wenger, C. Radio Frequency CMOS Chem-bio Viscosity Sensors based on Dielectric Spectroscopy. In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies; SCITEPRESS Science and Technology Publications, Porto, Portugal, 21 February 2017; pp. 142–148. [Google Scholar]
- Guha, S.; Schmalz, K.; Wenger, C.; Herzel, F. Self-calibrating highly sensitive dynamic capacitance sensor: Towards rapid sensing and counting of particles in laminar flow systems. Analyst 2015, 140, 3262–3272. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kabiri, S.; Sonkusale, S. CMOS dielectrophoretic Lab-on-Chip platform for manipulation and monitoring of cells. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBCMilan, Italy, 25–29 August 2015; Volume 2015, pp. 7530–7533. [Google Scholar]
- Otto, S.; Kaletta, U.; Bier, F.F.; Wenger, C.; Hölzel, R. Dielectrophoretic immobilisation of antibodies on microelectrode arrays. Lab. Chip 2014, 14, 998. [Google Scholar] [CrossRef] [PubMed]
- Manaresi, N.; Romani, A.; Medoro, G.; Altomare, L.; Leonardi, A.; Tartagni, M.; Guerrieri, R. A CMOS Chip for Individual Cell Manipulation and Detection. IEEE J. Solid-State Circuits 2003, 38, 2297–2305. [Google Scholar] [CrossRef]
- Ameri, A.; Zhang, L.; Gharia, A.; Anwar, M.; Niknejad, A.M. Dielectrophoretic-Assisted Biosensor for Single-Cell Characterization at Mmwave Frequencies in CMOS 28nm Technology. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII, Berlin, Germany, 23–27 June 2019; pp. 174–177. [Google Scholar]
- Iliescu, C.; Taylor, H.; Avram, M.; Miao, J.; Franssila, S. A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics 2012, 6, 016505–1650516. [Google Scholar] [CrossRef]
- Becker, H. Mind the gap! Lab. Chip 2010, 10, 271–273. [Google Scholar] [CrossRef]
- Matbaechi Ettehad, H.; Soltani Zarrin, P.; Hölzel, R.; Wenger, C. Dielectrophoretic Immobilization of Yeast Cells Using CMOS Integrated Microfluidics. Micromachines 2020, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, H.M.; Yadav, R.K.; Guha, S.; Wenger, C. Towards CMOS Integrated Microfluidics Using Dielectrophoretic Immobilization. Biosensors 2019, 9, 77. [Google Scholar] [CrossRef]
- Ettehad, H.M.; Guha, S.; Wenger, C. Simulation of CMOS Compatible Sensor Structures for Dielectrophoretic Biomolecule Immobilization. In Proceedings of the COMSOL-Bioscience and Bioengineering, Rotterdam, The Netherlands, 19 October 2017; p. 6. [Google Scholar]
- Lei, J.; Wan, J.T.K.; Yu, K.W.; Sun, H. First-principle approach to dielectric behavior of nonspherical cell suspensions. Phys. Rev. E 2001, 64, 012903. [Google Scholar] [CrossRef]
- Thomas, B. Jones Electromechanics of Particles; Cambridge University Press in Cambridge: New York, NY, USA, 1995; ISBN 0521431964. [Google Scholar]
- Cottet, J.; Fabregue, O.; Berger, C.; Buret, F.; Renaud, P.; Frénéa-Robin, M. MyDEP: A New Computational Tool for Dielectric Modeling of Particles and Cells. Biophys. J. 2019, 116, 12–18. [Google Scholar] [CrossRef]
- Huang, Y.; Holzel, R.; Pethig, R.; Wang, X.-B. Differences in the AC electrodynamics of viable and non-viable yeast cells determined through combined dielectrophoresis and electrorotation studies. Phys. Med. Biol. 1992, 37, 1499–1517. [Google Scholar] [CrossRef]
- Talary, M.S.; Burt, J.P.H.; Tame, J.A.; Pethig, R. Electromanipulation and separation of cells using travelling electric fields. J. Phys. D Appl. Phys. 1996, 29, 2198–2203. [Google Scholar] [CrossRef]



| Ref. | Purpose | Analyte | Microfluidic Material | DEP Microelectrode | DEP Parameters | Substrate |
|---|---|---|---|---|---|---|
| [56] | characterization, identification | Stem cells and their differentiated progeny | PDMS | IDE 1 | 25 kHz—10 MHz at 8 V | silicon wafers or glass slides |
| [57] | detection | Live Jurkat’s cytoplasm | PDMS | coplanar waveguide (CPW) | 10 MHz at 3 V | quartz |
| [58] | trapping, rotating, detecting | Hela cells and polystyrene particles | PDMS | IDE 1 | 1 MHz at 76/80 Vpp | PCB |
| [59] | detection, separation | Micro-nano particles 500 nm–10 µm | Glass | L-shaped electrode | 0–1 MHz at >−1.3 V and <1.4 | glass on PCB |
| [60] | trapping, manipulation | yeast cells | PDMS | 3D octa-pole | 100 Hz—5 MHz at 5 Vpp | CMOS |
| [61] | characterization, discrimination | cancer stem cells | PDMS | quadrupole electrode | 50–500 MHz at 2–4 Vpp | CMOS |
| Sample | DI-Water | Tap Water | KCL | PBS | D-PBS |
|---|---|---|---|---|---|
| Live yeast cells | 1.18 × 10−3 S/m | 7.82 × 10−2 S/m | 0.262 S/m | 1.430 S/m | 0.201 S/m |
| Dead yeast cells | 3.87 × 10−3 S/m | 7.85 × 10−2 S/m | 0.266 S/m S/m | 1.435 S/m | 0.206 S/m |
| MUT 1 | pDEP Range (Numerical Prediction) | pDEP Range (Empirical) | ||||
|---|---|---|---|---|---|---|
| Cell | Media | fc1 2 | fc2 | fc1 | fc2 | fopt 3 |
| Live Yeast cells | DIW | - | 65.4 MHz | 100 kHz | 12 MHz | 1–3 MHz |
| Tap water | 887 kHz | 68.2 MHz | 1 MHz | 40 MHz 4 | 10–14 MHz | |
| KCL (20 mM) | 11.6 MHz | 48 MHz | 11 MHz | not measured | 18–20 MHz | |
| PBS (0.1 M) | nDEP | nDEP | nDEP | nDEP | - | |
| D-PBS | 4.86 MHz | 59.98 MHz | 4 MHz | not measured | 13–20 MHz | |
| Dead Yeast cells | DIW | - | 2.5 MHz | 20 kHz | 2.3 MHz | 90–900 kHz |
| Tap water | nDEP | nDEP | nDEP | nDEP | - | |
| KCL (20 mM) | nDEP | nDEP | nDEP | nDEP | - | |
| PBS (0.1 M) | nDEP | nDEP | nDEP | nDEP | - | |
| D-PBS | nDEP | nDEP | nDEP | nDEP | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matbaechi Ettehad, H.; Wenger, C. Characterization and Separation of Live and Dead Yeast Cells Using CMOS-Based DEP Microfluidics. Micromachines 2021, 12, 270. https://doi.org/10.3390/mi12030270
Matbaechi Ettehad H, Wenger C. Characterization and Separation of Live and Dead Yeast Cells Using CMOS-Based DEP Microfluidics. Micromachines. 2021; 12(3):270. https://doi.org/10.3390/mi12030270
Chicago/Turabian StyleMatbaechi Ettehad, Honeyeh, and Christian Wenger. 2021. "Characterization and Separation of Live and Dead Yeast Cells Using CMOS-Based DEP Microfluidics" Micromachines 12, no. 3: 270. https://doi.org/10.3390/mi12030270
APA StyleMatbaechi Ettehad, H., & Wenger, C. (2021). Characterization and Separation of Live and Dead Yeast Cells Using CMOS-Based DEP Microfluidics. Micromachines, 12(3), 270. https://doi.org/10.3390/mi12030270






