Dynamic pH and Thermal Analysis of Paper-Based Microchip Electrophoresis
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
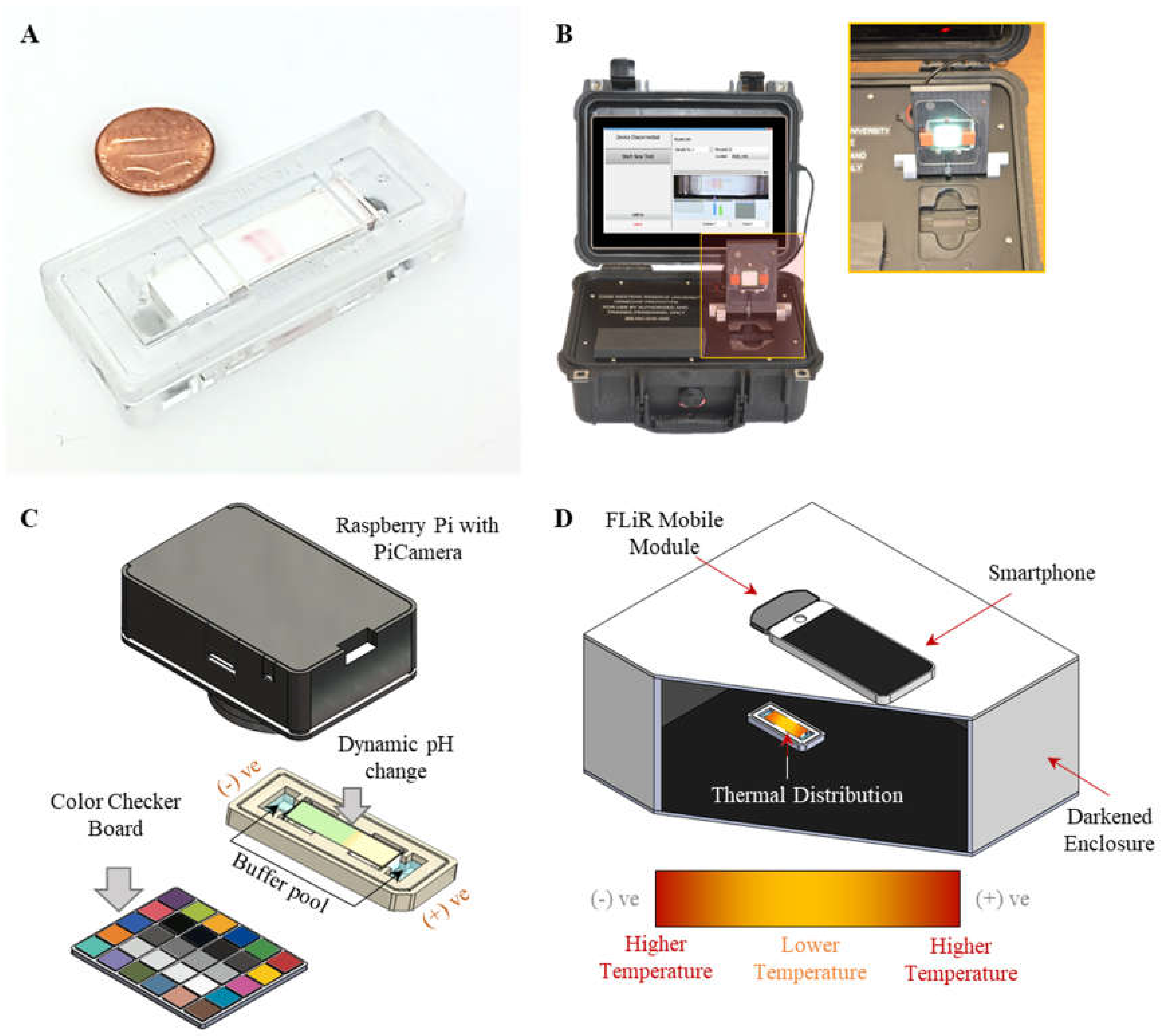
2.2. HemeChip Cartridge and Reader
2.3. Dynamic Track of pH Shift in Microchip Electrophoresis
2.4. Dynamic Tracking of Temperature Shifts in Microchip Electrophoresis
2.5. CA Paper Pre-Treatment
3. Result and Discussion
3.1. pH and Temperature-Tracking System Calibration
3.1.1. pH-Tracking System Calibration
3.1.2. Temperature-Tracking System Calibration
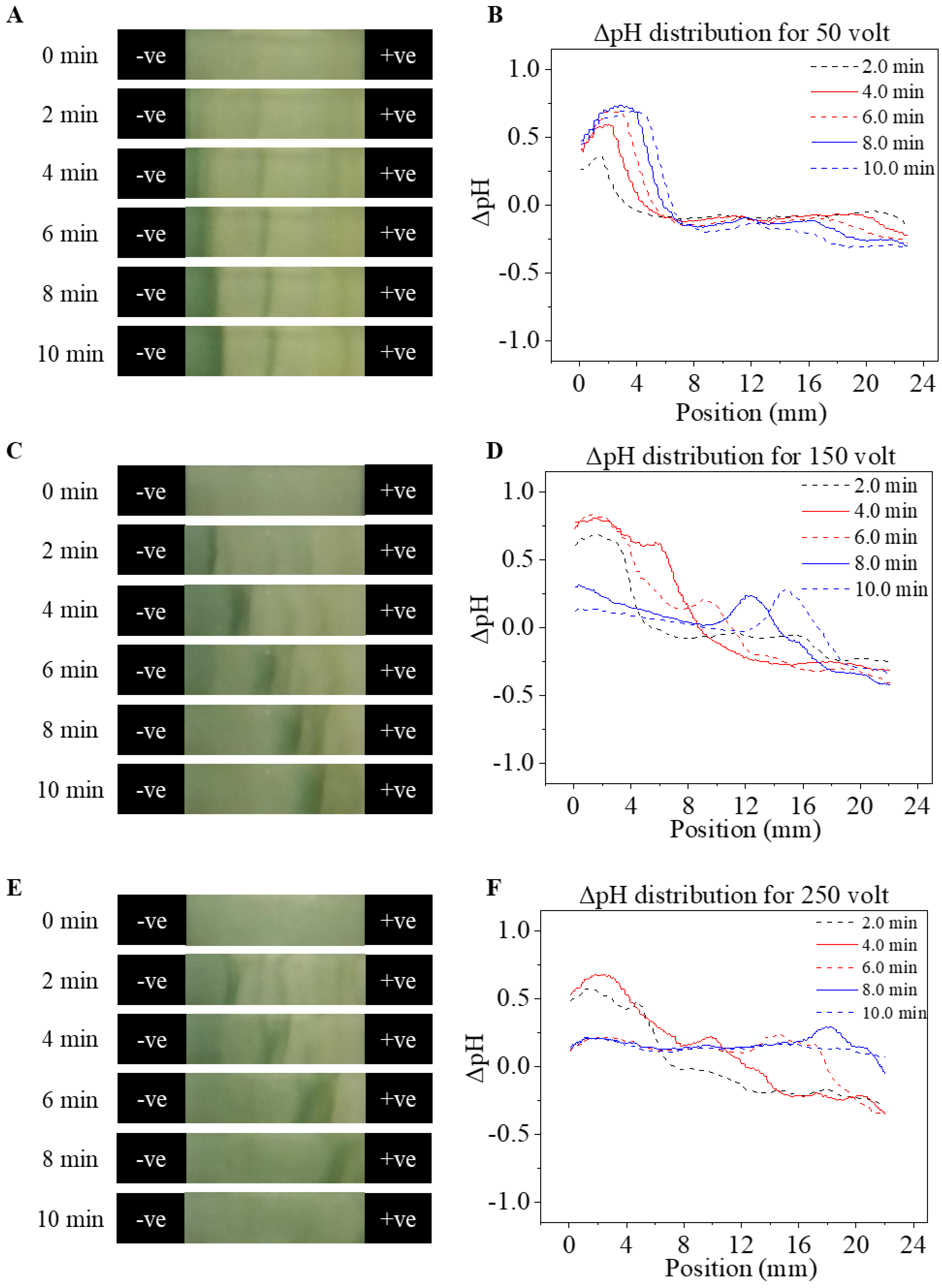
3.2. pH Shift during Paper-Based MicroChip Electrophoresis
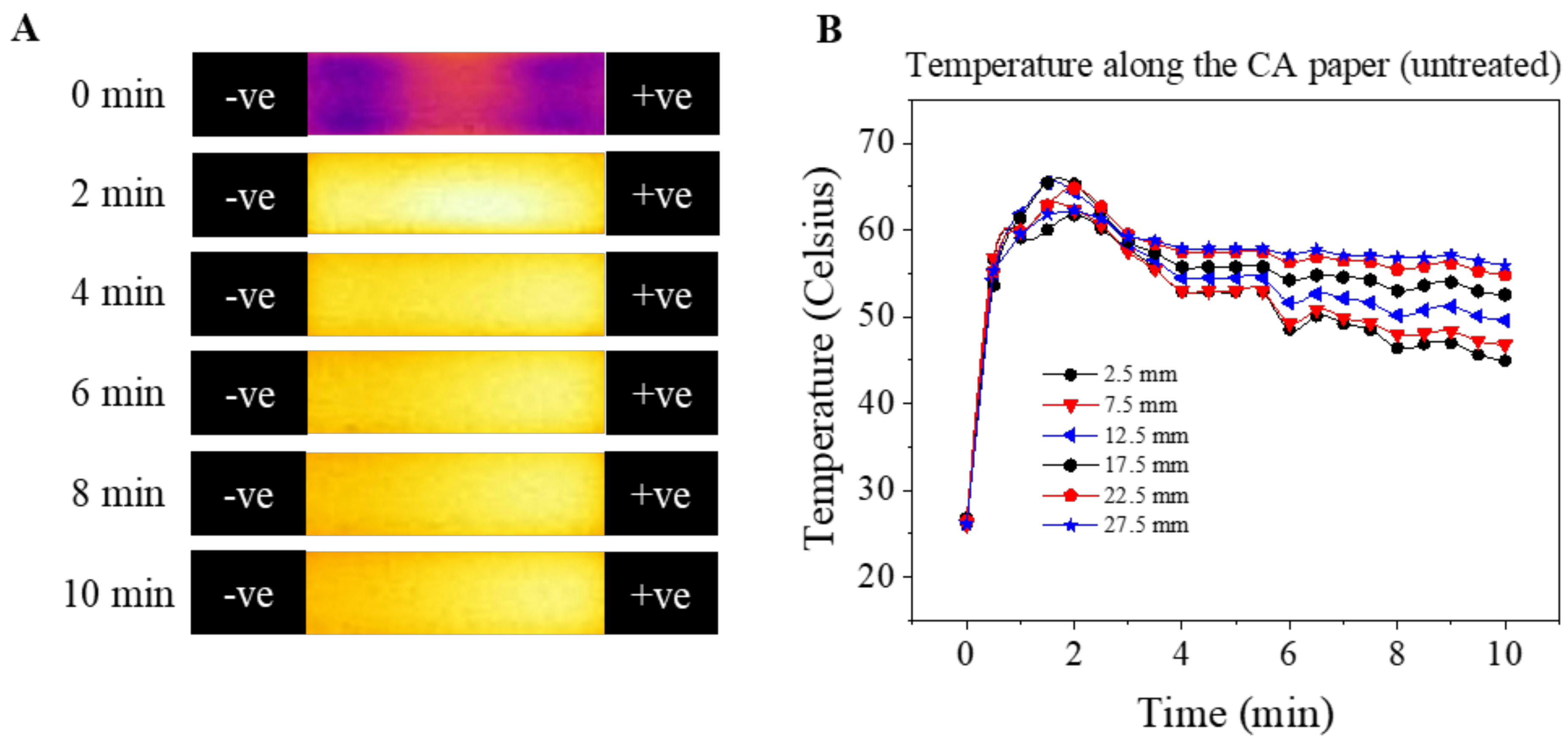
3.3. Temperature Shift during Paper-Based MicroChip Electrophoresis
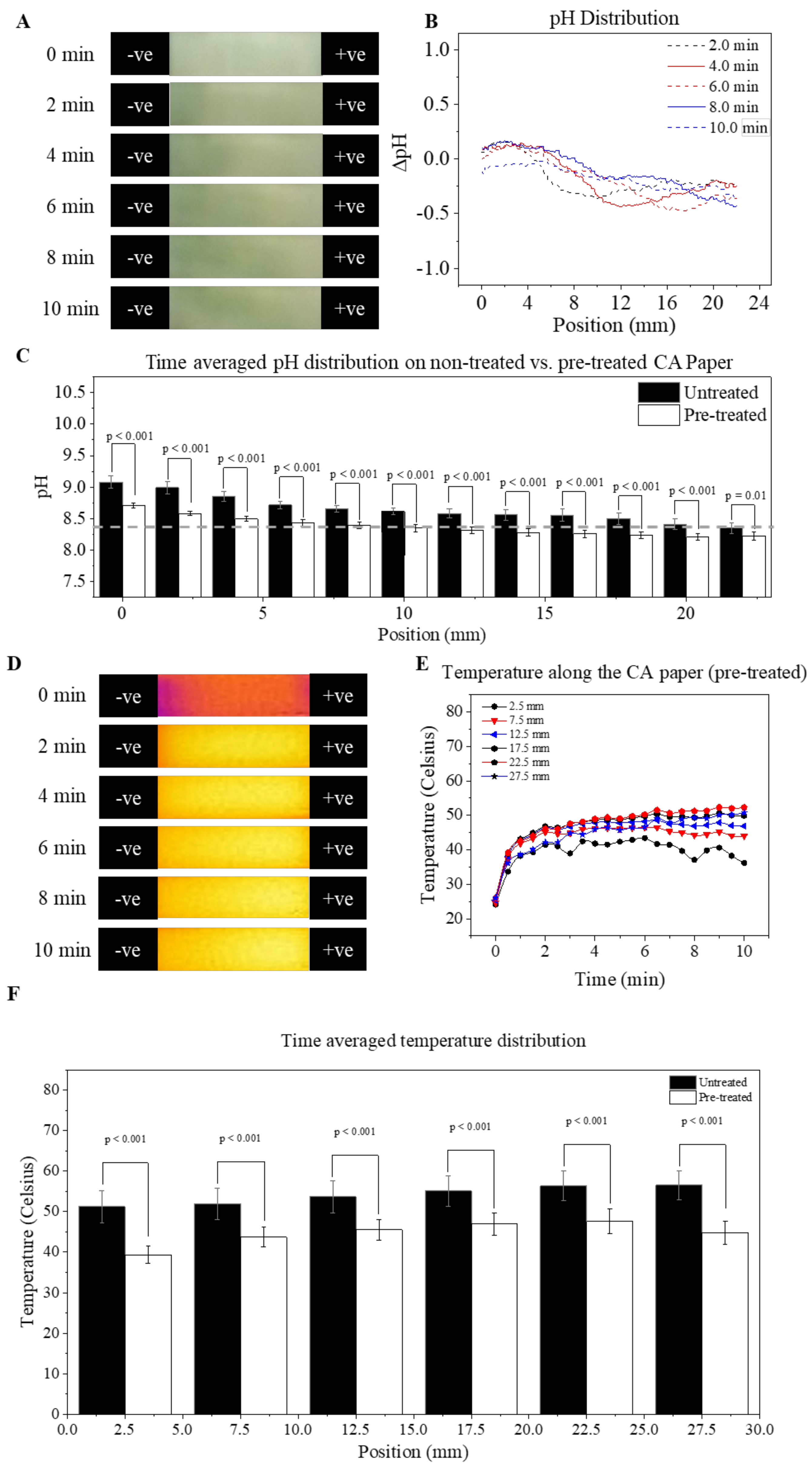
3.4. Mitigation of pH and Temperature Shifts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gong, M.M.; Sinton, D. Turning the Page: Advancing Paper-Based Microfluidics for Broad Diagnostic Application. Chem. Rev. 2017, 117, 8447–8480. [Google Scholar] [CrossRef]
- Li, X.; Ballerini, D.R.; Shen, W. A perspective on paper-based microfluidics: Current status and future trends. Biomicrofluidics 2012, 6, 011301. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yan, S.; Miao, C.; Li, L.; Shi, W.; Liu, X.; Luo, Y.; Liu, T.; Lin, B.; Wu, W.; et al. Paper Microfluidics for Cell Analysis. Adv. Heal. Mater. 2018, 8, e1801084. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, S.; Ge, S.; Yu, J.; Yan, M.; Li, N.; Huang, J. Electrophoretic separation in a microfluidic paper-based analytical device with an on-column wireless electrogenerated chemiluminescence detector. Chem. Commun. 2014, 50, 5699–5702. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, X.; Crooks, R.M. Low-Voltage Origami-Paper-Based Electrophoretic Device for Rapid Protein Separation. Anal. Chem. 2014, 86, 12390–12397. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Ma, B.; Xie, S.F.; Liu, K.; Fang, F. Simultaneous electrokinetic concentration and separation of proteins on a paper-based analytical device. RSC Adv. 2017, 7, 4011–4016. [Google Scholar] [CrossRef]
- An, R.; Man, Y.; Iram, S.; Kucukal, E.; Hasan, M.N.; Solis-Fuentes, A.; Bode, A.; Hill, A.; Cheng, K.; Huang, Y.; et al. Computer Vision and Deep Learning Assisted Microchip Electrophoresis for Integrated Anemia and Sickle Cell Disease Screening. Blood 2020, 136, 46–47. [Google Scholar] [CrossRef]
- Cetin, B.; Li, D. Effect of Joule heating on electrokinetic transport. Electrophoresis 2008, 29, 994–1005. [Google Scholar] [CrossRef]
- Corstjens, H.; Billiet, H.A.; Frank, J.; Luyben, K.C. Variation of the pH of the background electrolyte due to electrode reactions in capillary electropho-resis: Theoretical approach and in situ measurement. Electrophoresis 1996, 17, 137–143. [Google Scholar] [CrossRef]
- Bateman, K.P. Electrochemical properties of capillary electrophoresis-nanoelectrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 1999, 10, 309–317. [Google Scholar] [CrossRef][Green Version]
- Smith, A.D.; Moini, M. Control of Electrochemical Reactions at the Capillary Electrophoresis Outlet/Electrospray Emitter Electrode under CE/ESI-MS through the Application of Redox Buffers. Anal. Chem. 2001, 73, 240–246. [Google Scholar] [CrossRef]
- Bengtsson, K.; Nilsson, S.; Robinson, N.D. Conducting Polymer Electrodes for Gel Electrophoresis. PLoS ONE 2014, 9, e89416. [Google Scholar] [CrossRef]
- Erlandsson, P.G.; Robinson, N.D. Electrolysis-reducing electrodes for electrokinetic devices. Electrophoresis 2011, 32, 784–790. [Google Scholar] [CrossRef]
- Lacher, N.A.; Garrison, K.E.; Martin, R.S.; Lunte, S.M. Microchip capillary electrophoresis/electrochemistry. Electrophoresis 2001, 22, 2526–2536. [Google Scholar] [CrossRef]
- Evenhuis, C.J.; Haddad, P.R. Joule heating effects and the experimental determination of temperature during CE. Electrophoresis 2009, 30, 897–909. [Google Scholar] [CrossRef]
- Rathore, A.S. Joule heating and determination of temperature in capillary electrophoresis and capillary electrochromatography columns. J. Chromatogr. A 2004, 1037, 431–443. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Hasan, M.N.; Man, Y.; Gurkan, U.A. Integrated Anemia Detection and Hemoglobin Variant Identification Using Point-of-Care Microchip Electrophoresis. Blood 2019, 134 (Suppl. S1), 378. [Google Scholar] [CrossRef]
- Hasan, M.N.; Fraiwan, A.; An, R.; Alapan, Y.; Ung, R.; Akkus, A.; Xu, J.Z.; Rezac, A.J.; Kocmich, N.J.; Creary, M.S.; et al. Paper-based microchip electrophoresis for point-of-care hemoglobin testing. Analyst 2020, 145, 2525–2542. [Google Scholar] [CrossRef] [PubMed]
- Fraiwan, A.; Hasan, M.N.; An, R.; Xu, J.Z.; Rezac, A.J.; Kocmich, N.J.; Oginni, T.; Olanipekun, G.M.; Jibir, B.W.; Gambo, S.; et al. International Multi-Site Clinical Validation of Point-of-Care Microchip Electrophoresis Test for Hemoglobin Variant Identification. Blood 2019, 134 (Suppl. S1), 3373. [Google Scholar] [CrossRef]
- Hasan, M.N.; Fraiwan, A.; Little, J.A.; Gurkan, U.A. Hemechip: An Automated Portable Microchip Electrophoresis Platform for Point-of-Care Sickle Cell Disease Screening. Blood 2017, 130 (Suppl. S1), 3519. [Google Scholar]
- Hasan, M.N.; Fraiwan, A.; Gurkan, U.A.; Little, J.A. Live Demonstration: HemeChip—A Portable Microchip Electrophoresis Technology for Point-of-Care Sickle Cell Disease Screening. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; p. 1. [Google Scholar]
- Hasan, M.N.; Fraiwan, A.; Thota, P.; Oginni, T.; Olanipekun, G.M.; Hassan-Hanga, F.; Little, J.; Obaro, S.K.; Gurkan, U.A. Clinical Testing of Hemechip in Nigeria for Point-of-Care Screening of Sickle Cell Disease. Blood 2018, 132, 1095. [Google Scholar] [CrossRef]
- Ung, R.; Alapan, M.Y.; Hasan, M.M.N.; Romelfanger, M.; He, M.P.; Tam, A.; Rosanwo, B.T.; Akkus, A.; Cakar, M.A.; Icoz, K.; et al. Point-of-Care Screening for Sickle Cell Disease By a Mobile Micro-Electrophoresis Platform. Blood 2015, 126, 3379. [Google Scholar] [CrossRef]
- Man, Y.; Kucukal, E.; An, R.; Bode, A.; Little, J.A.; Gurkan, U.A. Standardized microfluidic assessment of red blood cell–mediated microcapillary occlusion: Association with clinical phenotype and hydroxyurea responsiveness in sickle cell disease. Microcirculation 2021, 28, e12662. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Kucukal, E.; An, R.; Watson, Q.D.; Bosch, J.; Zimmerman, P.A.; Little, J.A.; Gurkan, U.A. Microfluidic assessment of red blood cell mediated microvascular occlusion. Lab. Chip 2020, 20, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Goreke, U.; Kucukal, E.; Hill, A.; An, R.; Liu, S.; Bode, A.; Solis-Fuentes, A.; Nayak, L.V.; Little, J.A.; et al. Leukocyte adhesion to P-selectin and the inhibitory role of Crizanlizumab in sickle cell disease: A stand-ardized microfluidic assessment. Blood Cells Mol. Dis. 2020, 83, 102424. [Google Scholar] [CrossRef]
- Kucukal, E.; Man, Y.; Hill, A.; Liu, S.; Bode, A.; An, R.; Kadambi, J.; Little, J.A.; Gurkan, U.A. Whole blood viscosity and red blood cell adhesion: Potential biomarkers for targeted and curative therapies in sickle cell disease. Am. J. Hematol. 2020, 95, 1246–1256. [Google Scholar] [CrossRef]
- Noomuna, P.; Risinger, M.; Zhou, S.; Seu, K.; Man, Y.; An, R.; Sheik, D.A.; Wan, J.; Little, J.A.; Gurkan, U.A.; et al. Inhibition of Band 3 tyrosine phosphorylation: A new mechanism for treatment of sickle cell disease. Br. J. Haematol. 2020, 190, 599–609. [Google Scholar] [CrossRef]
- An, R.; Massa, K.; Wipf, D.O.; Minerick, A.R. Solution pH change in non-uniform alternating current electric fields at frequencies above the electrode charging frequency. Biomicrofluidics 2014, 8, 064126. [Google Scholar] [CrossRef]
- Scheinberg, H.I. The Structural Basis of Differences in Electrophoretic Behavior of Human Hemoglobins. In Proceedings of the Conference on Hemoglobin, Washington, DC, USA, 2–3 May 1957. Available online: https://www.ncbi.nlm.nih.gov/books/NBK224284/ (accessed on 30 September 2019).
- Miralles, V.; Huerre, A.; Malloggi, F.; Jullien, M.-C. A Review of Heating and Temperature Control in Microfluidic Systems: Techniques and Applications. Diagnostics 2013, 3, 33–67. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Bhaskaran, G.; Shuaib, N.; Saidur, R. Heat transfer and fluid flow characteristics in microchannels heat exchanger using nanofluids: A review. Renew. Sustain. Energy Rev. 2011, 15, 1502–1512. [Google Scholar] [CrossRef]
- Wang, Z.; Ivory, C.; Minerick, A.R. Surface isoelectric focusing (sIEF) with carrier ampholyte pH gradient. Electrophoresis 2017, 38, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Rogacs, A.; Santiago, J.G. Temperature Effects on Electrophoresis. Anal. Chem. 2013, 85, 5103–5113. [Google Scholar] [CrossRef] [PubMed]
- Kestin, J.; Sokolov, M.; Wakeham, W.A. Viscosity of liquid water in the range −8 °C to 150 °C. J. Phys. Chem. Ref. Data 1978, 7, 941–948. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.N.; An, R.; Akkus, A.; Akkaynak, D.; Minerick, A.R.; Kharangate, C.R.; Gurkan, U.A. Dynamic pH and Thermal Analysis of Paper-Based Microchip Electrophoresis. Micromachines 2021, 12, 1433. https://doi.org/10.3390/mi12111433
Hasan MN, An R, Akkus A, Akkaynak D, Minerick AR, Kharangate CR, Gurkan UA. Dynamic pH and Thermal Analysis of Paper-Based Microchip Electrophoresis. Micromachines. 2021; 12(11):1433. https://doi.org/10.3390/mi12111433
Chicago/Turabian StyleHasan, Muhammad Noman, Ran An, Asya Akkus, Derya Akkaynak, Adrienne R. Minerick, Chirag R. Kharangate, and Umut A. Gurkan. 2021. "Dynamic pH and Thermal Analysis of Paper-Based Microchip Electrophoresis" Micromachines 12, no. 11: 1433. https://doi.org/10.3390/mi12111433
APA StyleHasan, M. N., An, R., Akkus, A., Akkaynak, D., Minerick, A. R., Kharangate, C. R., & Gurkan, U. A. (2021). Dynamic pH and Thermal Analysis of Paper-Based Microchip Electrophoresis. Micromachines, 12(11), 1433. https://doi.org/10.3390/mi12111433







