Creating an Artificial 3-Dimensional Ovarian Follicle Culture System Using a Microfluidic System
Abstract
1. Introduction
2. Materials and Methods
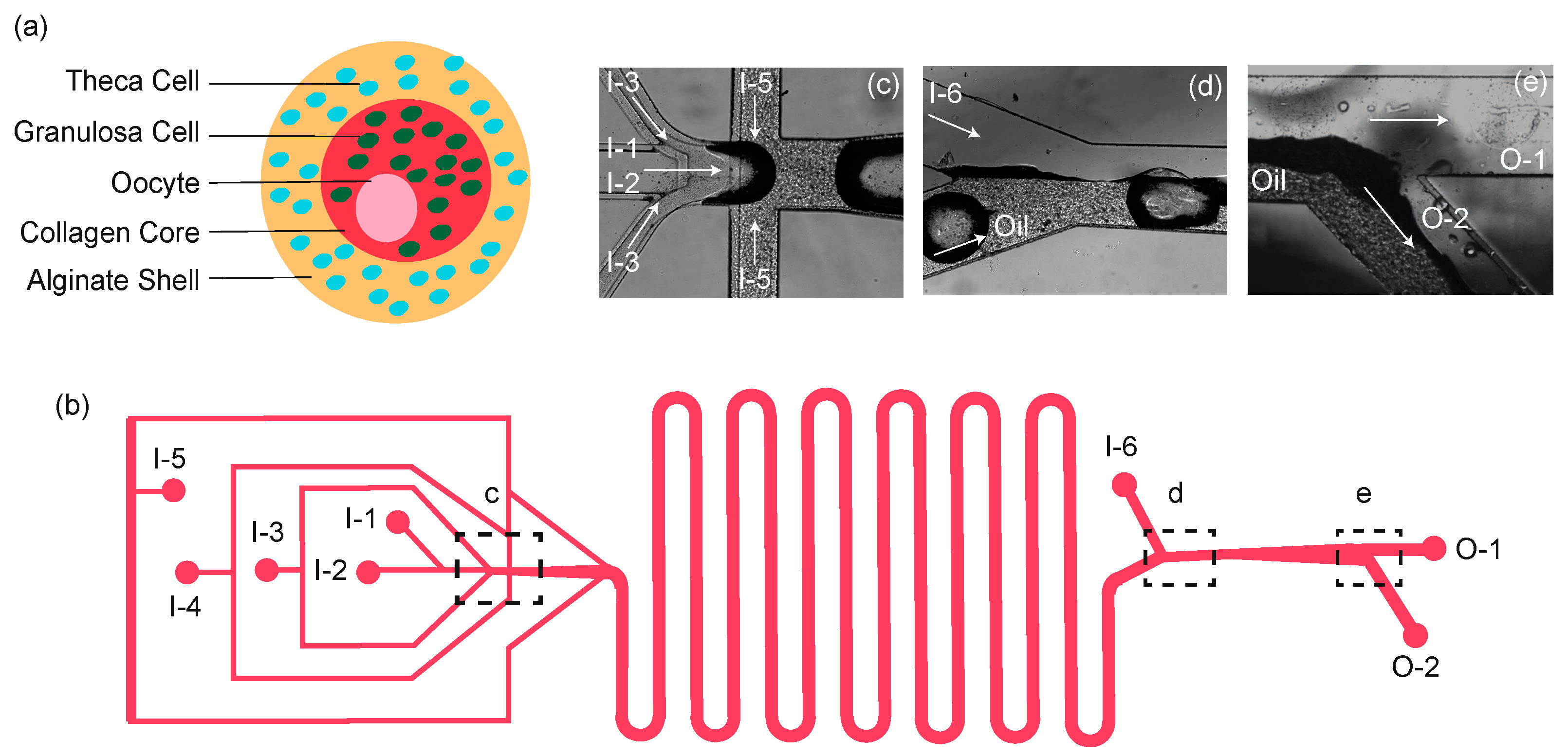
2.1. Design of 3-Dimensional Capsule
2.2. Granulosa and Theca Cells
2.3. Microfluidic Device Fabrication and Design
2.4. Encapsulation
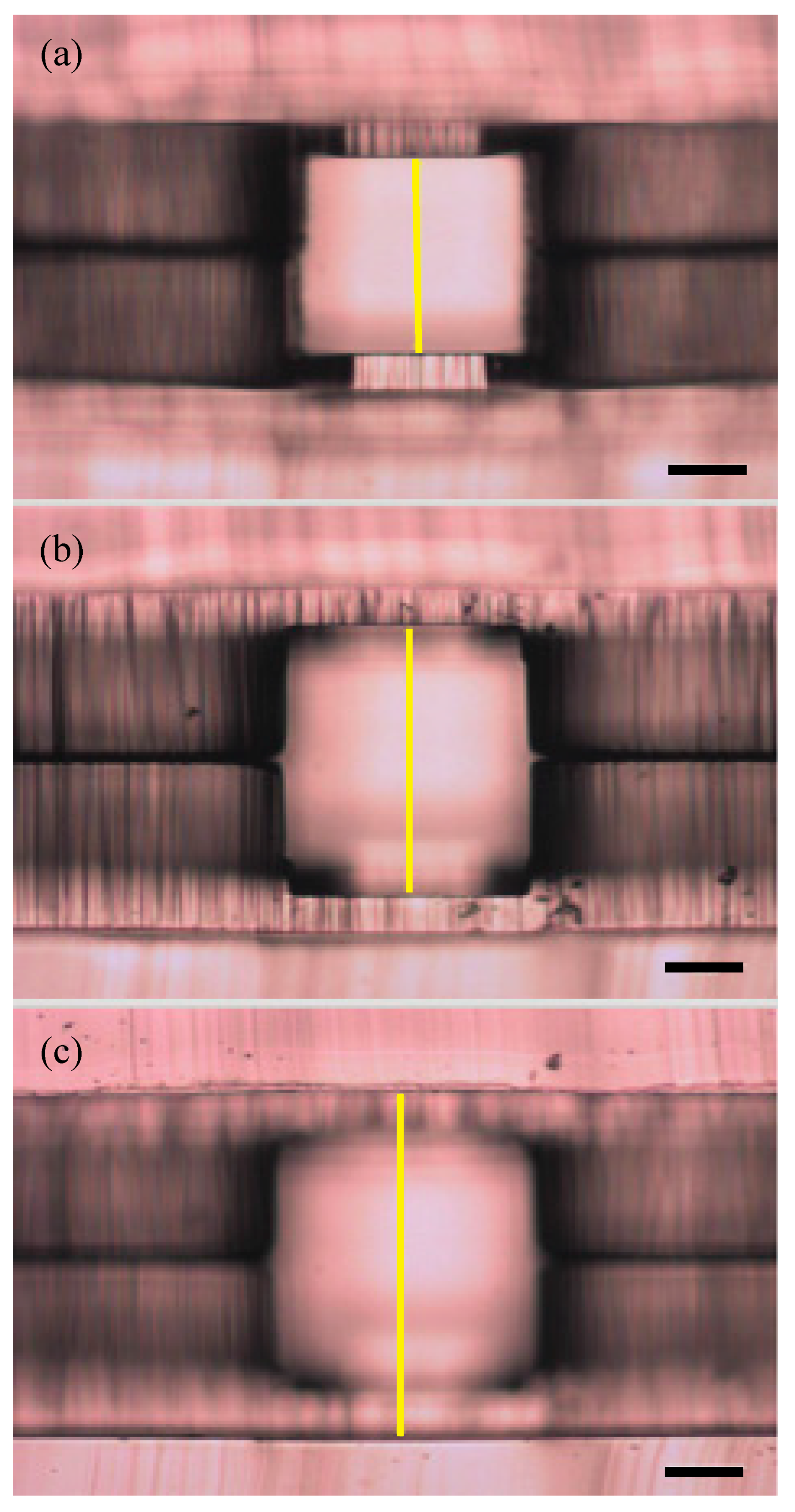
2.5. Spatial Confirmation
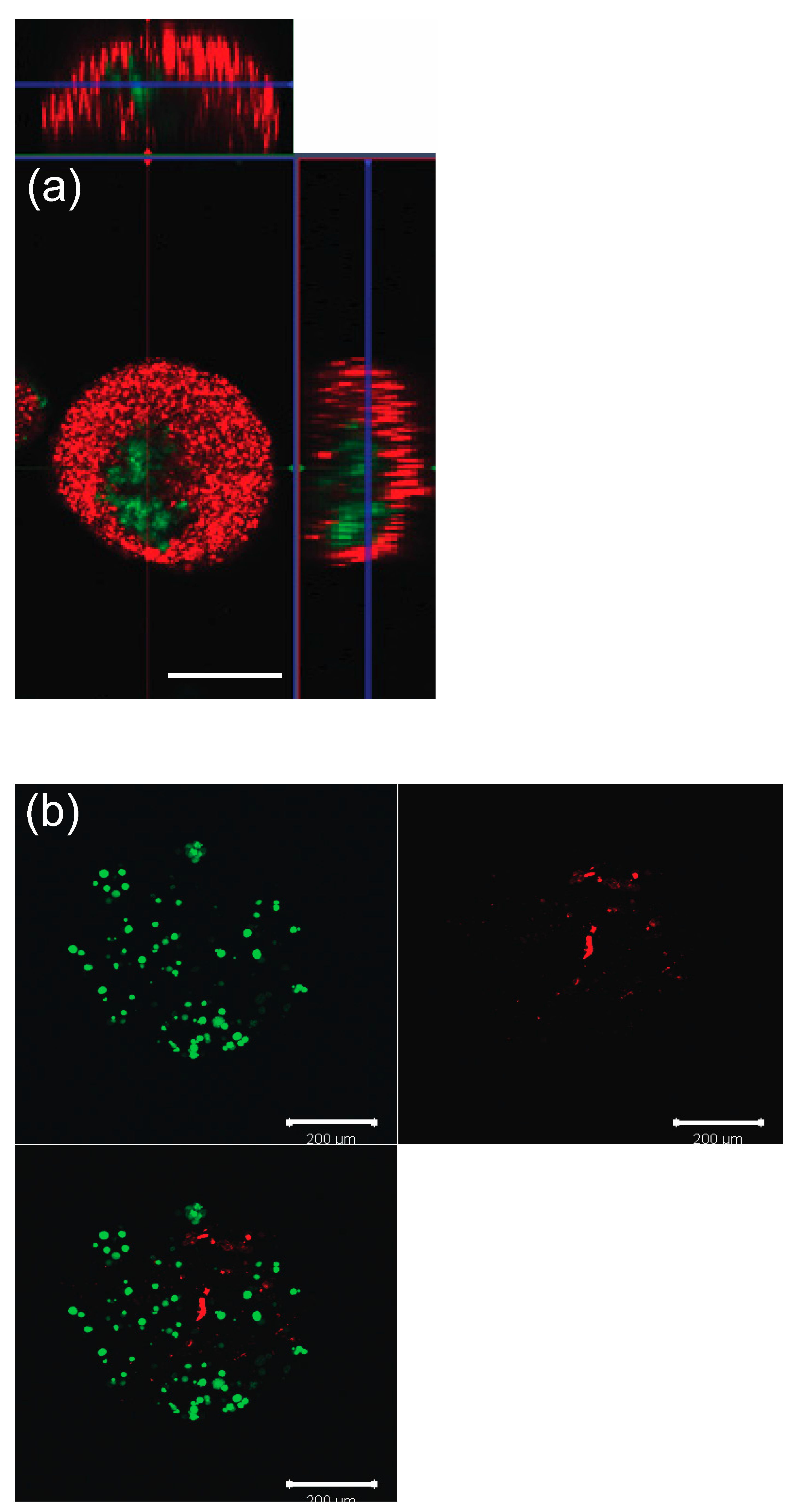
2.6. Viability for GCs and TCs within Microcapsules (No Oocytes)
2.7. Measurement of Hormones
2.8. Oocyte Isolation and Encapsulation
2.9. Statistical Analysis
3. Results
3.1. Spatial Confirmation
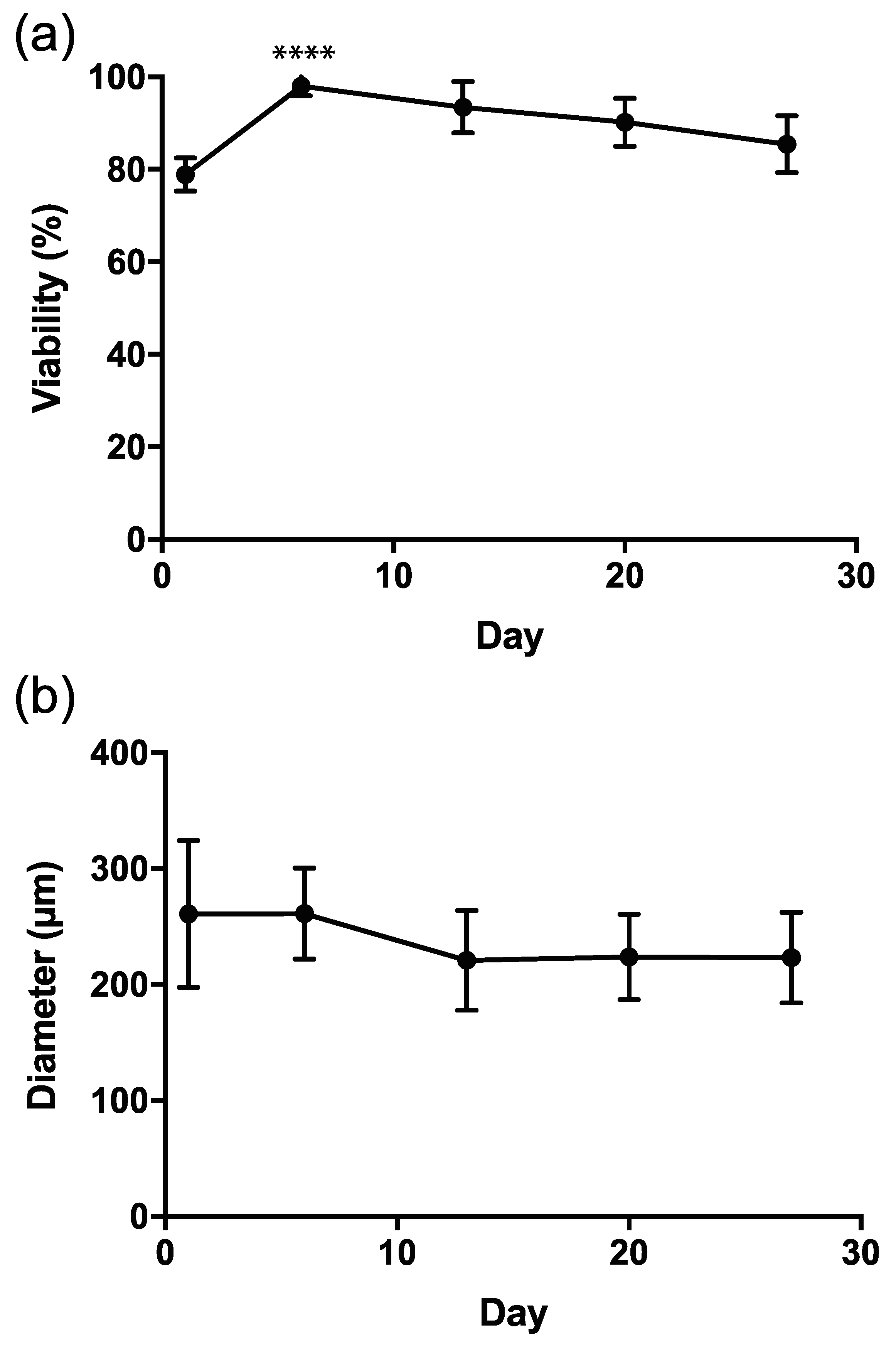
3.2. Viability Test for GC and TC within Microcapsules (No Oocytes)
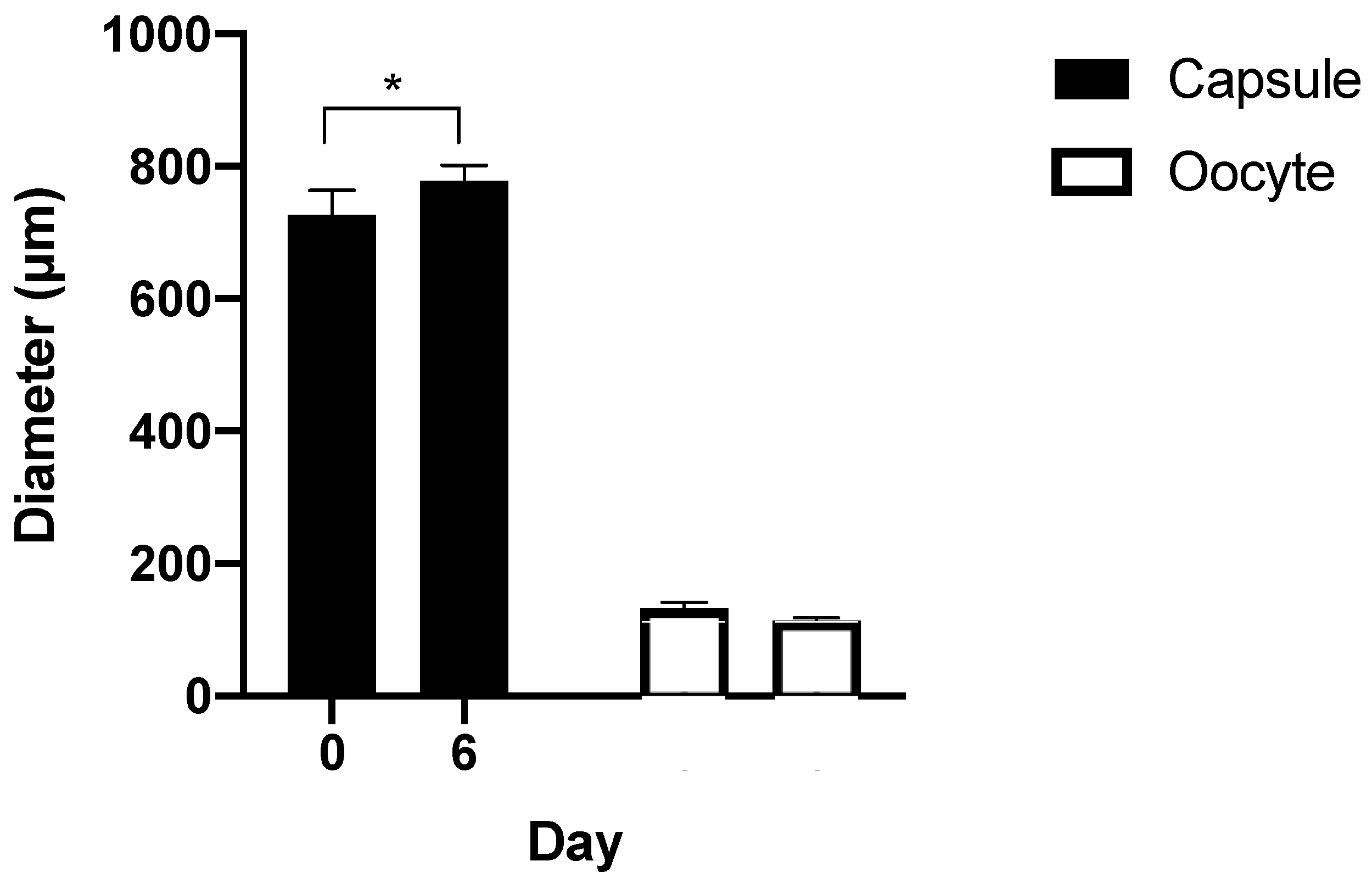
3.3. Capsule Size during Culture
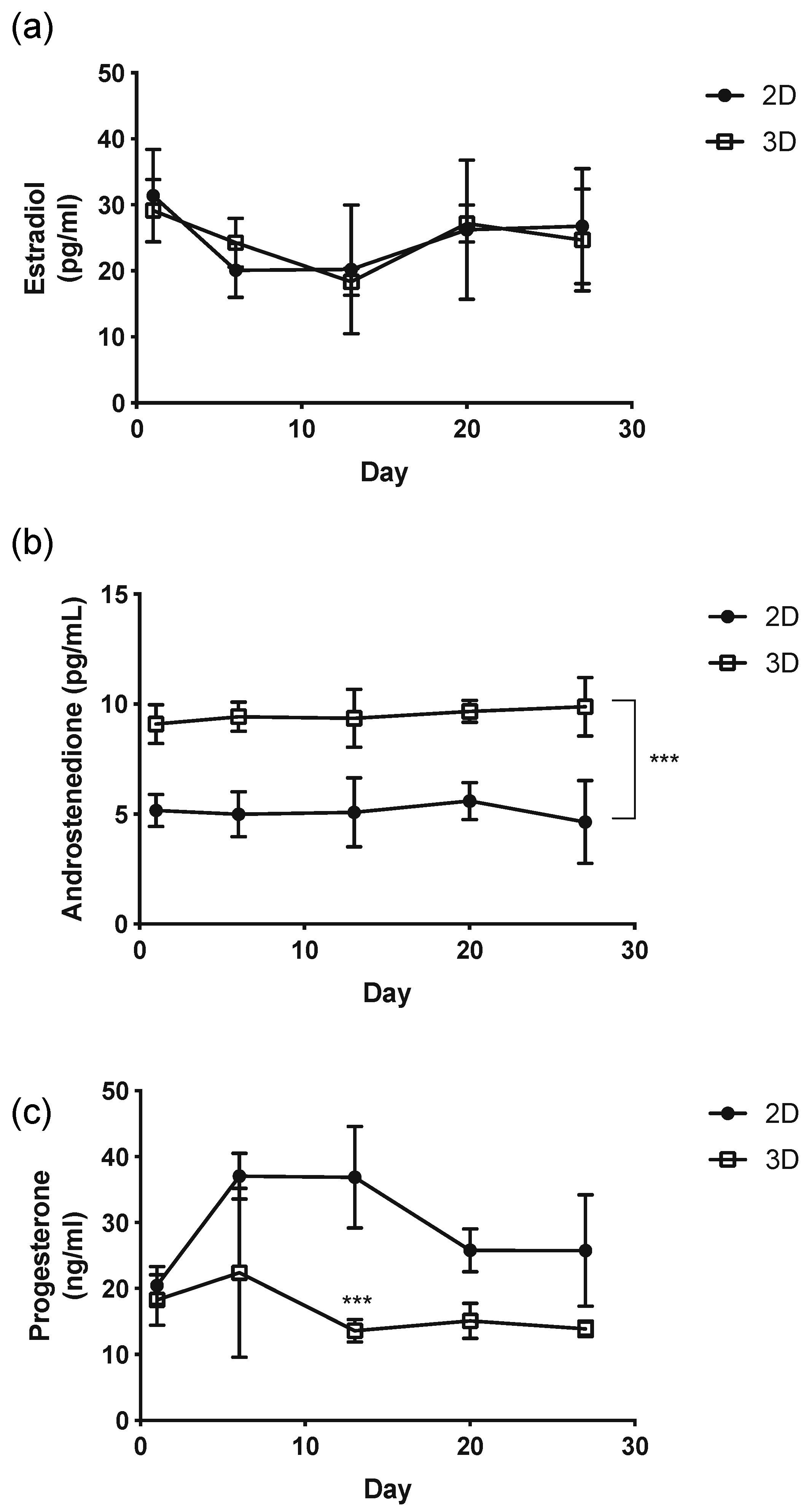
3.4. Measurement of Hormones
3.5. Encapsulated Oocytes with GCs and TCs
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.M. Reproductive outcomes for survivors of childhood cancer. Obstet. Gynecol. 2010, 116, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Oktem, O.; Oktay, K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007, 67, 10159–10162. [Google Scholar] [CrossRef] [PubMed]
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K. Fertility Preservation for Patients with Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2013. [Google Scholar] [CrossRef]
- Lambertini, M.; Moore, H.C.F.; Leonard, R.C.F.; Loibl, S.; Munster, P.; Bruzzone, M.; Boni, L.; Unger, J.M.; Anderson, R.A.; Mehta, K.; et al. Gonadotropin-Releasing Hormone Agonists During Chemotherapy for Preservation of Ovarian Function and Fertility in Premenopausal Patients With Early Breast Cancer: A Systematic Review and Meta-Analysis of Individual Patient-Level Data. J. Clin. Oncol. 2018, 36, 1981–1990. [Google Scholar] [CrossRef]
- Gook, D.A.; Edgar, D.H. Cryopreservation of female reproductive potential. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 55, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.H.; Offersen, B.V.; Andersen, C.Y.; Ernst, E. Legal termination of a pregnancy resulting from transplanted cryopreserved ovarian tissue due to cancer recurrence. J. Assist. Reprod. Genet. 2013, 30, 975–978. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Vos, M.; Smitz, J.; Woodruff, T.K. Fertility preservation in women with cancer. Lancet 2014, 384, 1302–1310. [Google Scholar] [CrossRef]
- Eppig, J.J.; O’Brien, M.J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 1996, 54, 197–207. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Pendola, J.K.; Eppig, J.J. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol. Reprod. 2003, 68, 1682–1686. [Google Scholar] [CrossRef]
- Hirao, Y.; Naruse, K.; Kaneda, M.; Somfai, T.; Iga, K.; Shimizu, M.; Akagi, S.; Cao, F.; Kono, T.; Nagai, T.; et al. Production of fertile offspring from oocytes grown in vitro by nuclear transfer in cattle. Biol. Reprod. 2013, 89, 57. [Google Scholar] [CrossRef]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.; Dolmans, M.M.; Vanacker, J.; Legat, C.; Fortuño Moya, C.; Donnez, J.; Amorim, C.A. A new step toward the artificial ovary: Survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 2014, 101, 1149–1156. [Google Scholar] [CrossRef]
- Chiti, M.C.; Dolmans, M.M.; Orellana, R.; Soares, M.; Paulini, F.; Donnez, J.; Amorim, C.A. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum. Reprod. 2016, 31, 427–435. [Google Scholar] [CrossRef]
- Paulini, F.; Vilela, J.M.; Chiti, M.C.; Donnez, J.; Jadoul, P.; Dolmans, M.M.; Amorim, C.A. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod. Biomed. Online 2016, 33, 425–432. [Google Scholar] [CrossRef]
- Laronda, M.M.; Jakus, A.E.; Whelan, K.A.; Wertheim, J.A.; Shah, R.N.; Woodruff, T.K. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 2015, 50, 20–29. [Google Scholar] [CrossRef]
- Mastrorocco, A.; Cacopardo, L.; Martino, N.A.; Fanelli, D.; Camillo, F.; Ciani, E.; Roelen, B.A.J.; Ahluwalia, A.; Dell’Aquila, M.E. One-step automated bioprinting-based method for cumulus-oocyte complex microencapsulation for 3D in vitro maturation. PLoS ONE 2020, 15, e0238812. [Google Scholar] [CrossRef] [PubMed]
- Laronda, M.M.; Rutz, A.L.; Xiao, S.; Whelan, K.A.; Duncan, F.E.; Roth, E.W.; Woodruff, T.K.; Shah, R.N. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 2017, 8, 15261. [Google Scholar] [CrossRef]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J.; et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017, 8, 14584. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.U.R.; Fu, M.; Deng, J.; Geng, C.; Luo, Y.; Lin, B.; Yu, X.; Liu, B. A Microfluidic Device for Culturing an Encapsulated Ovarian Follicle. Micromachines 2017, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Abir, R.; Nitke, S.; Ben-Haroush, A.; Fisch, B. In vitro maturation of human primordial ovarian follicles: Clinical significance, progress in mammals, and methods for growth evaluation. Histol. Histopathol. 2006, 21, 887–898. [Google Scholar] [CrossRef]
- Muruvi, W.; Picton, H.M.; Rodway, R.G.; Joyce, I.M. In vitro growth and differentiation of primary follicles isolated from cryopreserved sheep ovarian tissue. Anim. Reprod. Sci. 2009, 112, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Walls, M.L.; Hunter, T.; Ryan, J.P.; Keelan, J.A.; Nathan, E.; Hart, R.J. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: A comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum. Reprod. 2014, 30, 88–96. [Google Scholar] [CrossRef]
- Virant-Klun, I.; Bauer, C.; Ståhlberg, A.; Kubista, M.; Skutella, T. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod. Biomed. Online 2018, 36, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Campbell, L.; Allison, V.; Murray, A.; Spears, N. Culture and co-culture of mouse ovaries and ovarian follicles. J. Vis. Exp. 2015. [Google Scholar] [CrossRef] [PubMed]
- Tingen, C.M.; Kiesewetter, S.E.; Jozefik, J.; Thomas, C.; Tagler, D.; Shea, L.; Woodruff, T.K. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction 2011, 141, 809–820. [Google Scholar] [CrossRef]
- Casillas, F.; Teteltitla-Silvestre, M.; Ducolomb, Y.; Lemus, A.E.; Salazar, Z.; Casas, E.; Betancourt, M. Co-culture with granulosa cells improve the in vitro maturation ability of porcine immature oocytes vitrified with cryolock. Cryobiology 2014, 69, 299–304. [Google Scholar] [CrossRef] [PubMed]
- No, J.; Zhao, M.; Lee, S.; Ock, S.A.; Nam, Y.; Hur, T.Y. Enhanced in vitro maturation of canine oocytes by oviduct epithelial cell co-culture. Theriogenology 2018, 105, 66–74. [Google Scholar] [CrossRef]
- Li, S.K.; Hearn, M.T. Isolation of thecal cells: An assessment of purity and steroidogenic potential. J. Biochem. Biophys. Methods 2000, 45, 169–181. [Google Scholar] [CrossRef]
- Magoffin, D.A.; Erickson, G.F. Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology 1988, 122, 2345–2347. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Agarwal, P.; Huang, H.; Zhao, S.; He, X. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 2014, 35, 5122–5128. [Google Scholar] [CrossRef]
- Costa, R.R.; Girotti, A.; Santos, M.; Arias, F.J.; Mano, J.F.; Rodríguez-Cabello, J.C. Cellular uptake of multilayered capsules produced with natural and genetically engineered biomimetic macromolecules. Acta Biomater. 2014, 10, 2653–2662. [Google Scholar] [CrossRef]
- Choi, J.K.; Agarwal, P.; He, X. In vitro culture of early secondary preantral follicles in hanging drop of ovarian cell-conditioned medium to obtain MII oocytes from outbred deer mice. Tissue Eng. Part A 2013, 19, 2626–2637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Q.Y. Evaluation of oocyte quality: Morphological, cellular and molecular predictors. Reprod. Fertil. Dev. 2007, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Mouse oocyte development in vitro with various culture systems. Dev. Biol. 1977, 60, 371–388. [Google Scholar] [CrossRef]
- West, E.R.; Shea, L.D.; Woodruff, T.K. Engineering the follicle microenvironment. Semin. Reprod. Med. 2007, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Nayudu, P.L.; Osborn, S.M. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J. Reprod. Fertil. 1992, 95, 349–362. [Google Scholar] [CrossRef]
- Filatov, M.A.; Khramova, Y.V.; Semenova, M.L. In Vitro Mouse Ovarian Follicle Growth and Maturation in Alginate Hydrogel: Current State of the Art. Acta Nat. 2015, 7, 48–56. [Google Scholar] [CrossRef]
- Jamalzaei, P.; Valojerdi, M.R.; Montazeri, L.; Baharvand, H. Effects of Alginate Concentration and Ovarian Cells on In Vitro Development of Mouse Preantral Follicles: A Factorial Study. Int. J. Fertil. Steril. 2020, 13, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Alex, A.; AbdelHafez, F.; Calabro, A.; Goldfarb, J.; Fleischman, A.; Falcone, T. Three-dimensional in vitro follicle growth: Overview of culture models, biomaterials, design parameters and future directions. Reprod. Biol. Endocrinol. 2010, 8, 119. [Google Scholar] [CrossRef]
- Shea, L.D.; Woodruff, T.K.; Shikanov, A. Bioengineering the Ovarian Follicle Microenvironment. Annu. Rev. Biomed. Eng. 2014, 16, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M.; Woodruff, T.K.; Shea, L.D. Designing Follicle–Environment Interactions with Biomaterials. Cancer Treat. Res. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Xu, M.; West, E.; Shea, L.D.; Woodruff, T.K. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol. Reprod. 2006, 75, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Eiselt, P.; Yeh, J.; Latvala, R.K.; Shea, L.D.; Mooney, D.J. Porous carriers for biomedical applications based on alginate hydrogels. Biomaterials 2000, 21, 1921–1927. [Google Scholar] [CrossRef]
- Pangas, S.A.; Saudye, H.; Shea, L.D.; Woodruff, T.K. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003, 9, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Tamadon, A.; Park, K.H.; Kim, Y.Y.; Kang, B.C.; Ku, S.Y. Efficient biomaterials for tissue engineering of female reproductive organs. Tissue Eng. Regen. Med. 2016, 13, 447–454. [Google Scholar] [CrossRef]
- Xu, M.; Kreeger, P.K.; Shea, L.D.; Woodruff, T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006, 12, 2739–2746. [Google Scholar] [CrossRef]
- Kreeger, P.K.; Fernandes, N.N.; Woodruff, T.K.; Shea, L.D. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol. Reprod. 2005, 73, 942–950. [Google Scholar] [CrossRef]
- Oktay, K.; Nugent, D.; Newton, H.; Salha, O.; Chatterjee, P.; Gosden, R.G. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil. Steril. 1997, 67, 481–486. [Google Scholar] [CrossRef]
- Lenie, S.; Cortvrindt, R.; Adriaenssens, T.; Smitz, J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol. Reprod. 2004, 71, 1730–1738. [Google Scholar] [CrossRef]
- Sittadjody, S.; Saul, J.M.; Joo, S.; Yoo, J.J.; Atala, A.; Opara, E.C. Engineered multilayer ovarian tissue that secretes sex steroids and peptide hormones in response to gonadotropins. Biomaterials 2013, 34, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- Lenie, S.; Smitz, J. Functional AR signaling is evident in an in vitro mouse follicle culture bioassay that encompasses most stages of folliculogenesis. Biol. Reprod. 2009, 80, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Andoh, K.; Hagiwara, H.; Xiaowei, L.; Kikuchi, N.; Abe, Y.; Yamada, K.; Fatima, R.; Mizunuma, H. Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice. Endocrinology 2001, 142, 4930–4936. [Google Scholar] [CrossRef] [PubMed]

| Title | SU-8 Formulation | Maximum Spin Coating Speed (rpm) | Desired Total Height (μm) | Desired Total Half Height (μm) | Additional Height from Previous Layer (μm) |
|---|---|---|---|---|---|
| Adhesion Layer | 2000.5–2002 | 3000 | n/a | 1.4 | n/a |
| 20% Solids | |||||
| Layer 1 | 2100 | 2000 | 250 | 125 | 125 |
| (core) | |||||
| Layer 2 | 2050 | 3250 | 350 | 175 | 50 |
| (shell) | |||||
| Layer 3 | 2050 | 3250 | 450 | 225 | 50 |
| (oil) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Healy, M.W.; Dolitsky, S.N.; Villancio-Wolter, M.; Raghavan, M.; Tillman, A.R.; Morgan, N.Y.; DeCherney, A.H.; Park, S.; Wolff, E.F. Creating an Artificial 3-Dimensional Ovarian Follicle Culture System Using a Microfluidic System. Micromachines 2021, 12, 261. https://doi.org/10.3390/mi12030261
Healy MW, Dolitsky SN, Villancio-Wolter M, Raghavan M, Tillman AR, Morgan NY, DeCherney AH, Park S, Wolff EF. Creating an Artificial 3-Dimensional Ovarian Follicle Culture System Using a Microfluidic System. Micromachines. 2021; 12(3):261. https://doi.org/10.3390/mi12030261
Chicago/Turabian StyleHealy, Mae W., Shelley N. Dolitsky, Maria Villancio-Wolter, Meera Raghavan, Alexandra R. Tillman, Nicole Y. Morgan, Alan H. DeCherney, Solji Park, and Erin F. Wolff. 2021. "Creating an Artificial 3-Dimensional Ovarian Follicle Culture System Using a Microfluidic System" Micromachines 12, no. 3: 261. https://doi.org/10.3390/mi12030261
APA StyleHealy, M. W., Dolitsky, S. N., Villancio-Wolter, M., Raghavan, M., Tillman, A. R., Morgan, N. Y., DeCherney, A. H., Park, S., & Wolff, E. F. (2021). Creating an Artificial 3-Dimensional Ovarian Follicle Culture System Using a Microfluidic System. Micromachines, 12(3), 261. https://doi.org/10.3390/mi12030261






