Development of Cell-Carrying Magnetic Microrobots with Bioactive Nanostructured Titanate Surface for Enhanced Cell Adhesion
Abstract
:1. Introduction
2. Materials and Methods
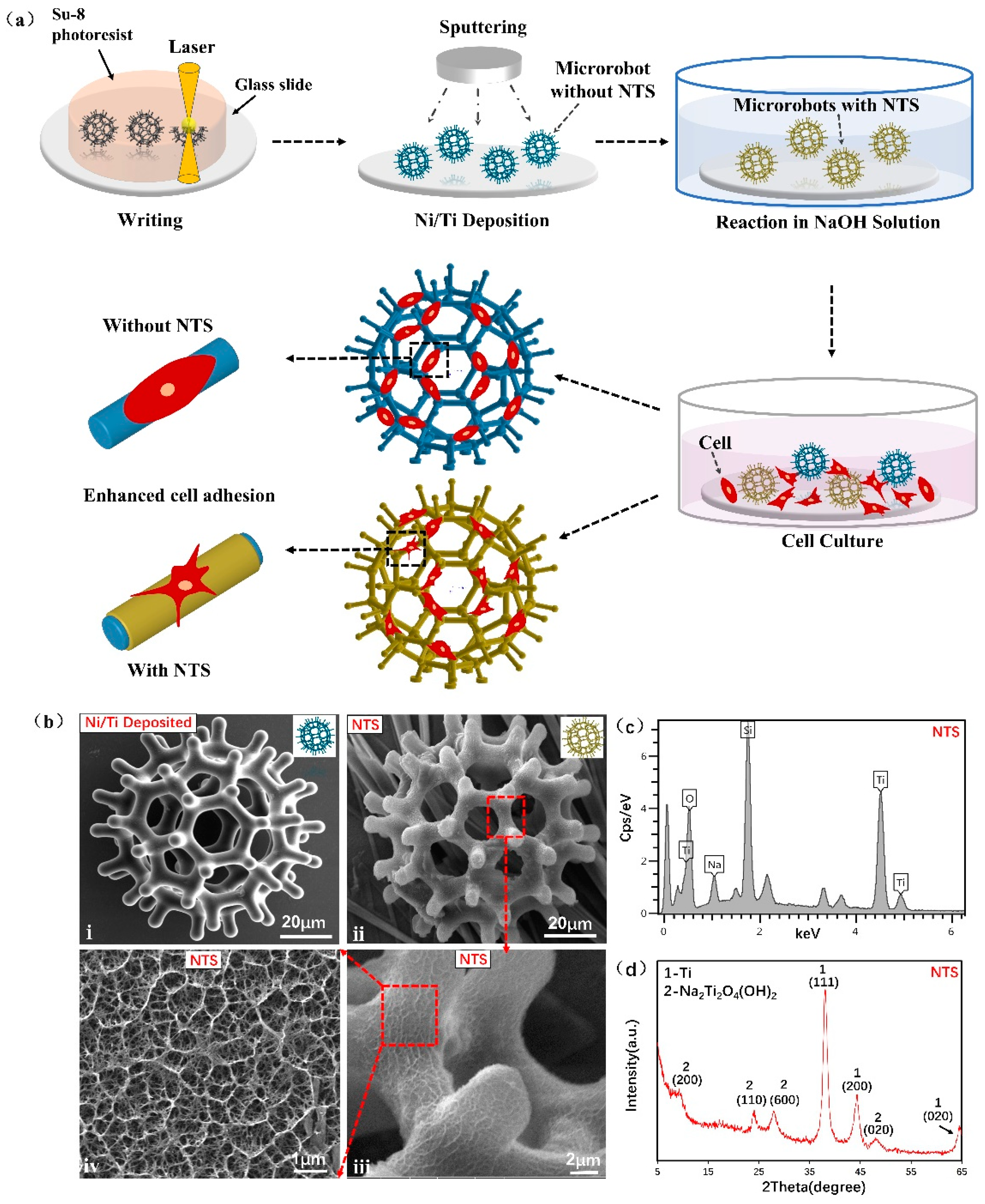
2.1. Microrobot Fabrication
2.2. Apparatus
2.3. Cell Culture on Microrobot
2.4. WCA Test
2.5. Morphology of Cells on NTS
2.6. Cell Viability Tests
2.7. Protein Adsorption Assay
2.8. Alkaline Phosphatase Activity Assay
2.9. Cell Adhesion Ability Test
3. Results
3.1. Characterizations
3.2. Surface Wettability
3.3. Cell Morphology Assay
3.4. Cell Biological Evaluation
3.5. Verification of Cell Adhesion Ability in Microfluidic Chip
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barati, D.; Watkins, K.; Wang, Z.; Yang, F. Injectable and Crosslinkable PLGA-Based Microribbons as 3D Macroporous Stem Cell Niche. Small 2020, 16, 1905820. [Google Scholar] [CrossRef]
- Go, G.; Han, J.; Zhen, J.; Zheng, S.; Yoo, A.; Jeon, M.-J.; Park, J.-O.; Park, S. A Magnetically Actuated Microscaffold Containing Mesenchymal Stem Cells for Articular Cartilage Repair. Adv. Healthc. Mater. 2017, 6, 1601378. [Google Scholar] [CrossRef]
- Zhang, S.; Burda, J.E.; Anderson, M.A.; Zhao, Z.; Ao, Y.; Cheng, Y.; Sun, Y.; Deming, T.J.; Sofroniew, M.V. Thermoresponsive Copolypeptide Hydrogel Vehicles for Central Nervous System Cell Delivery. ACS Biomater. Sci. Eng. 2015, 1, 705–717. [Google Scholar] [CrossRef] [Green Version]
- Stuckey, D.W.; Shah, K. Stem Cell-Based Therapies for Cancer Treatment: Separating Hope from Hype. Nat. Rev. Cancer 2014, 14, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Bender, E. Cell-Based Therapy: Cells on Trial. Nature 2016, 540, S106–S108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbern, J.C.; Lee, R.T. Cardiac Stem Cell Therapy and the Promise of Heart Regeneration. Cell Stem Cell 2013, 12, 689–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Xia, M.; Gao, Y.; Chen, Y.; Xu, Y. Human Umbilical Cord Mesenchymal Stem Cells: An Overview of Their Potential in Cell-Based Therapy. Expert Opin. Biol. Ther. 2015, 15, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V. Tisagenlecleucel—The First Approved CAR-T-Cell Therapy: Implications for Payers and Policy Makers. Nat. Rev. Clin. Oncol. 2018, 15, 11–12. [Google Scholar] [CrossRef]
- Cieślik, I.; Płocińska, M.; Płociński, T.; Zdunek, J.; Woźniak, M.J.; Bil, M.; Hirano, S. Influence of Polymeric Precursors on the Viability of Human Cells of Yttrium Aluminum Borates Nanoparticles Doped with Ytterbium Ions. Appl. Surf. Sci. 2019, 488, 874–886. [Google Scholar] [CrossRef]
- Newland, B.; Welzel, P.B.; Newland, H.; Renneberg, C.; Kolar, P.; Tsurkan, M.; Rosser, A.; Freudenberg, U.; Werner, C. Tackling Cell Transplantation Anoikis: An Injectable, Shape Memory Cryogel Microcarrier Platform Material for Stem Cell and Neuronal Cell Growth. Small 2015, 11, 5047–5053. [Google Scholar] [CrossRef] [Green Version]
- Vegas, A.J.; Veiseh, O.; Gürtler, M.; Millman, J.R.; Pagliuca, F.W.; Bader, A.R.; Doloff, J.C.; Li, J.; Chen, M.; Olejnik, K.; et al. Long-Term Glycemic Control Using Polymer-Encapsulated Human Stem Cell–Derived Beta Cells in Immune-Competent Mice. Nat. Med. 2016, 22, 306–311. [Google Scholar] [CrossRef]
- Orive, G.; Santos, E.; Poncelet, D.; Hernández, R.M.; Pedraz, J.L.; Wahlberg, L.U.; De Vos, P.; Emerich, D. Cell Encapsulation: Technical and Clinical Advances. Trends Pharmacol. Sci. 2015, 36, 537–546. [Google Scholar] [CrossRef]
- Liao, P.; Xing, L.; Zhang, S.; Sun, D. Magnetically Driven Undulatory Microswimmers Integrating Multiple Rigid Segments. Small 2019, 15, 1901197. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.; Yasa, I.C.; Yasa, O.; Tabak, A.F.; Giltinan, J.; Sitti, M. 3D-Printed Biodegradable Microswimmer for Theranostic Cargo Delivery and Release. ACS Nano 2019, 13, 3353–3362. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Huang, T.-Y.; Luo, Z.; Testa, P.; Gu, H.; Chen, X.-Z.; Nelson, B.J.; Heyderman, L.J. Nanomagnetic Encoding of Shape-Morphing Micromachines. Nature 2019, 575, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Fischer, P. Controlled Propulsion of Artificial Magnetic Nanostructured Propellers. Nano Lett. 2009, 9, 2243–2245. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, J.; Salehizadeh, M.; Onaizah, O.; Diller, E. Millimeter-Scale Flexible Robots with Programmable Three-Dimensional Magnetization and Motions. Sci. Robot. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhou, Q.; Vincent, M.; Deng, Y.; Yu, J.; Xu, J.; Xu, T.; Tang, T.; Bian, L.; Wang, Y.-X.J.; et al. Multifunctional Biohybrid Magnetite Microrobots for Imaging-Guided Therapy. Sci. Robot. 2017, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.C.M.; Liao, P.; Wei, T.; Zhang, L.; Sun, D. Magnetically Powered Biodegradable Microswimmers. Micromachines 2020, 11, 404. [Google Scholar] [CrossRef]
- Li, X.; Fukuda, T. Magnetically Guided Micromanipulation of Magnetic Microrobots for Accurate Creation of Artistic Patterns in Liquid Environment. Micromachines 2020, 11, 697. [Google Scholar] [CrossRef]
- Wei, T.; Li, J.; Zheng, L.; Wang, C.; Li, F.; Tian, H.; Sun, D. Development of a Cell-Loading Microrobot with Simultaneously Improved Degradability and Mechanical Strength for Performing In Vivo Delivery Tasks. Adv. Intell. Syst. 2021, 3, 2100052. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Kim, S.; Kim, J.-Y.; Moon, C.; Nelson, B.J.; Choi, H. A Capsule-Type Microrobot with Pick-and-Drop Motion for Targeted Drug and Cell Delivery. Adv. Healthc. Mater. 2018, 7, 1700985. [Google Scholar] [CrossRef] [PubMed]
- Yasa, I.C.; Tabak, A.F.; Yasa, O.; Ceylan, H.; Sitti, M. 3D-Printed Microrobotic Transporters with Recapitulated Stem Cell Niche for Programmable and Active Cell Delivery. Adv. Funct. Mater. 2019, 29, 1808992. [Google Scholar] [CrossRef]
- Gyak, K.-W.; Jeon, S.; Ha, L.; Kim, S.; Kim, J.; Lee, K.-S.; Choi, H.; Kim, D.-P. Magnetically Actuated SiCN-Based Ceramic Microrobot for Guided Cell Delivery. Adv. Healthc. Mater. 2019, 8, 1900739. [Google Scholar] [CrossRef] [PubMed]
- Steager, E.B.; Selman Sakar, M.; Magee, C.; Kennedy, M.; Cowley, A.; Kumar, V. Automated Biomanipulation of Single Cells Using Magnetic Microrobots. Int. J. Robot. Res. 2013, 32, 346–359. [Google Scholar] [CrossRef]
- Tottori, S.; Zhang, L.; Qiu, F.; Krawczyk, K.K.; Franco-Obregón, A.; Nelson, B.J. Magnetic Helical Micromachines: Fabrication, Controlled Swimming, and Cargo Transport. Adv. Mater. 2012, 24, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Qiu, F.; Kim, S.; Ghanbari, A.; Moon, C.; Zhang, L.; Nelson, B.J.; Choi, H. Fabrication and Characterization of Magnetic Microrobots for Three-Dimensional Cell Culture and Targeted Transportation. Adv. Mater. 2013, 25, 5863–5868. [Google Scholar] [CrossRef] [Green Version]
- Go, G.; Jeong, S.-G.; Yoo, A.; Han, J.; Kang, B.; Kim, S.; Nguyen, K.T.; Jin, Z.; Kim, C.-S.; Seo, Y.R.; et al. Human Adipose–Derived Mesenchymal Stem Cell–Based Medical Microrobot System for Knee Cartilage Regeneration in Vivo. Sci. Robot. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, S.; Ha, S.; Lee, S.; Kim, E.; Kim, S.Y.; Park, S.H.; Jeon, J.H.; Kim, S.W.; Moon, C.; et al. Magnetically Actuated Microrobots as a Platform for Stem Cell Transplantation. Sci. Robot. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Luo, T.; Wang, R.; Liu, C.; Chen, S.; Li, D.; Yue, J.; Cheng, S.; Sun, D. Development of a Magnetic Microrobot for Carrying and Delivering Targeted Cells. Sci. Robot. 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.Y.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. Enhanced in Vitro Biocompatibility of Ultrafine-Grained Titanium with Hierarchical Porous Surface. Appl. Surf. Sci. 2011, 257, 5634–5640. [Google Scholar] [CrossRef]
- Oh, S.; Daraio, C.; Chen, L.-H.; Pisanic, T.R.; Fiñones, R.R.; Jin, S. Significantly Accelerated Osteoblast Cell Growth on Aligned TiO2 Nanotubes. J. Biomed. Mater. Res. Part A 2006, 78, 97–103. [Google Scholar] [CrossRef]
- Pawlik, A.; Socha, R.P.; Hubalek Kalbacova, M.; Sulka, G.D. Surface Modification of Nanoporous Anodic Titanium Dioxide Layers for Drug Delivery Systems and Enhanced SAOS-2 Cell Response. Colloids Surf. B Biointerfaces 2018, 171, 58–66. [Google Scholar] [CrossRef]
- Yang, K.; Yu, S.J.; Lee, J.S.; Lee, H.-R.; Chang, G.-E.; Seo, J.; Lee, T.; Cheong, E.; Im, S.G.; Cho, S.-W. Electroconductive Nanoscale Topography for Enhanced Neuronal Differentiation and Electrophysiological Maturation of Human Neural Stem Cells. Nanoscale 2017, 9, 18737–18752. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tang, W.; Gregurec, D.; Yate, L.; Moya, S.E.; Wang, G. Layered Titanates with Fibrous Nanotopographic Features as Reservoir for Bioactive Ions to Enhance Osteogenesis. Appl. Surf. Sci. 2018, 436, 653–661. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Gao, H.; Ge, P.; Ren, K.; Gao, J.; Cao, Y.; Han, D.; Zhang, J. One-Step Fabrication of Functionalized Poly(Etheretherketone) Surfaces with Enhanced Biocompatibility and Osteogenic Activity. Mater. Sci. Eng. C 2018, 88, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, T.; Epstein, J.; Cooney, L.; Wang, H.; Li, Y.; Jiang, Y.-B.; Cogbill, A.; Varadan, V.; Tian, Z.R. Multifunctional Nanowire Bioscaffolds on Titanium. Chem. Mater. 2007, 19, 4454–4459. [Google Scholar] [CrossRef]
- Ren, N.; Li, R.; Chen, L.; Wang, G.; Liu, D.; Wang, Y.; Zheng, L.; Tang, W.; Yu, X.; Jiang, H.; et al. In Situ Construction of a Titanate–Silver Nanoparticle–Titanate Sandwich Nanostructure on a Metallic Titanium Surface for Bacteriostatic and Biocompatible Implants. J. Mater. Chem. 2012, 22, 19151–19160. [Google Scholar] [CrossRef]
- Ahn, H.H.; Lee, I.W.; Lee, H.B.; Kim, M.S. Cellular Behavior of Human Adipose-Derived Stem Cells on Wettable Gradient Polyethylene Surfaces. Int. J. Mol. Sci. 2014, 15, 2075–2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, W.; Cheng, L.; Li, H.; Zhou, Y.; Zhang, Y.; Chang, J. Preparation of Hydrophilic Poly(l-Lactide) Electrospun Fibrous Scaffolds Modified with Chitosan for Enhanced Cell Biocompatibility. Polymer 2012, 53, 2298–2305. [Google Scholar] [CrossRef]
- Birhanu, G.; Javar, H.A.; Seyedjafari, E.; Zandi-Karimi, A.; Telgerd, M.D. An Improved Surface for Enhanced Stem Cell Proliferation and Osteogenic Differentiation Using Electrospun Composite PLLA/P123 Scaffold. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Kandere-Grzybowska, K.; Soh, S.; Mahmud, G.; Komarova, Y.; Pilans, D.; Grzybowski, B.A. Short-Term Molecular Polarization of Cells on Symmetric and Asymmetric Micropatterns. Soft Matter 2010, 6, 3257–3268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix Nanotopography as a Regulator of Cell Function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, Q.; Lu, Q.; Xu, Y.; Yin, J.; Hu, J.; Wang, Z. Nanotopographical Guidance of C6 Glioma Cell Alignment and Oriented Growth. Biomaterials 2004, 25, 4215–4223. [Google Scholar] [CrossRef]
- Lavenus, S.; Ricquier, J.-C.; Louarn, G.; Layrolle, P. Cell Interaction with Nanopatterned Surface of Implants. Nanomedicine 2010, 5, 937–947. [Google Scholar] [CrossRef]
- Evans, N.D.; Gentleman, E.; Chen, X.; Roberts, C.J.; Polak, J.M.; Stevens, M.M. Extracellular Matrix-Mediated Osteogenic Differentiation of Murine Embryonic Stem Cells. Biomaterials 2010, 31, 3244–3252. [Google Scholar] [CrossRef]
- Lyu, Z.; Wang, H.; Wang, Y.; Ding, K.; Liu, H.; Yuan, L.; Shi, X.; Wang, M.; Wang, Y.; Chen, H. Maintaining the Pluripotency of Mouse Embryonic Stem Cells on Gold Nanoparticle Layers with Nanoscale but Not Microscale Surface Roughness. Nanoscale 2014, 6, 6959–6969. [Google Scholar] [CrossRef]
- Hidajat, N.; Kreuschner, M.; Röttgen, R.; Schröder, R.-J.; Schmidt, S.; Felix, R. Placement of Transjugular Intrahepatic Portosystemic Shunt via the Left Hepatic Vein under Sonographic Guidance in a Patient with Right Hemihepatectomy. Acta Radiol. 2003, 44, 363–365. [Google Scholar] [CrossRef]
- Wei, T.; Liu, J.; Li, D.; Chen, S.; Zhang, Y.; Li, J.; Fan, L.; Guan, Z.; Lo, C.-M.; Wang, L.; et al. Development of Magnet-Driven and Image-Guided Degradable Microrobots for the Precise Delivery of Engineered Stem Cells for Cancer Therapy. Small 2020, 16, 1906908. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Fan, L.; Li, Y.; Wei, T.; Wang, C.; Li, F.; Tian, H.; Sun, D. Development of Cell-Carrying Magnetic Microrobots with Bioactive Nanostructured Titanate Surface for Enhanced Cell Adhesion. Micromachines 2021, 12, 1572. https://doi.org/10.3390/mi12121572
Li J, Fan L, Li Y, Wei T, Wang C, Li F, Tian H, Sun D. Development of Cell-Carrying Magnetic Microrobots with Bioactive Nanostructured Titanate Surface for Enhanced Cell Adhesion. Micromachines. 2021; 12(12):1572. https://doi.org/10.3390/mi12121572
Chicago/Turabian StyleLi, Junyang, Lei Fan, Yanfang Li, Tanyong Wei, Cheng Wang, Feng Li, Hua Tian, and Dong Sun. 2021. "Development of Cell-Carrying Magnetic Microrobots with Bioactive Nanostructured Titanate Surface for Enhanced Cell Adhesion" Micromachines 12, no. 12: 1572. https://doi.org/10.3390/mi12121572
APA StyleLi, J., Fan, L., Li, Y., Wei, T., Wang, C., Li, F., Tian, H., & Sun, D. (2021). Development of Cell-Carrying Magnetic Microrobots with Bioactive Nanostructured Titanate Surface for Enhanced Cell Adhesion. Micromachines, 12(12), 1572. https://doi.org/10.3390/mi12121572






