Low Temperature Hydrophilic SiC Wafer Level Direct Bonding for Ultrahigh-Voltage Device Applications
Abstract
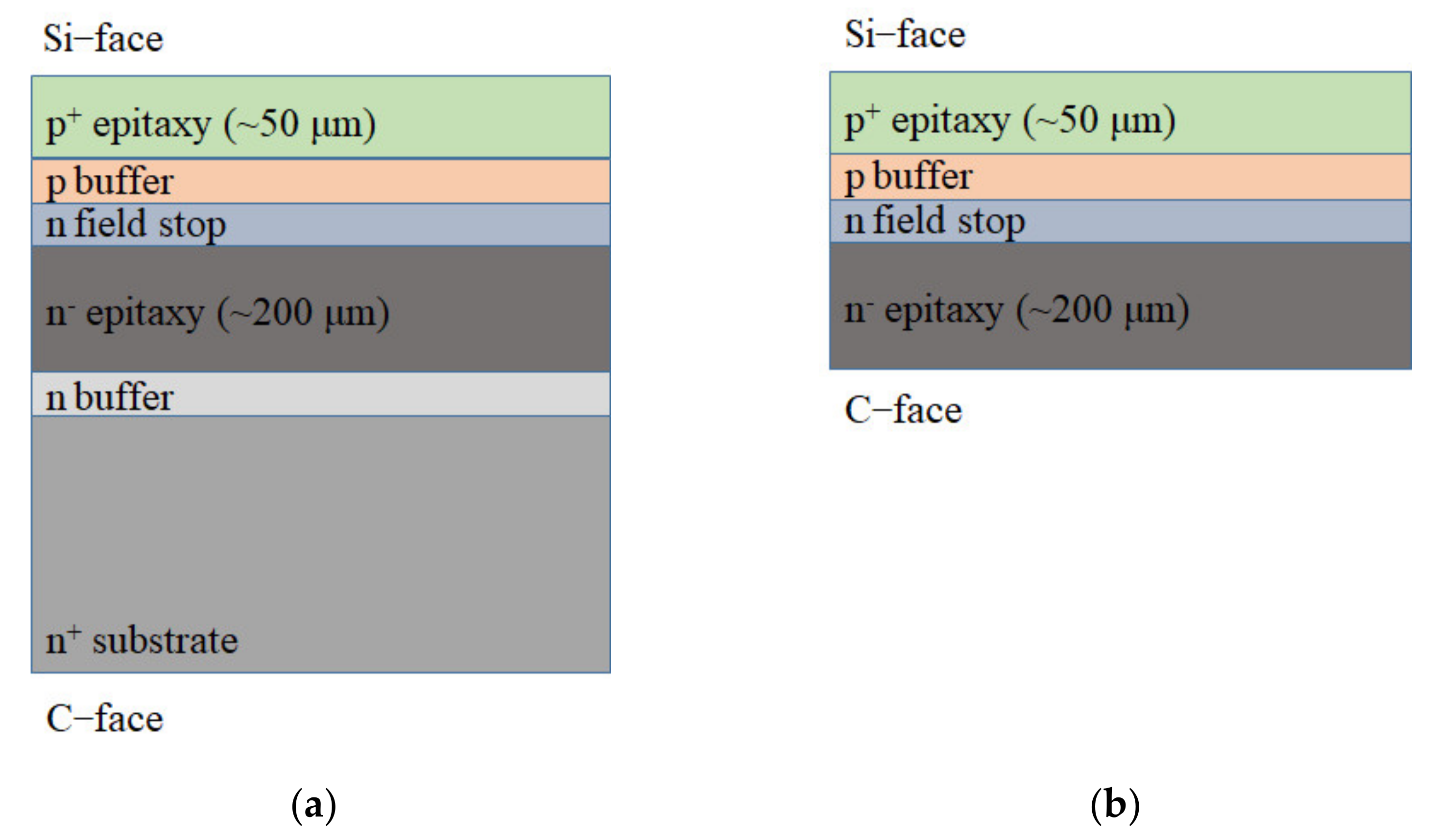
:1. Introduction
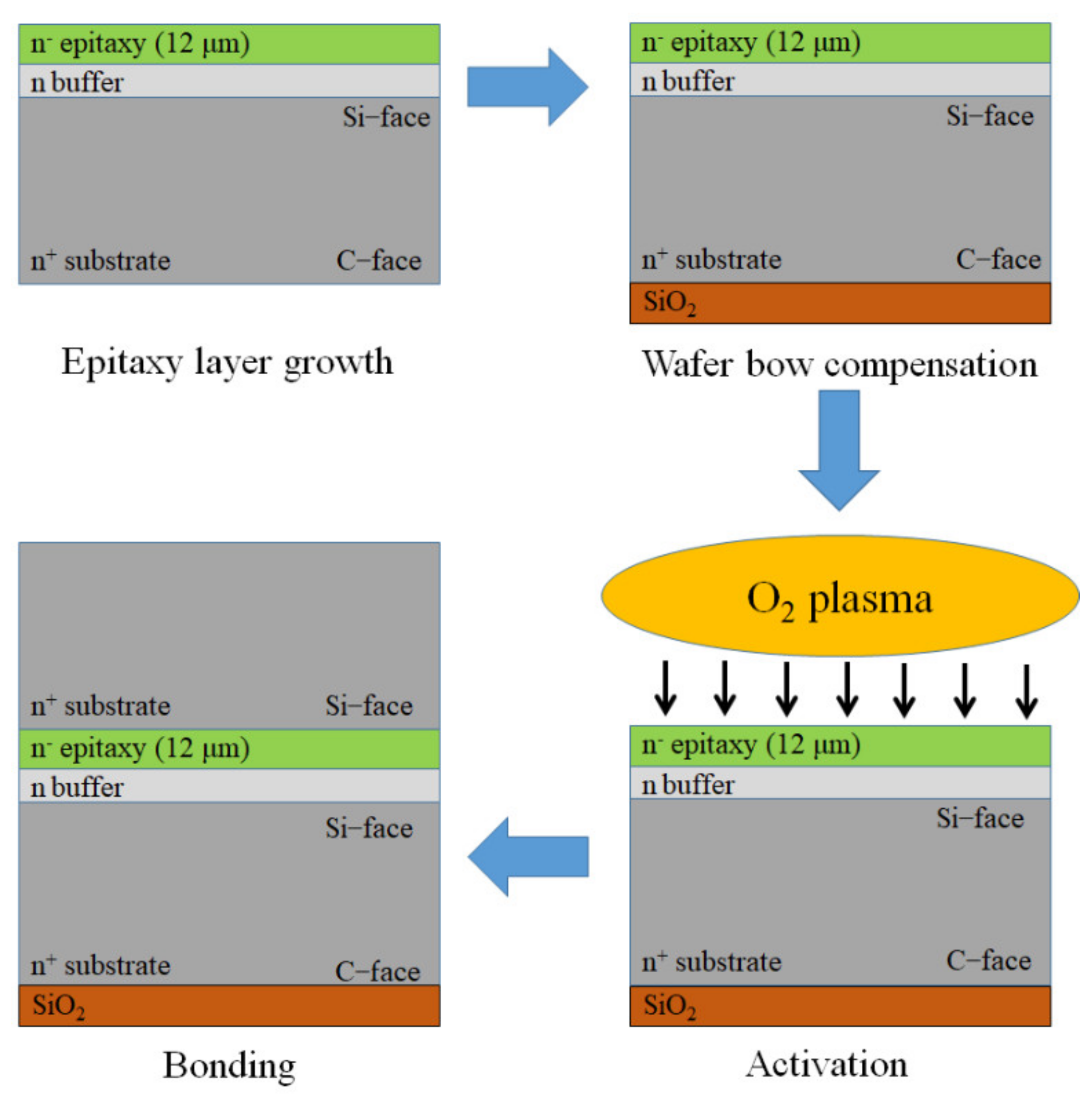
2. Experimental Details
3. Experiment Results and Discussions
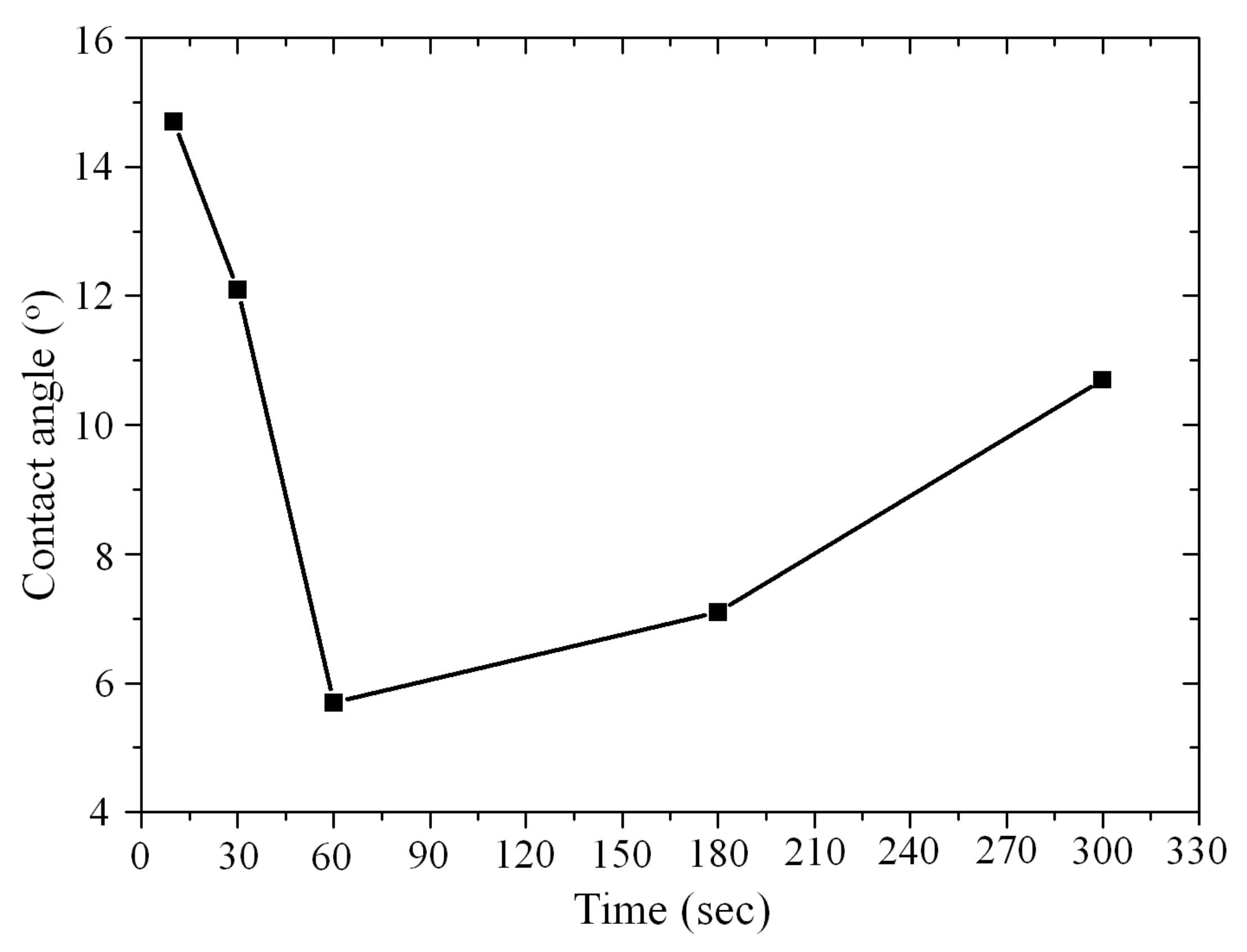
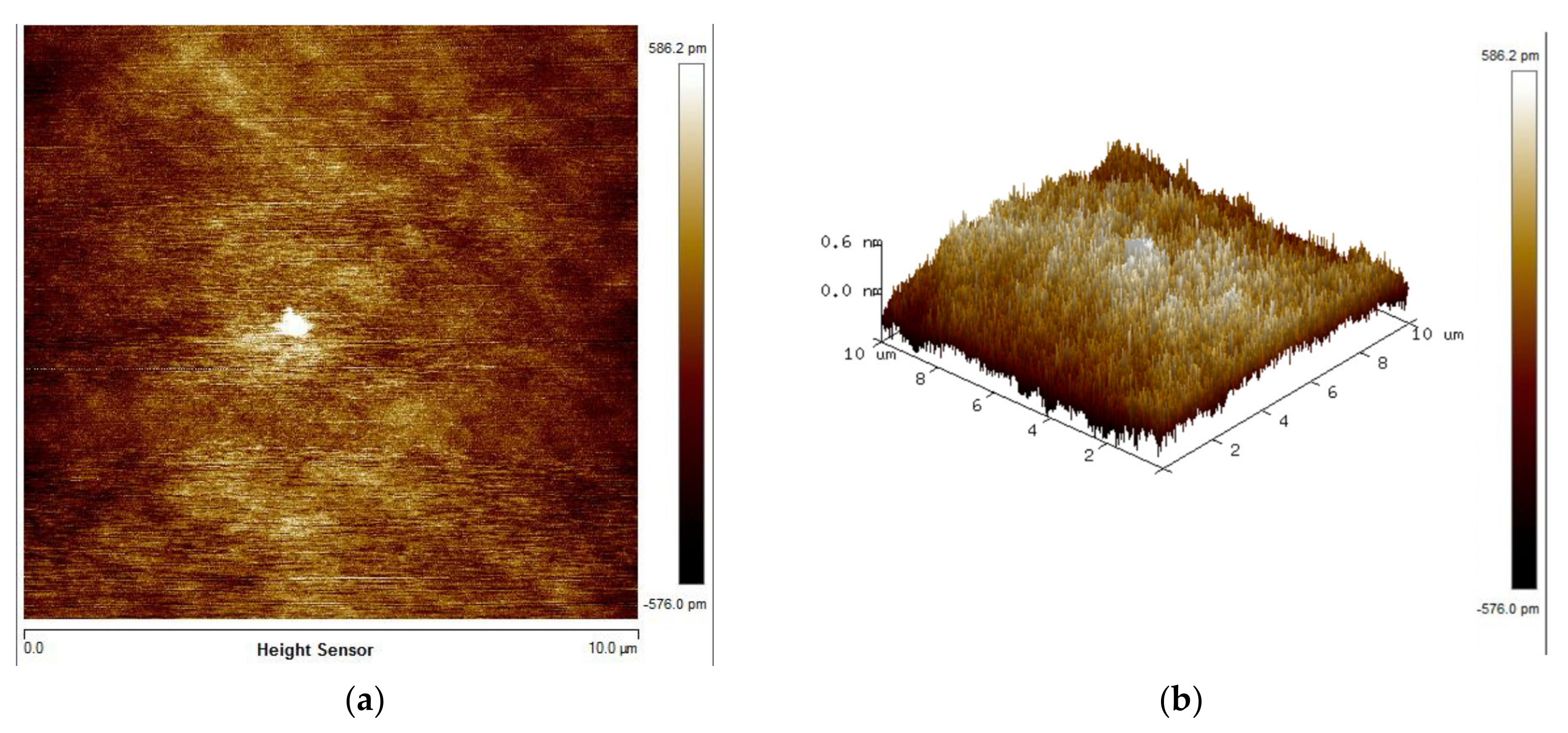
3.1. Surface Activation
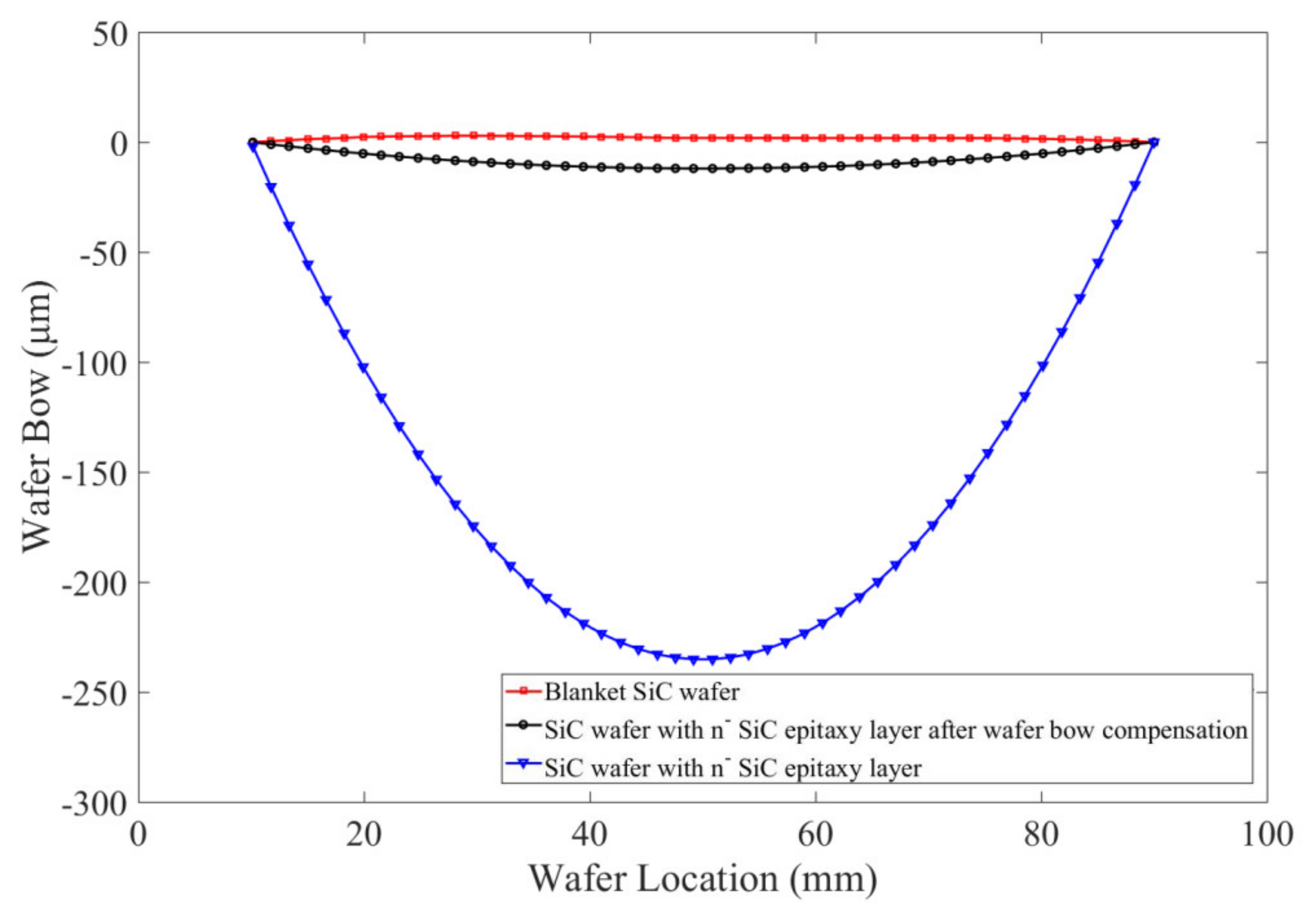
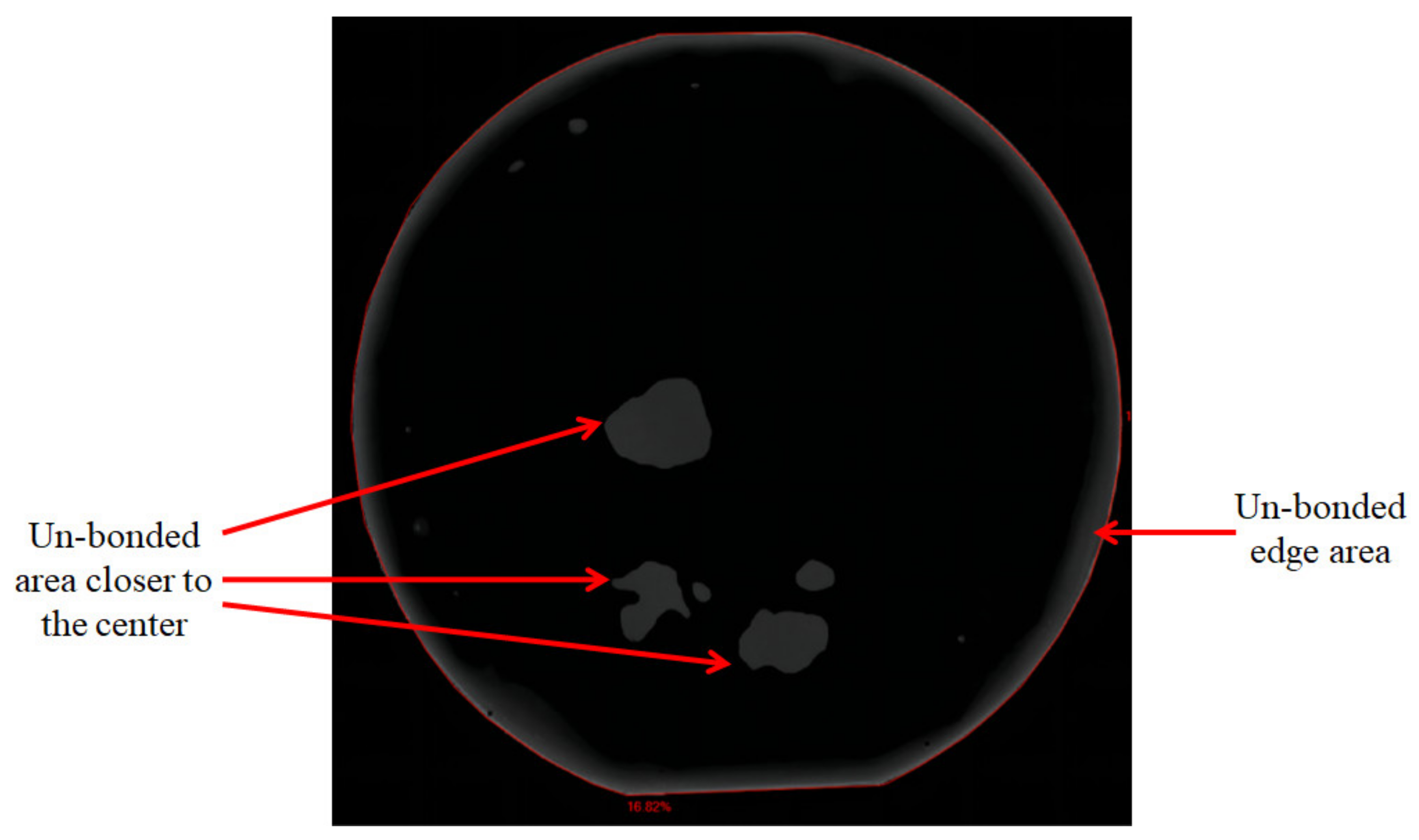
3.2. Bonding Uniformity and Bonding Strength
3.3. Bonding Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moghe, R.; Kandula, R.P.; Iyer, A.; Divan, D. Loss Comparison between SiC, Hybrid Si/SiC, and Si Devices in Direct AC/AC Converters. In Proceedings of the 2012 IEEE Energy Conversion Congress and Exposition, Raleigh, NC, USA, 15–20 September 2012; pp. 3848–3855. [Google Scholar]
- Suga, T.; Mu, F.; Fujino, M.; Takahashi, Y.; Nakazawa, H.; Iguchi, K. Silicon carbide wafer bonding by modified surface activated bonding method. Jpn. J. Appl. Phys. 2015, 54, 030214. [Google Scholar] [CrossRef] [Green Version]
- Mu, F.; Iguchi, K.; Nakazawa, H.; Takahashi, Y.; Fujino, M.; Suga, T. Direct Wafer Bonding of SiC-SiC at Room Temperature by SAB Method. In Semiconductor Wafer Bonding: Science, Technology and Applications 14; ECS Transactions Volume 75; Suga, T., Baumgart, H., Fournel, F., Goorsky, M.S., Hobart, K.D., Knechtel, R., Seng Tan, C., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2016; pp. 77–83. [Google Scholar]
- Mu, F.; Uomoto, M.; Shimatsu, T.; Wang, Y.; Iguchi, K.; Nakazawa, H.; Takahashi, Y.; Higurashi, E.; Suga, T. De-bondable SiC-SiC wafer bonding via an intermediate Ni nano-film. Appl. Surf. Sci. 2019, 465, 591–595. [Google Scholar] [CrossRef]
- Chu, K.-W.; Lee, W.-S.; Cheng, C.-Y.; Huang, C.-F.; Zhao, F.; Lee, L.-S.; Chen, Y.-S.; Lee, C.-Y.; Tsai, M.-J. Demonstration of Lateral IGBTs in 4H-SiC. IEEE Electron Device Lett. 2013, 34, 286–288. [Google Scholar] [CrossRef]
- Ryu, S.-H.; Capell, C.; Cheng, L.; Jonas, C.; Gupta, A.; Donofrio, M.; Clayton, J.; O’Loughlin, M.; Burk, A.; Grider, D.; et al. High Performance, Ultra High Voltage 4H-SiC IGBTs. In Proceedings of the 2012 IEEE Energy Conversion Congress and Exposition, Raleigh, NC, USA, 15–20 September 2012; pp. 3603–3608. [Google Scholar]
- Ryu, S.-H.; Capell, C.; Cheng, L.; Jonas, C.; Gupta, A.; Donofrio, M.; Clayton, J.; O’Loughlin, M.; Burk, A.; Grider, D.; et al. Ultra High Voltage (>12 kV), High Performance 4H-SiC IGBTs. In Proceedings of the 2012 24th International Symposium on Power Semiconductor Devices and Ics, Bruges, Belgium, 3–7 June 2012; pp. 257–260. [Google Scholar]
- Usman, M.; Nawaz, M. Device design assessment of 4H-SiC n-IGBT—A simulation study. Solid-State Electron. 2014, 92, 5–11. [Google Scholar] [CrossRef]
- Wang, X.; Cooper, J.A. High-Voltage n-Channel IGBTs on Free-Standing 4H-SiC Epilayers. IEEE Trans. Electron Devices 2010, 57, 511–515. [Google Scholar] [CrossRef]
- Miyazawa, T.; Ji, S.Y.; Kojima, K.; Ishida, Y.; Nakayama, K.; Tanaka, A.; Asano, K.; Tsuchida, H. Epitaxial Growth of Thick Multi-Layer 4H-SiC for the Fabrication of Very High-Voltage C-Face n-Channel IGBT. Mater. Sci. Forum 2014, 778–780, 135–138. [Google Scholar] [CrossRef]
- Fukuda, K.; Okamoto, D.; Okamoto, M.; Deguchi, T.; Mizushima, T.; Takenaka, K.; Fujisawa, H.; Harada, S.; Tanaka, Y.; Yonezawa, Y.; et al. Development of Ultrahigh-Voltage SiC Devices. IEEE Trans. Electron Devices 2015, 62, 396–404. [Google Scholar] [CrossRef]
- Yushin, G.N.; Kvit, A.V.; Sitar, Z. Transmission electron microscopy studies of the bonded SiC-SiC interface. J. Mater. Sci. 2005, 40, 4369–4371. [Google Scholar] [CrossRef]
- Grekhov, I.V.; Kostina, L.S.; Argunova, T.S.; Belyakova, E.I.; Je, J.H.; Ivanov, P.A.; Samsonova, T.P. Direct bonding of silicon carbide wafers with a regular relief at the interface. Tech. Phys. Lett. 2006, 32, 453–455. [Google Scholar] [CrossRef]
- Wei, J.; Xie, H.; Nai, M.L.; Wong, C.K.; Lee, L.C. Low Temperature Wafer Anodic Bonding. J. Micromech. Microeng. 2003, 13, 217–222. [Google Scholar] [CrossRef]
- Lin, X.H.; Liao, G.L.; Tang, Z.R.; Shi, T.L. UV Surface Exposure for Low Temperature Hydrophilic Silicon Direct Bonding. Microsyst. Technol. 2009, 15, 317–321. [Google Scholar] [CrossRef]
- Mu, F.; Iguchi, K.; Nakazawa, H.; Takahashi, Y.; Fujino, M.; Suga, T. Direct Wafer Bonding of SiC-SiC by SAB for Monolithic Integration of SiC MEMS and Electronics. ECS J. Solid State Sci. Technol. 2016, 5, P451–P456. [Google Scholar] [CrossRef]
- Mu, F.; Wang, Y.; Suga, T. Room Temperature Wafer Bonding of Wide Bandgap Semiconductors. In Semiconductor Wafer Bonding: Science, Technology, and Applications 15; ECS Transactions Volume 86; Tan, C.S., Suga, T., Baumgart, H., Fournel, F., Goorsky, M., Hobart, K.D., Knechtel, R., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2018; pp. 3–21. [Google Scholar]
- Luan, P.; Knoll, A.J.; Bruggeman, P.J.; Oehrlein, G.S. Plasma–surface interaction at atmospheric pressure: A case study of polystyrene etching and surface modification by Ar/O2 plasma jet. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 05C315. [Google Scholar] [CrossRef]
- Clugston, M.; Flemming, R. Advanced Chemistry; Oxford University Press: Oxford, UK, 2000; p. 355. ISBN 978-0-19-914633-8. [Google Scholar]
- Tan, C.S.; Chen, K.N.; Fan, A.; Reif, R. Low-temperature Direct CVD Oxides to Thermal Oxide Wafer Bonding in Silicon Layer Transfer. Electrochem. Solid-State Lett. 2005, 8, G1–G4. [Google Scholar] [CrossRef]
- Wiemer, M.; Wünsch, D.; Bruer, J.; Gessner, T. Plasma-activated bonding. In Handbook of Wafer Bonding; Wiley Online Library: Hoboken, NJ, USA, 2012; pp. 101–106. [Google Scholar]
- Maszara, W.P.; Goetz, G.; Caviglia, A.; McKitterick, J.B. Bonding of Silicon Wafers for Silicon-On-Insulator. J. Appl. Phys. 1988, 64, 4943–4950. [Google Scholar] [CrossRef]
- Kikuchi, H.; Kalia, R.K.; Nakano, A.; Vashishta, P.; Branicio, P.S.; Shimojo, F. Brittle dynamic fracture of crystalline cubic silicon carbide (3C-SiC) via molecular dynamics simulation. J. Appl. Phys. 2005, 98, 103524. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.S.; Reif, R. Microelectronics Thin Film Handling and Transfer Using Low-temperature Wafer Bonding. Electrochem. Solid-State Lett. 2005, 8, G362–G366. [Google Scholar] [CrossRef]
- Shen, X.; Oxley, M.P.; Puzyrev, Y.; Tuttle, B.R.; Duscher, G.; Pantelides, S.T. Excess carbon in silicon carbide. J. Appl. Phys. 2010, 108, 123705. [Google Scholar] [CrossRef]
- Urresti, J.; Arith, F.; Olsen, S.; Wright, N.; O’Neill, A. Design and analysis of high mobility enhancement-mode 4H-SiC MOSFETs using a thin-SiO2/Al2O3 gate-stack. IEEE Trans. Electron Devices 2019, 66, 1710–1716. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Di Ventra, M.; Kim, S.G.; Pantelides, S.T. Atomic-scale dynamics of the formation and dissolution of carbon clusters in SiO2. Phys. Rev. Lett. 2001, 86, 5946–5949. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| SiC Wafer | n− SiC Epitaxy Growth Layer | |||
|---|---|---|---|---|
| Contact Angle | Rms Roughness | Contact Angle | Rms Roughness | |
| Before activation | (52.7 ± 3.57)° | (6.15 ± 0.0986) nm | 55.7° | 9.5 nm |
| After activation | (3.59 ± 0.469)° | (0.184 ± 0.0351) nm | 7° | 3.5 nm |
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Contact angle before activation | 51.8° | 47.8° | 53.5° | 57.8° | 52.4° |
| Contact angle after activation | 3.3° | 3.98° | 3.67° | 3.34° | 3.67° |
| RMS roughness before activation | 5.98 nm | 6.20 nm | 6.16 nm | 6.22 nm | 6.20 nm |
| RMS roughness after activation | 0.131 nm | 0.216 nm | 0.201 nm | 0.166 nm | 0.206 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhang, C.; Wu, J.; Yang, F.; An, Y.; Hu, F.; Fan, J. Low Temperature Hydrophilic SiC Wafer Level Direct Bonding for Ultrahigh-Voltage Device Applications. Micromachines 2021, 12, 1575. https://doi.org/10.3390/mi12121575
Zhang W, Zhang C, Wu J, Yang F, An Y, Hu F, Fan J. Low Temperature Hydrophilic SiC Wafer Level Direct Bonding for Ultrahigh-Voltage Device Applications. Micromachines. 2021; 12(12):1575. https://doi.org/10.3390/mi12121575
Chicago/Turabian StyleZhang, Wenting, Caorui Zhang, Junmin Wu, Fei Yang, Yunlai An, Fangjing Hu, and Ji Fan. 2021. "Low Temperature Hydrophilic SiC Wafer Level Direct Bonding for Ultrahigh-Voltage Device Applications" Micromachines 12, no. 12: 1575. https://doi.org/10.3390/mi12121575
APA StyleZhang, W., Zhang, C., Wu, J., Yang, F., An, Y., Hu, F., & Fan, J. (2021). Low Temperature Hydrophilic SiC Wafer Level Direct Bonding for Ultrahigh-Voltage Device Applications. Micromachines, 12(12), 1575. https://doi.org/10.3390/mi12121575






