Development of a Microfluidic Device to Form a Long Chemical Gradient in a Tissue from Both Ends with an Analysis of Its Appearance and Content
Abstract
:1. Introduction
2. Materials and Methods
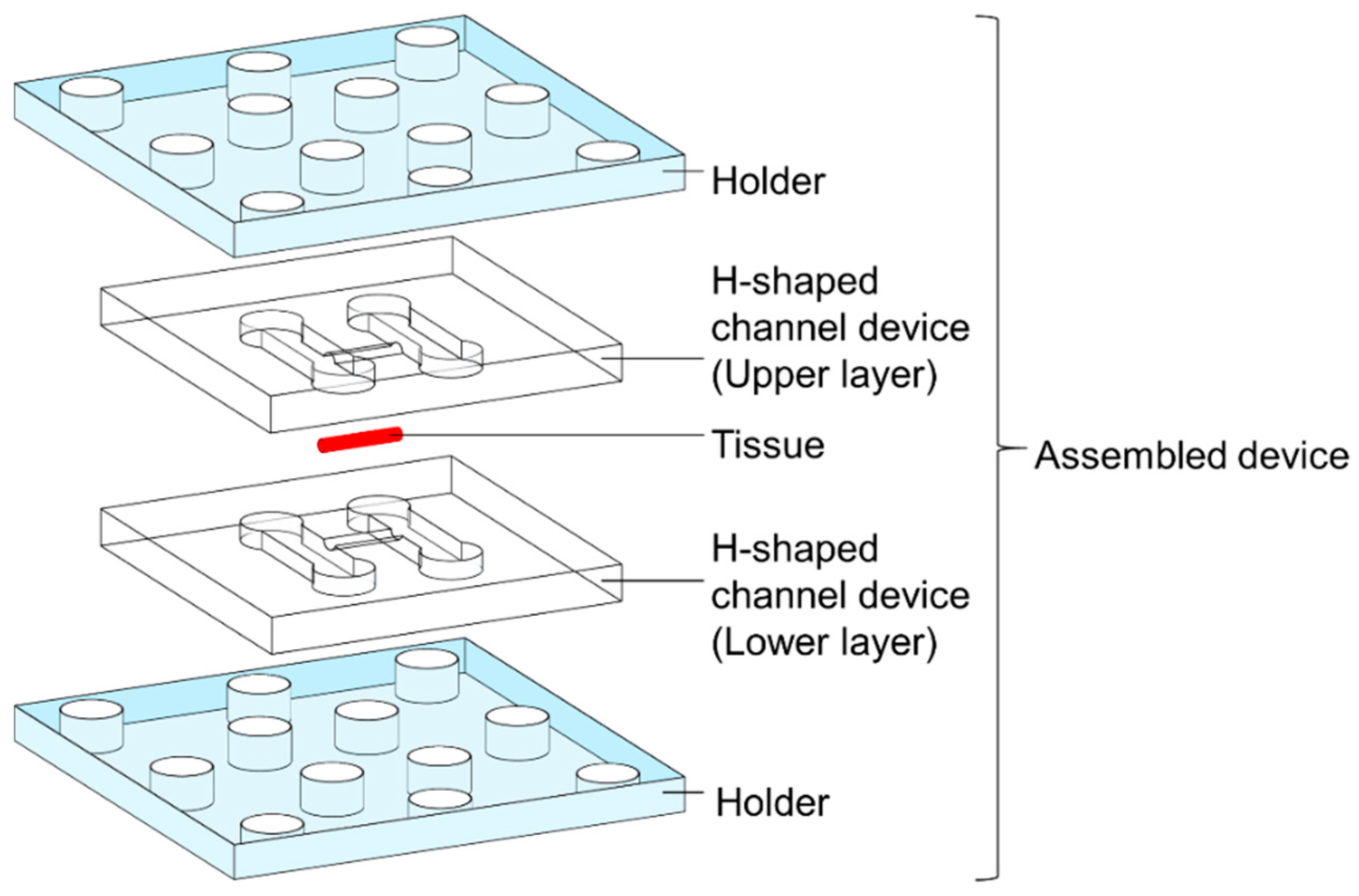
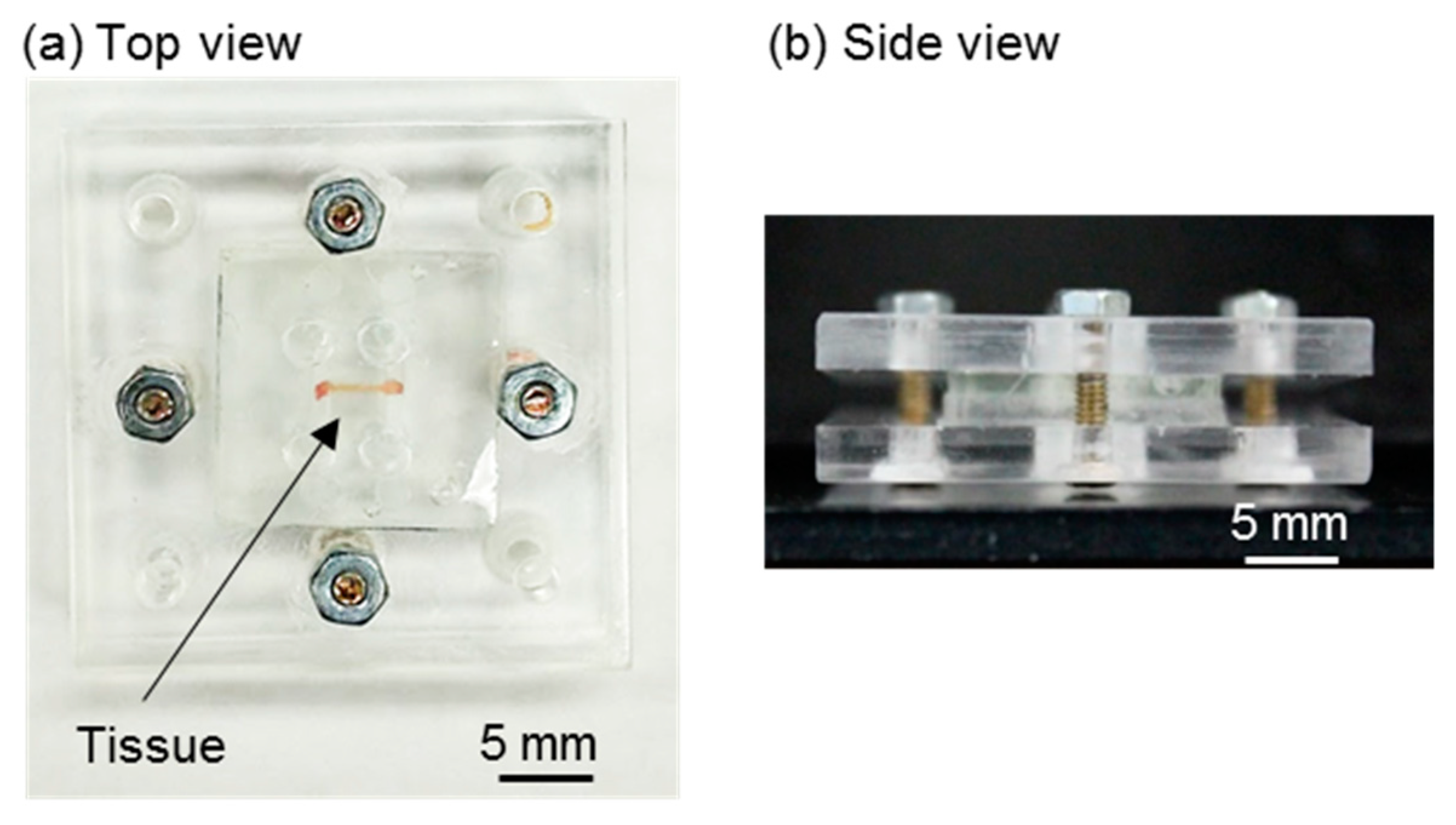
2.1. H-Shaped Channel Device and Its Peripherals
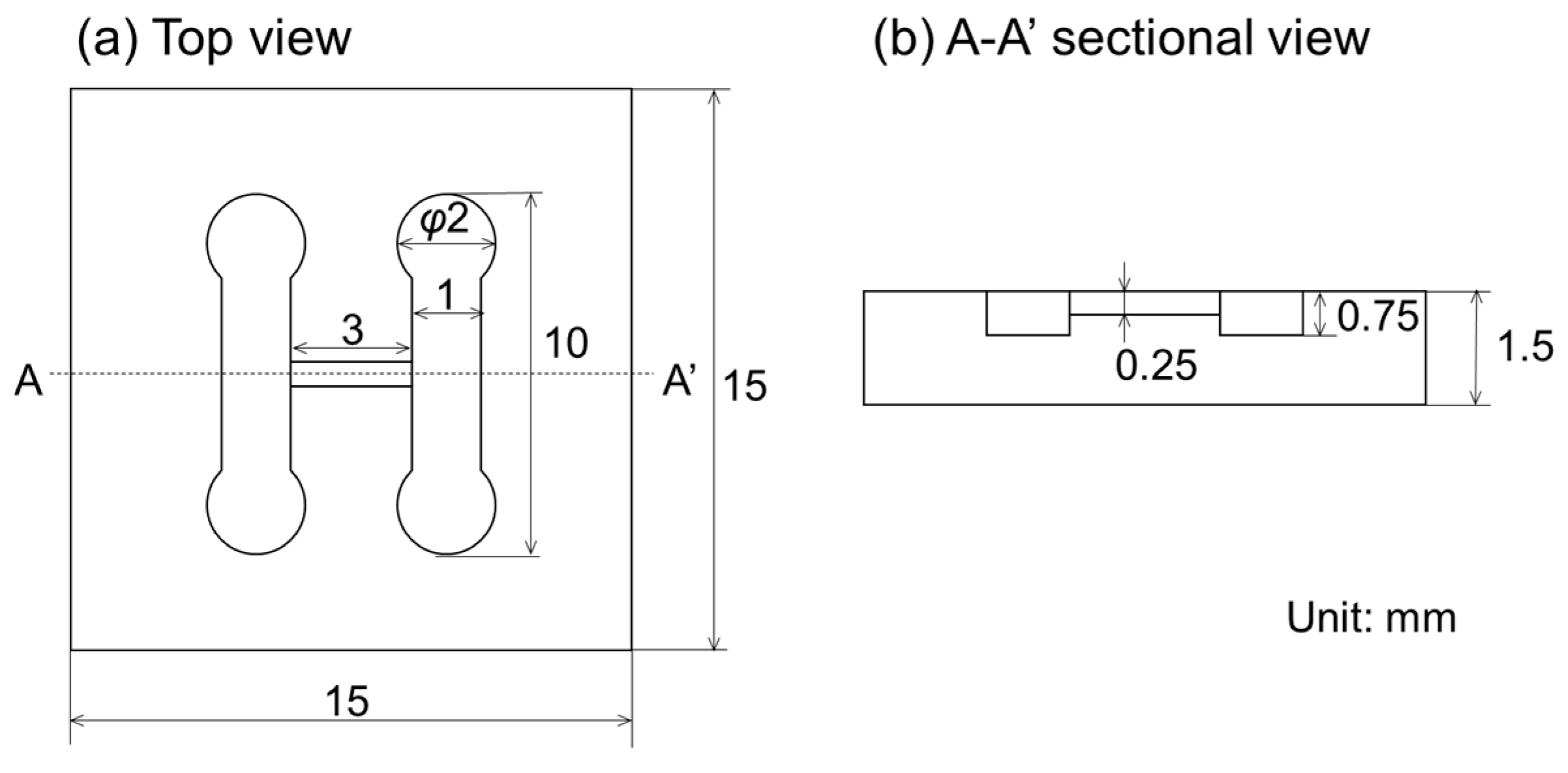
2.2. Design
2.3. Fabrication Process
2.4. Assembly Process
2.4.1. Experimental Setup
2.4.2. Sectioning
2.4.3. Image Analysis
3. Results and Discussion
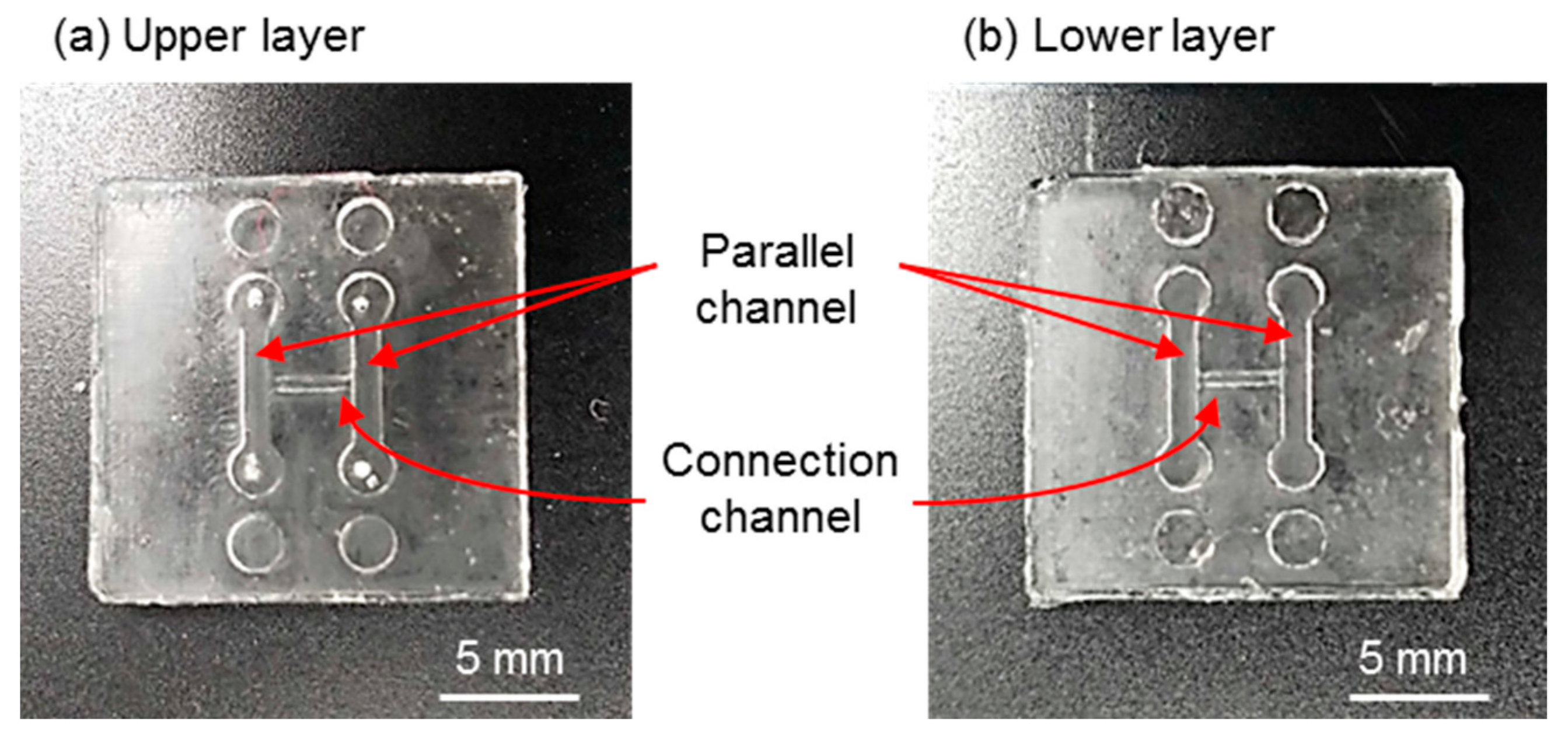
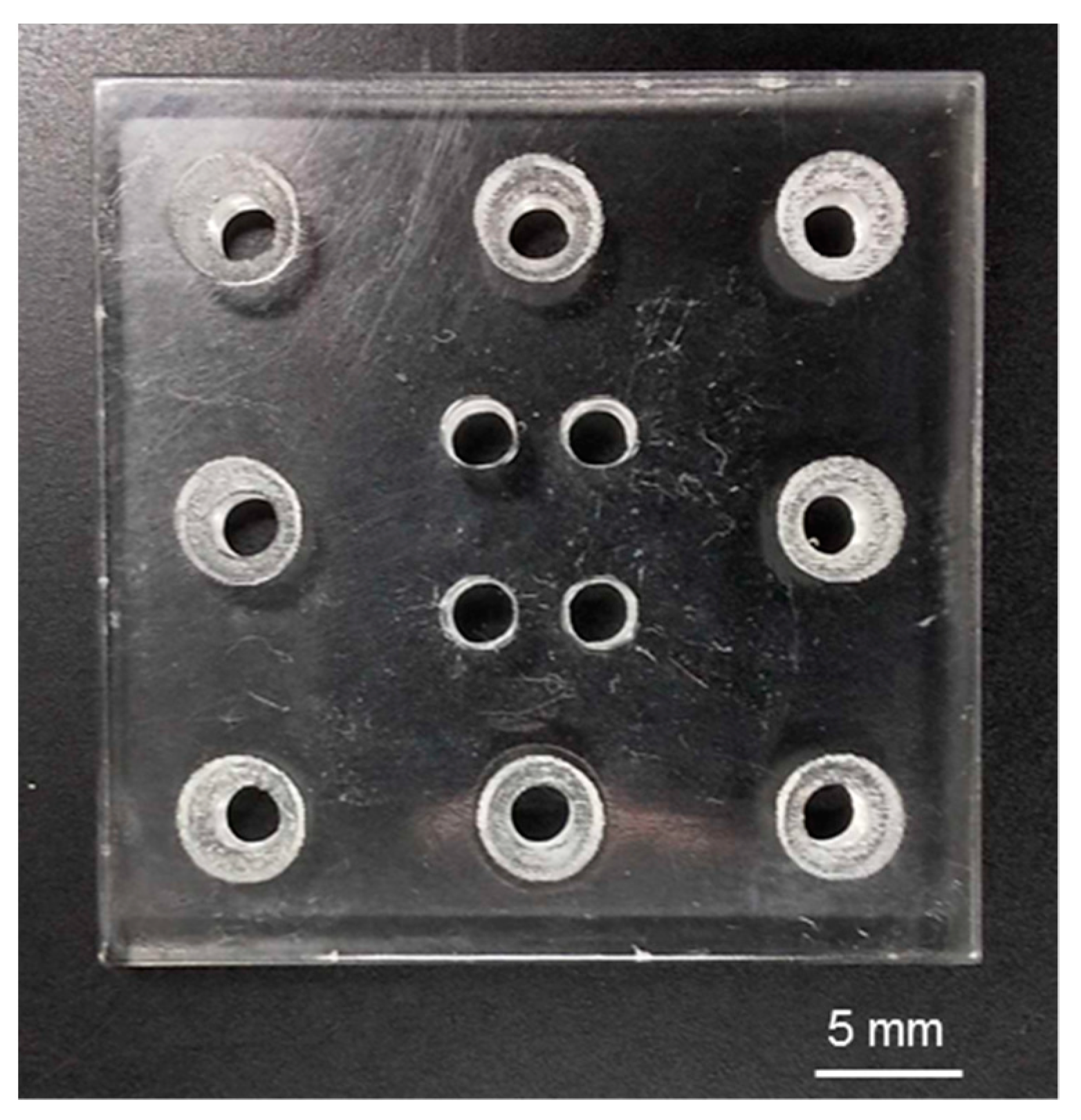
3.1. Fabricated Components and Assembly
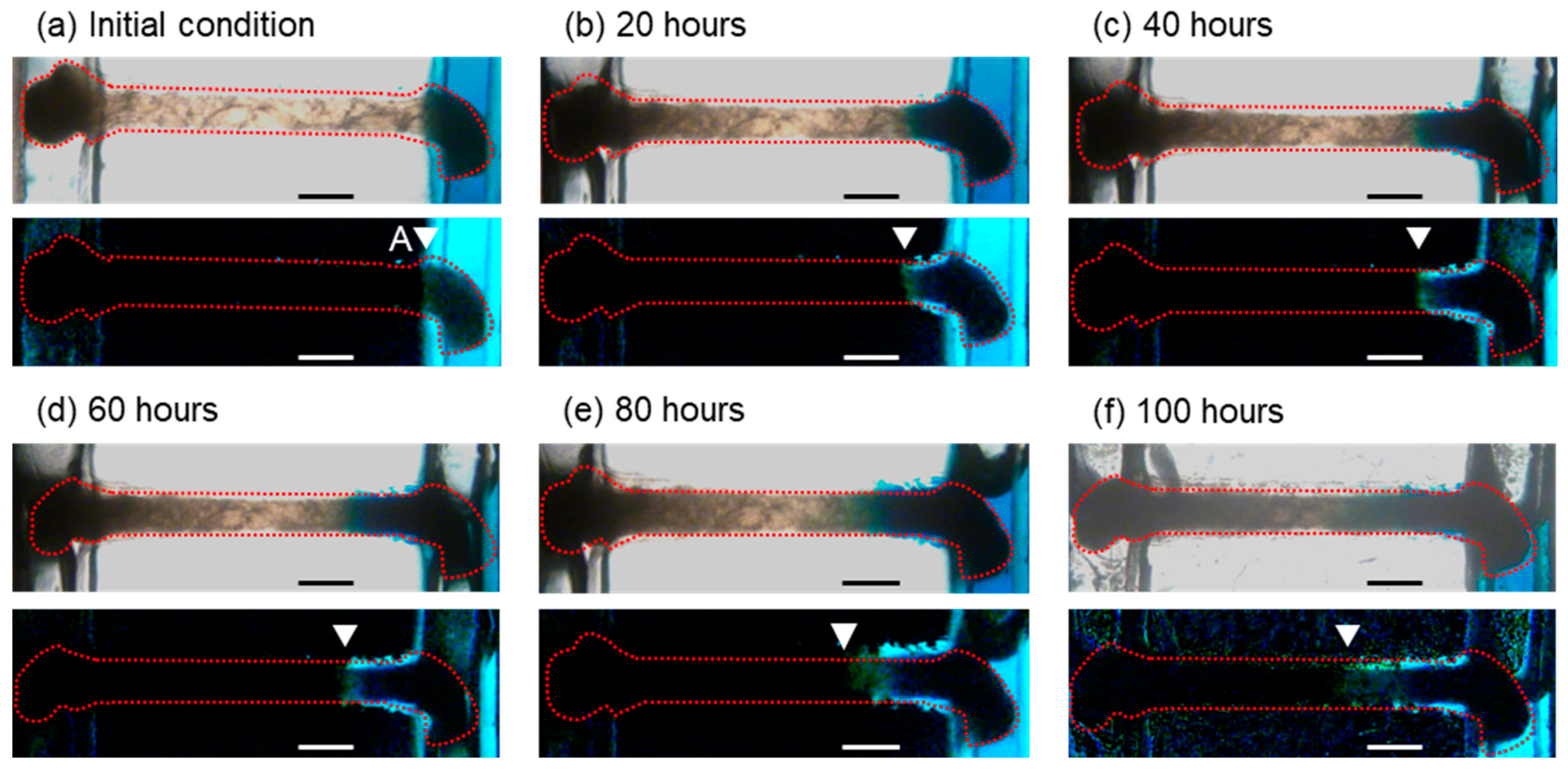
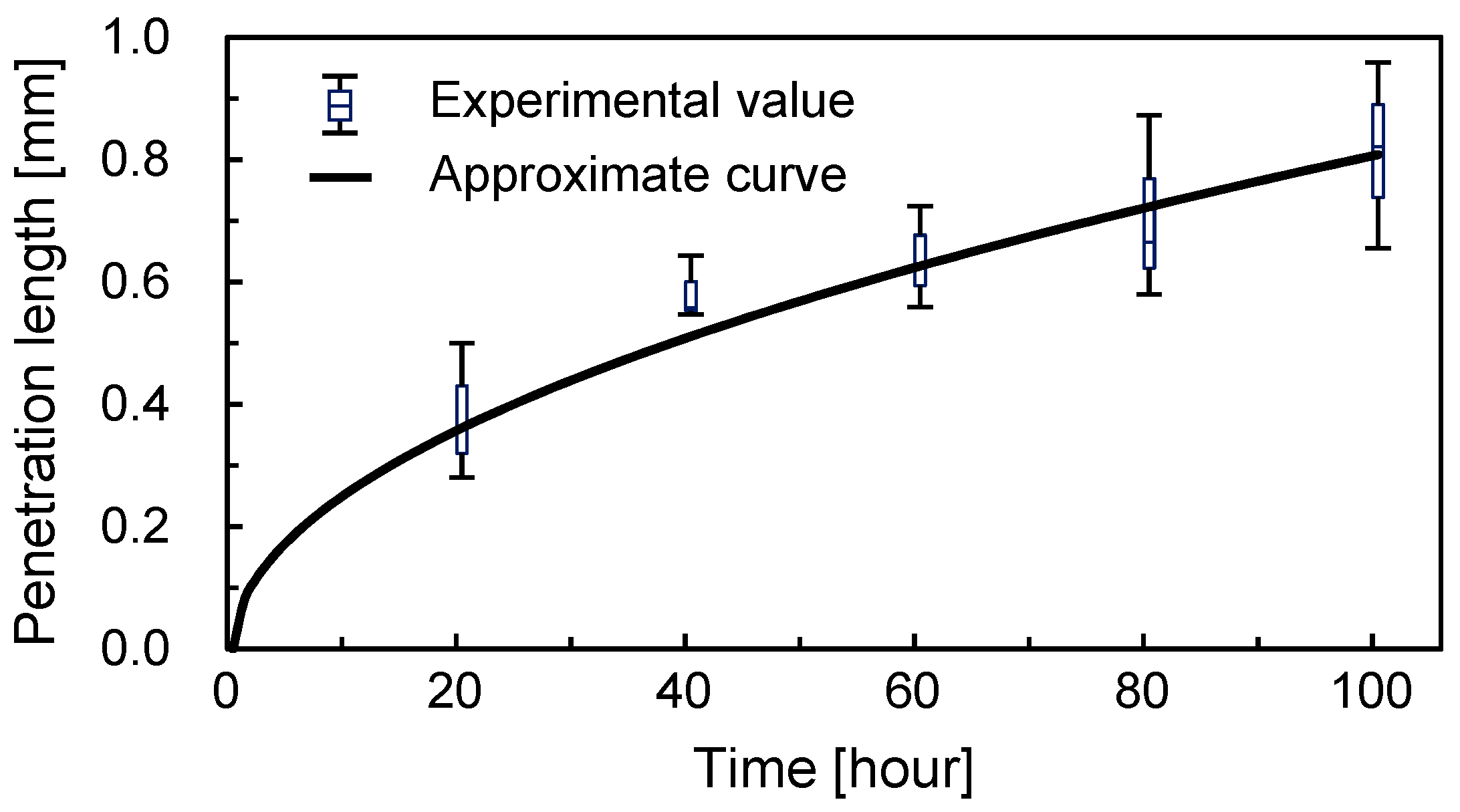
3.2. Concentration Gradient Formed by Long-Term Perfusion and Real-Time Observation
3.3. Color Gradient Evaluation
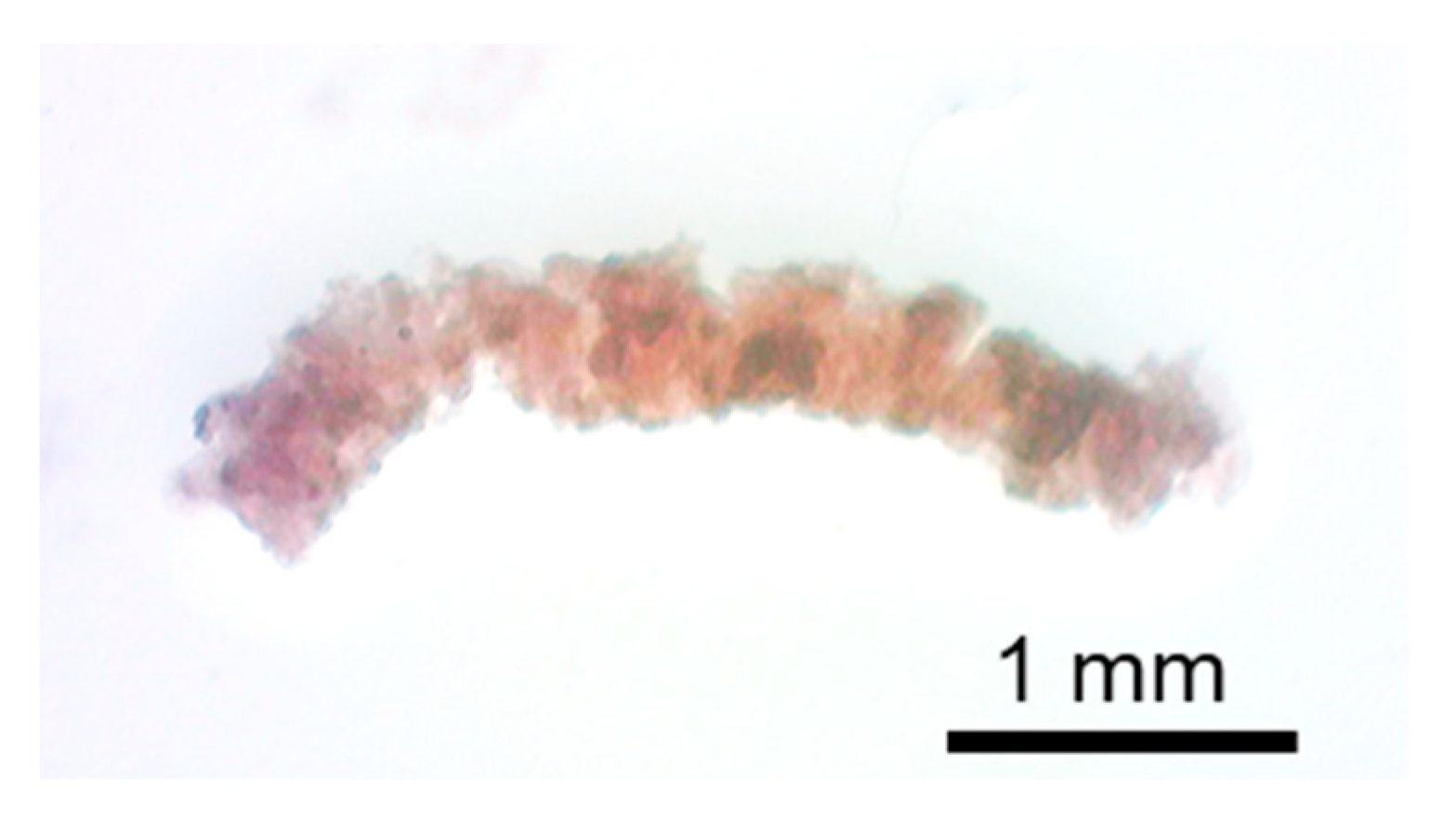
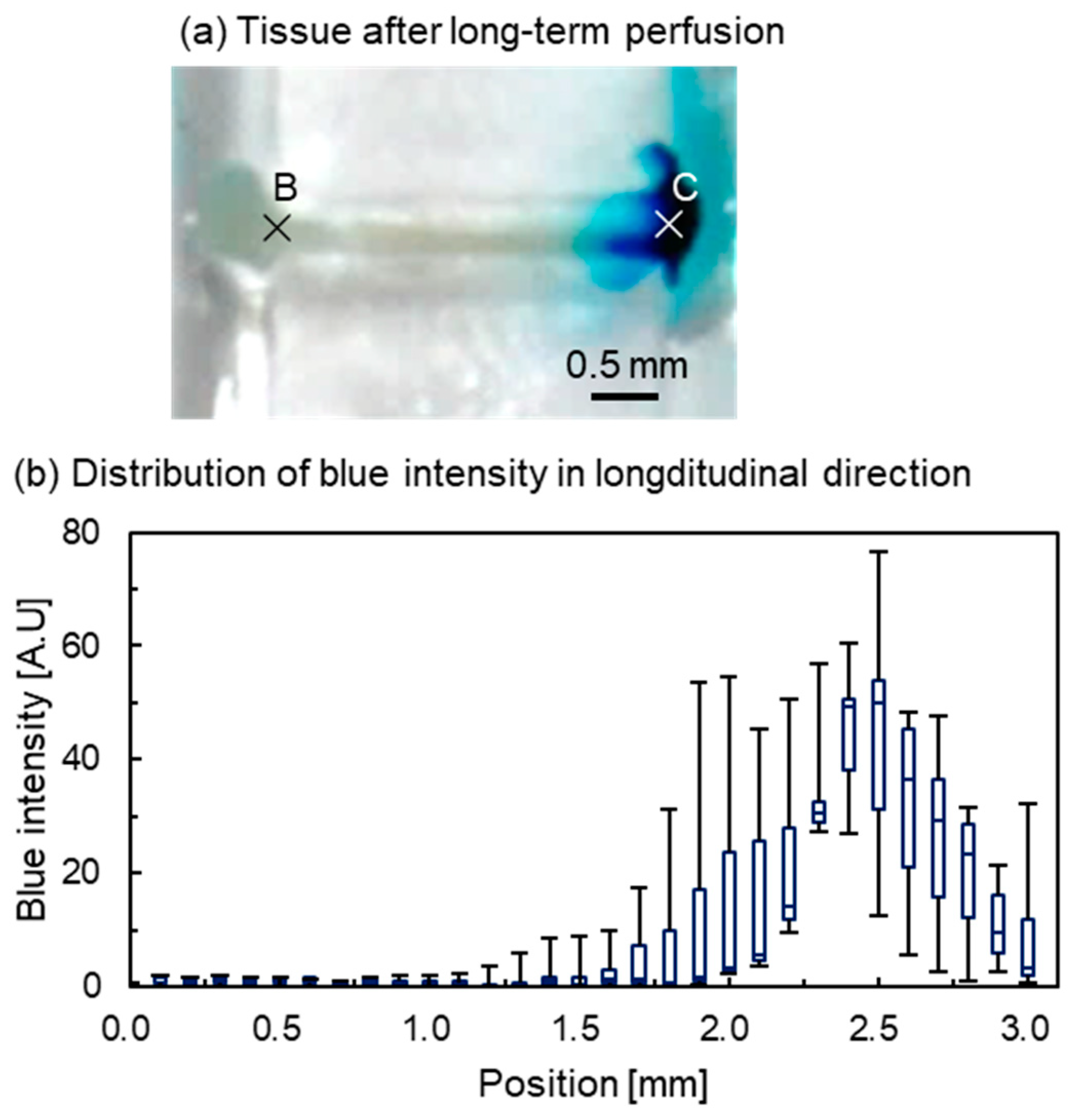
3.3.1. Intensity Distribution of Tissue from the Appearance
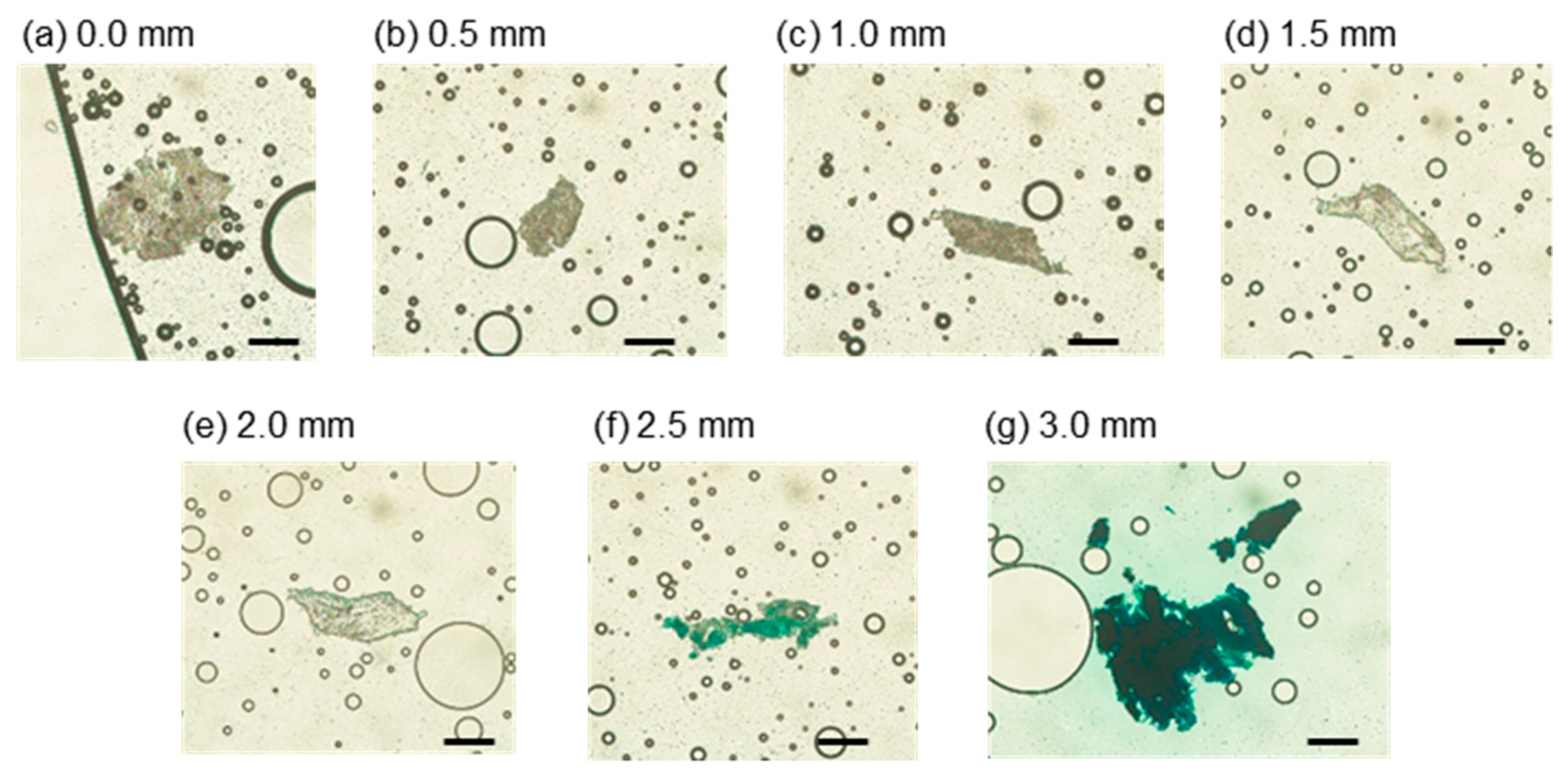
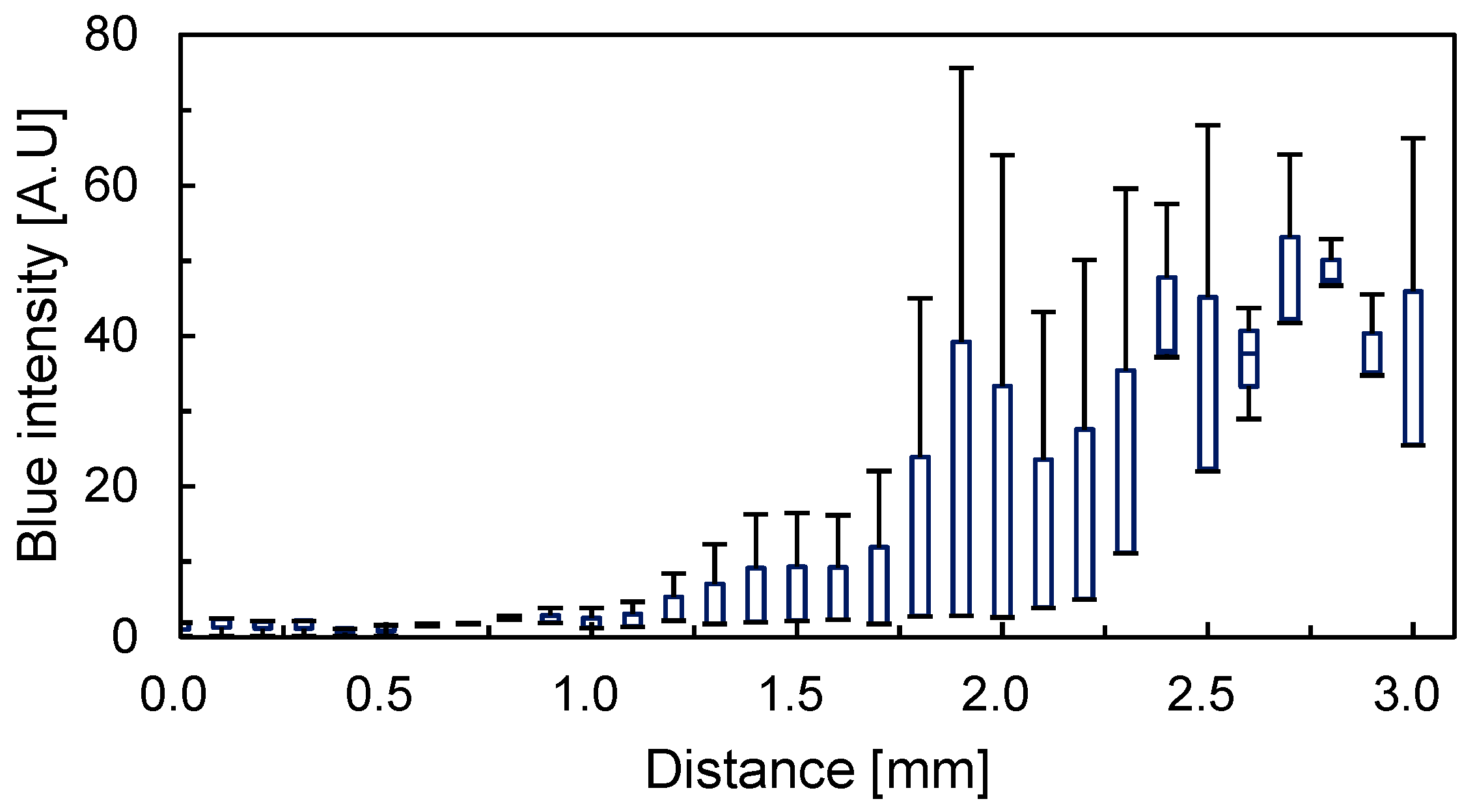
3.3.2. Intensity Distribution of Tissue from Slices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Chatzinikolaidou, M. Cell spheroids: The new frontiers in in vitro models for cancer drug validation. Drug Discov. 2016, 21, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Wang, L. In vitro human cell line models to predict clinical response to anticancer drugs. Pharmacogenomics 2015, 16, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokhari, M.; Carnachan, R.J.; Cameron, N.R.; Przyborski, S.A. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J. Anat. 2007, 211, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Perche, F.; Torchilin, V.P. Cancer cell spheroids as a model to evaluate chemotherapy protocols. Cancer Biol. Ther. 2012, 13, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updat. 2011, 14, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Mayer, A.; Höckel, M. Tumor hypoxia and malignant progression. Meth. Enzymol. 2004, 381, 335–354. [Google Scholar] [CrossRef]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [Green Version]
- Griffith, L.; Swartz, M. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.; Stelzer, E. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.; Coward, J.I.; Bast, R.C., Jr.; Berchuck, A.; Berek, J.S.; Brenton, J.D.; Coukos, G.; Crum, C.C.; Drapkin, R.; Etemadmoghadam, D.; et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat. Rev. Cancer 2011, 11, 719–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, J.; Pollard, J. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Moses, H.L. Tumor–stroma interactions. Curr. Opin. Genet. Dev. 2005, 15, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.; Liu, W.; Wang, Y.; Wang, J.C.; Tu, Q.; Liu, R.; Xu, J.; Shen, S.; Wang, J. Microvalve and liquid membrane double-controlled integrated microfluidics for observing the interaction of breast cancer cells. Microfluid. Nanofluidics 2013, 14, 515–526. [Google Scholar] [CrossRef]
- Ma, Y.H.; Middleton, K.; You, L.; Sun, Y. A review of microfluidic approaches for investigating cancer extravasation during metastasis. Microsyst. Nanoeng. 2018, 4, 17104. [Google Scholar] [CrossRef] [Green Version]
- Korenchuk, S.; Lehr, J.E.; Mclean, L.; Lee, Y.G.; Whitney, S.; Vessella, R.; Lin, D.L.; Pienta, K.J. VCaP, a cell-based model system of human prostate cancer. In Vivo 2001, 15, 163–168. [Google Scholar]
- Rotem, A.; Janzer, A.; Izar, B.; Ji, Z.; Doench, J.G.; Garraway, L.A.; Struhl, K. Alternative to the soft-agar assay that permits high-throughput drug and genetic screens for cellular transformation. Proc. Natl. Acad. Sci. USA 2015, 112, 5708–5713. [Google Scholar] [CrossRef] [Green Version]
- Oliver, A.W.; Marc, M. Role of tissue stroma in cancer cell invasion. J. Pathol. 2003, 200, 429–447. [Google Scholar] [CrossRef]
- Khong, Y.M.; Zhang, J.; Zhou, S.; Cheung, C.; Doberstein, K.; Samper, V.; Yu, H. Novel intra-tissue perfusion system for culturing thick liver tissue. Tissue Eng. 2007, 13, 2345–2356. [Google Scholar] [CrossRef]
- Schumacher, K.; Khong, Y.M.; Chang, S.; Ni, J.; Sun, W.; Yu, H. Perfusion culture improves the maintenance of cultured liver tissue slices. Tissue Eng. 2007, 13, 197–205. [Google Scholar] [CrossRef]
- Sonveaux, P.; Végran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Valerio, G.; Martínez-Lozano, M.; Bassani, N.; Vidal, A.; Ochoa-de-Olza, M.; Suárez, C.; García-Del-Muro, X.; Carles, J.; Viñals, F.; Graupera, M.; et al. Resistance to antiangiogenic therapies by metabolic symbiosis in renal cell carcinoma PDX models and patients. Cell Rep. 2016, 15, 1134–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bock, K.; Mazzone, M.; Carmeliet, P. Antiangiogenic therapy, hypoxia, and metastasis: Risky liaisons, or not? Nat. Rev. Clin. Oncol. 2011, 8, 393–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Li, C.; Li, H.; Zeng, S.; Qin, J.; Lin, B. A microfluidic device for characterizing the invasion of cancer cells in 3-D matrix. Electrophoresis 2009, 30, 4285–4291. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Park, S. A microfluidic spheroid culture device with a concentration gradient generator for high-throughput screening of drug efficacy. Molecules 2018, 23, 3355. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Nagrath, S. Microfluidics and cancer: Are we there yet? Biomed. Microdevices 2013, 15, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Van Midwoud, P.M.; Groothuis, G.M.; Merema, M.T.; Verpoorte, E. Microfluidic biochip for the perifusion of precision-cut rat liver slices for metabolism and toxicology studies. Biotechnol. Bioeng. 2010, 105, 184–194. [Google Scholar] [CrossRef]
- Horowitz, L.F.; Rodriguez, A.D.; Au-Yeung, A.; Bishop, K.W.; Barner, L.A.; Mishra, G.; Raman, A.; Delgado, P.; Liu, J.T.C.; Gujral, T.S.; et al. Microdissected “cuboids” for microfluidic drug testing of intact tissues. Lab. Chip 2021, 21, 122–142. [Google Scholar] [CrossRef]
- Astolfi, M.; Péant, B.; Lateef, M.A.; Rousset, N.; Kendall-Dupont, J.; Carmona, E.; Monet, F.; Saad, F.; Provencher, D.M.; Mes-Masson, A.; et al. Micro-dissected tumor tissues on chip: An ex vivo method for drug testing and personalized therapy. Lab. Chip 2016, 16, 312–325. [Google Scholar] [CrossRef]
- Griffith, C.K.; Miller, C.; Sainson, R.C.; Calvert, J.W.; Jeon, N.L.; Hughes, C.C.; George, S.C. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005, 11, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image J: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dietsche, G.; Iftime, D.; Skipetrov, S.E.; Maret, G.; Elbert, T.; Rockstroh, B.; Gisler, T. Noninvasive detection of functional brain activity with near-infrared diffusing-wave spectroscopy. J. Biomed. Opt. 2005, 10, 044002. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokuoka, Y.; Kondo, K.; Nakaigawa, N.; Ishida, T. Development of a Microfluidic Device to Form a Long Chemical Gradient in a Tissue from Both Ends with an Analysis of Its Appearance and Content. Micromachines 2021, 12, 1482. https://doi.org/10.3390/mi12121482
Tokuoka Y, Kondo K, Nakaigawa N, Ishida T. Development of a Microfluidic Device to Form a Long Chemical Gradient in a Tissue from Both Ends with an Analysis of Its Appearance and Content. Micromachines. 2021; 12(12):1482. https://doi.org/10.3390/mi12121482
Chicago/Turabian StyleTokuoka, Yasunori, Keiichi Kondo, Noboru Nakaigawa, and Tadashi Ishida. 2021. "Development of a Microfluidic Device to Form a Long Chemical Gradient in a Tissue from Both Ends with an Analysis of Its Appearance and Content" Micromachines 12, no. 12: 1482. https://doi.org/10.3390/mi12121482
APA StyleTokuoka, Y., Kondo, K., Nakaigawa, N., & Ishida, T. (2021). Development of a Microfluidic Device to Form a Long Chemical Gradient in a Tissue from Both Ends with an Analysis of Its Appearance and Content. Micromachines, 12(12), 1482. https://doi.org/10.3390/mi12121482





