Research on Sapphire Deep Cavity Corrosion and Mask Selection Technology
Abstract
1. Introduction
2. Preparation of Sensitive Cavity Structure
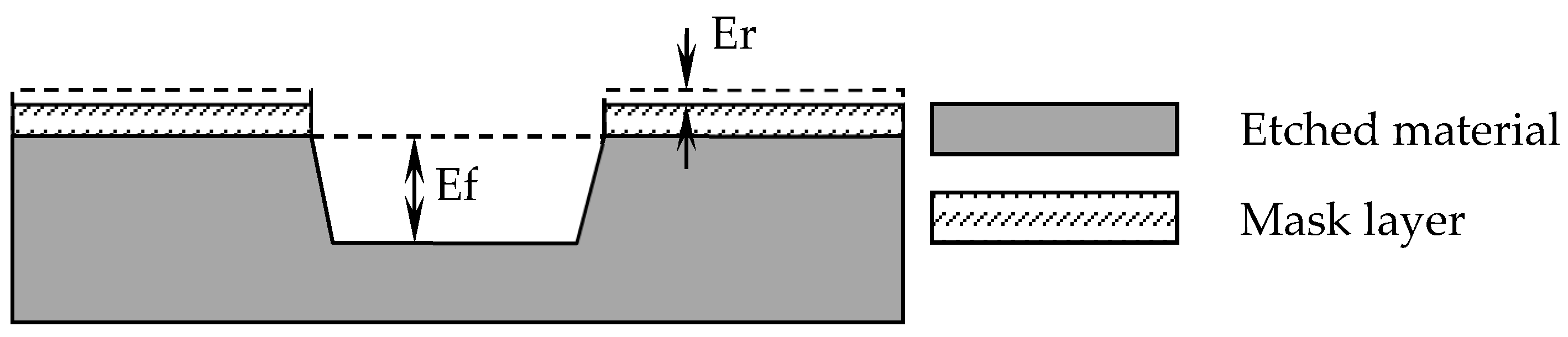
2.1. Sapphire Etching Principle
2.2. The Selection Ratio
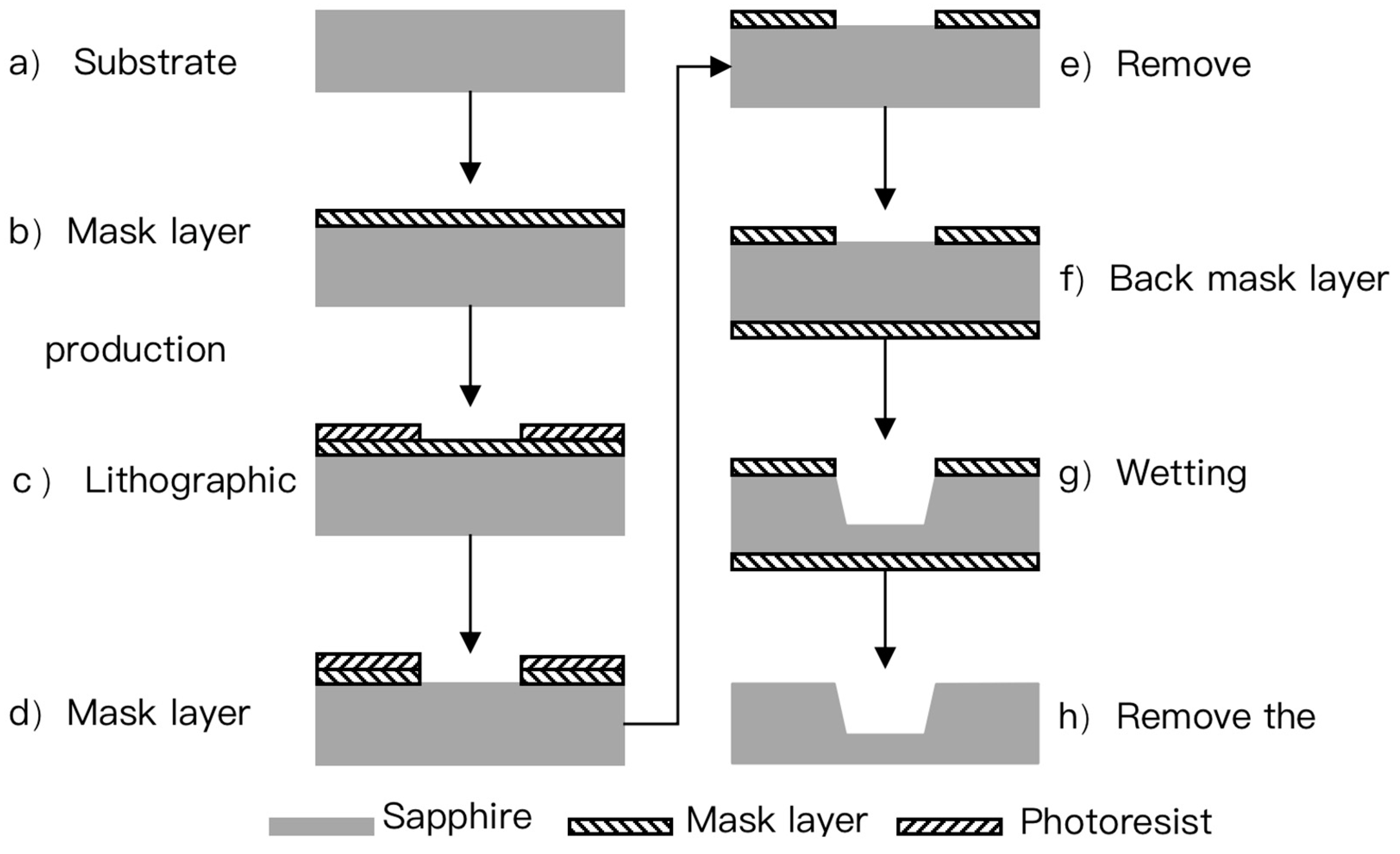
2.3. Sapphire Wet Etching Process
- (a)
- The mask layer is made by a deposition or plating process, which serves as the mask layer for the corrosion of the sensitive cavity.
- (b)
- The photolithography process is used to realize the patterning of the mask layer.
- (c)
- The wet corrosion process is used to achieve the corrosion of the sensitive cavity.
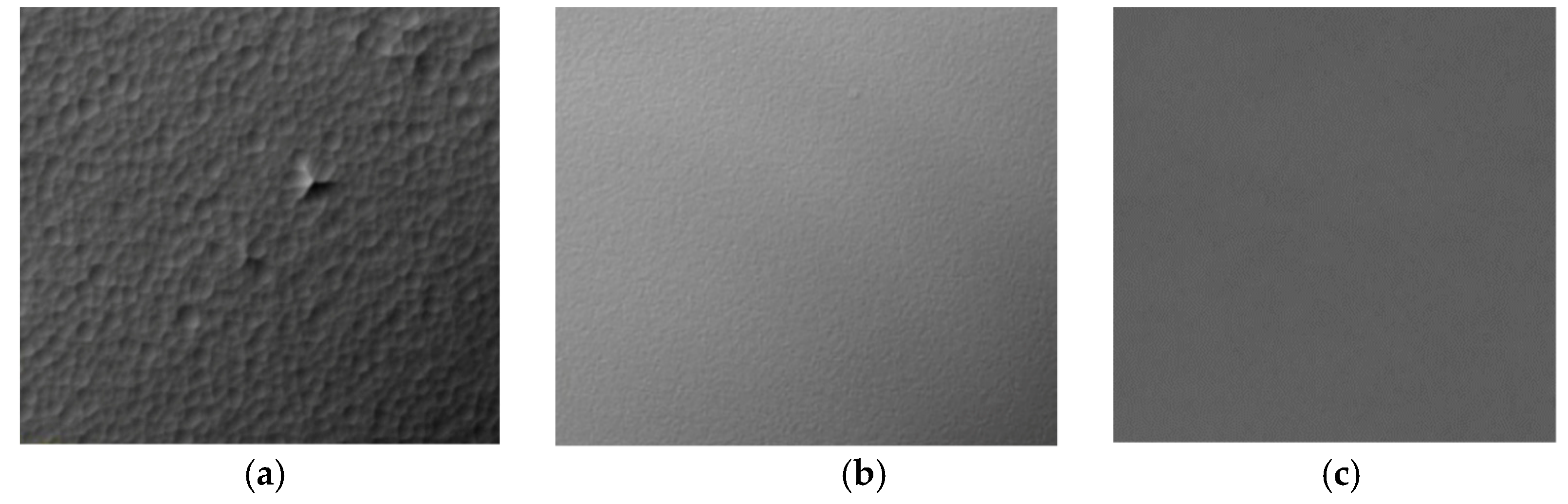
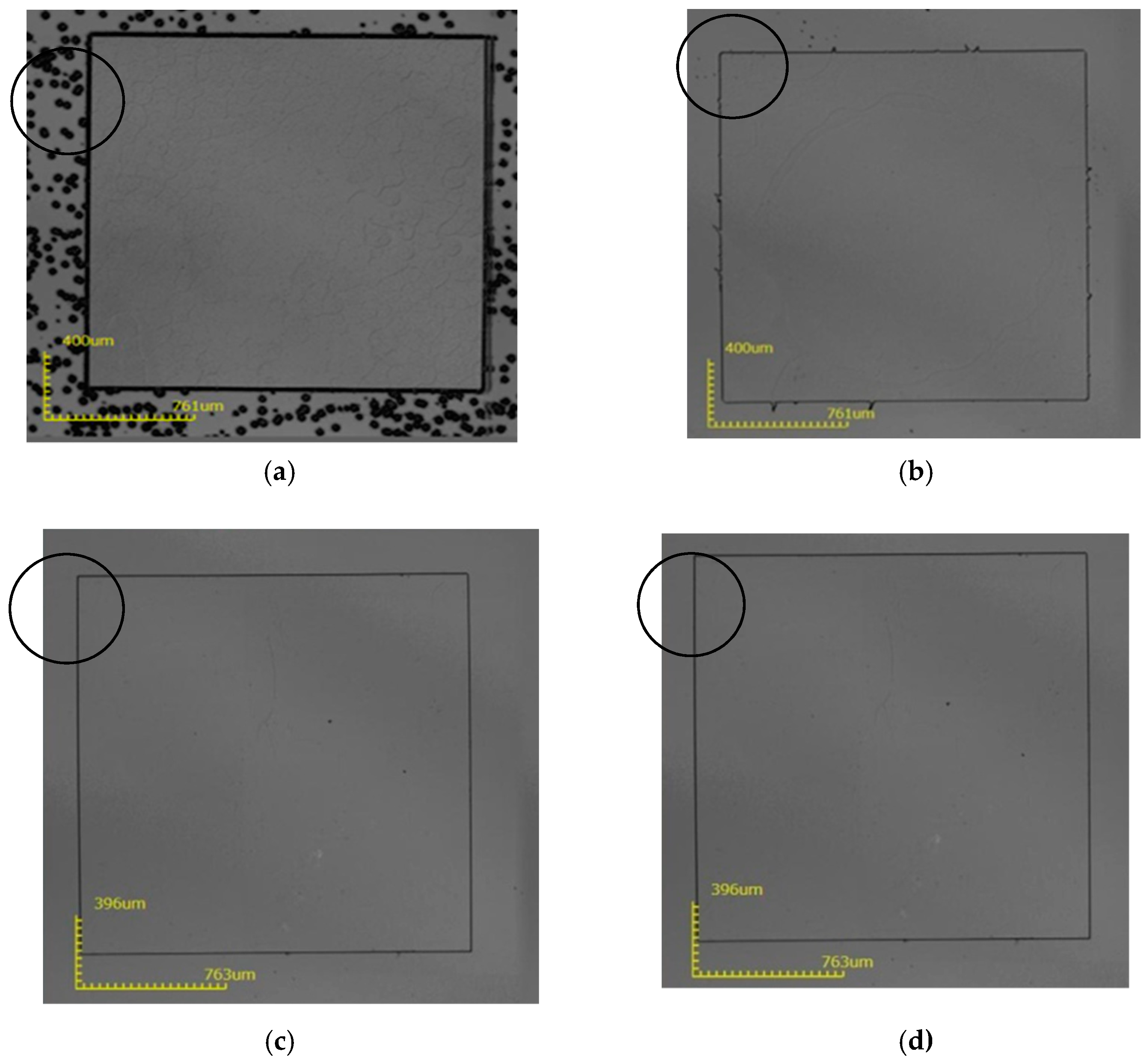
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kurtz, A.D.; Ned, A.A.; Goodman, S.; Epstein, A.H. Latest Ruggedized High Temperature Piezoresistive Transducers; NASA 2003 Propulsion Measurement Sensor Development Workshop: Huntsville, AP, USA, 2003.
- Zhu, Y.Z.; Cooper, K.L.; Pickrell, G.R.; Wang, A. High-temperature fiber-tip pressure sensor. J. Light Wave Technol. 2006, 24, 861–869. [Google Scholar]
- Wang, A.; Gollapudi, S.; Murphy, K.A.; May, R.G.; Claus, R.O. Sapphire-Fiber-Based Intrinsic Fabry-Perot-Interferometer. Opt. Lett. 1992, 17, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, J.; Chen, W.; Yin, L.; Liu, X. A Novel High-Precision Digital Tunneling Magnetic Resistance-Type Sensor for the Nanosatellites’ Space Application. Micromachines 2018, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.M.; Shen, Y.H.; Chen, F.M.; Ye, L. Plastic bending of sapphire fibers for infrared sensing and power delivery applications. Appl. Opt. 2000, 39, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Hu, J.P.; Liu, A.X. Harmonic Distortion Optimization for Sigma–Delta Modulators Based on TMR Sensors. Sensors 2020, 20, 1041. [Google Scholar] [CrossRef]
- Lally, E.M.; Xu, Y.; Wang, A. Sapphire direct bonding as a platform for pressure sensing at extreme high temperatures. Fiber Opt. Sens. Appl. 2009. [Google Scholar] [CrossRef]
- Chengqiang, H.; Yang, X. Study Progress of the Manufacturing Process of Patterned Sapphire Substrates. Semicond. Technol. 2012, 37, 497–581. [Google Scholar]
- Valipour, M. Optimization of neural networks for precipitation analysis in a humid region to detect drought and wet year alarms. Meteorol. Appl. 2016, 23, 91–100. [Google Scholar] [CrossRef]
- Yannopoulos, S.; Lyberatos, G.; Theodossiou, N.; Li, W. Evolution of Water Lifting Devices (Pumps) over the Centuries Worldwide. Water 2015, 7, 5031–5060. [Google Scholar] [CrossRef]
- Valipour, M. Analysis of potential evapotranspiration using limited weather data. Appl. Water Sci. 2014, 6, 1–11. [Google Scholar] [CrossRef]
- Shang, Y.Q.; Hong, Q.; Yun-Long, W.; Ya-Lin, W. Study on sapphire microstructure processing technology based on wet etching. Int. J. Mod. Phys. B 2016, 30, 1741004. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, Z.; Jiang, S.; Shen, H.; Cao, F.; Ning, Z.; Huang, Y.; Xing, D.; Zuo, H.; Han, J.; et al. Cavity etching evolution on the A-plane of sapphire crystal in molten KOH etchant. J. Cryst. Growth 2020, 552, 125926. [Google Scholar] [CrossRef]
- Xie, X.; Huang, X.; Jiang, W.; Wei, X.; Hu, W.; Ren, Q. Three dimensional material removal model of laser-induced backside wet etching of sapphire substrate with CuSO 4 solutions. Opt. Laser Technol. 2017, 89, 59–68. [Google Scholar] [CrossRef]
- Kimura, Y.; Takubo, S. Corrosion fatigue of bio-ceramic sapphire in isotonic sodium chloride solution. Int. J. Fatigue 2000, 22, 899–904. [Google Scholar] [CrossRef]
- Capuano, L.; Tiggelaar, R.; Berenschot, J.; Gardeniers, J.; Römer, G. Fabrication of millimeter-long structures in sapphire using femtosecond infrared laser pulses and selective etching. Opt. Lasers Eng. 2020, 133, 106114. [Google Scholar] [CrossRef]
- Kumar, P.; Lee, J.; Lee, G.; Rao, S.; Singh, D.; Singh, R.K. Low temperature wet etching to reveal sub-surface damage in sapphire substrates. Appl. Surf. Sci. 2013, 273, 58–61. [Google Scholar] [CrossRef]
- Yang, D.; Liang, H.; Qiu, Y.; Shen, R.; Liu, Y.; Xia, X.; Song, S.; Zhang, K.; Yu, Z.; Zhang, Y.; et al. Evolution of the crystallographic planes of cone-shaped patterned sapphire substrate treated by wet etching. Appl. Surf. Sci. 2014, 295, 26–30. [Google Scholar] [CrossRef]
- Xing, Y.; Guo, Z.; Gosálvez, M.A.; Wu, G.; Qiu, X. Characterization of anisotropic wet etching of single-crystal sapphire. Sens. Actuators A Phys. 2020, 303, 111667. [Google Scholar] [CrossRef]
- Zheng, J. Study on Wet Etching Cleaning Technology of Sapphire Wafer for LED. Equip. Electron. Prod. Manuf. 2013, 223, 8–58. [Google Scholar]
- Yan, T. Submicron Fine Cuting-Surface of Sapphire Obtained by Chemical Corrosion Assisted Pico second Laser Fulmination Technology. Chin. J. Lasers 2017, 44, 1002002. [Google Scholar]
- Ma, R. Experimental Study on the Measurement of Subsurface Damage of Ground Sapphire by Corrosion Method. J. Synth. Cryst. 2019, 49, 2216–2227. [Google Scholar]
- Xu, W. Research on a Paterned Sapphire Substrate for GaN-Based LED and the Fabrication by Wet Etching. Microelectron. Comput. 2015, 32, 102–106. [Google Scholar]
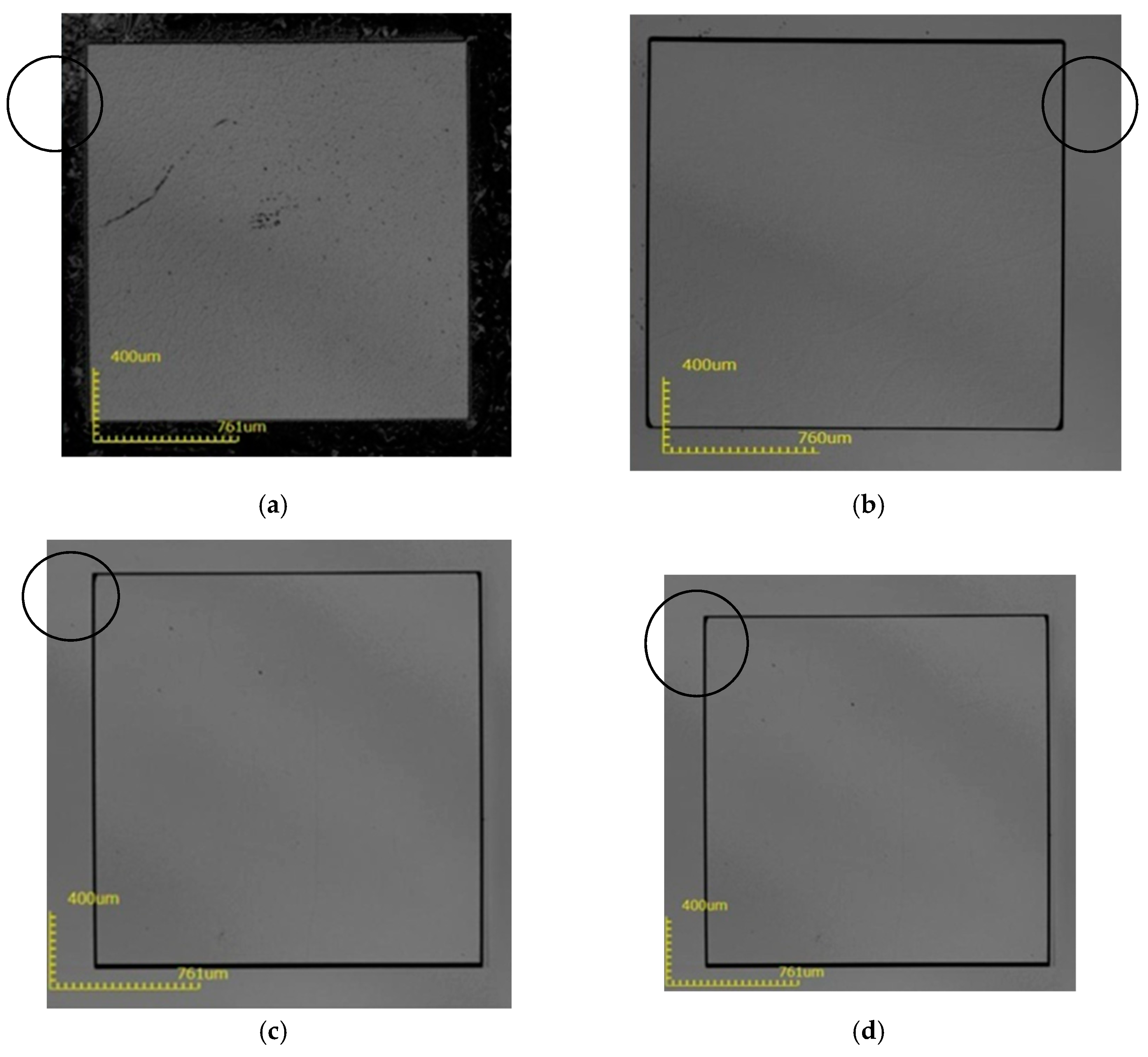
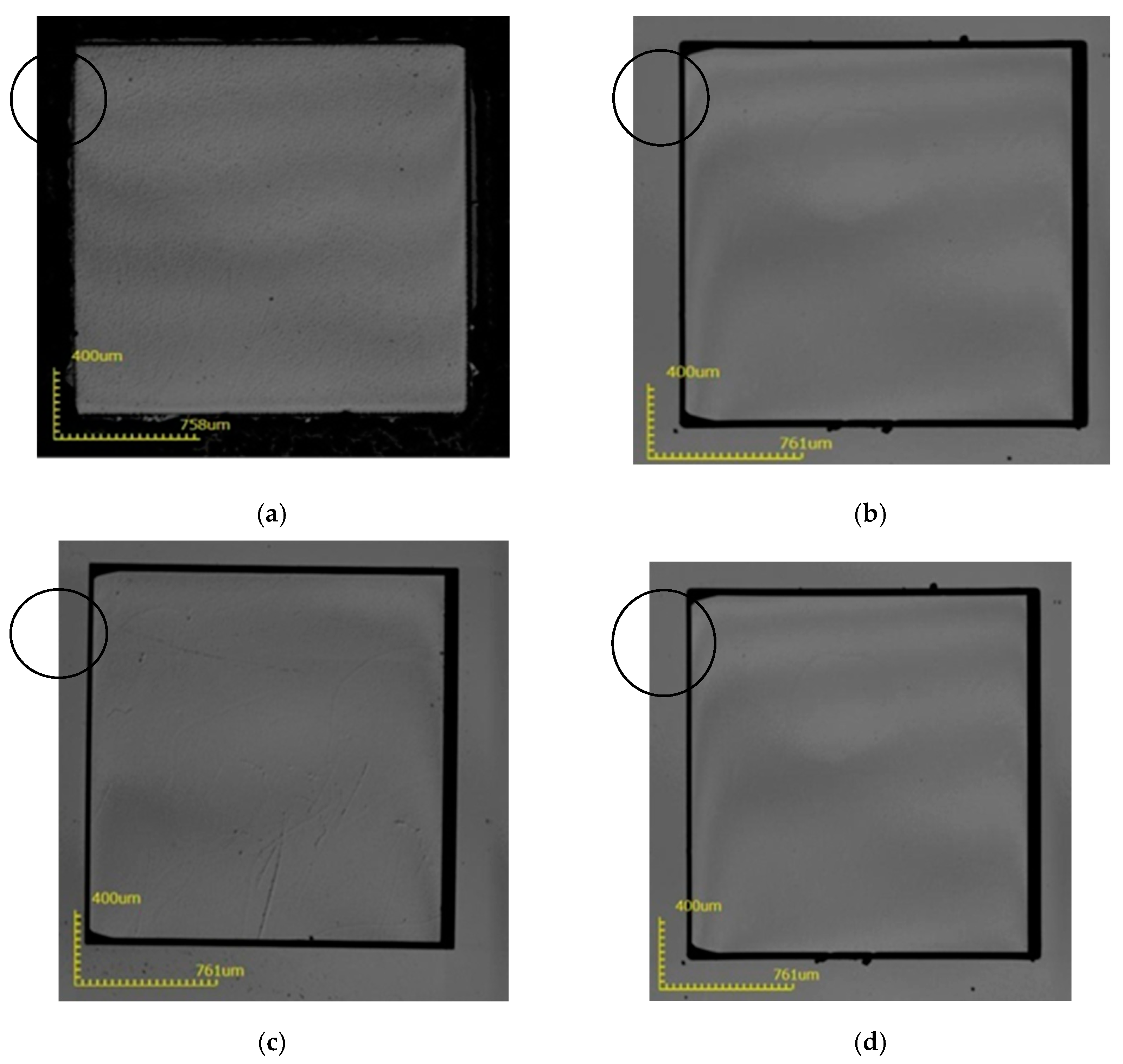
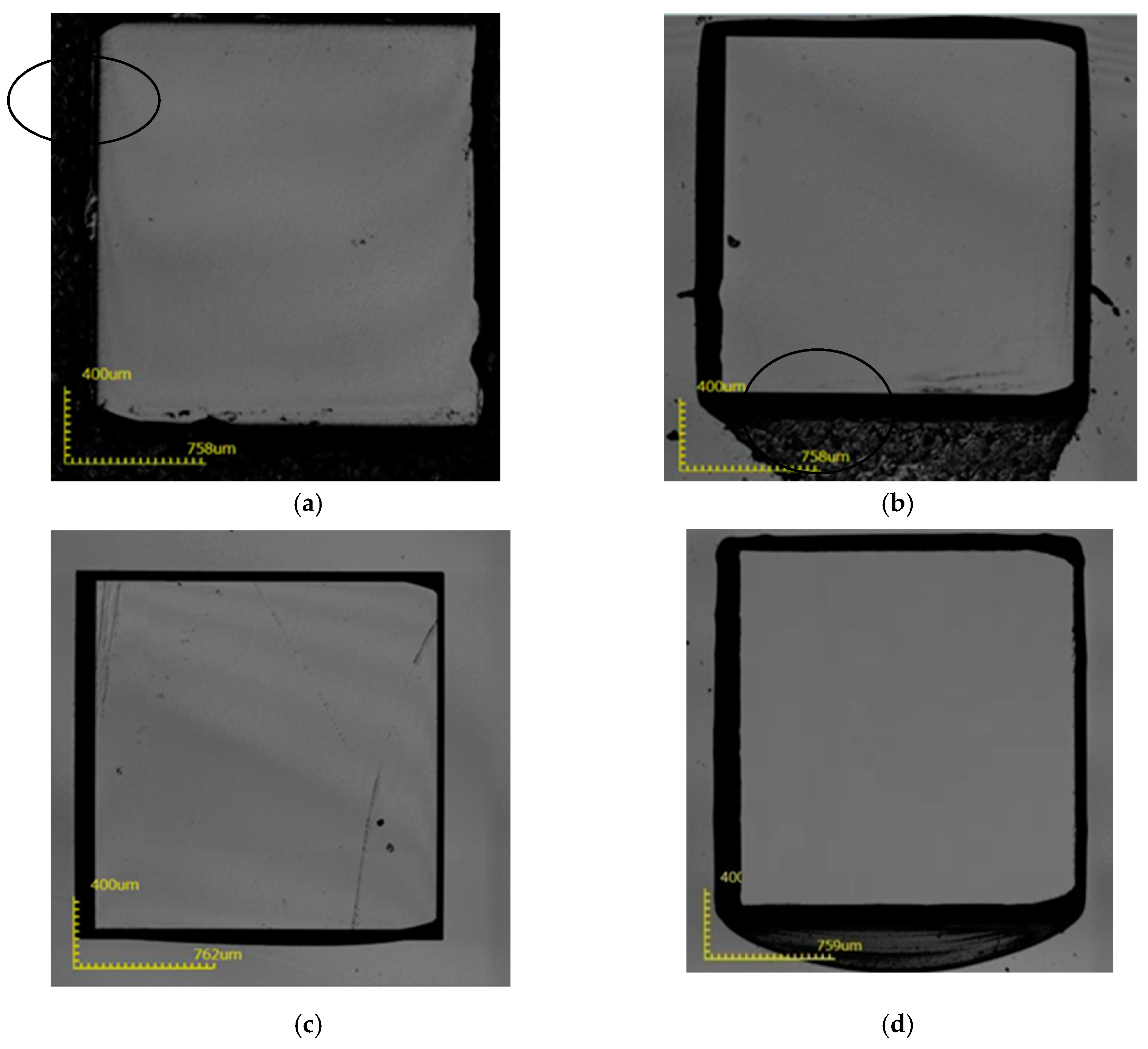
| Scheme | Process Conditions (H3PO4:H2SO4 = 1:3) | ||||
|---|---|---|---|---|---|
| Corrosive Liquid Temperature (°C) | |||||
| 200 | 250 | 275 | 300 | ||
| Mask layer material | 1 | Cr/Au | / | / | / |
| 2 | SiO2/Cr/Au | SiO2/Cr/Au | SiO2/Cr/Au | SiO2/Cr/Au | |
| 3 | SiO2/SiN | SiO2/SiN | SiO2/SiN | SiO2/SiN | |
| 4 | SiO2 | SiO2 | SiO2 | SiO2 | |
| Scheme | Process Conditions (H3PO4:H2SO4 = 1:3) Corrosive Liquid Temperature (200 °C), Time (1 h) | ||
|---|---|---|---|
| Mask Thickness before Etching (nm) | Mask Thickness after Etching (nm) | Sensitive Cavity Depth (nm) | |
| 1-1# | 245 | ||
| 2-1# | 1215 | 1056 | 540 |
| 3-1# | 1966 | 1926 | 541 |
| 4-1# | 999 | 971 | 540 |
| Sample | Process Conditions (H3PO4:H2SO4 = 1:3) Corrosive Liquid Temperature (250 °C), Time (1 h) | ||
|---|---|---|---|
| Mask Thickness before Etching (nm) | Mask Thickness after Etching (nm) | Sensitive Cavity Depth (μm) | |
| 1-2# | 245 | ||
| 2-2# | 1215 | 1024 | 13.8 |
| 3-2# | 1966 | 1965 | 13.9 |
| 4-2# | 999 | 990 | 13.9 |
| Scheme | Process Conditions (H3PO4:H2SO4 = 1:3) Corrosive Liquid Temperature (275 °C), Time (1 h) | ||
|---|---|---|---|
| Mask Thickness before Etching (nm) | Mask Thickness after Etching (nm) | Sensitive Cavity Depth (μm) | |
| 1-3# | 245 | / | / |
| 2-3# | 1215 | 685 | 49 |
| 3-3# | 1966 | 970 | 49 |
| 4-3# | 999 | 612 | 49 |
| Scheme | Process Conditions (H3PO4:H2SO4 = 1:3) Corrosive Liquid Temperature (300 °C), Time (1 h) | ||
|---|---|---|---|
| Mask Thickness before Etching (nm) | Mask Thickness after Etching (nm) | Sensitive Cavity Depth (μm) | |
| 1-4# | 245 | / | / |
| 2-4# | 1215 | 175 | 72 |
| 3-4# | 1966 | 153 | 72 |
| 4-4# | 999 | 115 | 72 |
| Material | Process Conditions (H3PO4:H2SO4 = 1:3) | ||
|---|---|---|---|
| 250 °C | 275 °C | 300 °C | |
| Corrosion rate of SiO2 (nm/h) | 9 | 387 | 884 |
| Corrosion rate of SiN (nm/h) | 1 | 1045 | 22,495 |
| Corrosion rate of sapphire (μm/h) | 13.8 | 48.8 | 72.3 |
| Material | Process Conditions (H3PO4:H2SO4 = 1:3) | ||
|---|---|---|---|
| 250 °C | 275 °C | 300 °C | |
| SiO2: sapphire | 1533.3 | 126.1 | 81.8 |
| SiN: sapphire | 13,800.0 | 46.7 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, Y.-Q.; Zhang, H.-Q.; Zhang, Y. Research on Sapphire Deep Cavity Corrosion and Mask Selection Technology. Micromachines 2021, 12, 136. https://doi.org/10.3390/mi12020136
Shang Y-Q, Zhang H-Q, Zhang Y. Research on Sapphire Deep Cavity Corrosion and Mask Selection Technology. Micromachines. 2021; 12(2):136. https://doi.org/10.3390/mi12020136
Chicago/Turabian StyleShang, Ying-Qi, Hong-Quan Zhang, and Yan Zhang. 2021. "Research on Sapphire Deep Cavity Corrosion and Mask Selection Technology" Micromachines 12, no. 2: 136. https://doi.org/10.3390/mi12020136
APA StyleShang, Y.-Q., Zhang, H.-Q., & Zhang, Y. (2021). Research on Sapphire Deep Cavity Corrosion and Mask Selection Technology. Micromachines, 12(2), 136. https://doi.org/10.3390/mi12020136




