Micro/Nanopatterned Superhydrophobic Surfaces Fabrication for Biomolecules and Biomaterials Manipulation and Analysis
Abstract
:1. Introduction
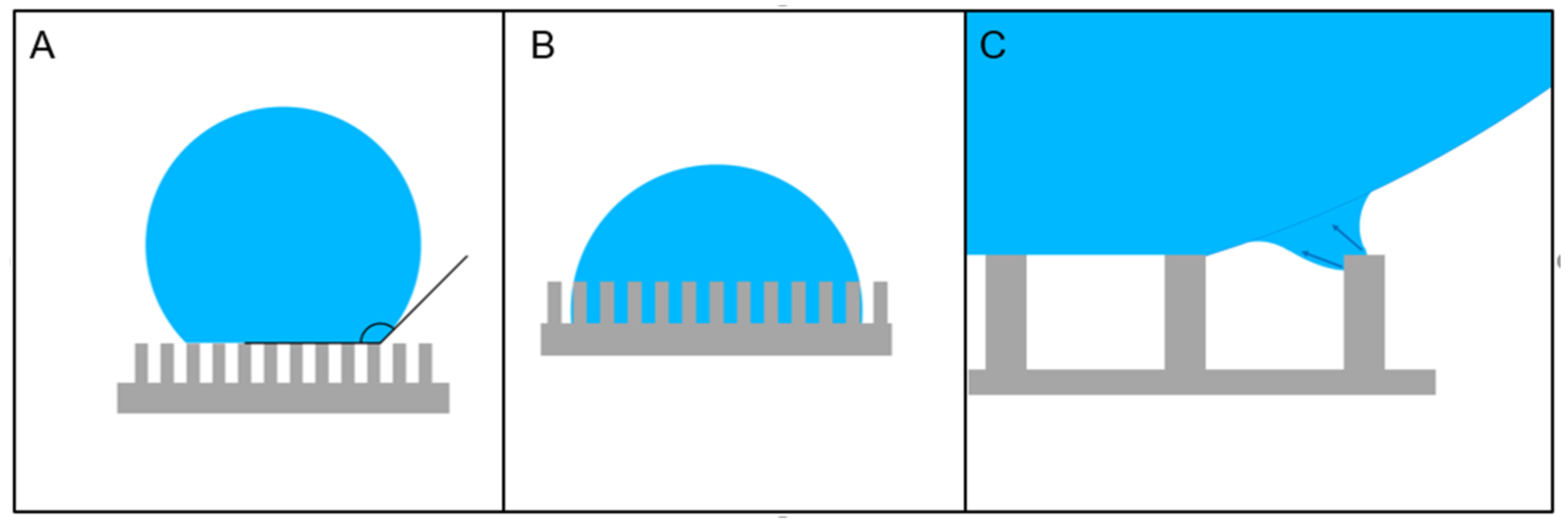
2. Phenomenology of Superhydrophobicity
3. Fabrication Approaches for the Realization of Ordered SHS
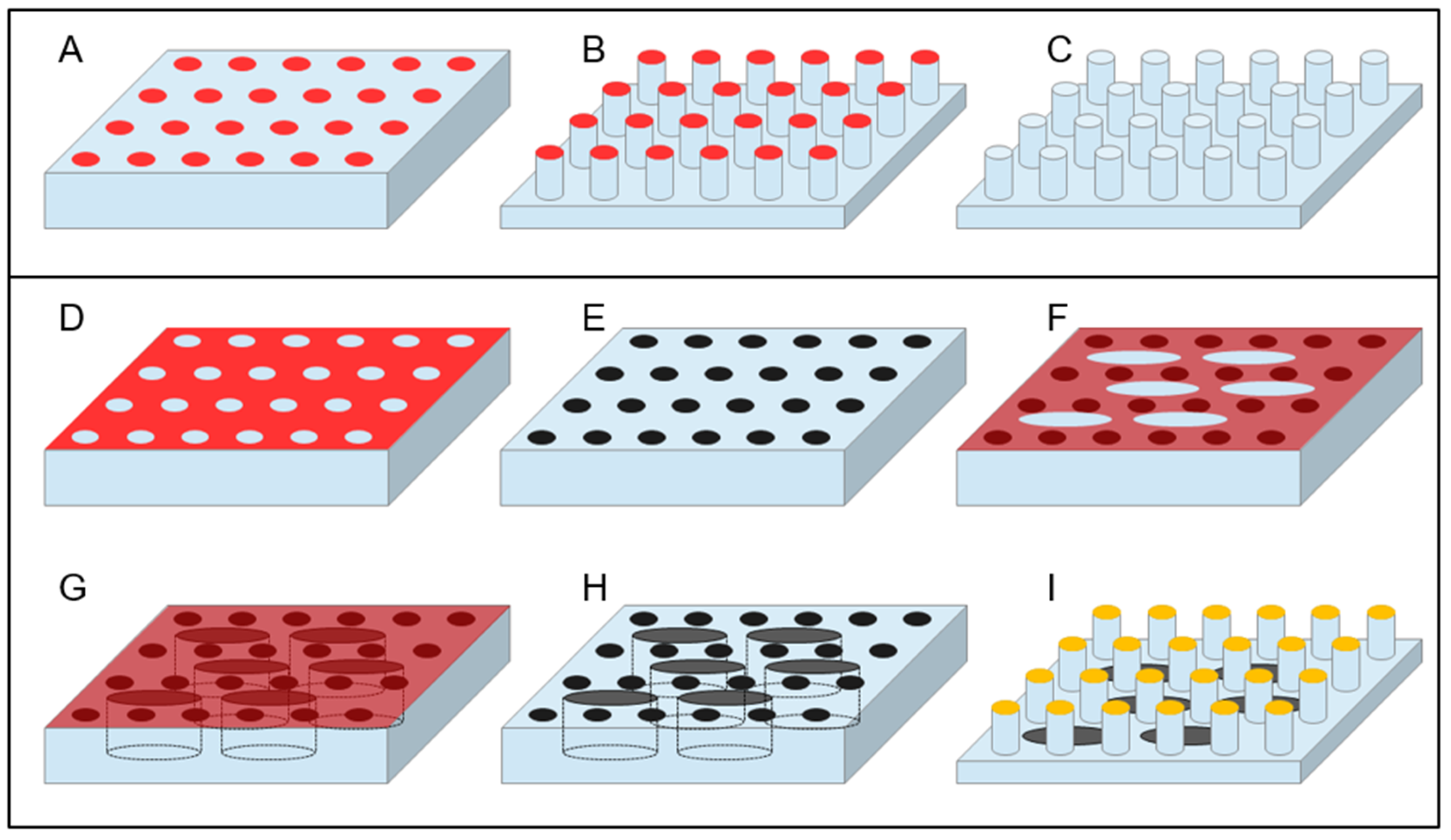
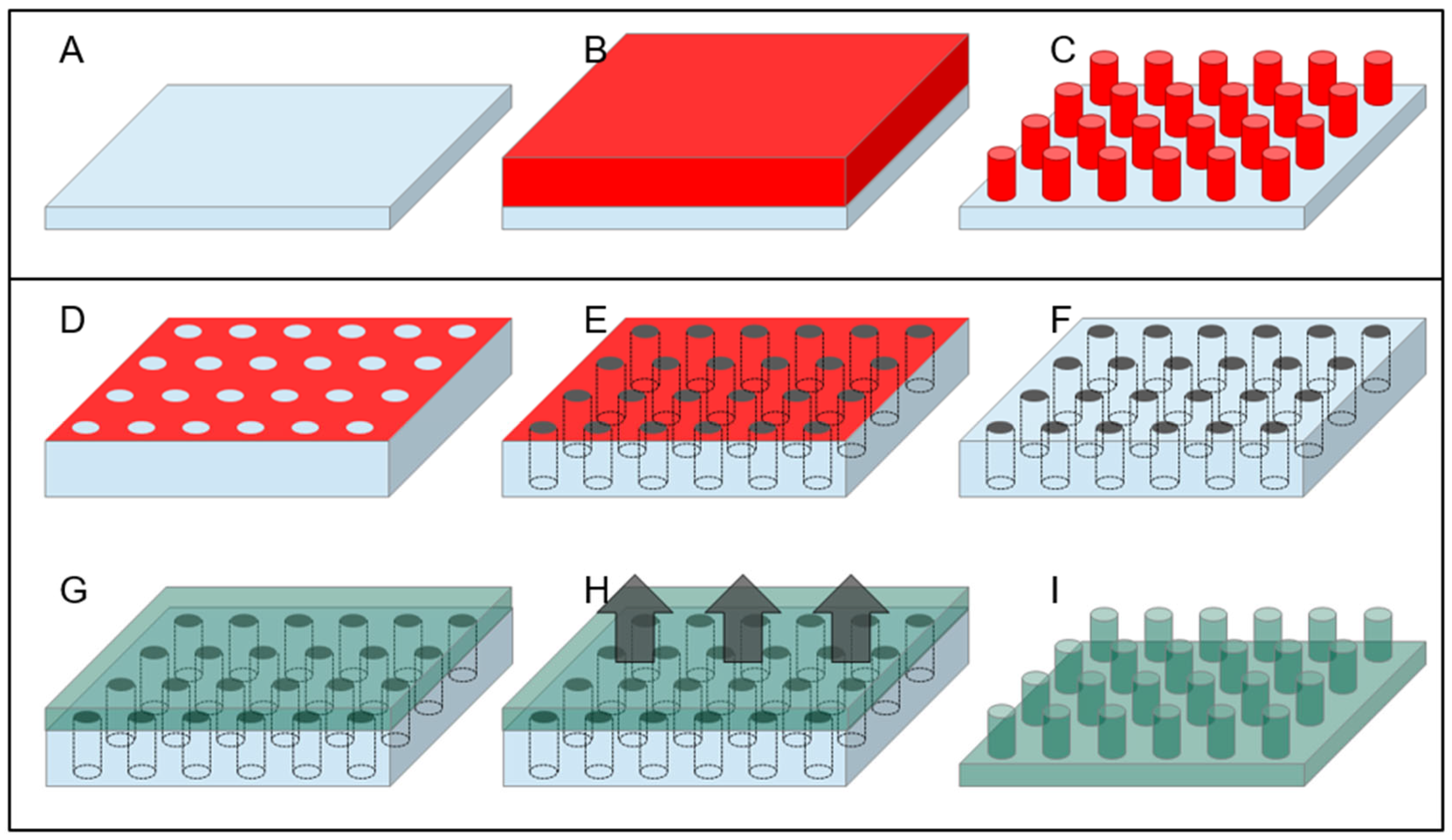
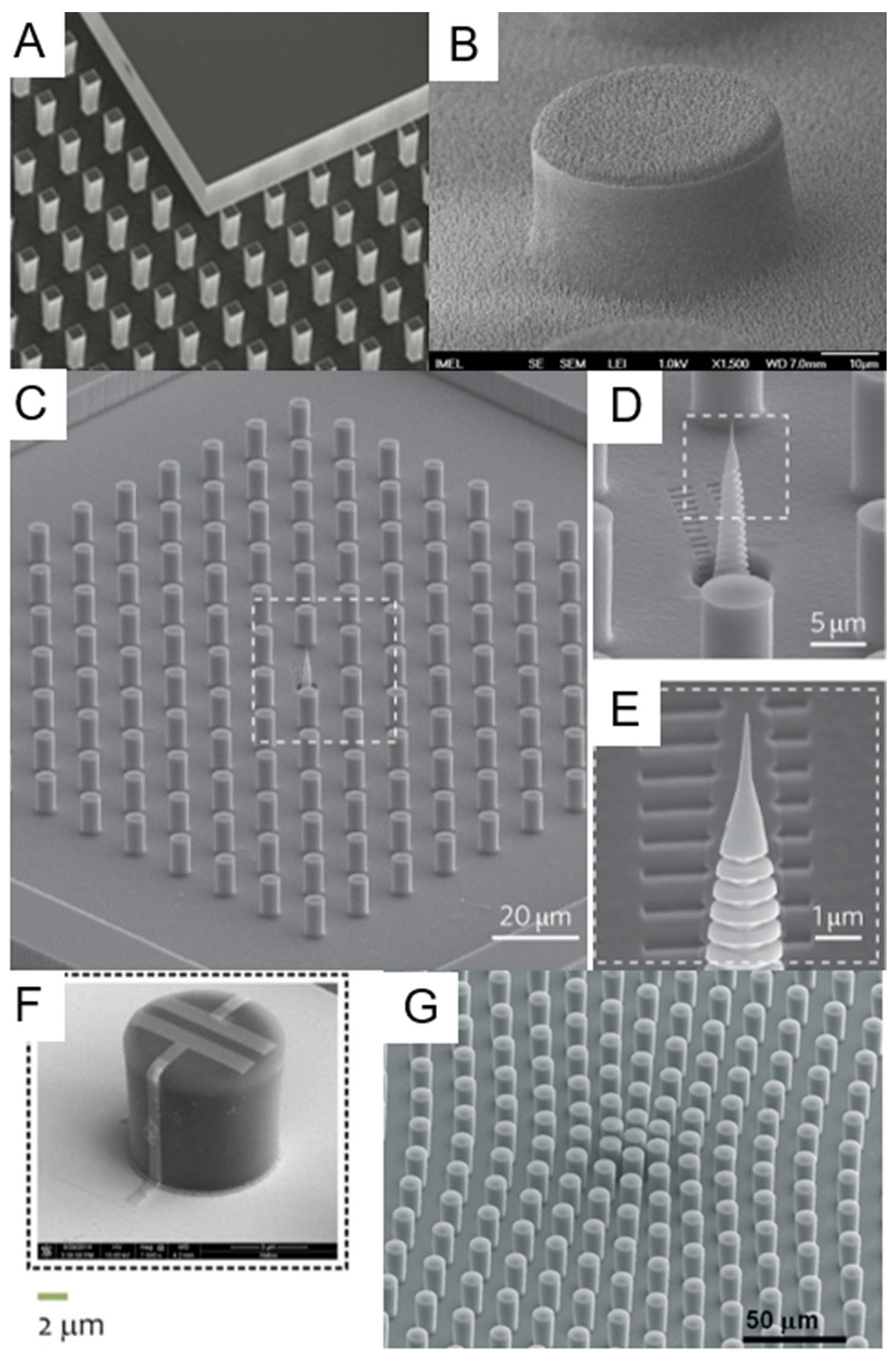
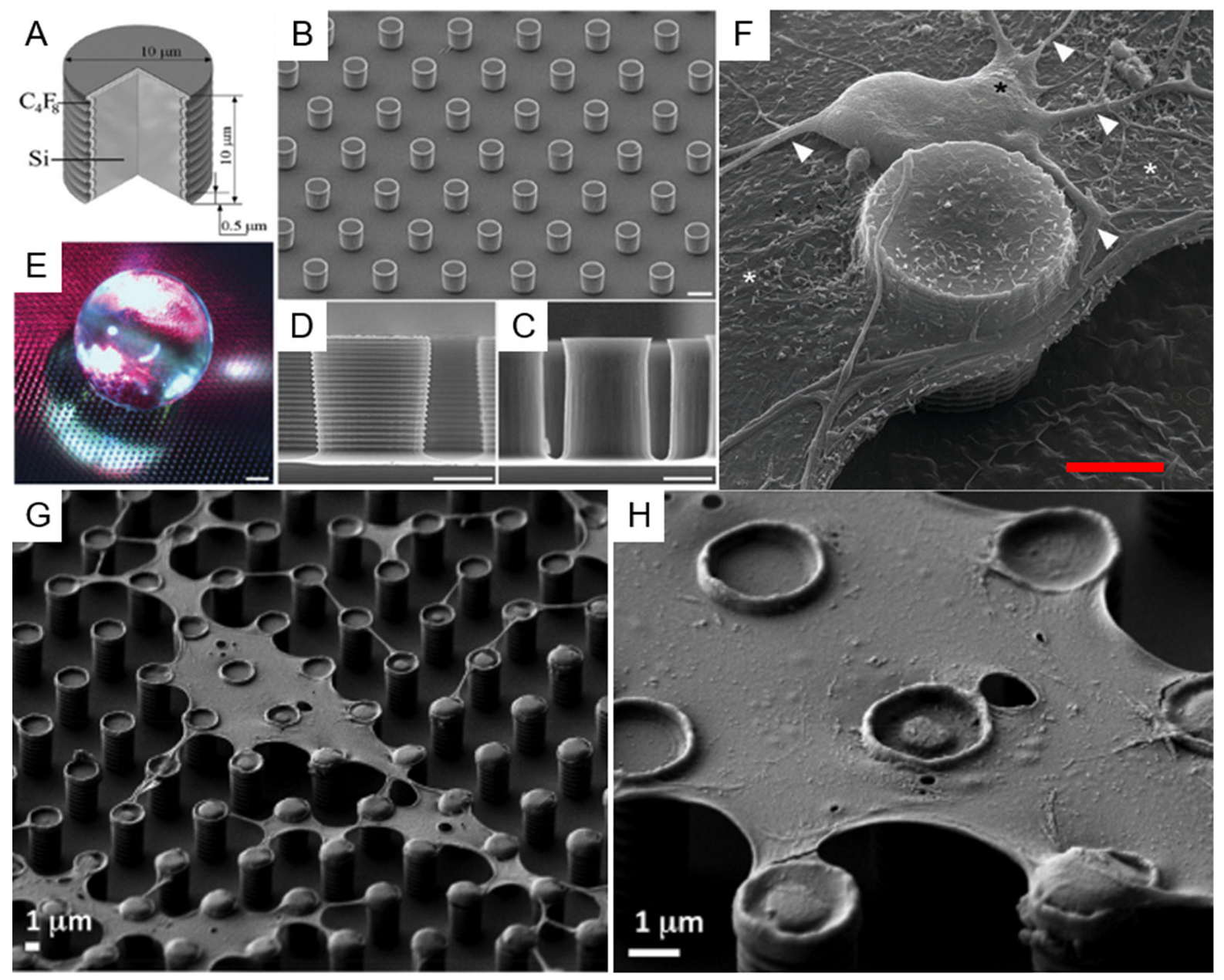
3.1. Top-Down Fabrication Methods
3.2. Bottom-Up Fabrication Methods and Combined Approaches
4. The Hydrophobic Coating of Surfaces
5. SHS for Manipulation of DNA and Proteins
6. SHS for Manipulation of Cells and Cellular Derived Structures
7. Outlook and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Wong, T.S.; Sun, T.; Feng, L.; Aizenberg, J. Interfacial materials with special wettability. MRS Bull. 2013, 38, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; He, J. Substrate-versatile approach to robust antireflective and superhydrophobic coatings with excellent self-cleaning property in varied environments. ACS Appl. Mater. Interfaces 2017, 9, 34367–34376. [Google Scholar] [CrossRef] [PubMed]
- Blossey, R.; Scientifique, C. 2003_Self-cleaning surfaces–virtual realities_Nature. Nat. Publ. Gr. 2003, 2, 301–306. [Google Scholar]
- Ghasemlou, M.; Le, P.H.; Daver, F.; Murdoch, B.J.; Ivanova, E.P.; Adhikari, B. Robust and Eco-Friendly Superhydrophobic Starch Nanohybrid Materials with Engineered Lotus Leaf Mimetic Multiscale Hierarchical Structures. ACS Appl. Mater. Interfaces 2021, 13, 36558–36573. [Google Scholar] [CrossRef]
- Mi, Y.; Liu, X.; Gao, Z.; Wang, M.; Shi, L.; Zhang, X.; Gao, K.; Mugisha, E.R.; Yan, W. 3D Photovoltaic Router of Water Microdroplets Aiming at Free-Space Microfluidic Transportation. ACS Appl. Mater. Interfaces 2021, 13, 45018–45032. [Google Scholar] [CrossRef]
- Lv, X.S.; Qin, Y.; Liang, H.; Zhao, B.; He, Y.; Cui, X. A facile method for constructing a superhydrophobic zinc coating on a steel surface with anti-corrosion and drag-reduction properties. Appl. Surf. Sci. 2021, 562, 150192. [Google Scholar] [CrossRef]
- Zhao, X.; Soper, S.A.; Murphy, M.C. A high-adhesion binding strategy for silica nanoparticle-based superhydrophobic coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126810. [Google Scholar] [CrossRef]
- Das, A.; Dhar, M.; Manna, U. Small molecules derived Tailored-Superhydrophobicity on fibrous and porous Substrates—With superior tolerance. Chem. Eng. J. 2021, 430, 132597. [Google Scholar] [CrossRef]
- Fihri, A.; Bovero, E.; Al-Shahrani, A.; Al-Ghamdi, A.; Alabedi, G. Recent progress in superhydrophobic coatings used for steel protection: A review. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 378–390. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xia, H.; Kim, E.; Sun, H.B. Recent developments in superhydrophobic surfaces with unique structural and functional properties. Soft Matter 2012, 8, 11217–11231. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Britto, J.G.; Durairaj, R.B.; Mageshwaran, G. Superhydrophobic surfaces: A review on fundamentals, applications, and challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Liravi, M.; Pakzad, H.; Moosavi, A.; Nouri-Borujerdi, A. A comprehensive review on recent advances in superhydrophobic surfaces and their applications for drag reduction. Prog. Org. Coat. 2020, 140, 105537. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Celia, E.; Darmanin, T.; Taffin de Givenchy, E.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef]
- Gogolides, E.; Ellinas, K.; Tserepi, A. Hierarchical micro and nano structured, hydrophilic, superhydrophobic and superoleophobic surfaces incorporated in microfluidics, microarrays and lab on chip microsystems. Microelectron. Eng. 2015, 132, 135–155. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic Coatings on Cellulose-Based Materials: Fabrication, Properties, and Applications. Adv. Mater. Interfaces 2014, 1, 1–20. [Google Scholar] [CrossRef]
- Khanmohammadi, C.K.; Sohrabi, B.; Rahmanzadeh, A. Superhydrophobicity: Advanced biological and biomedical applications. Biomater. Sci. 2019, 7, 3110–3137. [Google Scholar] [CrossRef]
- Lima, A.C.; Mano, J.F. Micro/nano-structured superhydrophobic surfaces in the biomedical field: Part II: Applications overview. Nanomedicine 2015, 10, 271–297. [Google Scholar] [CrossRef]
- Lima, A.C.; Mano, J.F. Micro-/nano-structured superhydrophobic surfaces in the biomedical field: Part I: Basic concepts and biomimetic approaches. Nanomedicine 2015, 10, 103–119. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.D.; Yang, B. Patterned surfaces for biological applications: A new platform using two dimensional structures as biomaterials. Chin. Chem. Lett. 2017, 28, 675–690. [Google Scholar] [CrossRef]
- Celik, N.; Sahin, F.; Ruzi, M.; Yay, M.; Unal, E.; Onses, M.S. Blood repellent superhydrophobic surfaces constructed from nanoparticle-free and biocompatible materials. Colloids Surf. B Biointerfaces 2021, 205, 111864. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.; Zhang, A.; Jin, S.; Liang, X.; Tang, Z.; Liu, X.; Chen, H. Vascular cell behavior on heparin-like polymers modified silicone surfaces: The prominent role of the lotus leaf-like topography. J. Colloid Interface Sci. 2021, 603, 501–510. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, Y.; Lou, X.; Xia, F. Bioinspired superwetting surfaces for biosensing. View 2021, 2, 20200053. [Google Scholar] [CrossRef]
- Kumar, R.; Sahani, A.K. Role of superhydrophobic coatings in biomedical applications. Mater. Today Proc. 2021, 45, 5655–5659. [Google Scholar] [CrossRef]
- Lim, J.I.; Kim, S.I.; Jung, Y.; Kim, S.H. Fabrication and medical applications of lotus-leaf-like structured superhydrophobic surfaces. Polym. Korea 2013, 37, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.; Seo, J.; Han, H.; Kang, S.; Kim, H.; Lee, T. Bio-inspired extreme wetting surfaces for biomedical applications. Materials (Basel) 2016, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Ciasca, G.; Papi, M.; Businaro, L.; Campi, G.; Ortolani, M.; Palmieri, V.; Cedola, A.; De Ninno, A.; Gerardino, A.; Maulucci, G.; et al. Recent advances in superhydrophobic surfaces and their relevance to biology and medicine. Bioinspiration Biomim. 2016, 11, 011001. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Pedraza, F.; Mahadik, S.S.; Relekar, B.P.; Thorat, S.S. Biocompatible superhydrophobic coating material for biomedical applications. J. Sol-Gel Sci. Technol. 2017, 81, 791–796. [Google Scholar] [CrossRef]
- Kefallinou, D.; Ellinas, K.; Speliotis, T.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Optimization of antibacterial properties of “hybrid” metal-sputtered superhydrophobic surfaces. Coatings 2020, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Regenerable superhydrophobic coatings for biomedical fabrics. Coatings 2020, 10, 578. [Google Scholar] [CrossRef]
- Gayani, B.; Dilhari, A.; Kottegoda, N.; Ratnaweera, D.R.; Weerasekera, M.M. Reduced Crystalline Biofilm Formation on Superhydrophobic Silicone Urinary Catheter Materials. ACS Omega 2021, 6, 11488–11496. [Google Scholar] [CrossRef] [PubMed]
- Geyer, F.; D’Acunzi, M.; Yang, C.Y.; Müller, M.; Baumli, P.; Kaltbeitzel, A.; Mailänder, V.; Encinas, N.; Vollmer, D.; Butt, H.J. How to Coat the Inside of Narrow and Long Tubes with a Super-Liquid-Repellent Layer—A Promising Candidate for Antibacterial Catheters. Adv. Mater. 2019, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kasapgil, E.; Badv, M.; Cantú, C.A.; Rahmani, S.; Erbil, H.Y.; Anac, S.I.; Weitz, J.I.; Hosseini-Doust, Z.; Didar, T.F. Polysiloxane Nanofilaments Infused with Silicone Oil Prevent Bacterial Adhesion and Suppress Thrombosis on Intranasal Splints. ACS Biomater. Sci. Eng. 2021, 7, 541–552. [Google Scholar] [CrossRef]
- Parbat, D.; Bhunia, B.K.; Mandal, B.B.; Manna, U. Bio-inspired Underwater Super-Oil-Wettability for Controlling Platelet Adhesion. Chem. Asian J. 2021, 16, 1081–1085. [Google Scholar] [CrossRef]
- Salimi, E. Superhydrophobic blood-compatible surfaces: State of the art. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 363–372. [Google Scholar] [CrossRef]
- Montgomerie, Z.; Popat, K.C. Improved hemocompatibility and reduced bacterial adhesion on superhydrophobic titania nanoflower surfaces. Mater. Sci. Eng. C 2021, 119, 111503. [Google Scholar] [CrossRef]
- Wu, X.H.; Liew, Y.K.; Mai, C.W.; Then, Y.Y. Potential of superhydrophobic surface for blood-contacting medical devices. Int. J. Mol. Sci. 2021, 22, 3341. [Google Scholar] [CrossRef]
- Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for tunable drug release: Using displacement of air to control delivery rates. J. Am. Chem. Soc. 2012, 134, 2016–2019. [Google Scholar] [CrossRef] [Green Version]
- Falde, E.J.; Freedman, J.D.; Herrera, V.L.M.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Layered superhydrophobic meshes for controlled drug release. J. Control. Release 2015, 214, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Song, B.; Zhu, J. Bioinspired Design of Chitosan Spheres for Controlled Drug Delivery. J. Nanosci. Nanotechnol. 2017, 17, 3873–3879. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.; Kim, S.H.; Kim, E.; Jang, J.H. Superhydrophobic, Reversibly Elastic, Moldable, and Electrospun (SupREME) Fibers with Multimodal Functions: From Oil Absorbents to Local Drug Delivery Adjuvants. Adv. Funct. Mater. 2017, 27, 1–12. [Google Scholar] [CrossRef]
- Nor, Y.A.; Niu, Y.; Karmakar, S.; Zhou, L.; Xu, C.; Zhang, J.; Zhang, H.; Yu, M.; Mahony, D.; Mitter, N.; et al. Shaping nanoparticles with hydrophilic compositions and hydrophobic properties as nanocarriers for antibiotic delivery. ACS Cent. Sci. 2015, 1, 328–334. [Google Scholar] [CrossRef]
- Costa, A.M.S.; Alatorre-Meda, M.; Alvarez-Lorenzo, C.; Mano, J.F. Superhydrophobic Surfaces as a Tool for the Fabrication of Hierarchical Spherical Polymeric Carriers. Small 2015, 11, 3648–3652. [Google Scholar] [CrossRef]
- Lima, A.C.; Song, W.; Blanco-Fernandez, B.; Alvarez-Lorenzo, C.; Mano, J.F. Synthesis of temperature-responsive Dextran-MA/PNIPAAm particles for controlled drug delivery using superhydrophobic surfaces. Pharm. Res. 2011, 28, 1294–1305. [Google Scholar] [CrossRef] [Green Version]
- De Gennes, P.G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827–863. [Google Scholar] [CrossRef]
- Rafiee, J.; Mi, X.; Gullapalli, H.; Thomas, A.V.; Yavari, F.; Shi, Y.; Ajayan, P.M.; Koratkar, N.A. Wetting transparency of graphene. Nat. Mater. 2012, 11, 217–222. [Google Scholar] [CrossRef]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, M.; Ueda, Y. The lowest surface free energy based on -CF3 alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Quére, D.; Lafuma, A.; Bico, J. Slippy and sticky microtextured solids. Nanotechnology 2003, 14, 1109–1112. [Google Scholar] [CrossRef]
- Bartolo, D.; Bouamrirene, F.; Verneuil, É.; Buguin, A.; Silberzan, P.; Moulinet, S. Bouncing or sticky droplets: Impalement transitions on superhydrophobic micropatterned surfaces. Europhys. Lett. 2006, 74, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Moulinet, S.; Bartolo, D. Life and death of a fakir droplet: Impalement transitions on superhydrophobic surfaces. Eur. Phys. J. E 2007, 24, 251–260. [Google Scholar] [CrossRef]
- Reyssat, M.; Yeomans, J.M.; Quéré, D. Impalement of fakir drops. EPL 2008, 81, 1–6. [Google Scholar] [CrossRef]
- Limongi, T.; Tirinato, L.; Pagliari, F.; Giugni, A.; Allione, M.; Perozziello, G.; Candeloro, P.; Di Fabrizio, E. Fabrication and Applications of Micro/Nanostructured Devices for Tissue Engineering. Nano-Micro Lett. 2017, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rahmawan, Y.; Xu, L.; Yang, S. Self-assembly of nanostructures towards transparent, superhydrophobic surfaces. J. Mater. Chem. A 2013, 1, 2955–2969. [Google Scholar] [CrossRef]
- Yeom, J.; Wu, Y.; Selby, J.C.; Shannon, M.A. Maximum achievable aspect ratio in deep reactive ion etching of silicon due to aspect ratio dependent transport and the microloading effect. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2005, 23, 2319. [Google Scholar] [CrossRef]
- Sökmen, U.; Stranz, A.; Fündling, S.; Wehmann, H.H.; Bandalo, V.; Bora, A.; Tornow, M.; Waag, A.; Peiner, E. Capabilities of ICP-RIE cryogenic dry etching of silicon: Review of exemplary microstructures. J. Micromech. Microeng. 2009, 19, 105005. [Google Scholar] [CrossRef]
- Jansen, H.V.; De Boer, M.J.; Unnikrishnan, S.; Louwerse, M.C.; Elwenspoek, M.C. Black silicon method X: A review on high speed and selective plasma etching of silicon with profile control: An in-depth comparison between Bosch and cryostat DRIE processes as a roadmap to next generation equipment. J. Micromech. Microeng. 2009, 19, 33001. [Google Scholar] [CrossRef]
- Zeniou, A.; Ellinas, K.; Olziersky, A.; Gogolides, E. Ultra-high aspect ratio Si nanowires fabricated with plasma etching: Plasma processing, mechanical stability analysis against adhesion and capillary forces and oleophobicity. Nanotechnology 2014, 25, 35302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Park, K.C.; Law, K.Y. Effect of surface texturing on superoleophobicity, contact angle hysteresis, and “robustness. ” Langmuir 2012, 28, 14925–14934. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Falqui, A.; Moretti, M.; Limongi, T.; Allione, M.; Genovese, A.; Lopatin, S.; Tirinato, L.; Das, G.; Torre, B.; et al. The structure of DNA by direct imaging. Sci. Adv. 2015, 1, e1500734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limongi, T.; Cesca, F.; Gentile, F.; Marotta, R.; Ruffilli, R.; Barberis, A.; Dal Maschio, M.; Petrini, E.M.; Santoriello, S.; Benfenati, F.; et al. Nanostructured superhydrophobic substrates trigger the development of 3D neuronal networks. Small 2013, 9, 402–412. [Google Scholar] [CrossRef]
- Gentile, F.; Coluccio, M.L.; Zaccaria, R.P.; Francardi, M.; Cojoc, G.; Perozziello, G.; Raimondo, R.; Candeloro, P.; Di Fabrizio, E. Selective on site separation and detection of molecules in diluted solutions with super-hydrophobic clusters of plasmonic nanoparticles. Nanoscale 2014, 6, 8208–8225. [Google Scholar] [CrossRef]
- De Angelis, F.; Gentile, F.; Mecarini, F.; Das, G.; Moretti, M.; Candeloro, P.; Coluccio, M.L.; Cojoc, G.; Accardo, A.; Liberale, C.; et al. Breaking the diffusion limit with super-hydrophobic delivery of molecules to plasmonic nanofocusing SERS structures. Nat. Photonics 2011, 5, 682–687. [Google Scholar] [CrossRef]
- Malara, N.; Gentile, F.; Coppedè, N.; Coluccio, M.L.; Candeloro, P.; Perozziello, G.; Ferrara, L.; Giannetto, M.; Careri, M.; Castellini, A.; et al. Superhydrophobic lab-on-chip measures secretome protonation state and provides a personalized risk assessment of sporadic tumour. NPJ Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Coppedè, N.; Ferrara, L.; Bifulco, P.; Villani, M.; Iannotta, S.; Zappettini, A.; Cesarelli, M.; Di Fabrizio, E.; Gentile, F. Multiscale modification of the conductive PEDOT:PSS polymer for the analysis of biological mixtures in a super-hydrophobic drop. Microelectron. Eng. 2016, 158, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Gentile, F.; Moretti, M.; Limongi, T.; Falqui, A.; Bertoni, G.; Scarpellini, A.; Santoriello, S.; Maragliano, L.; Proietti Zaccaria, R.; Di Fabrizio, E. Direct imaging of DNA fibers: The visage of double helix. Nano Lett. 2012, 12, 6453–6458. [Google Scholar] [CrossRef]
- Marini, M.; Allione, M.; Lopatin, S.; Moretti, M.; Giugni, A.; Torre, B.; di Fabrizio, E. Suspended DNA structural characterization by TEM diffraction. Microelectron. Eng. 2018, 187–188, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Accardo, A.; Gentile, F.; Mecarini, F.; De Angelis, F.; Burghammer, M.; Di Fabrizio, E.; Riekel, C. In situ X-ray scattering studies of protein solution droplets drying on micro-and nanopatterned superhydrophobic PMMA surfaces. Langmuir 2010, 26, 15057–15064. [Google Scholar] [CrossRef]
- Accardo, A.; Gentile, F.; Mecarini, F.; De Angelis, F.; Burghammer, M.; Di Fabrizio, E.; Riekel, C. Ultrahydrophobic PMMA micro- and nano-textured surfaces fabricated by optical lithography and plasma etching for X-ray diffraction studies. Microelectron. Eng. 2011, 88, 1660–1663. [Google Scholar] [CrossRef]
- Marinaro, G.; Accardo, A.; De Angelis, F.; Dane, T.; Weinhausen, B.; Burghammer, M.; Riekel, C. A superhydrophobic chip based on SU-8 photoresist pillars suspended on a silicon nitride membrane. Lab Chip 2014, 14, 3705–3709. [Google Scholar] [CrossRef] [Green Version]
- Tropmann, A.; Tanguy, L.; Koltay, P.; Zengerle, R.; Riegger, L. Completely superhydrophobic PDMS surfaces for microfluidics. Langmuir 2012, 28, 8292–8295. [Google Scholar] [CrossRef]
- Draper, M.C.; Crick, C.R.; Orlickaite, V.; Turek, V.A.; Parkin, I.P.; Edel, J.B. Superhydrophobic surfaces as an on-chip microfluidic toolkit for total droplet control. Anal. Chem. 2013, 85, 5405–5410. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Ge, Q.; Yang, L.L.; Shi, X.J.; Li, J.J.; Yang, D.Q.; Sacher, E. Durable superhydrophobic PTFE films through the introduction of micro- And nanostructured pores. Appl. Surf. Sci. 2015, 339, 151–157. [Google Scholar] [CrossRef]
- Zhan, Y.L.; Ruan, M.; Li, W.; Li, H.; Hu, L.Y.; Ma, F.M.; Yu, Z.L.; Feng, W. Fabrication of anisotropic PTFE superhydrophobic surfaces using laser microprocessing and their self-cleaning and anti-icing behavior. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 8–15. [Google Scholar] [CrossRef]
- Sreekantan, S.; Hassan, M.; Murthe, S.S.; Seeni, A. Biocompatibility and cytotoxicity study of polydimethylsiloxane (Pdms) and palm oil fuel ash (pofa) sustainable super-hydrophobic coating for biomedical applications. Polymers 2020, 12, 3034. [Google Scholar] [CrossRef]
- Hwang, S.; Chen, X.; Zhou, G.; Su, D. In Situ Transmission Electron Microscopy on Energy-Related Catalysis. Adv. Energy Mater. 2020, 10, 1–24. [Google Scholar] [CrossRef]
- Stricker, E.A.; Ke, X.; Wainright, J.S.; Unocic, R.R.; Savinell, R.F. Current Density Distribution in Electrochemical Cells with Small Cell Heights and Coplanar Thin Electrodes as Used in ec-S/TEM Cell Geometries. J. Electrochem. Soc. 2019, 166, H126–H134. [Google Scholar] [CrossRef]
- Gogolides, E.; Constantoudis, V.; Kokkoris, G.; Kontziampasis, D.; Tsougeni, K.; Boulousis, G.; Vlachopoulou, M.; Tserepi, A. Controlling roughness: From etching to nanotexturing and plasma-directed organization on organic and inorganic materials. J. Phys. D. Appl. Phys. 2011, 44, 174021. [Google Scholar] [CrossRef] [Green Version]
- Kokkoris, G.; Constantoudis, V.; Angelikopoulos, P.; Boulousis, G.; Gogolides, E. Dual nanoscale roughness on plasma-etched Si surfaces: Role of etch inhibitors. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 1–4. [Google Scholar] [CrossRef]
- Shieh, J.; Hou, F.J.; Chen, Y.C.; Chen, H.M.; Yang, S.P.; Cheng, C.C.; Chen, H.L. Robust airlike superhydrophobic surfaces. Adv. Mater. 2010, 22, 597–601. [Google Scholar] [CrossRef]
- Liu, X.; Coxon, P.R.; Peters, M.; Hoex, B.; Cole, J.M.; Fray, D.J. Black silicon: Fabrication methods, properties and solar energy applications. Energy Environ. Sci. 2014, 7, 3223–3263. [Google Scholar] [CrossRef] [Green Version]
- Otto, M.; Algasinger, M.; Branz, H.; Gesemann, B.; Gimpel, T.; Füchsel, K.; Käsebier, T.; Kontermann, S.; Koynov, S.; Li, X.; et al. Black silicon photovoltaics. Adv. Opt. Mater. 2015, 3, 147–164. [Google Scholar] [CrossRef]
- Tan, Q.; Lu, F.; Xue, C.; Zhang, W.; Lin, L.; Xiong, J. Nano-fabrication methods and novel applications of black silicon. Sens. Actuators A Phys. 2019, 295, 560–573. [Google Scholar] [CrossRef]
- Marquez-Velasco, J.; Vlachopoulou, M.E.; Tserepi, A.; Gogolides, E. Stable superhydrophobic surfaces induced by dual-scale topography on SU-8. Microelectron. Eng. 2010, 87, 782–785. [Google Scholar] [CrossRef]
- Vourdas, N.; Tserepi, A.; Gogolides, E. Nanotextured super-hydrophobic transparent poly(methyl methacrylate) surfaces using high-density plasma processing. Nanotechnology 2007, 18, 125304. [Google Scholar] [CrossRef]
- Salapare, H.S.; Guittard, F.; Noblin, X.; Taffin de Givenchy, E.; Celestini, F.; Ramos, H.J. Stability of the hydrophilic and superhydrophobic properties of oxygen plasma-treated poly(tetrafluoroethylene) surfaces. J. Colloid Interface Sci. 2013, 396, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.M.; Ribeiro, C.; Botelho, G.; Borges, J.; Lopes, C.; Vaz, F.; Carabineiro, S.A.C.; MacHado, A.V.; Lanceros-Méndez, S. Superhydrophilic poly(l-lactic acid) electrospun membranes for biomedical applications obtained by argon and oxygen plasma treatment. Appl. Surf. Sci. 2016, 371, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Tsougeni, K.; Tserepi, A.; Boulousis, G.; Constantoudis, V.; Gogolides, E. Control of nanotexture and wetting properties of polydimethylsiloxane from very hydrophobic to super-hydrophobic by plasma processing. Plasma Process. Polym. 2007, 4, 398–405. [Google Scholar] [CrossRef]
- Chen, M.H.; Hsu, T.H.; Chuang, Y.J.; Tseng, F.G. Dual hierarchical biomimic superhydrophobic surface with three energy states. Appl. Phys. Lett. 2009, 95, 1–4. [Google Scholar] [CrossRef]
- Chen, M.H.; Chuang, Y.J.; Tseng, F.G. Self-masked high-aspect-ratio polymer nanopillars. Nanotechnology 2008, 19, 505301. [Google Scholar] [CrossRef]
- Vourdas, N.; Kontziampasis, D.; Kokkoris, G.; Constantoudis, V.; Goodyear, A.; Tserepi, A.; Cooke, M.; Gogolides, E. Plasma directed assembly and organization: Bottom-up nanopatterning using top-down technology. Nanotechnology 2010, 21, 85302. [Google Scholar] [CrossRef]
- Arukalam, I.O.; Oguzie, E.E.; Li, Y. Fabrication of FDTS-modified PDMS-ZnO nanocomposite hydrophobic coating with anti-fouling capability for corrosion protection of Q235 steel. J. Colloid Interface Sci. 2016, 484, 220–228. [Google Scholar] [CrossRef]
- Maboudian, R.; Ashurst, W.R.; Carraro, C. Self-assembled monolayers as anti-stiction coatings for MEMS: Characteristics and recent developments. Sens. Actuators A Phys. 2000, 82, 219–223. [Google Scholar] [CrossRef]
- Pan, Z.; Shahsavan, H.; Zhang, W.; Yang, F.K.; Zhao, B. Superhydro-oleophobic bio-inspired polydimethylsiloxane micropillared surface via FDTS coating/blending approaches. Appl. Surf. Sci. 2015, 324, 612–620. [Google Scholar] [CrossRef]
- Ellinas, K.; Pujari, S.P.; Dragatogiannis, D.A.; Charitidis, C.A.; Tserepi, A.; Zuilhof, H.; Gogolides, E. Plasma micro-nanotextured, scratch, water and hexadecane resistant, superhydrophobic, and superamphiphobic polymeric surfaces with perfluorinated monolayers. ACS Appl. Mater. Interfaces 2014, 6, 6510–6524. [Google Scholar] [CrossRef]
- Xiu, Y.; Hess, D.W.; Wong, C.P. A novel method to prepare superhydrophobic, self-cleaning and transparent coatings for biomedical applications. In Proceedings of the 2007 Proceedings 57th Electronic Components and Technology Conference, Sparks, NV, USA, 29 May–1 June 2007; pp. 1218–1223. [Google Scholar]
- Wang, E.N.; Bucaro, M.A.; Taylor, J.A.; Kolodner, P.; Aizenberg, J.; Krupenkin, T. Droplet mixing using electrically tunable superhydrophobic nanostructured surfaces. Microfluid. Nanofluidics 2009, 7, 137–140. [Google Scholar] [CrossRef]
- Pan, Z.; Dash, S.; Weibel, J.A.; Garimella, S.V. Assessment of water droplet evaporation mechanisms on hydrophobic and superhydrophobic substrates. Langmuir 2013, 29, 15831–15841. [Google Scholar] [CrossRef]
- Yin, Q.; Sun, F.; Wang, X.; Gao, S.; Zhang, S.; Qi, J.; Wang, K.; Qi, D. Suppressing the Ring Stain Effect with Superhydrophilic/Superhydrophobic Patterned Surfaces. ACS Omega 2020, 5, 11235–11240. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Kim, C.J. Droplet evaporation of pure water and protein solution on nanostructured superhydrophobic surfaces of varying heights. Langmuir 2009, 25, 7561–7567. [Google Scholar] [CrossRef] [PubMed]
- Moradi Mehr, S.; Businaro, L.; Habibi, M.; Moradi, A.R. Collective behavior of evaporating droplets on superhydrophobic surfaces. AIChE J. 2020, 66, 1–6. [Google Scholar] [CrossRef]
- Gentile, F.; Das, G.; Coluccio, M.L.; Mecarini, F.; Accardo, A.; Tirinato, L.; Tallerico, R.; Cojoc, G.; Liberale, C.; Candeloro, P.; et al. Ultra low concentrated molecular detection using super hydrophobic surface based biophotonic devices. Microelectron. Eng. 2010, 87, 798–801. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Campion, A.; Kambhampati, P.; Campion, A.; Harris, C. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Surface-enhanced Raman scattering and biophysics. J. Phys. Condens. Matter 2002, 14, R597. [Google Scholar] [CrossRef] [Green Version]
- Battista, E.; Coluccio, M.L.; Alabastri, A.; Barberio, M.; Causa, F.; Netti, P.A.; Di Fabrizio, E.; Gentile, F. Metal enhanced fluorescence on super-hydrophobic clusters of gold nanoparticles. Microelectron. Eng. 2017, 175, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Coluccio, M.L.; Gentile, F.; Francardi, M.; Perozziello, G.; Malara, N.; Candeloro, P.; Di Fabrizio, E. Electroless deposition and nanolithography can control the formation of materials at the nano-scale for plasmonic applications. Sensors 2014, 14, 6056–6083. [Google Scholar] [CrossRef] [Green Version]
- Gentile, F.; Coluccio, M.L.; Accardo, A.; Asande, M.; Cojoc, G.; Mecarini, F.; Das, G.; Liberale, C.; De Angelis, F.; Candeloro, P.; et al. Nanoporous-micropatterned-superhydrophobic surfaces as harvesting agents for few low molecular weight molecules. Microelectron. Eng. 2011, 88, 1749–1752. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Dak, P.; Salm, E.; Dash, S.; Garimella, S.V.; Bashir, R.; Alam, M.A. Nanotextured superhydrophobic electrodes enable detection of attomolar-scale DNA concentration within a droplet by non-faradaic impedance spectroscopy. Lab Chip 2013, 13, 4248–4256. [Google Scholar] [CrossRef] [Green Version]
- Ciasca, G.; Businaro, L.; De Ninno, A.; Cedola, A.; Notargiacomo, A.; Campi, G.; Papi, M.; Ranieri, A.; Carta, S.; Giovine, E.; et al. Wet sample confinement by superhydrophobic patterned surfaces for combined X-ray fluorescence and X-ray phase contrast imaging. Microelectron. Eng. 2013, 111, 304–309. [Google Scholar] [CrossRef]
- De Ninno, A.; Ciasca, G.; Gerardino, A.; Calandrini, E.; Papi, M.; De Spirito, M.; Nucara, A.; Ortolani, M.; Businaro, L.; Baldassarre, L. An integrated superhydrophobic-plasmonic biosensor for mid-infrared protein detection at the femtomole level. Phys. Chem. Chem. Phys. 2015, 17, 21337–21342. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, Y.; Sun, Y.; Shi, Y.; Wen, Z.; Li, Z. Silver nanoparticles coated zinc oxide nanorods array as superhydrophobic substrate for the amplified SERS effect. J. Phys. Chem. C 2011, 115, 9977–9983. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, N.; You, T.; Zhang, C.; Yin, P. Superhydrophobic “wash free” 3D nanoneedle array for rapid, recyclable and sensitive SERS sensing in real environment. Sens. Actuators B Chem. 2018, 267, 129–135. [Google Scholar] [CrossRef]
- Ren, H.X.; Chen, X.; Huang, X.J.; Im, M.; Kim, D.H.; Lee, J.H.; Yoon, J.B.; Gu, N.; Liu, J.H.; Choi, Y.K. A conventional route to scalable morphology-controlled regular structures and their superhydrophobic/hydrophilic properties for biochips application. Lab Chip 2009, 9, 2140–2144. [Google Scholar] [CrossRef]
- Xu, L.P.; Chen, Y.; Yang, G.; Shi, W.; Dai, B.; Li, G.; Cao, Y.; Wen, Y.; Zhang, X.; Wang, S. Ultratrace DNA Detection Based on the Condensing-Enrichment Effect of Superwettable Microchips. Adv. Mater. 2015, 27, 6878–6884. [Google Scholar] [CrossRef]
- Beyazkilic, P.; Saateh, A.; Bayindir, M.; Elbuken, C. Evaporation-Induced Biomolecule Detection on Versatile Superhydrophilic Patterned Surfaces: Glucose and DNA Assay. ACS Omega 2018, 3, 13503–13509. [Google Scholar] [CrossRef]
- Marini, M.; Limongi, T.; Falqui, A.; Genovese, A.; Allione, M.; Moretti, M.; Lopatin, S.; Tirinato, L.; Das, G.; Torre, B.; et al. Imaging and structural studies of DNA-protein complexes and membrane ion channels. Nanoscale 2017, 9, 2768–2777. [Google Scholar] [CrossRef] [Green Version]
- Ciasca, G.; Businaro, L.; Papi, M.; Notargiacomo, A.; Chiarpotto, M.; De Ninno, A.; Palmieri, V.; Carta, S.; Giovine, E.; Gerardino, A.; et al. Self-assembling of large ordered DNA arrays using superhydrophobic patterned surfaces. Nanotechnology 2013, 24, 495302. [Google Scholar] [CrossRef]
- Ciasca, G.; Papi, M.; Palmieri, V.; Chiarpotto, M.; Di Claudio, S.; De Ninno, A.; Giovine, E.; Campi, G.; Gerardino, A.; Businaro, L.; et al. Controlling DNA bundle size and spatial rrangement in selfassembled arrays on superhydrophobic surface. Nano-Micro Lett. 2015, 7, 146–151. [Google Scholar] [CrossRef]
- Ciasca, G.; Papi, M.; Chiarpotto, M.; De Ninno, A.; Giovine, E.; Campi, G.; Gerardino, A.; De Spirito, M.; Businaro, L. Controlling the Cassie-to-Wenzel Transition: An Easy Route towards the Realization of Tridimensional Arrays of Biological Objects. Nano-Micro Lett. 2014, 6, 280–286. [Google Scholar] [CrossRef]
- Marini, M.; Allione, M.; Torre, B.; Moretti, M.; Limongi, T.; Tirinato, L.; Giugni, A.; Das, G.; di Fabrizio, E. Raman on suspended DNA: Novel super-hydrophobic approach for structural studies. Microelectron. Eng. 2017, 175, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Marini, M.; Allione, M.; di Fabrizio, E. DNA aggregation controlled by super-hydrophobic devices: From structural studies to sensing. Mater. Today 2018, 21, 455–456. [Google Scholar] [CrossRef]
- Stassi, S.; Marini, M.; Allione, M.; Lopatin, S.; Marson, D.; Laurini, E.; Pricl, S.; Pirri, C.F.C.F.; Ricciardi, C.; Di Fabrizio, E. Nanomechanical DNA resonators for sensing and structural analysis of DNA-ligand complexes. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borin, D.; Melli, M.; Dal Zilio, S.; Toffoli, V.; Scoles, G.; Toffoli, G.; Lazzarino, M. How to engineer superhydrophobic micromechanical sensors preserving mass resolution. Sens. Actuators B Chem. 2014, 199, 62–69. [Google Scholar] [CrossRef]
- Tardivo, M.; Toffoli, V.; Fracasso, G.; Borin, D.; Dal Zilio, S.; Colusso, A.; Carrato, S.; Scoles, G.; Meneghetti, M.; Colombatti, M.; et al. Parallel optical read-out of micromechanical pillars applied to prostate specific membrane antigen detection. Biosens. Bioelectron. 2015, 72, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Melli, M.; Scoles, G.; Lazzarino, M. Fast detection of biomolecules in diffusion-limited regime using micromechanical pillars. ACS Nano 2011, 5, 7928–7935. [Google Scholar] [CrossRef]
- Gao, A.; Wu, Q.; Wang, D.; Ha, Y.; Chen, Z.; Yang, P. A Superhydrophobic Surface Templated by Protein Self-Assembly and Emerging Application toward Protein Crystallization. Adv. Mater. 2016, 28, 579–587. [Google Scholar] [CrossRef]
- Yang, B.; Adams, D.J.; Marlow, M.; Zelzer, M. Surface-Mediated Supramolecular Self-Assembly of Protein, Peptide, and Nucleoside Derivatives: From Surface Design to the Underlying Mechanism and Tailored Functions. Langmuir 2018, 34, 15109–15125. [Google Scholar] [CrossRef] [Green Version]
- Shiu, J.Y.; Chen, P. Addressable protein patterning via switchable superhydrophobic microarrays. Adv. Funct. Mater. 2007, 17, 2680–2686. [Google Scholar] [CrossRef]
- Renberg, B.; Andersson-Svahn, H.; Hedhammar, M. Mimicking silk spinning in a microchip. Sens. Actuators B Chem. 2014, 195, 404–408. [Google Scholar] [CrossRef]
- Andersson, M.; Jia, Q.; Abella, A.; Lee, X.Y.; Landreh, M.; Purhonen, P.; Hebert, H.; Tenje, M.; Robinson, C.V.; Meng, Q.; et al. Biomimetic spinning of artificial spider silk from a chimeric minispidroin. Nat. Chem. Biol. 2017, 13, 262–264. [Google Scholar] [CrossRef]
- Knight, D.P.; Vollrath, F. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar]
- Gustafsson, L.; Jansson, R.; Hedhammar, M.; van der Wijngaart, W. Structuring of Functional Spider Silk Wires, Coatings, and Sheets by Self-Assembly on Superhydrophobic Pillar Surfaces. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef]
- Chiou, N.R.; Lu, C.; Guan, J.; Lee, L.J.; Epstein, A.J. Growth and alignment of polyaniline nanofibres with superhydrophobic, superhydrophilic and other properties. Nat. Nanotechnol. 2007, 2, 354–357. [Google Scholar] [CrossRef]
- Shirahama, T.; Cohen, A.S. Structure of amyloid fibrils after negative staining and high-resolution electron microscopy. Nature 1965, 206, 737–738. [Google Scholar] [CrossRef]
- Makin, O.S.; Serpell, L.C. Structures for amyloid fibrils. FEBS J. 2005, 272, 5950–5961. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Sambashivan, S.; Nelson, R.; Ivanova, M.I.; Sievers, S.A.; Apostol, M.I.; Thompson, M.J.; Balbirnie, M.; Wiltzius, J.J.W.; McFarlane, H.T.; et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature 2007, 447, 453–457. [Google Scholar] [CrossRef]
- Kurouski, D.; Van Duyne, R.P.; Lednev, I.K. Exploring the structure and formation mechanism of amyloid fibrils by Raman spectroscopy: A review. Analyst 2015, 140, 4967–4980. [Google Scholar] [CrossRef]
- Antzutkin, O.N.; Leapman, R.D.; Balbach, J.J.; Tycko, R. Supramolecular structural constraints on Alzheimer’s β-amyloid fibrils from electron microscopy and solid-state nuclear magnetic resonance. Biochemistry 2002, 41, 15436–15450. [Google Scholar] [CrossRef] [PubMed]
- Adamcik, J.; Mezzenga, R. Study of amyloid fibrils via atomic force microscopy. Curr. Opin. Colloid Interface Sci. 2012, 17, 369–376. [Google Scholar] [CrossRef]
- Goldsbury, C.; Kistler, J.; Aebi, U.; Arvinte, T.; Cooper, G.J.S. Watching amyloid fibrils grow by time-lapse atomic force microscopy. J. Mol. Biol. 1999, 285, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Akhremitchev, B.B. Packing density and structural heterogeneity of insulin amyloid fibrils measured by AFM nanoindentation. Biomacromolecules 2006, 7, 1630–1636. [Google Scholar] [CrossRef]
- Tang, M.; Comellas, G.; Rienstra, C.M. Advanced solid-state NMR approaches for structure determination of membrane proteins and amyloid fibrils. Acc. Chem. Res. 2013, 46, 2080–2088. [Google Scholar] [CrossRef]
- Loquet, A.; El Mammeri, N.; Stanek, J.; Berbon, M.; Bardiaux, B.; Pintacuda, G.; Habenstein, B. 3D structure determination of amyloid fibrils using solid-state NMR spectroscopy. Methods 2018, 138–139, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Petkova, A.T.; Ishii, Y.; Balbach, J.J.; Antzutkin, O.N.; Leapman, R.D.; Delaglio, F.; Tycko, R. A structural model for Alzheimer’s β-amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. USA 2002, 99, 16742–16747. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef] [Green Version]
- Moretti, M.; Allione, M.; Marini, M.; Giugni, A.; Torre, B.; Das, G.; Di Fabrizio, E. Confined laminar flow on a super-hydrophobic surface drives the initial stages of tau protein aggregation. Microelectron. Eng. 2018, 191, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Marinaro, G.; Accardo, A.; Benseny-Cases, N.; Burghammer, M.; Castillo-Michel, H.; Cotte, M.; Dante, S.; De Angelis, F.; Di Cola, E.; Di Fabrizio, E.; et al. Probing droplets with biological colloidal suspensions on smart surfaces by synchrotron radiation micro- and nano-beams. Opt. Lasers Eng. 2016, 76, 57–63. [Google Scholar] [CrossRef]
- Accardo, A.; Shalabaeva, V.; Hesse, B.; Cotte, M.; Krahne, R.; Riekel, C.; Dante, S. Temperature, surface morphology and biochemical cues: A combined approach to influence the molecular conformation of Alpha-synuclein. Microelectron. Eng. 2016, 158, 64–68. [Google Scholar] [CrossRef]
- Accardo, A.; Trevisiol, E.; Cerf, A.; Thibault, C.; Laurell, H.; Buscato, M.; Lenfant, F.; Arnal, J.-F.; Fontaine, C.; Vieu, C. Versatile multicharacterization platform involving tailored superhydrophobic SU-8 micropillars for the investigation of breast cancer estrogen receptor isoforms. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2016, 34, 06K201. [Google Scholar] [CrossRef]
- Zhang, P.; Moretti, M.; Allione, M.; Tian, Y.; Ordonez-Loza, J.; Altamura, D.; Giannini, C.; Torre, B.; Das, G.; Li, E.; et al. A droplet reactor on a super-hydrophobic surface allows control and characterization of amyloid fibril growth. Commun. Biol. 2020, 3, 1–13. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 3676478. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Marinaro, G.; Burghammer, M.; Costa, L.; Dane, T.; De Angelis, F.; Di Fabrizio, E.; Riekel, C. Directed Growth of Virus Nanofilaments on a Superhydrophobic Surface. ACS Appl. Mater. Interfaces 2015, 7, 12373–12379. [Google Scholar] [CrossRef]
- Tirinato, L.; Gentile, F.; Di Mascolo, D.; Coluccio, M.L.; Das, G.; Liberale, C.; Pullano, S.A.; Perozziello, G.; Francardi, M.; Accardo, A.; et al. SERS analysis on exosomes using super-hydrophobic surfaces. Microelectron. Eng. 2012, 97, 337–340. [Google Scholar] [CrossRef]
- Accardo, A.; Fabrizio, D.; Limongi, T.; Di Fabrizio, E.; Limongi, T.; Marinaro, G.; Riekel, C. Probing droplets on superhydrophobic surfaces by synchrotron radiation scattering techniques. J. Synchrotron Radiat. 2014, 21, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Accardo, A.; Mecarini, F.; Leoncini, M.; Brandi, F.; Di Cola, E.; Burghammer, M.; Riekel, C.; Di Fabrizio, E. Fast, active droplet interaction: Coalescence and reactive mixing controlled by electrowetting on a superhydrophobic surface. Lab Chip 2013, 13, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Accardo, A.; Tirinato, L.; Altamura, D.; Sibillano, T.; Giannini, C.; Riekel, C.; Di Fabrizio, E. Superhydrophobic surfaces allow probing of exosome self organization using X-ray scattering. Nanoscale 2013, 5, 2295–2299. [Google Scholar] [CrossRef] [PubMed]
- Malara, N.; Gentile, F.; Ferrara, L.; Villani, M.; Iannotta, S.; Zappettini, A.; Di Fabrizio, E.; Trunzo, V.; Mollace, V.; Coppedé, N. Tailoring super-hydrophobic properties of electrochemical biosensor for early cancer detection. MRS Adv. 2016, 1, 3545–3552. [Google Scholar] [CrossRef] [Green Version]
- Gentile, F.; Ferrara, L.; Villani, M.; Bettelli, M.; Iannotta, S.; Zappettini, A.; Cesarelli, M.; Di Fabrizio, E.; Coppedè, N. Geometrical Patterning of Super-Hydrophobic Biosensing Transistors Enables Space and Time Resolved Analysis of Biological Mixtures. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gentile, F.; Coppedè, N.; Tarabella, G.; Villani, M.; Calestani, D.; Candeloro, P.; Iannotta, S.; Di Fabrizio, E. Microtexturing of the conductive PEDOT: PSS Polymer for superhydrophobic organic electrochemical transistors. Biomed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Hsiao, Y.S.; Luo, S.C.; Hou, S.; Zhu, B.; Sekine, J.; Kuo, C.W.; Chueh, D.Y.; Yu, H.H.; Tseng, H.R.; Chen, P. 3D bioelectronic interface: Capturing circulating tumor cells onto conducting polymer-based micro/nanorod arrays with chemical and topographical control. Small 2014, 10, 3012–3017. [Google Scholar] [CrossRef]
- Ferrari, A.; Cecchini, M.; Dhawan, A.; Micera, S.; Tonazzini, I.; Stabile, R.; Pisignano, D.; Beltram, F. Nanotopographic control of neuronal polarity. Nano Lett. 2011, 11, 505–511. [Google Scholar] [CrossRef]
- Marino, A.; Ciofani, G.; Filippeschi, C.; Pellegrino, M.; Pellegrini, M.; Orsini, P.; Pasqualetti, M.; Mattoli, V.; Mazzolai, B. Two-Photon Polymerization of Sub-micrometric Patterned Surfaces: Investigation of Cell-Substrate Interactions and Improved Di ff erentiation of Neuron-like Cells. ACS Appl. Mater. Interfaces 2013, 5, 13012–13021. [Google Scholar] [CrossRef]
- Cutarelli, A.; Ghio, S.; Zasso, J.; Speccher, A.; Scarduelli, G.; Roccuzzo, M.; Crivellari, M.; Maria Pugno, N.; Casarosa, S.; Boscardin, M.; et al. Vertically-Aligned Functionalized Silicon Micropillars for 3D Culture of Human Pluripotent Stem Cell-Derived Cortical Progenitors. Cells 2019, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Tullii, G.; Giona, F.; Lodola, F.; Bonfadini, S.; Bossio, C.; Varo, S.; Desii, A.; Criante, L.; Sala, C.; Pasini, M.; et al. High-Aspect-Ratio Semiconducting Polymer Pillars for 3D Cell Cultures. ACS Appl. Mater. Interfaces 2019, 11, 28125–28137. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, B.N.; Marchioli, G.; Song, W.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; van Apeldoorn, A.; Mano, J.F. Wettability influences cell behavior on superhydrophobic surfaces with different topographies. Biointerphases 2012, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Jin, J.; Zheng, D.; Guan, W.; Guo, Y.; Chen, A.; Peng, Y.; Gao, Q.; Zheng, Y.; Huang, H. Influence of super-hydrophobic silicone rubber substrate on the growth and differentiation of human lens epithelial cells. J. Mater. Sci. Mater. Med. 2018, 29, 1–10. [Google Scholar] [CrossRef]
- Alves, N.M.; Shi, J.; Oramas, E.; Santos, J.L.; Tomás, H.; Mano, J.F. Bioinspired superhydrophobic poly(L-lactic acid) surfaces control bone marrow derived cells adhesion and proliferation. J. Biomed. Mater. Res. Part A 2009, 91, 480–488. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, M.; Huang, W.; Zhou, L.; Wong, M.S.; Wang, Y. Superhydrophobic/hydrophobic nanofibrous network with tunable cell adhesion: Fabrication, characterization and cellular activities. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 482, 718–723. [Google Scholar] [CrossRef]
- Lai, Y.; Lin, L.; Pan, F.; Huang, J.; Song, R.; Huang, Y.; Lin, C.; Fuchs, H.; Chi, L. Bioinspired patterning with extreme wettability contrast on TiO2 nanotube array surface: A versatile platform for biomedical applications. Small 2013, 9, 2945–2953. [Google Scholar] [CrossRef]
- Naeemabadi, N.; Seyfi, J.; Hejazi, E.; Hejazi, I.; Khonakdar, H.A. Investigation on surface properties of superhydrophobic nanocomposites based on polyvinyl chloride and correlation with cell adhesion behavior. Polym. Adv. Technol. 2019, 30, 1027–1035. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Song, W.; Alves, N.M.; Mano, J.F. Chemical modification of bioinspired superhydrophobic polystyrene surfaces to control cell attachment/proliferation. Soft Matter 2011, 7, 8932–8941. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Alves, N.M.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2014, 28, 843–863. [Google Scholar] [CrossRef]
- Sousa, C.; Rodrigues, D.; Oliveira, R.; Song, W.; Mano, J.F.; Azeredo, J. Superhydrophobic poly(l-lactic acid) surface as potential bacterial colonization substrate. AMB Express 2011, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.B.; Salgado, C.L.; Song, W.; Mano, J.F. Combinatorial on-chip study of miniaturized 3D porous scaffolds using a patterned superhydrophobic platform. Small 2013, 9, 768–778. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Neto, A.I.; Song, W.; Mano, J.F. Two-dimensional open microfluidic devices by tuning the wettability on patterned superhydrophobic polymeric surface. Appl. Phys. Express 2010, 3, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Song, W.; Sun, X.; Zhang, S. Inkjet-Printing Patterned Chip on Sticky Superhydrophobic Surface for High-Efficiency Single-Cell Array Trapping and Real-Time Observation of Cellular Apoptosis. ACS Appl. Mater. Interfaces 2018, 10, 31054–31060. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.I.; Custódio, C.A.; Song, W.; Mano, J.F. High-throughput evaluation of interactions between biomaterials, proteins and cells using patterned superhydrophobic substrates. Soft Matter 2011, 7, 4147–4151. [Google Scholar] [CrossRef] [Green Version]
- Ballester-Beltrán, J.; Rico, P.; Moratal, D.; Song, W.; Mano, J.F.; Salmerón-Sánchez, M. Role of superhydrophobicity in the biological activity of fibronectin at the cell-material interface. Soft Matter 2011, 7, 10803–10811. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Neto, A.I.; Correia, C.R.; Rial-Hermida, M.I.; Alvarez-Lorenzo, C.; Mano, J.F. Superhydrophobic chips for cell spheroids high-throughput generation and drug screening. ACS Appl. Mater. Interfaces 2014, 6, 9488–9495. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.I.; Levkin, P.A.; Mano, J.F. Patterned superhydrophobic surfaces to process and characterize biomaterials and 3D cell culture. Mater. Horiz. 2018, 5, 379–393. [Google Scholar] [CrossRef]
- Song, W.; Mano, J.F. Interactions between cells or proteins and surfaces exhibiting extreme wettabilities. Soft Matter 2013, 9, 2985–2999. [Google Scholar] [CrossRef] [Green Version]
- Ueda, E.; Feng, W.; Levkin, P.A. Superhydrophilic–Superhydrophobic Patterned Surfaces as High-Density Cell Microarrays: Optimization of Reverse Transfection. Adv. Healthc. Mater. 2016, 5, 2646–2654. [Google Scholar] [CrossRef]
- Pereira, D.R.; Silva-Correia, J.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Salgado, A.J.; Oliveira, J.M.; Mano, J.F.; Sousa, N.; Reis, R.L. Development of gellan gum-based microparticles/hydrogel matrices for application in the intervertebral disc regeneration. Tissue Eng. Part C Methods 2011, 17, 961–972. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.L.; Hung, S.C.; Lin, S.Y.; Chen, Y.L.; Lo, W.H. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J. Biomed. Mater. Res. Part A 2003, 64, 273–281. [Google Scholar] [CrossRef]
- Hu, M.; Kurisawa, M.; Deng, R.; Teo, C.M.; Schumacher, A.; Thong, Y.X.; Wang, L.; Schumacher, K.M.; Ying, J.Y. Cell immobilization in gelatin-hydroxyphenylpropionic acid hydrogel fibers. Biomaterials 2009, 30, 3523–3531. [Google Scholar] [CrossRef]
- Lima, A.C.; Batista, P.; Valente, T.A.M.; Silva, A.S.; Correia, I.J.; Mano, J.F. Novel methodology based on biomimetic superhydrophobic substrates to immobilize cells and proteins in hydrogel spheres for applications in bone regeneration. Tissue Eng. Part A 2013, 19, 1175–1187. [Google Scholar] [CrossRef] [Green Version]
- Sibillano, T.; De Caro, L.; Altamura, D.; Siliqi, D.; Ramella, M.; Boccafoschi, F.; Ciasca, G.; Campi, G.; Tirinato, L.; Di Fabrizio, E.; et al. An optimized table-top small-angle X-ray scattering set-up for the nanoscale structural analysis of soft matter. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sereda, V.; Lednev, I.K. Polarized Raman spectroscopy of aligned insulin fibrils. J. Raman Spectrosc. 2014, 45, 665–671. [Google Scholar] [CrossRef]
- Lefèvre, T.; Rousseau, M.E.; Pézolet, M. Protein secondary structure and orientation in silk as revealed by Raman spectromicroscopy. Biophys. J. 2007, 92, 2885–2895. [Google Scholar] [CrossRef] [Green Version]
- Reimer, L. Transmission Electron Microscopy: Physics of Image Formation and Microanalysis; Springer: Cham, Switzerland, 2013; Volume 36, ISBN 3662135531. [Google Scholar]
- Tanaka, N. Present status and future prospects of spherical aberration corrected TEM/STEM for study of nanomaterials. Sci. Technol. Adv. Mater. 2008, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.; Rose, H.; Uhlemann, S.; Schwan, E.; Kabius, B.; Urban, K. A spherical-aberration-corrected 200 kV transmission electron microscope. Ultramicroscopy 1998, 75, 53–60. [Google Scholar] [CrossRef]
- Sasaki, T.; Sawada, H.; Hosokawa, F.; Sato, Y.; Suenaga, K. Aberration-corrected STEM/TEM imaging at 15kV. Ultramicroscopy 2014, 145, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.E.; Hetherington, C.; Kirkland, A.; Chang, L.Y.; Stahlberg, H.; Browning, N. Low-dose aberration corrected cryo-electron microscopy of organic specimens. Ultramicroscopy 2008, 108, 1636–1644. [Google Scholar] [CrossRef] [Green Version]
- Pawley, J.; Schatten, H. Biological Low-Voltage Scanning Electron Microscopy; Springer: Cham, Switzerland, 2007; ISBN 0387729720. [Google Scholar]
- Liu, C.; Liao, S.C.; Song, J.; Mauk, M.G.; Li, X.; Wu, G.; Ge, D.; Greenberg, R.M.; Yang, S.; Bau, H.H. A high-efficiency superhydrophobic plasma separator. Lab Chip 2016, 16, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Miele, E.; Accardo, A.; Falqui, A.; Marini, M.; Giugni, A.; Leoncini, M.; De Angelis, F.; Krahne, R.; Fabrizio, E. Di Writing and functionalisation of suspended DNA nanowires on superhydrophobic pillar arrays. Small 2015, 11, 134–140. [Google Scholar] [CrossRef]
- Miele, E.; Malerba, M.; Dipalo, M.; Rondanina, E.; Toma, A.; Angelis, F. De Controlling wetting and self-assembly dynamics by tailored hydrophobic and oleophobic surfaces. Adv. Mater. 2014, 26, 4179–4183. [Google Scholar] [CrossRef]

| Application | Deposited Material | Substrate Material | Coating | Notes | Ref. |
|---|---|---|---|---|---|
| Superhydrophobic concentrator | DNA, Rhodamine 6G | Silicon, DRIE-machined | PI-PTFE 1 | Detection by SERS and focusing of plasmons on tips | [66] |
| DNA | Electroplated Ni on SiO2 insulating layer | Surface chemical roughening of Ni | Detection of material by impedance spectroscopy | [112] | |
| apoferritin | Silicon, DRIE-machined | Silanization by TMCS 2 | Detection by IR spectroscopy, enhanced by nanoantennas on sensing area | [115] | |
| ferritin | Silicon, DRIE-machined | Silanization by TMCS | Detection by X-ray fluorescence and X-ray phase contrast imaging | [116] | |
| Material stretching across gaps | DNA | Silicon, DRIE-machined with holes | PI-PTFE | Observation of DNA periodic structure by HRTEM | [69] |
| DNA | Silicon, DRIE-machined with holes, top Au coating | PI-PTFE | First TEM observation of single isolated DNA molecule | [63] | |
| DNA+rad51, blood cells membranes | Silicon, DRIE-machined with holes, different Au coatings | Vapor phase deposited FDTS 3 | Characterization by HRTEM | [120] | |
| DNA | Silicon, DRIE-machined | Silanization by TMCS | Control of orientation and vertical positioning of filaments | [121,122] | |
| Spider silk proteins | Silicon, DRIE-machined, reentrant profile | PI-PTFE | First reported structuring of recombinant spider silk on SHS | [136] | |
| lysozyme amyloid fibrils, PHF6 peptide solution, and Tau441 proteins | Silicon, DRIE-machined with holes | Vapor phase deposited FDTS | Self-aggregation of protein fibrils induced by Marangoni convection. Raman, X-ray diffraction, and atomic force microscopy characterization of depositions. | [154] | |
| Tobacco mosaic virus | Silicon, DRIE-machined, and SU8 grown on Si3N4 suspended membranes | PI-PTFE on both types | Creation of crystallized aggregates and stretched filaments, analyzed by X-ray diffraction, atomic force Microscopy, and optical and electron microscopy | [159] | |
| Controlled deposition and aggregation | proteins | Polymethyl methacrylate | Plasma-induced surface roughening | Synchrotron X-ray diffraction of protein aggregate | [71] |
| Tau proteins | Silicon, DRIE-machined | Vapor phase deposited FDTS | Induction of formation of protein fibrils in the suspended droplet | [150] | |
| Estrogen receptor proteins | SU8, supported on CaF2 or Si3N4 suspended membranes | SU8 roughening by CF4/02 plasma followed by PI-PTFE | Combined Raman and X-ray diffraction analysis of deposited filaments across pillars and evaporation residuals | [153] | |
| Sensing | DNA | Silicon, DRIE-machined, top Au coating | Vapor-phase-deposited FDTS | Detection of stiffness of DNA and presence of intercalants by laserDoppler vibrometry | [126] |
| DNA | Silicon, DRIE-machined | PI-PTFE | Controlled realization of DNA monolayers on pillars, detection via vibrometry on single pillars | [127,129] | |
| Blood clinical samples | Silicon, DRIE-machined | PI-PTFE, followed by a PEDOT 4 coating | Incorporate electrodes for conductivity measurements.Application in tumoral risk assessment | [67] | |
| Circulating tumor cells | PEDOT, after transfer from PDMS mold | Transparent, mass-producible, and conductive substrate for biosensing applications | [167] | ||
| Tissue engineering and cell manipulation | Fibroblasts and osteoblasts cells | Polystyrene | UV light and Ozone selective treatments | Flat biocompatible platform for 3D cell scaffolding | [181] |
| Human embryonic kidney and cervical carcinoma cells | HEMA-EDMA 5 on glass | UV-induced photopatterning | Selective superhydrophobic patterning to avoid cell interference in different cultured spots | [189] | |
| Neuronal cells | Silicon, DRIE-machined, completely Au coated | PI-PTFE | Growing of neurons stretched across structured substrate | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allione, M.; Limongi, T.; Marini, M.; Torre, B.; Zhang, P.; Moretti, M.; Perozziello, G.; Candeloro, P.; Napione, L.; Pirri, C.F.; et al. Micro/Nanopatterned Superhydrophobic Surfaces Fabrication for Biomolecules and Biomaterials Manipulation and Analysis. Micromachines 2021, 12, 1501. https://doi.org/10.3390/mi12121501
Allione M, Limongi T, Marini M, Torre B, Zhang P, Moretti M, Perozziello G, Candeloro P, Napione L, Pirri CF, et al. Micro/Nanopatterned Superhydrophobic Surfaces Fabrication for Biomolecules and Biomaterials Manipulation and Analysis. Micromachines. 2021; 12(12):1501. https://doi.org/10.3390/mi12121501
Chicago/Turabian StyleAllione, Marco, Tania Limongi, Monica Marini, Bruno Torre, Peng Zhang, Manola Moretti, Gerardo Perozziello, Patrizio Candeloro, Lucia Napione, Candido Fabrizio Pirri, and et al. 2021. "Micro/Nanopatterned Superhydrophobic Surfaces Fabrication for Biomolecules and Biomaterials Manipulation and Analysis" Micromachines 12, no. 12: 1501. https://doi.org/10.3390/mi12121501
APA StyleAllione, M., Limongi, T., Marini, M., Torre, B., Zhang, P., Moretti, M., Perozziello, G., Candeloro, P., Napione, L., Pirri, C. F., & Di Fabrizio, E. (2021). Micro/Nanopatterned Superhydrophobic Surfaces Fabrication for Biomolecules and Biomaterials Manipulation and Analysis. Micromachines, 12(12), 1501. https://doi.org/10.3390/mi12121501









