Microfluidic Cell Transport with Piezoelectric Micro Diaphragm Pumps
Abstract
:1. Introduction
2. Materials and Methods
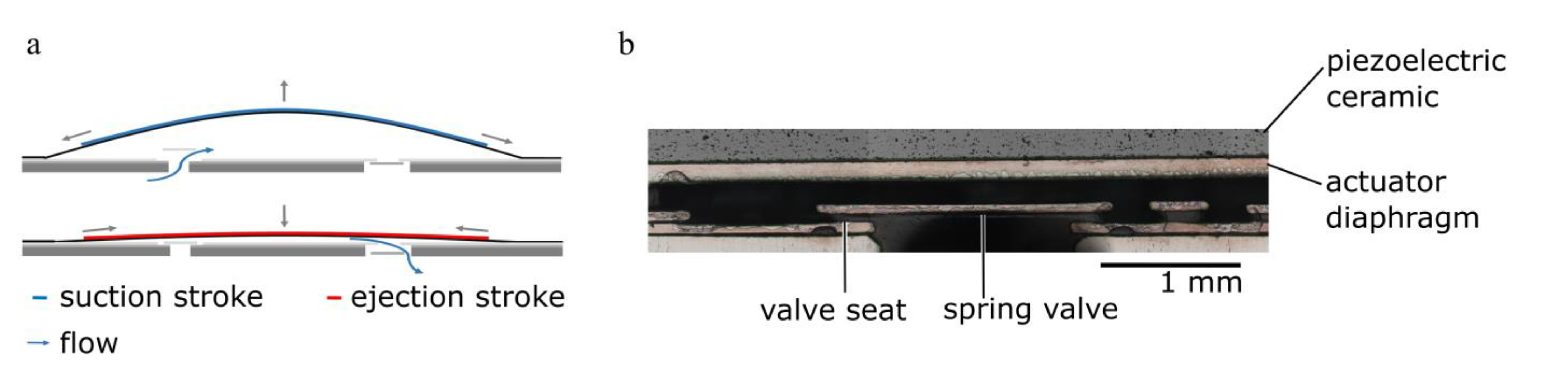
2.1. Piezoelectric Micro Diaphragm Pumps
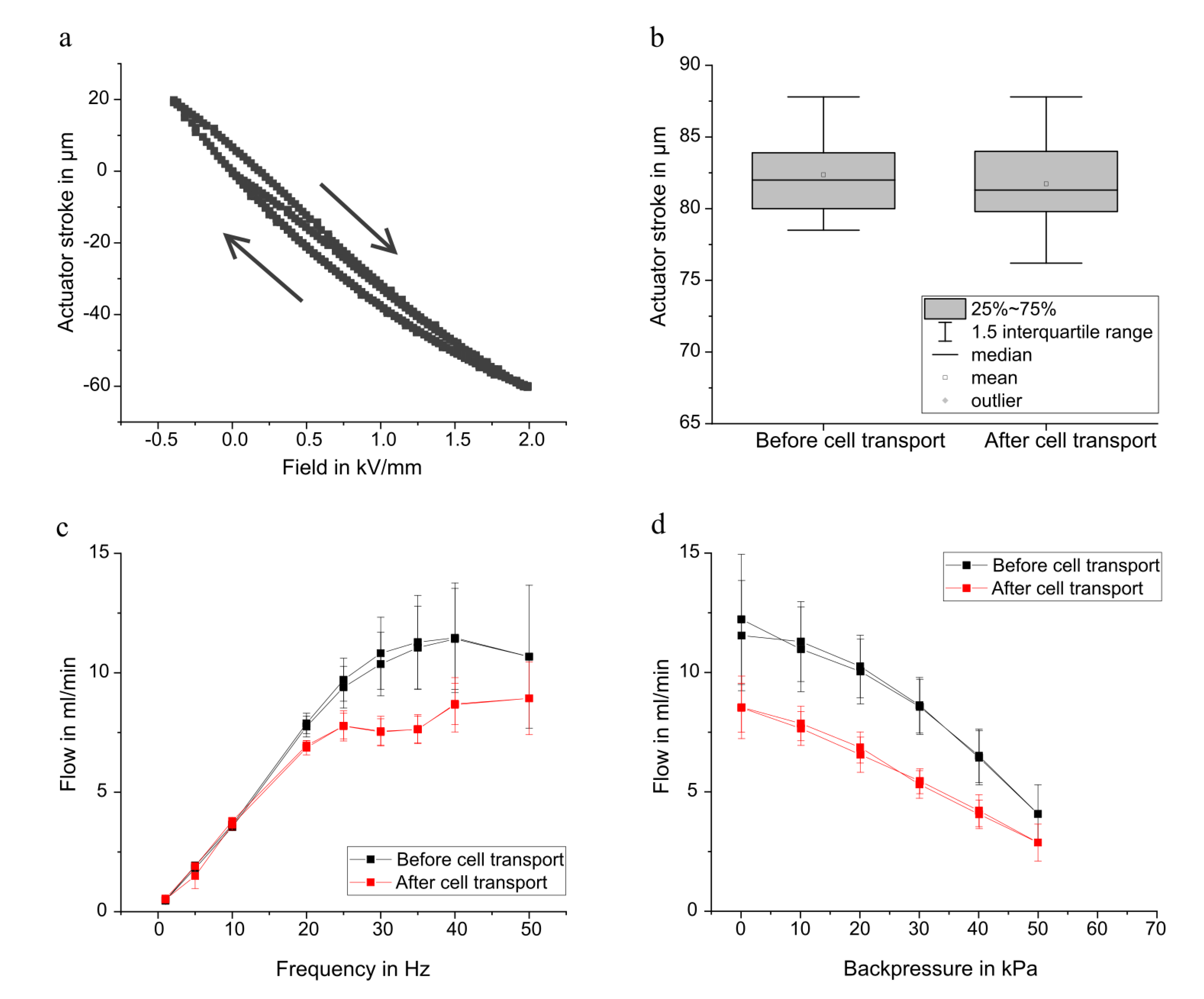
2.1.1. Actuator Stroke Measurements
2.1.2. Fluidic Characterisation
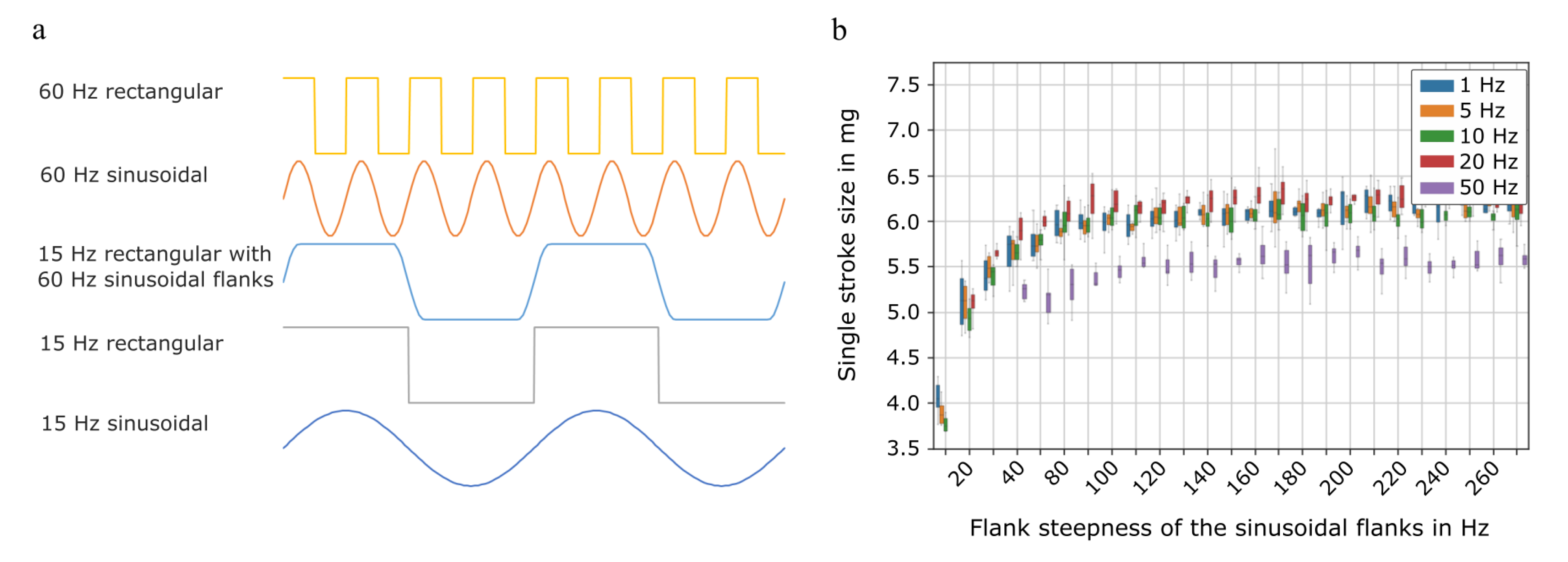
2.1.3. Single Stroke Characterisation
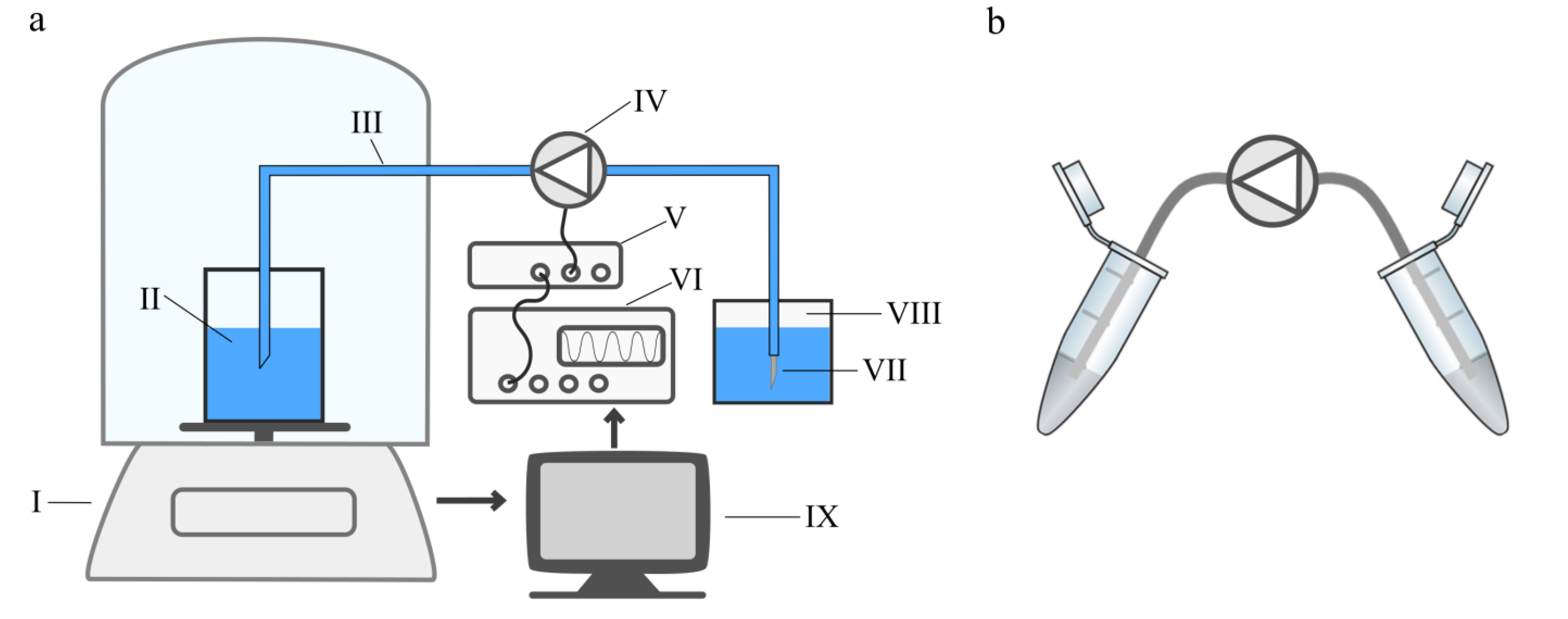
2.2. Cell Transport
Propidium Iodide Cell Viability by Flow Cytometry
3. Results and Discussion
3.1. Pump Characterisation
3.1.1. General Micro Diaphragm Pump Characterisation
3.1.2. Single Stroke Characterisation
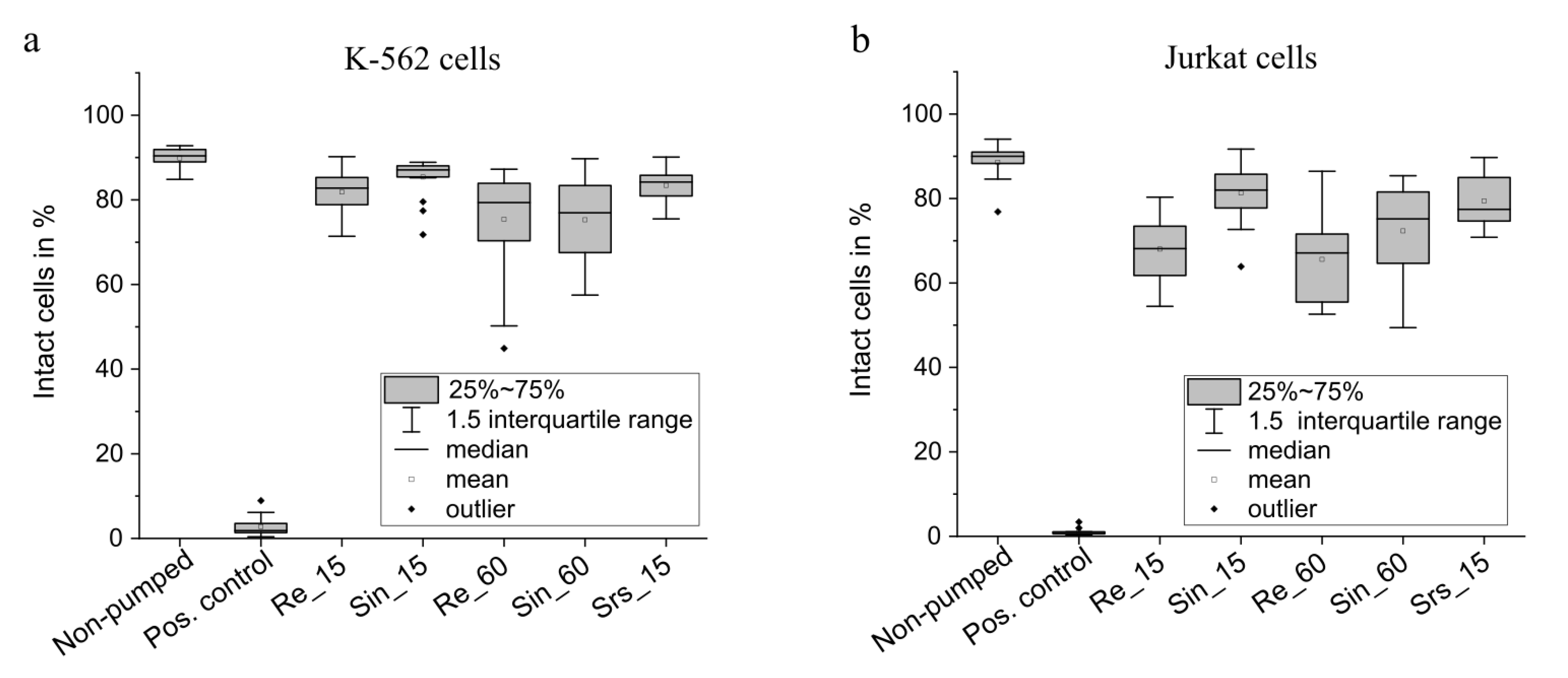
3.2. Cell Transport
3.2.1. Consideration of Effects Influencing the Cell Viability
3.2.2. Viability of K-562 and Jurkat Cells after Transport
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Experimental Setup for Cell Transport and Gravimetric Measurement
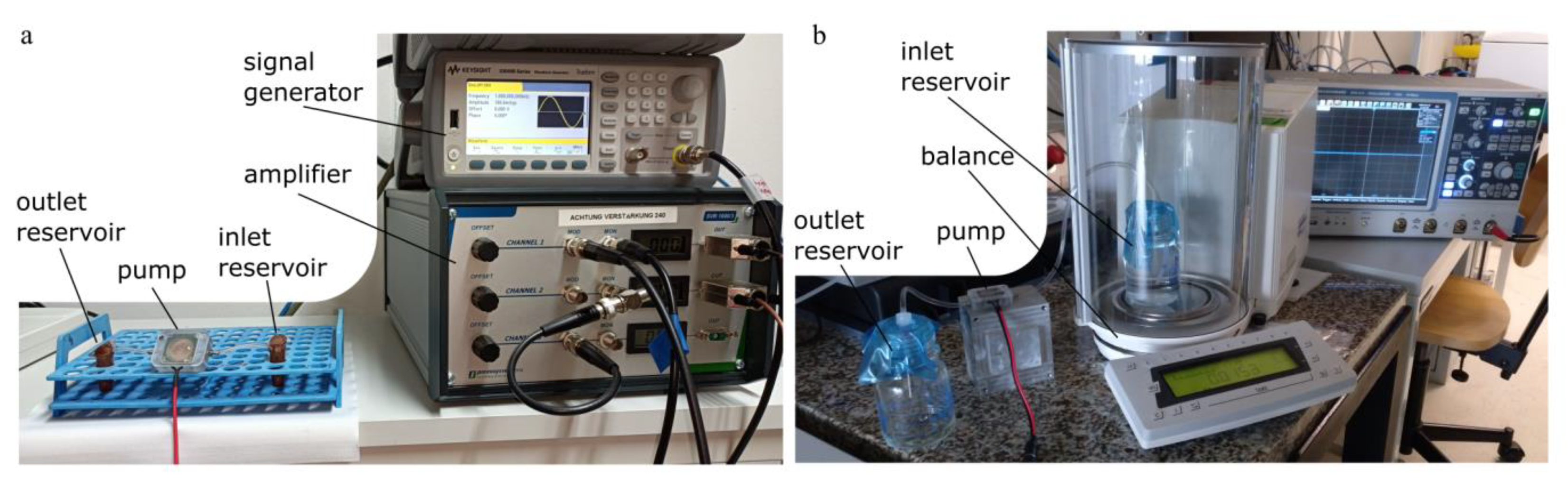
Appendix B
Flow Characterisation with Different Actuation Waveforms
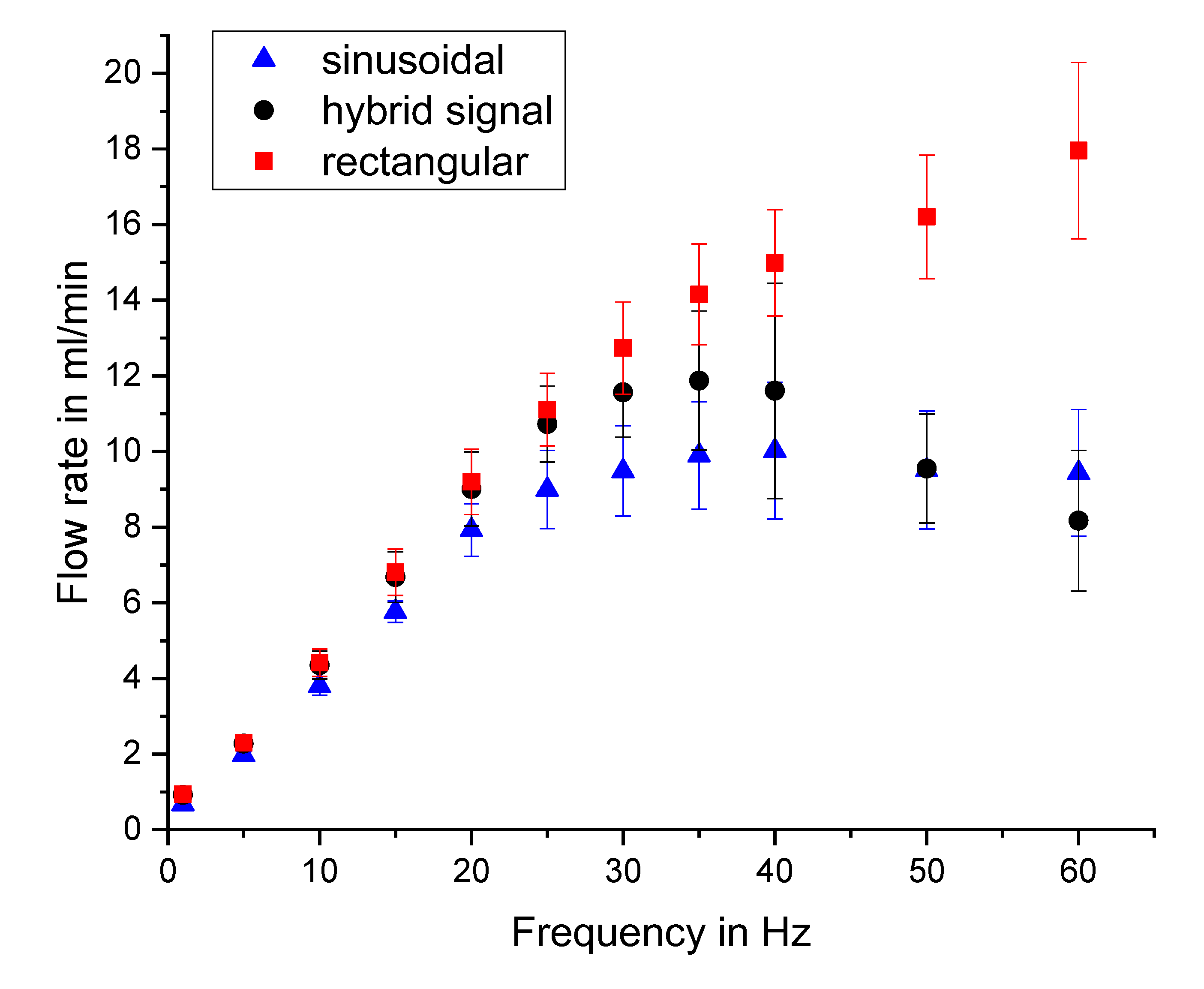
References
- Kurniawan, Y.S. Micro Total Analysis System Application for Biomedicals: A Mini-Review. Biomed. J. Sci. Tech. Res. 2019, 12, 1–2. [Google Scholar] [CrossRef]
- Patabadige, D.E.W.; Jia, S.; Sibbitts, J.; Sadeghi, J.; Sellens, K.; Culbertson, C. Micro Total Analysis Systems: Fundamental Advances and Applications. Anal. Chem. 2016, 88, 320–338. [Google Scholar] [CrossRef]
- Bancroft, G.N.; Sikavitsas, V.I.; Mikos, A.G. Technical Note: Design of a Flow Perfusion Bioreactor System for Bone Tissue-Engineering Applications. Tissue Eng. 2003, 9, 549–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khait, L.; Hecker, L.; Radnoti, D.; Birla, R.K. Micro-Perfusion for Cardiac Tissue Engineering: Development of a Bench-Top System for the Culture of Primary Cardiac Cells. Ann. Biomed. Eng. 2008, 36, 713–725. [Google Scholar] [CrossRef]
- Chung, B.G.; Flanagan, L.A.; Rhee, S.W.; Schwartz, P.H.; Lee, A.P.; Monuki, E.S.; Jeon, N.L. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 2005, 5, 401–406. [Google Scholar] [CrossRef]
- Tourovskaia, A.; Figueroa-Masot, X.; Folch, A. Differentiation-on-a-chip: A microfluidic platform for long-term cell culture studies. Lab Chip 2005, 5, 14–19. [Google Scholar] [CrossRef]
- Elemoso, A.; Shalunov, G.; Balakhovsky, Y.M.; Ostrovskiy, A.Y.; Khesuani, Y.D. 3D Bioprinting: The Roller Coaster Ride to Commercialization. Int. J. Bioprint. 2020, 6, 301. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Peng, W.; Ozbolat, V. Application areas of 3D bioprinting. Drug Discov. Today 2016, 21, 1257–1271. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Unutmaz, D.; Ozbolat, I.T. Bioprinting towards Physiologically Relevant Tissue Models for Pharmaceutics. Trends Biotechnol. 2016, 34, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Datta, P.; Ayan, B.; Ozbolat, V.; Sosnoski, D.; Ozbolat, I.T. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017, 57, 26–46. [Google Scholar] [CrossRef]
- Butzelaar, L.; Ulrich, M.; van der Molen, A.M.; Niessen, F.; Beelen, R. Currently known risk factors for hypertrophic skin scarring: A review. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 163–169. [Google Scholar] [CrossRef]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef]
- Henderson, J.; Arya, R.; Gillespie, P. Skin graft meshing, over-meshing and cross-meshing. Int. J. Surg. 2012, 10, 547–550. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.-C.; Davoodi, P.; Vijayavenkataraman, S.; Tian, Y.; Ng, W.C.; Fuh, J.Y.; Robinson, K.S.; Wang, C.-H. 3D bioprinting of skin tissue: From pre-processing to final product evaluation. Adv. Drug Deliv. Rev. 2018, 132, 270–295. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Günther, A. Handheld skin printer: In situ formation of planar biomaterials and tissues. Lab Chip 2018, 18, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Laser, D.J.; Santiago, J.G. A review of micropumps. J. Micromech. Microeng. 2004, 14, R35. [Google Scholar] [CrossRef]
- Mohith, S.; Karanth, P.N.; Kulkarni, S. Recent trends in mechanical micropumps and their applications: A review. Mechatronics 2019, 60, 34–55. [Google Scholar] [CrossRef]
- Bußmann, A.B.; Grünerbel, L.M.; Durasiewicz, C.P.; Thalhofer, T.A.; Wille, A.; Richter, M. Microdosing for drug delivery application—A review. Sens. Actuators A Phys. 2021, 330, 112820. [Google Scholar] [CrossRef]
- Lai, B.-K.; Kahn, H.; Phillips, S.M.; Heuer, A.H. A Comparison of PZT-Based and TiNi Shape Memory Alloy-Based MEMS Microactuators. Ferroelectrics 2004, 306, 221–226. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Liu, H.-K. Innovative Composite PDMS Micropump with Electromagnetic Drive. Sens. Mater. 2010, 22, 85–100. [Google Scholar]
- Yamahata, C.; Vandevyver, C.; Lacharme, F.; Izewska, P.; Vogel, H.; Freitag, R.; Gijs, M.A.M. Pumping of mammalian cells with a nozzle-diffuser micropump. Lab Chip 2005, 5, 1083–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamahata, C.; Lotto, C.; Al-Assaf, E.; Gijs, M.A.M. A PMMA valveless micropump using electromagnetic actuation. Microfluid. Nanofluid. 2005, 1, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Bußmann, A.B.; Durasiewicz, C.P.; Kibler, S.H.A.; Wald, C.K. Piezoelectric titanium based microfluidic pump and valves for implantable medical applications. Sens. Actuators A Phys. 2021, 323, 112649. [Google Scholar] [CrossRef]
- Thalhofer, T.; Bussmann, A.; Durasiewicz, C.; Hayden, O. Effect of Actuation Signal on Single Stroke Volume in Metal Micro Diaphragm Pumps. In Actuator, Proceedings of the International Conference and Exhibition on New Actuator Systems and Applications, online, February 2021; VDE Verlag GmbH: Berlin, Germany, 2021; pp. 1–4. [Google Scholar]
- Drexler, H.G. Leukemia cell lines: In vitro models for the study of chronic myeloid leukemia. Leuk. Res. 1994, 18, 919–927. [Google Scholar] [CrossRef]
- Schneider, U.; Schwenk, H.-U.; Bornkamm, G. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int. J. Cancer 1977, 19, 621–626. [Google Scholar] [CrossRef]
- Zengerle, R.; Geiger, W.; Richter, M.; Ulrich, J.; Kluge, S.; Richter, A. Transient measurements on miniaturized diaphragm pumps in microfluid systems. Sens. Actuators A Phys. 1995, 47, 557–561. [Google Scholar] [CrossRef]
- Jenke, C.; Kager, S.; Richter, M.; Kutter, C. Flow rate influencing effects of micropumps. Sens. Actuators A Phys. 2018, 276, 335–345. [Google Scholar] [CrossRef]
- Ozbey, A.; Karimzadehkhouei, M.; Kocaturk, N.M.; Bilir, S.E.; Kutlu, O.; Gozuacik, D.; Kosar, A. Inertial focusing of cancer cell lines in curvilinear microchannels. Micro Nano Eng. 2019, 2, 53–63. [Google Scholar] [CrossRef]
- Connolly, S.; McGourty, K.; Newport, D. The in vitro inertial positions and viability of cells in suspension under different in vivo flow conditions. Sci. Rep. 2020, 10, 1711. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Oskooei, A.; i Solvas, X.C.; Demello, A.; Kaigala, G.V. Hydrodynamics in Cell Studies. Chem. Rev. 2018, 118, 2042–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herz, M.; Horsch, D.; Wachutka, G.; Lueth, T.C.; Richter, M. Design of ideal circular bending actuators for high performance micropumps. Sens. Actuators A Phys. 2010, 163, 231–239. [Google Scholar] [CrossRef]
| Waveform | Frequency in Hz | Abbreviation | |
|---|---|---|---|
| Setting 1 | Rectangular | 15 | Re_15 |
| Setting 2 | Sinusoidal | 15 | Sin_15 |
| Setting 3 | Rectangular with 60 Hz sinusoidal flanks (hybrid actuation) | 15 | Srs_15 |
| Setting 4 | Rectangular | 60 | Re_60 |
| Setting 5 | Sinusoidal | 60 | Sin_60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bußmann, A.; Thalhofer, T.; Hoffmann, S.; Daum, L.; Surendran, N.; Hayden, O.; Hubbuch, J.; Richter, M. Microfluidic Cell Transport with Piezoelectric Micro Diaphragm Pumps. Micromachines 2021, 12, 1459. https://doi.org/10.3390/mi12121459
Bußmann A, Thalhofer T, Hoffmann S, Daum L, Surendran N, Hayden O, Hubbuch J, Richter M. Microfluidic Cell Transport with Piezoelectric Micro Diaphragm Pumps. Micromachines. 2021; 12(12):1459. https://doi.org/10.3390/mi12121459
Chicago/Turabian StyleBußmann, Agnes, Thomas Thalhofer, Sophie Hoffmann, Leopold Daum, Nivedha Surendran, Oliver Hayden, Jürgen Hubbuch, and Martin Richter. 2021. "Microfluidic Cell Transport with Piezoelectric Micro Diaphragm Pumps" Micromachines 12, no. 12: 1459. https://doi.org/10.3390/mi12121459
APA StyleBußmann, A., Thalhofer, T., Hoffmann, S., Daum, L., Surendran, N., Hayden, O., Hubbuch, J., & Richter, M. (2021). Microfluidic Cell Transport with Piezoelectric Micro Diaphragm Pumps. Micromachines, 12(12), 1459. https://doi.org/10.3390/mi12121459







