Fundamental Studies of Rapidly Fabricated On-Chip Passive Micromixer for Modular Microfluidics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
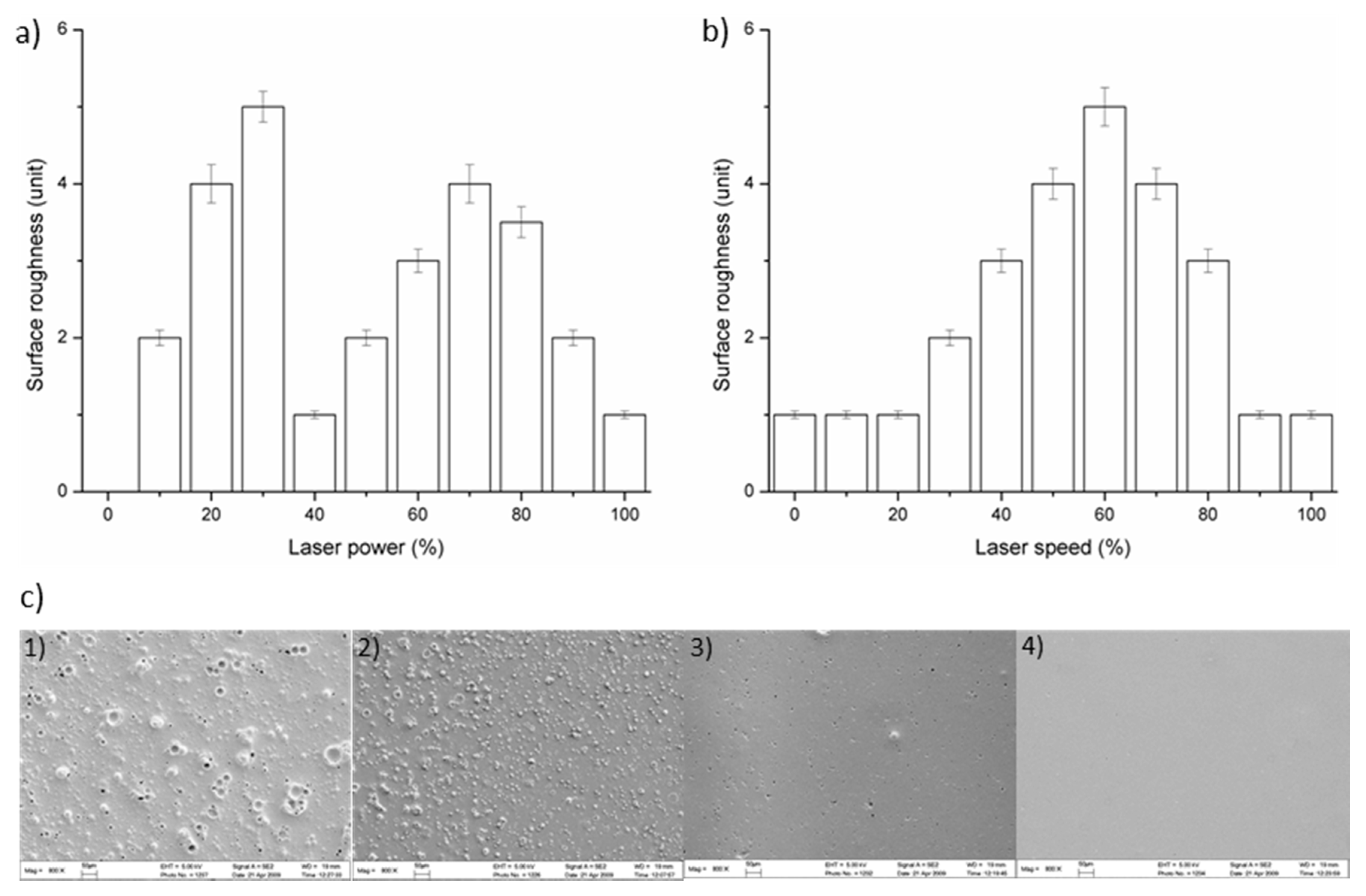
2.2. Ablation of Microspheric Particles on PMMA Surface as Mixing Structures
2.3. Numerical Simulations of Mixing Structures
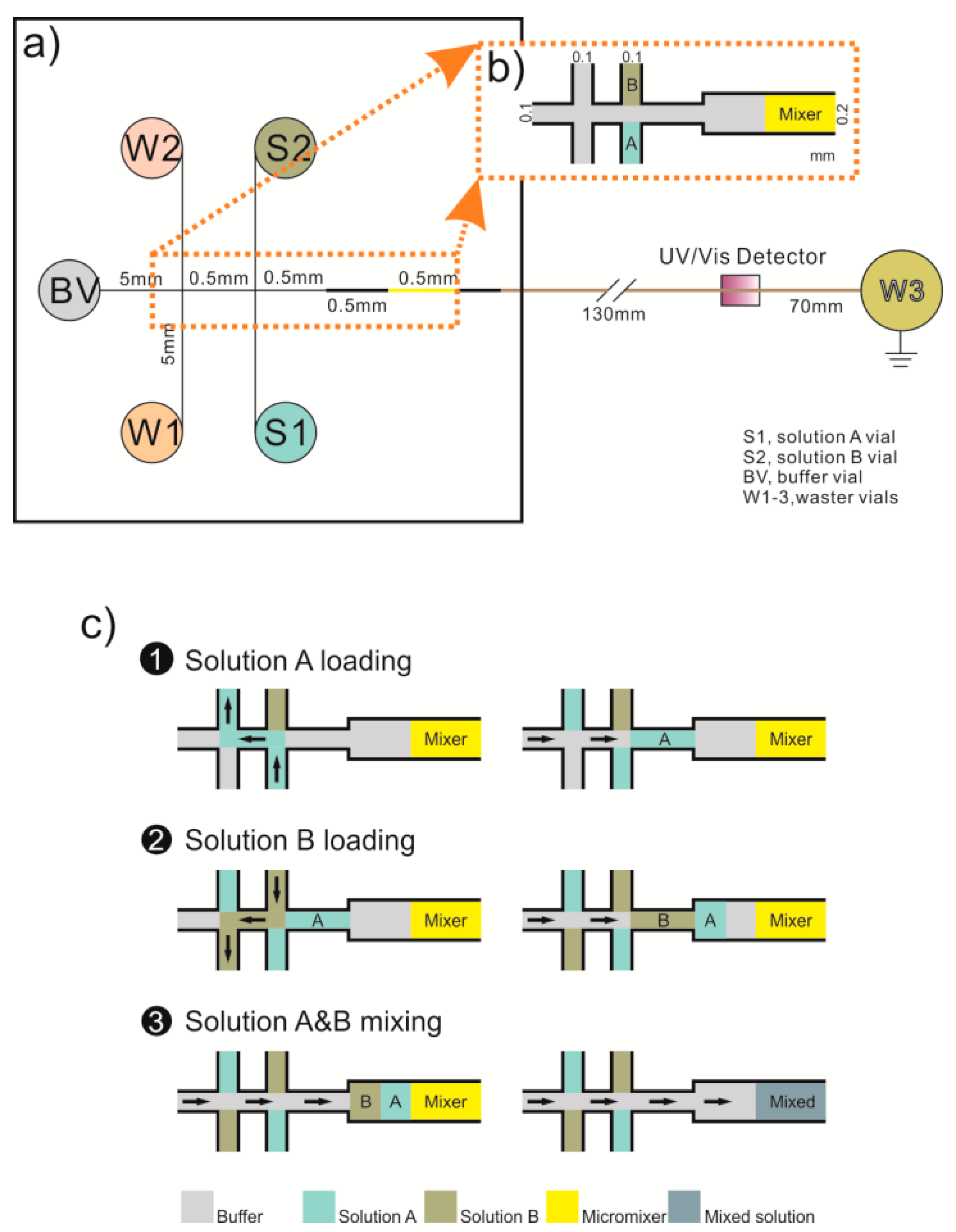
2.4. Design and Fabrication of Micromixer with Irregular Microspheric Particles
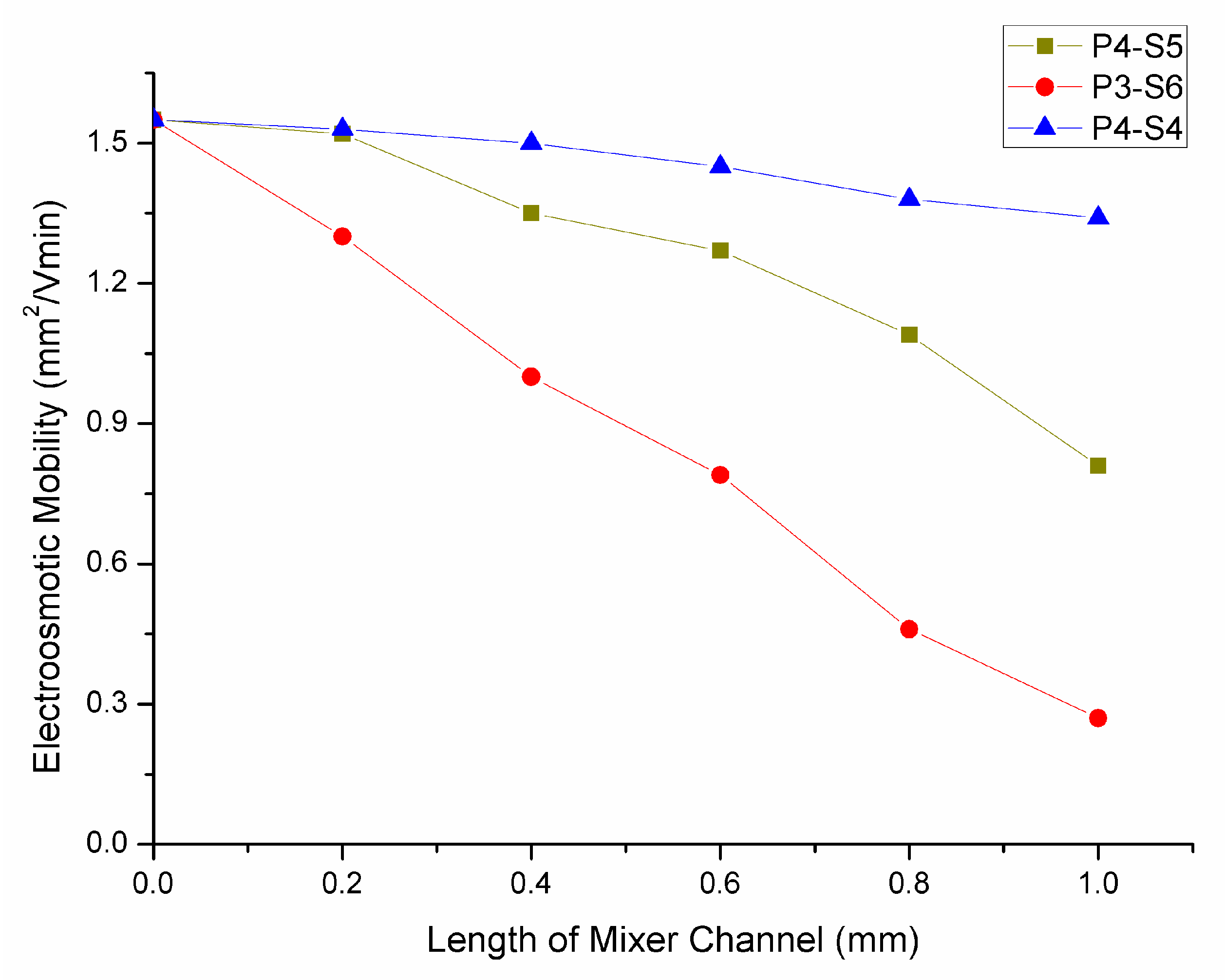
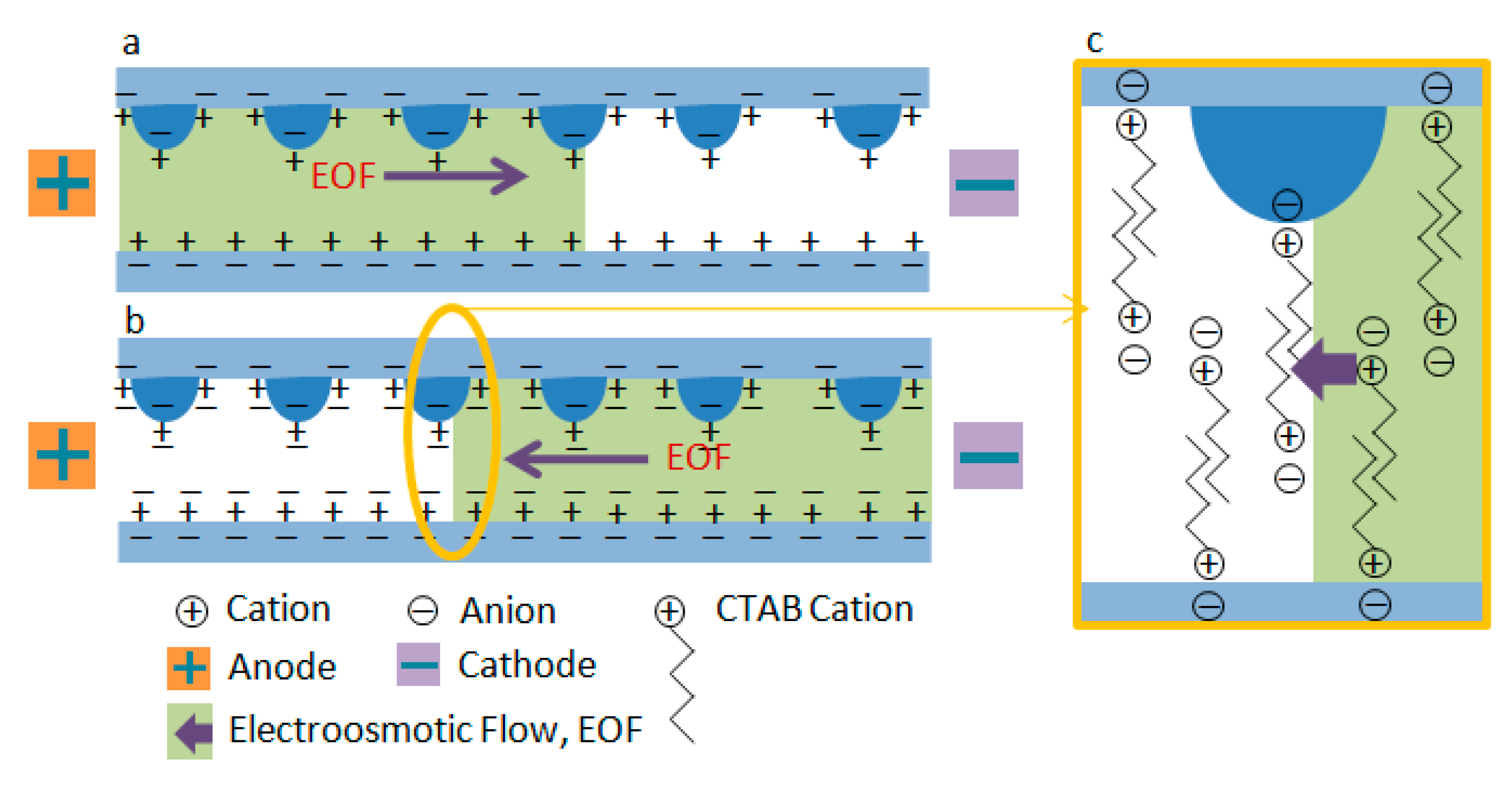
2.5. Dynamic Coating and Electroosmotic Flow Measurements on PMMA Microfluidic Chip
2.6. Operation Procedure for the Microfluidic Chip with On-chip Sequential Mixing
3. Results
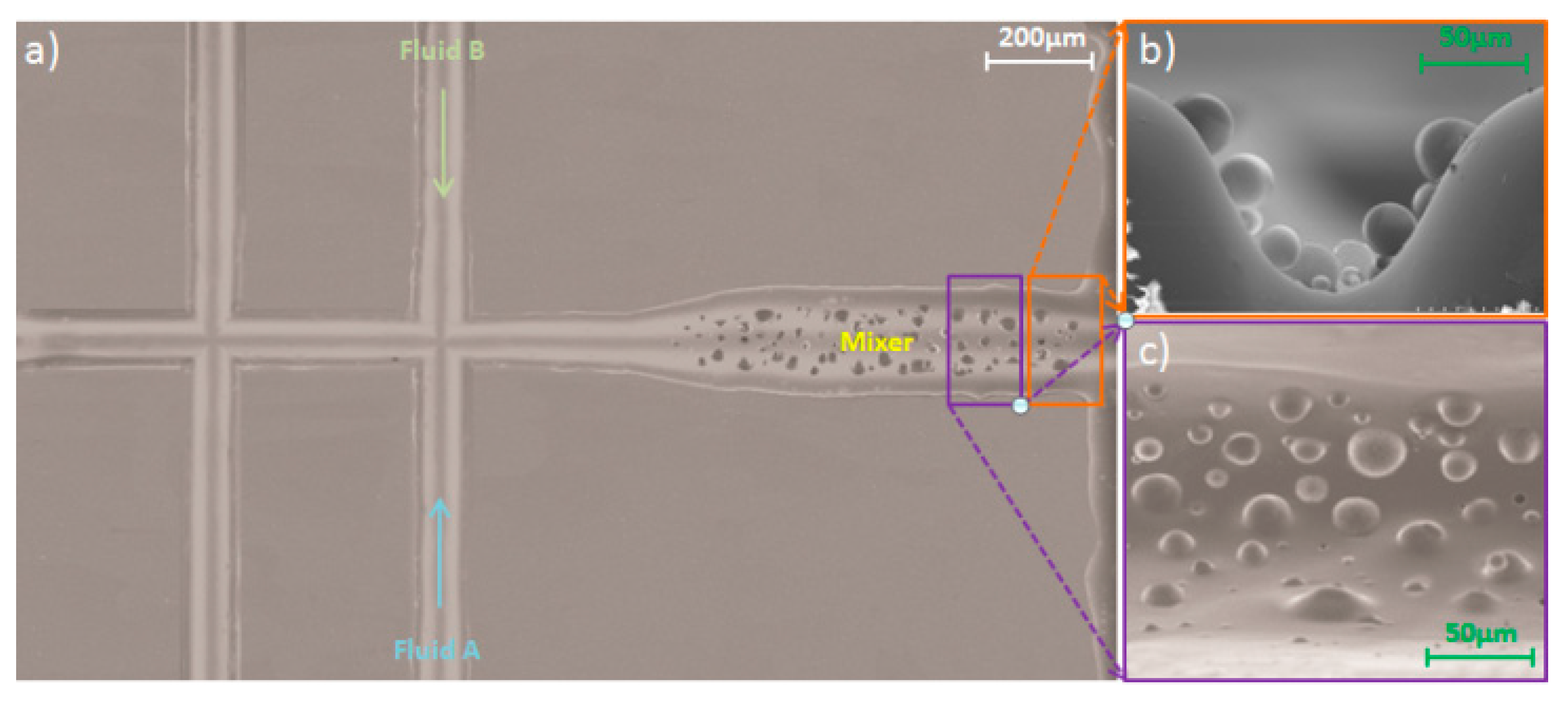
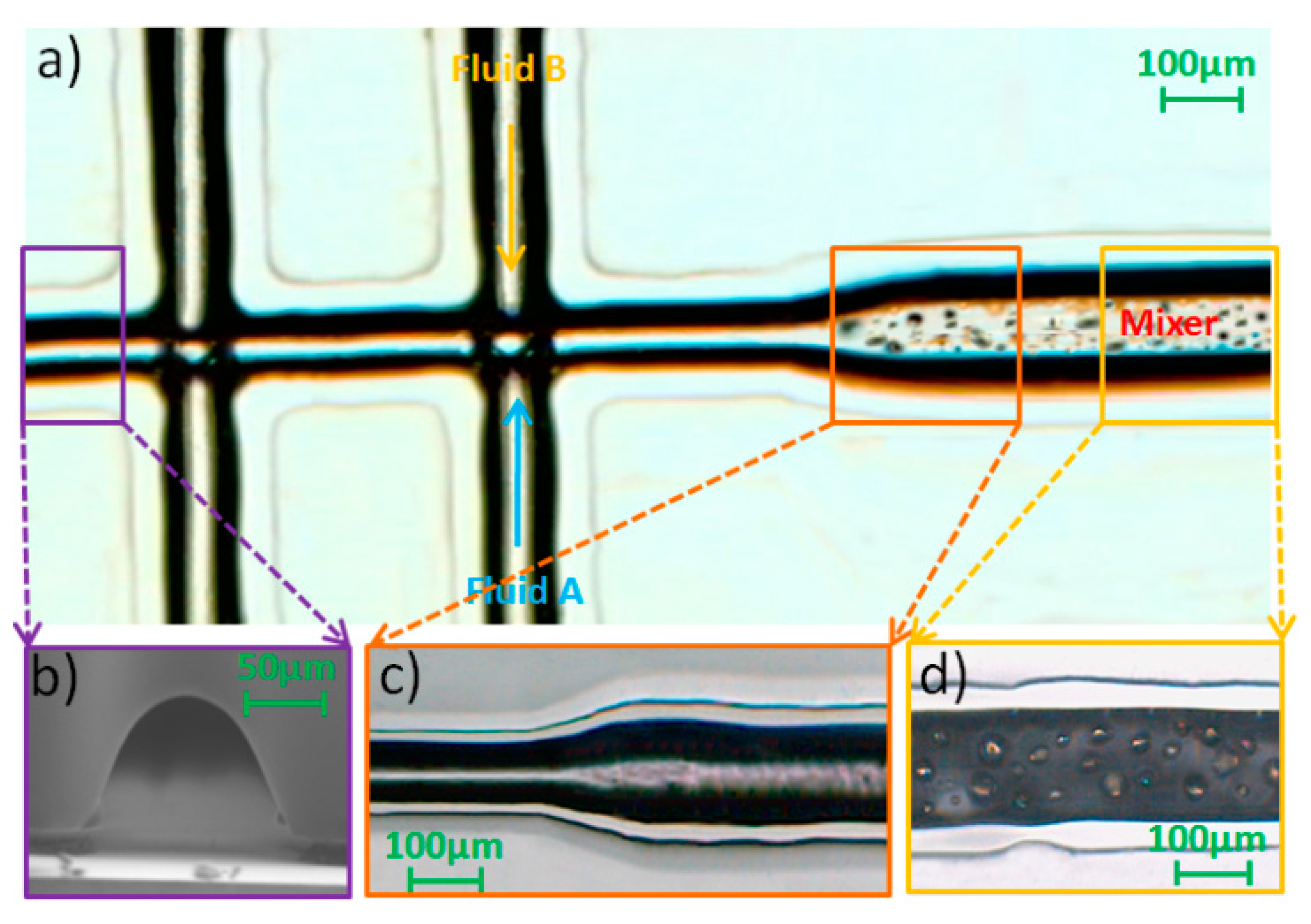
3.1. Microfabrication and Surface Patterning of Mixer
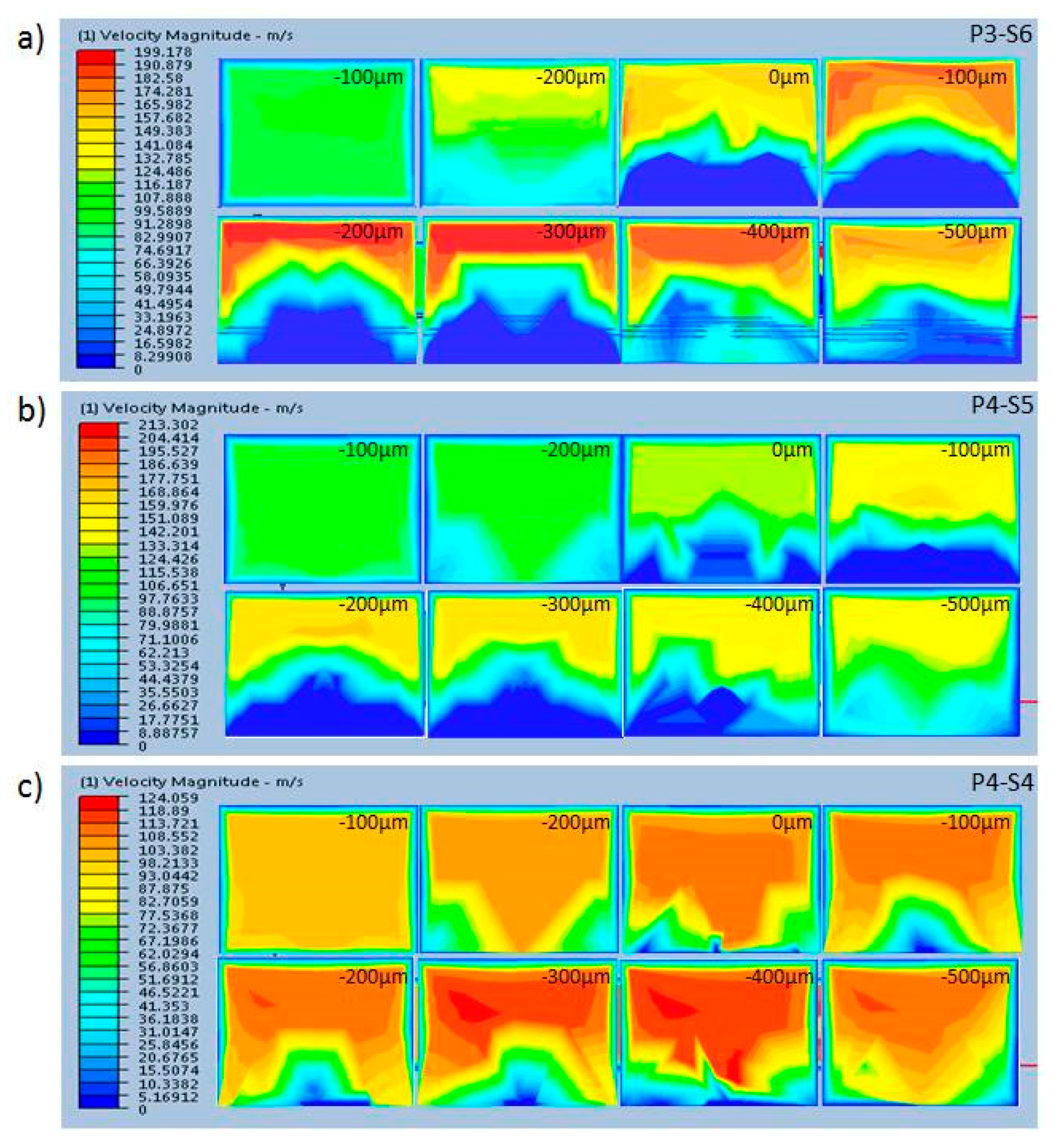
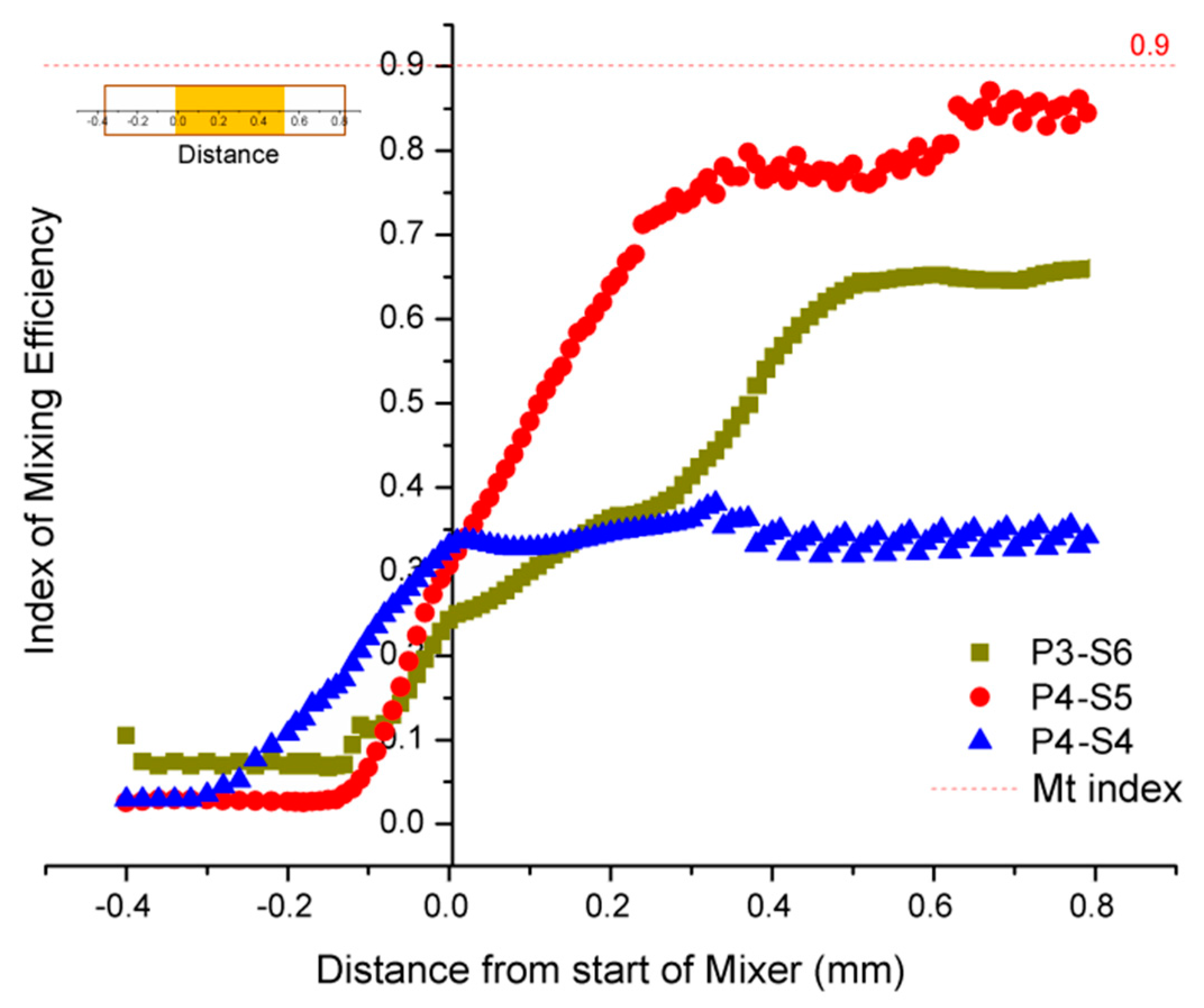
3.2. CFD Simulation of Three Patterns of the Mixing Channel
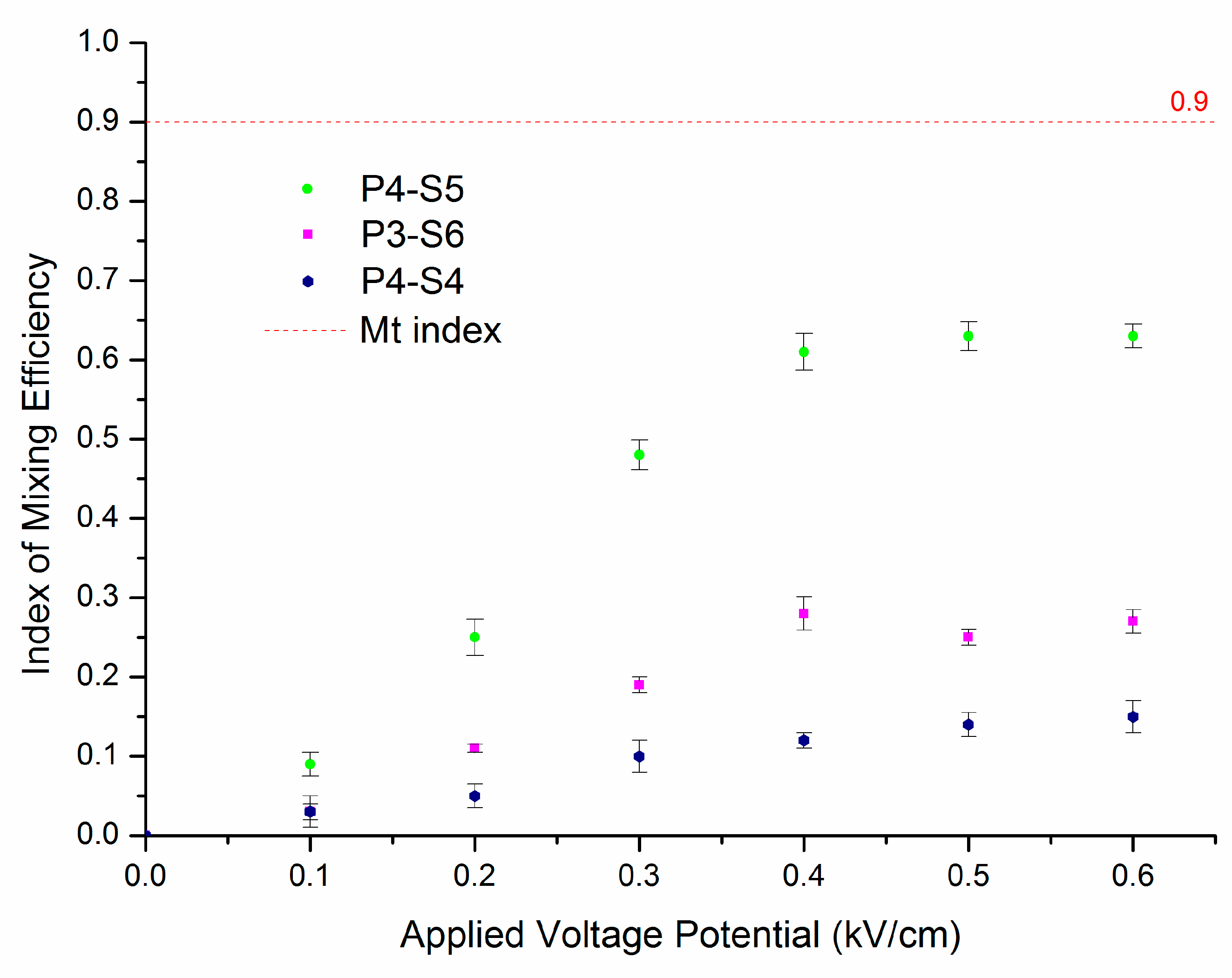
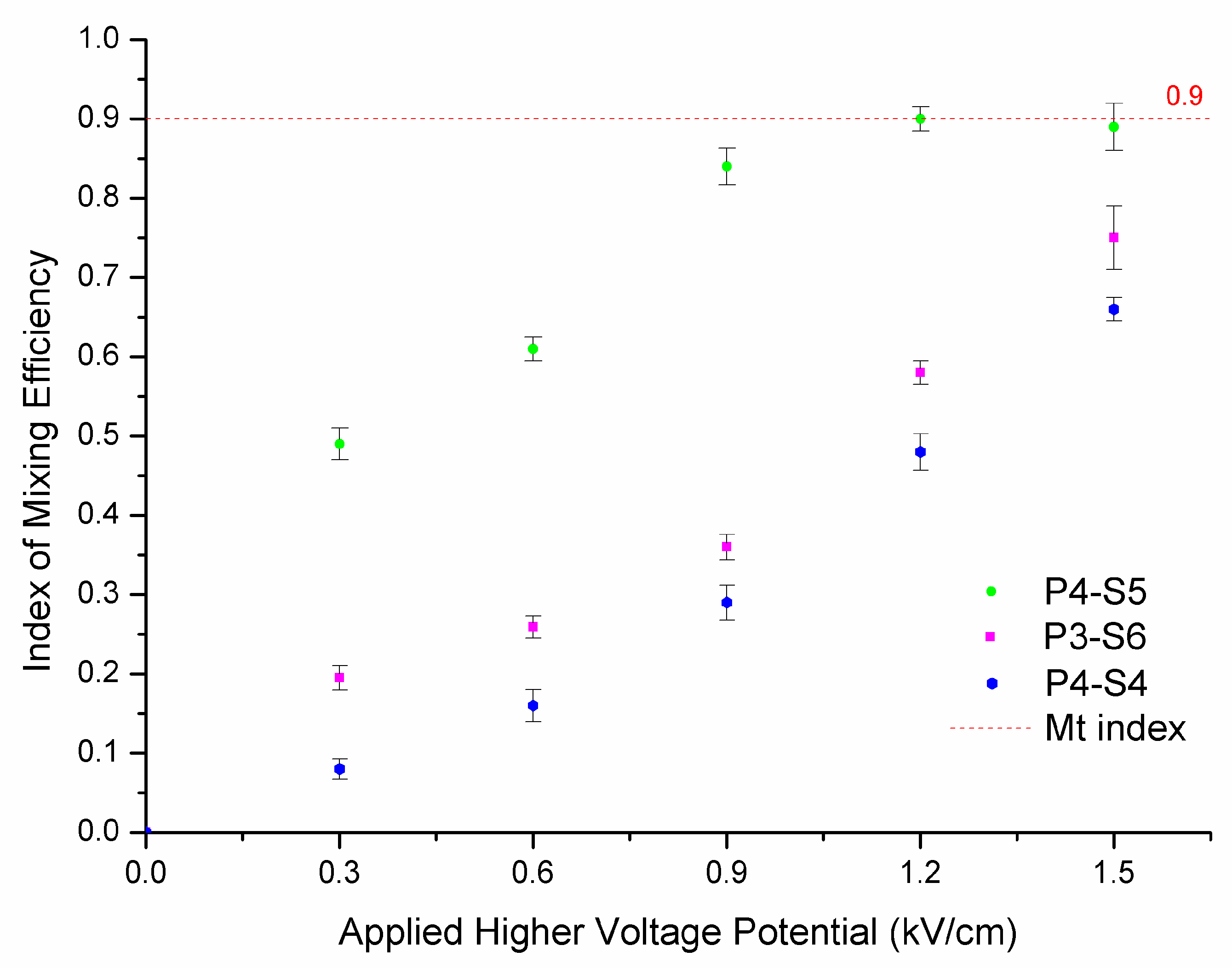
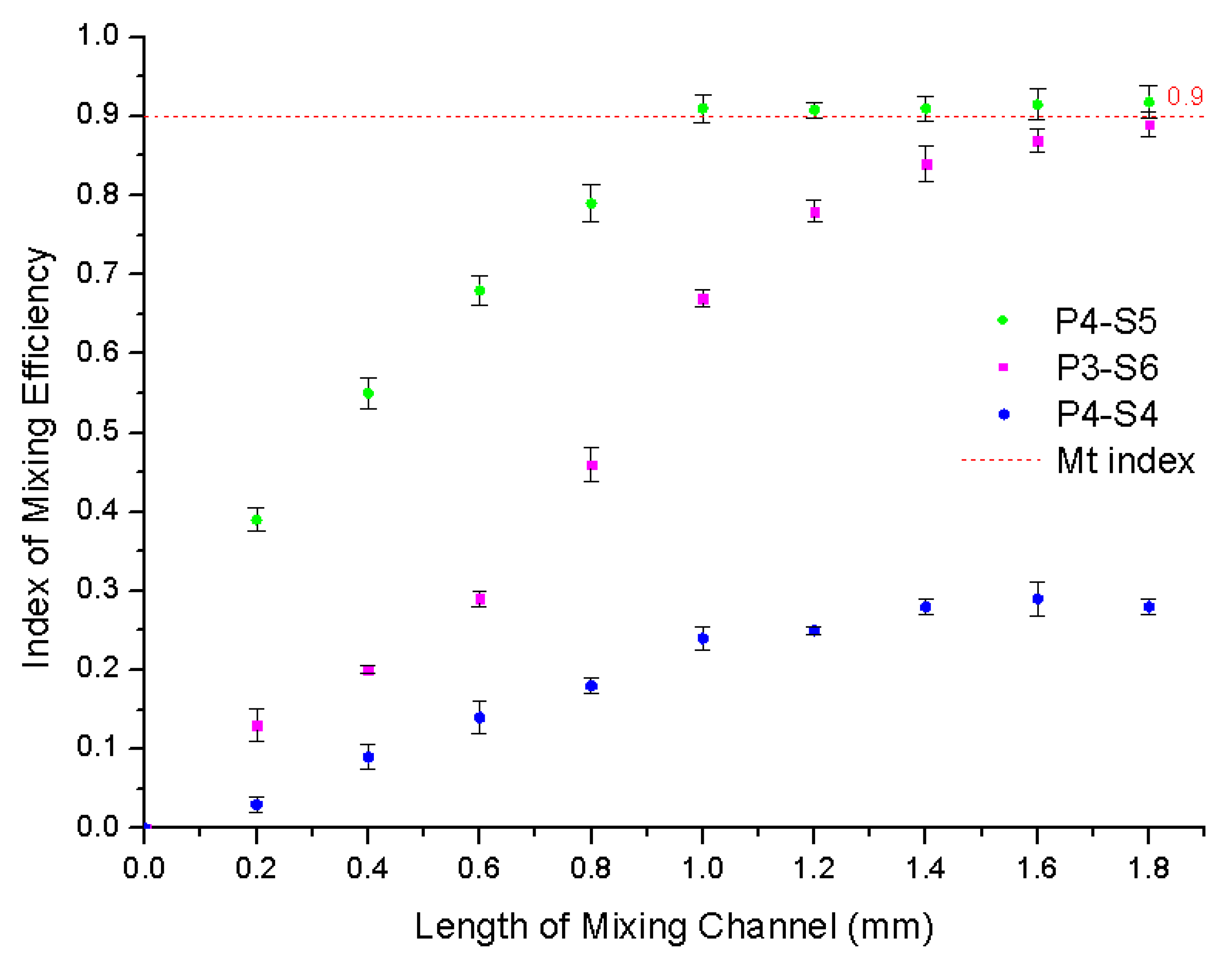
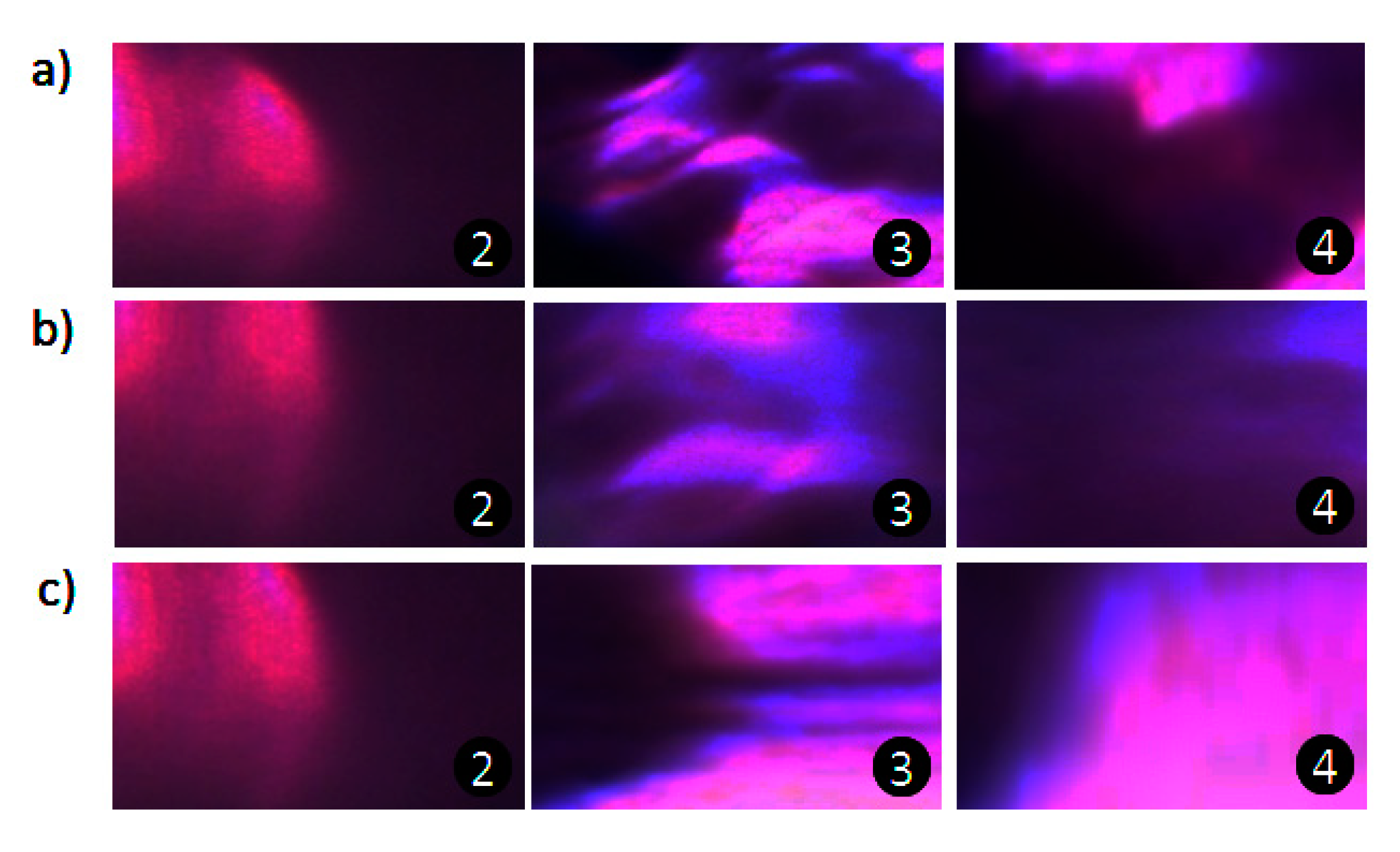
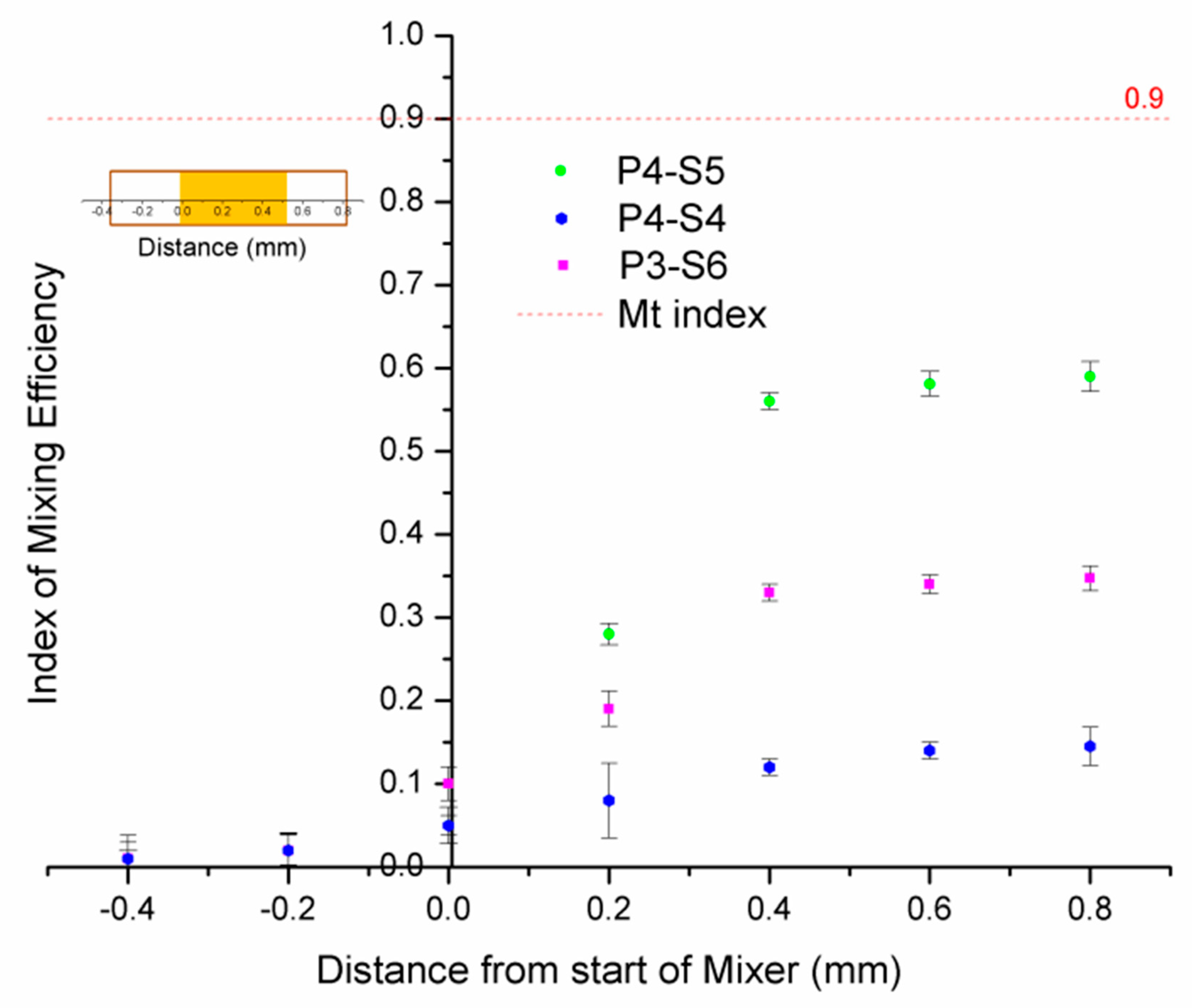
3.3. Effect of Microchip Parameters on Mixing Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ottino, J.M.; Wiggins, S. Passive micromixer using by convection and surface tension effects with air-liquid interface. Science 2004, 305, 485–486. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Muthuramalingam, K.; Tung, K.-H.; Chuan, H.-H.; Liang, K.-Y.; Hsu, C.-P.; Cheng, C.-M. Portable device for quick detection of viable bacteria in water. Micromachines 2020, 11, 1079. [Google Scholar] [CrossRef]
- Gidde, R.R. Design optimization of micromixer with circular mixing chambers (M-CMC) using Taguchi-based grey relational analysis. Int. J. Chem. React. Eng. 2020, 18, 20200057. [Google Scholar] [CrossRef]
- Sudarsan, A.P.; Ugaz, V.M. Multivortex micromixing. Proc. Natl. Acad. Sci. USA 2006, 103, 7228–7233. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Joung, Y.-H.; Ahn, S.; Park, C.-K.; Choi, J.; Koo, C. Monolithic 3D micromixer with impeller for glass microfluidic systems. Lab Chip 2020, 20, 4474–4485. [Google Scholar] [CrossRef]
- Fuwad, A.; Hossain, S.; Ryu, H.; Ansari, M.A.; Khan, M.S.I.; Kim, K.-Y.; Jeon, T.-J.; Kim, S.M. Numerical and experimental study on mixing in chaotic micromixers with crossing structures. Chem. Eng. Technol. 2020, 43, 1866–1875. [Google Scholar] [CrossRef]
- Schudel, B.R.; Choi, C.J.; Cunningham, B.T.; Kenis, P.J.A. Microfluidic chip for combinatorial mixing and screening of assays. Lab Chip 2009, 9, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Kefou, N.; Karvelas, E.; Karamanos, K.; Karakasidis, T.; Sarris, I.E. Water purification in micromagnetofluidic devices: Mixing in MHD micromixers. Procedia Eng. 2016, 162, 593–600. [Google Scholar] [CrossRef]
- Guo, W.P.; Fung, Y.-S. Microfluidic chip-capillary electrophoresis with dynamic multi-segment standard addition for rapidly identifying nephrolithiasis markers in urine. Electrophoresis 2011, 32, 3437–3445. [Google Scholar] [CrossRef] [PubMed]
- Liosis, C.; Karvelas, E.G.; Karakasidis, T.; Sarris, I.E. Numerical study of magnetic particles mixing in waste water under an external magnetic field. J. Water Supply Res. Technol. 2020, 69, 266–275. [Google Scholar] [CrossRef]
- Glasgow, I.; Batton, J.; Aubry, N. Electroosmotic mixing in microchannels. Lab Chip 2004, 4, 558–562. [Google Scholar] [CrossRef]
- Zhan, X.; Jing, D. Influence of geometric parameters on the fluidic and mixing characteristics of T-shaped micromixer. Microsyst. Technol. 2020, 26, 2989–2996. [Google Scholar] [CrossRef]
- Dehghani, T.; Sadegh, M.F.; Vajdi, M.; Shahedi, A.M.; Shokouhimehr, M.; Mohammadi, M. Mixing enhancement through a micromixer using topology optimization. Chem. Eng. Res. Des. 2020, 161, 187–196. [Google Scholar] [CrossRef]
- Ahmed, F.; Kim, K.Y. Analysis of a two-layer nozzle-and-diffuser electroosmotic micromixer. Chem. Eng. Technol. 2019, 42, 2164–2170. [Google Scholar] [CrossRef]
- Kawazumi, H.; Tashiro, A.; Ogino, K.; Maeda, H. Observation of fluidic behavior in a polymethylmethacrylate-fabricated micro-channel by a simple spectroscopic analysis. Lab Chip 2002, 2, 8–10. [Google Scholar] [CrossRef]
- Hsieh, S.-S.; Lin, H.-C.; Lin, C.-Y. Electroosmotic flow velocity measurements in a square microchannel. Colloid Polym. Sci. 2006, 284, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Mersal, G.A.M.; Bilitewski, U. Manipulation of the electroosmotic flow in glass und PMMA microchips with respect to specific enzymatic glucose determinations. Microchim. Acta 2005, 151, 29–38. [Google Scholar] [CrossRef]
- Juraeva, M.; Kang, D.J. Mixing performance of a cross-channel split-and-recombine micro-mixer combined with mixing cell. Micromachines 2020, 11, 685. [Google Scholar] [CrossRef]
- Kim, N.; Chan, W.X.; Ng, S.H.; Yoon, Y.-J.; Allen, J.B. Understanding interdependencies between mechanical velocity and electrical voltage in electromagnetic micromixers. Micromachines 2020, 11, 636. [Google Scholar] [CrossRef]
- Raza, W.; Hossain, S.; Kim, K.-Y. A review of passive micromixers with a comparative analysis. Micromachines 2020, 11, 455. [Google Scholar] [CrossRef]
- Bijsterbosch, B. Characterization of silica surfaces by adsorption from solution. Investigations into the mechanism of adsorption of cationic surfactants. J. Colloid Interface Sci. 1974, 47, 186–198. [Google Scholar] [CrossRef]
- Rahmannezhad, J.; Mirbozorgi, S.A. CFD analysis and RSM-based design optimization of novel grooved micromixers with obstructions. Int. J. Heat Mass Transf. 2019, 140, 483–497. [Google Scholar] [CrossRef]
- Chen, X.; Li, T. A novel design for passive misscromixers based on topology optimization method. Biomed. Microdevices 2016, 18, 57. [Google Scholar] [CrossRef]
- Cai, G.; Xue, L.; Zhang, H.; Lin, J. A review on micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Lai, B.-H. Numerical Study of T-Shaped Micromixers with Vortex-Inducing Obstacles in the Inlet Channels. Micromachines 2020, 11, 1122. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, H.; Shi, L.; Liu, Z.; Joo, S.W. An enhanced electroosmotic micromixer with an efficient asymmetric lateral structure. Micromachines 2016, 7, 218. [Google Scholar] [CrossRef]
- Clark, J.; Kaufman, M.; Fodor, P.S. Mixing enhancement in serpentine micromixers with a non-rectangular cross-section. Micromachines 2018, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, T.; Kothapalli, C.R.; Fodor, P.S. Mixing optimization in grooved serpentine microchannels. Micromachines 2020, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Karvelas, E.; Liosis, C.; Benos, L.; Karakasidis, T.; Sarris, I.E. Micromixing efficiency of particles in heavy metal removal processes under various inlet conditions. Water 2019, 11, 1135. [Google Scholar] [CrossRef]

| Plug A (Length) | Plug B (Length) | Plug A + B (Volume a) | Mixing Parameters | |||
|---|---|---|---|---|---|---|
| Efficiency b | RSD c | Time d | RSD c | |||
| 1 mm | 1 mm | ~30 nL | 0.05 | 1.5% | 3.12 s | 3.24% |
| 0.9 mm | 0.9 mm | ~27 nL | 0.12 | 1.8% | 2.87 s | 3.67% |
| 0.8 mm | 0.8 mm | ~24 nL | 0.24 | 1.9% | 2.43 s | 3.75% |
| 0.7 mm | 0.7 mm | ~21 nL | 0.38 | 2.3% | 1.91 s | 4.10% |
| 0.6 mm | 0.6 mm | ~18 nL | 0.51 | 2.5% | 1.24 s | 3.89% |
| 0.5 mm | 0.5 mm | ~15 nL | 0.62 | 2.6% | 1.03 s | 4.51% |
| 0.4 mm | 0.4 mm | ~12 nL | 0.70 | 2.4% | 0.97 s | 4.07% |
| 0.3 mm | 0.3 mm | ~9 nL | 0.79 | 2.2% | 0.93 s | 4.22% |
| 0.2 mm | 0.2 mm | ~6 nL | 0.87 | 1.3% | 0.89 s | 4.91% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Tang, L.; Zhou, B.; Fung, Y. Fundamental Studies of Rapidly Fabricated On-Chip Passive Micromixer for Modular Microfluidics. Micromachines 2021, 12, 153. https://doi.org/10.3390/mi12020153
Guo W, Tang L, Zhou B, Fung Y. Fundamental Studies of Rapidly Fabricated On-Chip Passive Micromixer for Modular Microfluidics. Micromachines. 2021; 12(2):153. https://doi.org/10.3390/mi12020153
Chicago/Turabian StyleGuo, Wenpeng, Li Tang, Biqiang Zhou, and Yingsing Fung. 2021. "Fundamental Studies of Rapidly Fabricated On-Chip Passive Micromixer for Modular Microfluidics" Micromachines 12, no. 2: 153. https://doi.org/10.3390/mi12020153
APA StyleGuo, W., Tang, L., Zhou, B., & Fung, Y. (2021). Fundamental Studies of Rapidly Fabricated On-Chip Passive Micromixer for Modular Microfluidics. Micromachines, 12(2), 153. https://doi.org/10.3390/mi12020153





