Synthesis and Characteristics of Double-Shell Mesoporous Hollow Silica Nanomaterials to Improve CO2 Adsorption Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
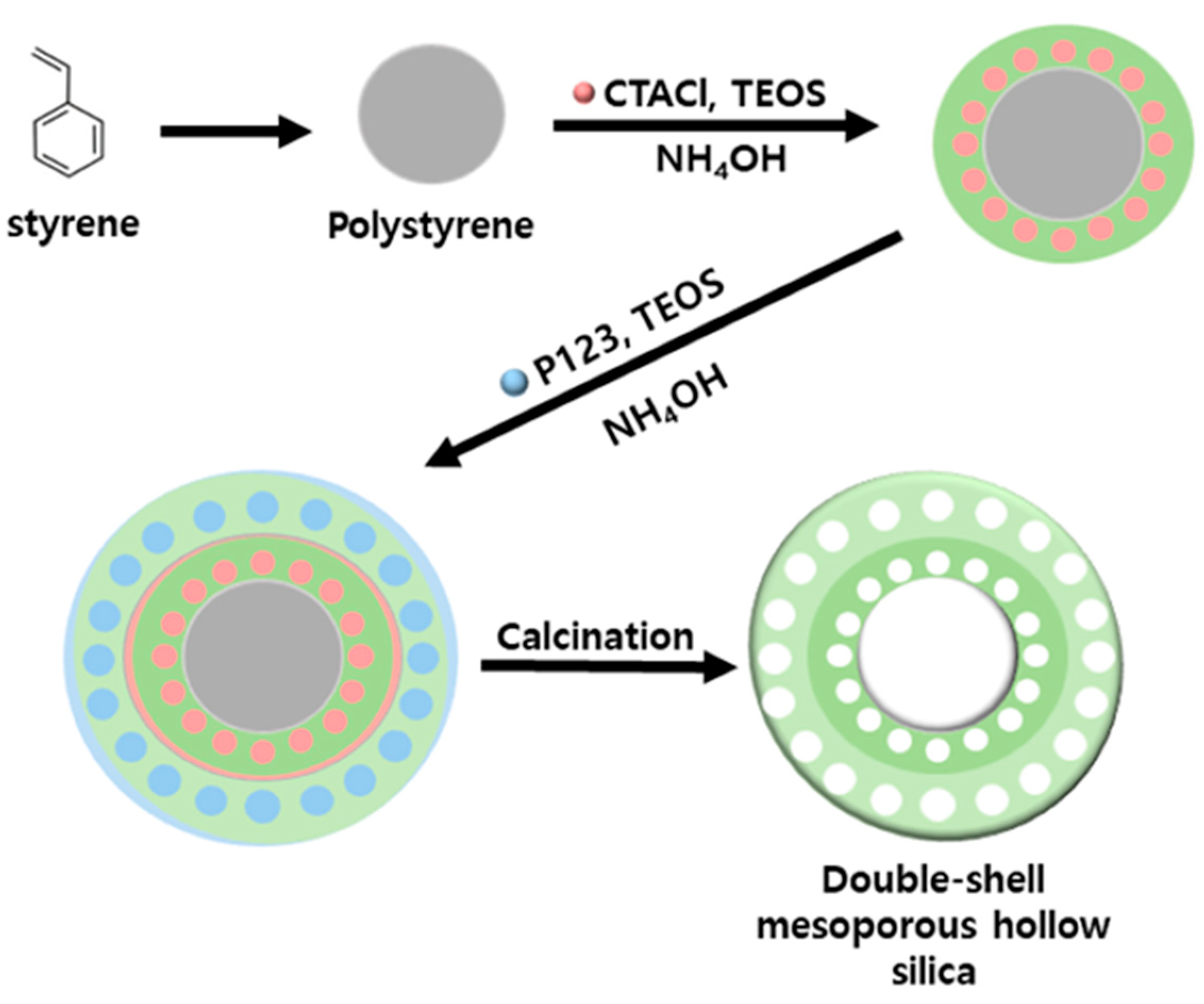
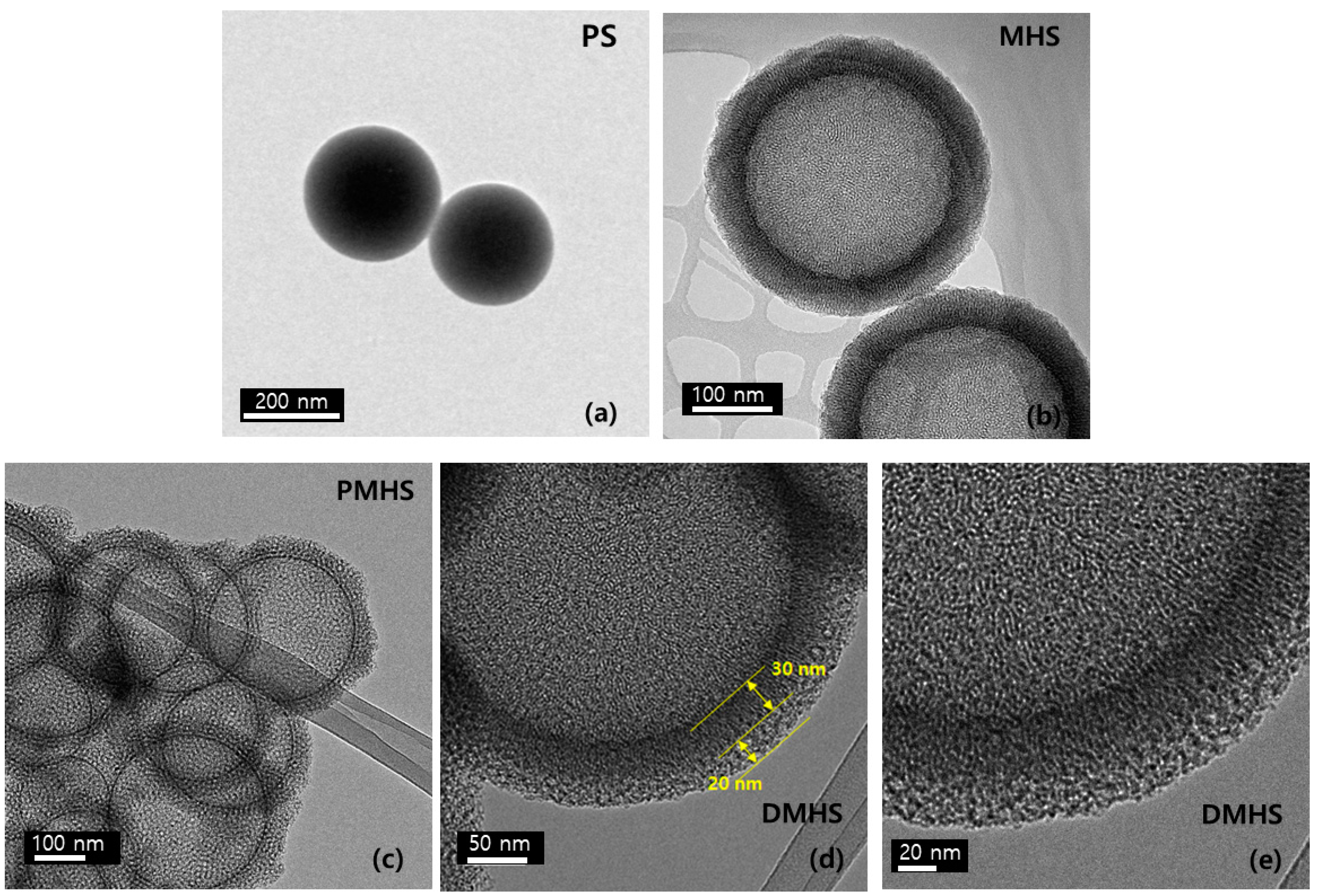
2.2. Polystyrene Synthesis
2.3. Synthesis of Mesoporous Hollow Silica
2.4. Synthesis of the Double-Shell Mesoporous Hollow Silica
2.5. Introduction of the Amine Group
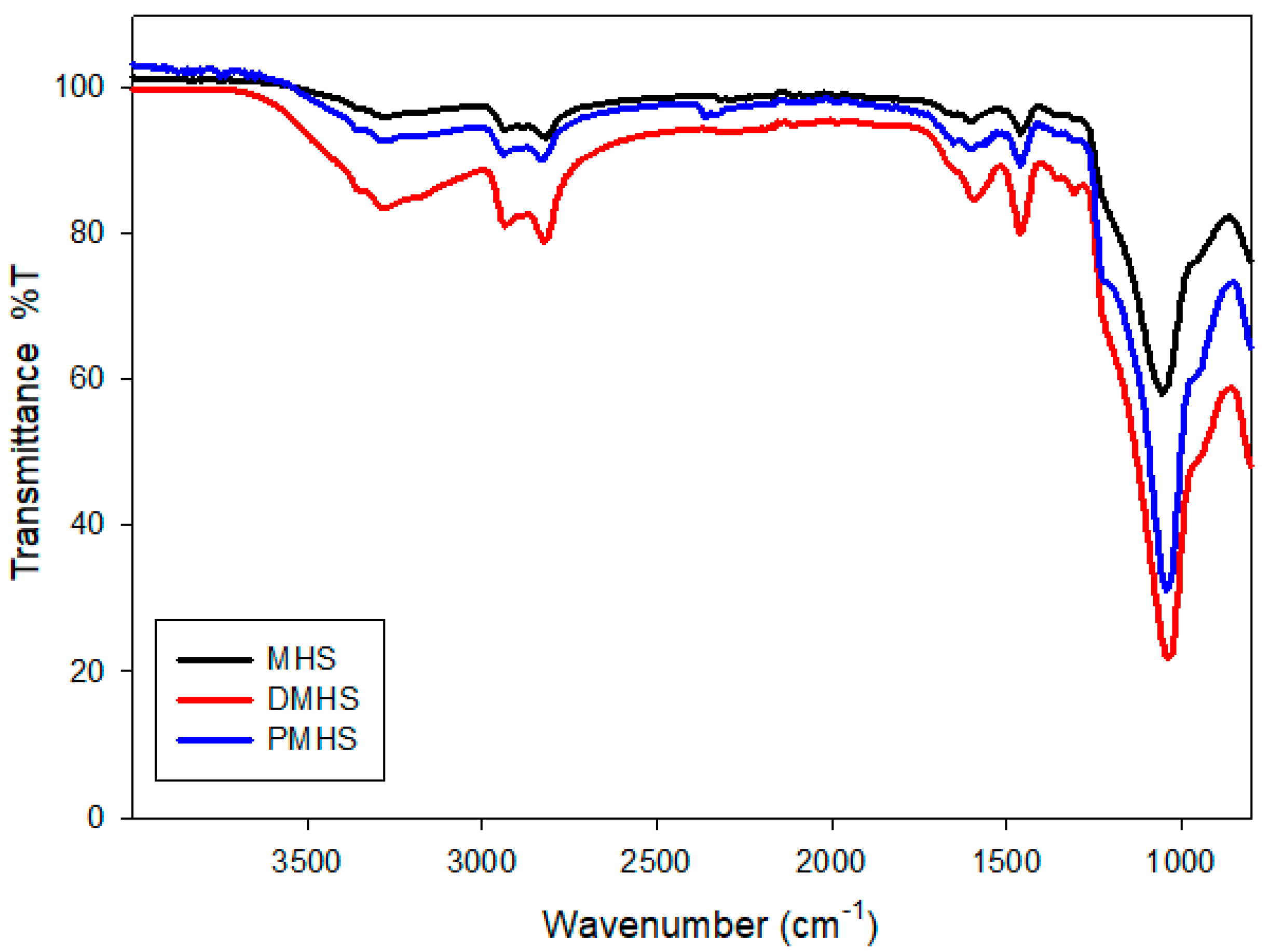
2.6. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Galan-Mascaros, J.R. Photoelectrochemical solar fuels from carbon dioxide, water and sunlight. Catal. Sci. Technol. 2020, 10, 1967–1974. [Google Scholar] [CrossRef]
- Siegelman, R.L.; Kim, E.J.; Long, J.R. Porous materials for carbon dioxide separations. Nat. Mat. 2021, 20, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Hussin, F.; Aroua, M.K. Recent trends in the development of adsorption technologies for carbon dioxide capture: A brief literature and patent reviews (2014–2018). J. Clean. Prod. 2020, 253, 119707–119732. [Google Scholar] [CrossRef]
- Sperry, J.S.; Venturas, M.D.; Todd, H.N.; Trugman, A.T.; Anderegg, W.R.L.; Wang, Y.; Tai, X. The impact of rising CO2 and acclimation on the response of US forests to global warming. Proc. Natl. Acad. Sci. USA 2019, 116, 25734–25744. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon dioxide adsorption based on porous materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203–143214. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Zaharioiu, A.; Oancea, S.; Bucura, F.; Raboaca, M.S.; Filote, C.; Iomete, R.E.; Niculescu, V.C.; Constantinescu, M. Sewage sludge derived materials for CO2 adsorption. Appl. Sci. 2021, 11, 7139. [Google Scholar] [CrossRef]
- Liu, S.; Han, X.; Chai, Y.; Wu, G.; Li, W.; Li, J.; Manuel, I.S.P.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; et al. Efficient Separation of Acetylene and Carbon Dioxide in a Decorated Zeolite. Angew. Chem. Int. Ed. 2021, 60, 6526–6532. [Google Scholar] [CrossRef] [PubMed]
- Harlick, P.; Tezel, F.H. An experimental adsorbent screening study for CO2 removal from N2. Microporous Mesoporous Mater. 2004, 76, 71–79. [Google Scholar] [CrossRef]
- Karimi, M.; Rodrigues, A.E.; Silva, J.A.C. Designing a simple volumetric apparatus for measuring gas adsorption equilibria and kinetics of sorption. Application and validation for CO2, CH4 and N2 adsorption in binder-free beads of 4A zeolite. Chem. Eng. J. 2021, 425, 130538–130549. [Google Scholar] [CrossRef]
- Yoo, H.M.; Lee, S.Y.; Park, S.J. Ordered nanoporous carbon for increasing CO2 capture. J. Solid State Chem. 2013, 197, 361–365. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Zheng, Q.; Qiao, Y.; Wang, Q. Layered double hydroxides/oxidized carbon nanotube nanocomposites for CO2 capture. J. Ind. Eng. Chem. 2016, 36, 255–262. [Google Scholar] [CrossRef]
- Bae, J.Y. Analysis of CO2 adsorption characteristics of activated carbon loaded with alkali and alkaline earth metal. J. Nanosci. Nanotechnol. 2018, 18, 6101–6105. [Google Scholar] [CrossRef]
- Rasjidi, N.A.; Bokhari, A.; Yusup, S. Evaluation of kinetics and mechanism properties of CO2 adsorption onto the palm kernel shell activated carbon. Environ. Sci. Pollut. Res. 2021, 28, 33967–33979. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Ou, C.; Dong, X. Porous metal−organic frameworks for carbon dioxide adsorption and separation at low pressure. ACS Sustain. Chem. Eng. 2020, 8, 15378–15404. [Google Scholar] [CrossRef]
- Chen, S.; Lucier, B.E.G.; Boyle, P.D.; Huang, Y. Understanding the fascinating origins of CO2 adsorption and dynamics in MOFs. Chem. Mater. 2016, 28, 5829–5846. [Google Scholar] [CrossRef]
- Xue, D.X.; Cairns, A.J.; Belmabkhout, Y.; Wojtas, L.; Liu, Y.; Alkordi, M.H.; Eddaoudi, M. Tunable RE-fcu-MOFs: A platform for systematic enhancement of CO2 adsorption energetics and uptake. J. Am. Chem. Soc. 2013, 135, 7660–7667. [Google Scholar] [CrossRef]
- Ramyashree, M.S.; Priya, S.S.; Freudenberg, N.C.; Sudhakar, K.; Tahir, M. Metal-organic framework-based photocatalysts for carbon dioxide reduction to methanol: A review on progress and application. J. CO2 Util. 2021, 43, 101374–101396. [Google Scholar] [CrossRef]
- Castaldo, R.; Avolio, R.; Cocca, M.; Errico, M.E.; Avella, M.; Gentile, G. Amino-functionalized hyper-crosslinked resins for enhanced adsorption of carbon dioxide and polar dyes. Chem. Eng. J. 2021, 418, 129463–129470. [Google Scholar] [CrossRef]
- Henao, W.; Jaramillo, L.Y.; Lopez, D.; Romero-Saez, M. Buitrago-Sierra, R. Insights into the CO2 capture over amine-functionalized mesoporous silica adsorbents derived from rice husk ash. J. Environ. Chem. Eng. 2020, 8, 104362–104372. [Google Scholar] [CrossRef]
- Guo, X.; Ding, L.; Kanamori, K.; Nakanishi, K.; Yang, H. Functionalization of hierarchically porous silica monoliths with polyethyleneimine (PEI) for CO2 adsorption. Microporous Mesoporous Mater. 2017, 245, 51–57. [Google Scholar] [CrossRef]
- Popa, A.; Borcanescu, S.; Holclajtner-Antunovic, I.; Bajuk-Bogdanovic, D.; Uskokovic-Markovic, S. Preparation and characterisation of amino-functionalized pore-expanded mesoporous silica for carbon dioxide capture. J. Porous Mater. 2021, 28, 143–156. [Google Scholar] [CrossRef]
- Bae, J.Y.; Jang, S.G. Characteristics of CO2 capture by tetraethylenepentamine modified mesoporous silica morphology. J. Nanosci. Nanotechnol. 2019, 19, 6291–6296. [Google Scholar] [CrossRef]
- Chang, A.C.C.; Chuang, S.S.C.; Gray, M.; Soong, Y. In-Situ infrared study of CO2 adsorption on SBA-15 grafted with γ-(Aminopropyl)triethoxysilane. Energy Fuels 2003, 17, 468–473. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Yogo, K.; Yashima, T. Adsorption of carbon dioxide on amine modified SBA-15 in the presence of water vapor. Chem. Lett. 2004, 33, 510–511. [Google Scholar] [CrossRef]
- Bae, J.Y. CO2 Capture by amine-functionalized mesoporous hollow silica. J. Nanosci. Nanotechnol. 2017, 17, 7418–7422. [Google Scholar] [CrossRef]
- Jang, H.R.; Oh, H.J.; Kim, J.H.; Jung, K.Y. Synthesis of mesoporous spherical silica via spray pyrolysis: Pore size control and evaluation of performance in paclitaxel pre-purification. Microporous Mesoporous Mater. 2013, 165, 219–227. [Google Scholar] [CrossRef]
- Yun, J.; Jun, J.; Lee, J.; Ryu, J.; Lee, K.; Yu, H.; Jang, J. Fabrication of monodisperse nitrogen-doped carbon double-shell hollow nanoparticles for supercapacitors. RSC Adv. 2017, 7, 20694–20699. [Google Scholar] [CrossRef] [Green Version]
- Kishor, R.; Ghoshal, A.K. Amine-modified mesoporous silica for CO2 adsorption: The role of structural parameters. Ind. Eng. Chem. Res. 2017, 56, 6078–6087. [Google Scholar] [CrossRef]
- Zheng, F.; Tran, D.N.; Busche, B.J.; Fryxell, G.E.; Addleman, R.S.; Zemanian, T.S.; Aardahl, C.L. Ethylenediamine-modified SBA-15 as regenerable CO2 sorbent. Ind. Eng. Chem. Res. 2005, 44, 3099–3105. [Google Scholar] [CrossRef]
- Yan, X.L.; Zhang, L.; Zhang, Y.; Qiao, K.; Yan, Z.F.; Komarneni, S. Amine-modified mesocellular silica foams for CO2 capture. Chem. Eng. J. 2011, 168, 918–924. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, S.W. Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-modified mesoporous molecular sieve MCM-41. Microporous Mesoporous Mater. 2003, 62, 29–45. [Google Scholar] [CrossRef]

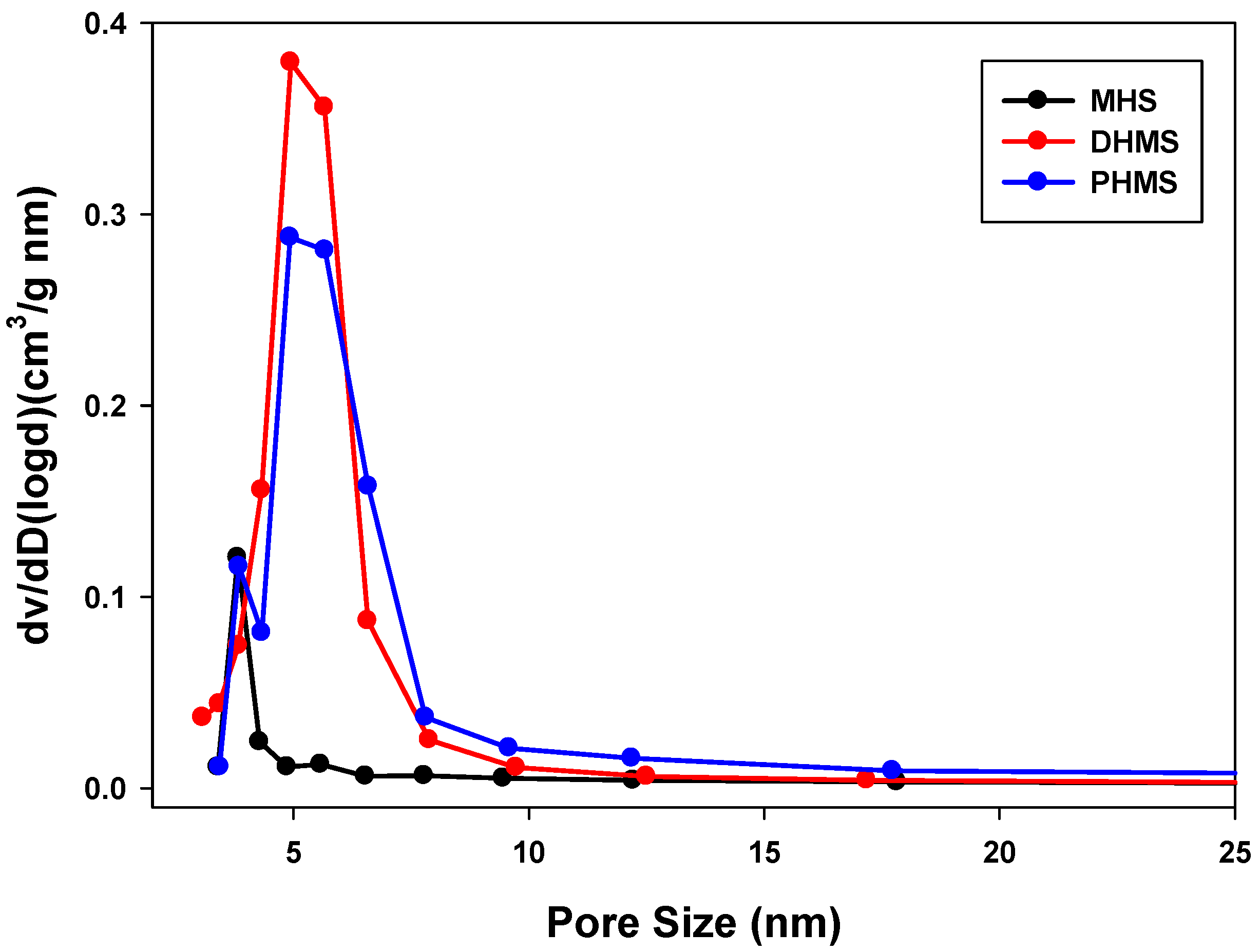
| Sample Name | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size a (nm) |
|---|---|---|---|
| MHS | 1164 | 0.321 | 3.309 |
| PHMS | 609 | 0.933 | 5.679 |
| DMHS | 690 | 1.012 | 4.944 |
| Sample Name | Pore Volume (cm3/g) | Atom Si (%) | Atom N (%) | Atom C (%) | Atom O (%) |
|---|---|---|---|---|---|
| MHS | 0.321 | 12.35 | 7.97 | 44.17 | 35.51 |
| PMHS | 0.933 | 18.65 | 8.33 | 37.21 | 35.81 |
| DMHS | 1.012 | 10.82 | 13.30 | 44.27 | 31.60 |
| Sample Name | Before Amine Modification (g) | After Amine Modification (g) | Amine Modification Percent (%) |
|---|---|---|---|
| MHS | 0.5 | 0.8897 | 77.94 |
| PHMS | 0.5 | 0.9097 | 81.94 |
| DMHS | 0.5 | 0.9984 | 99.68 |
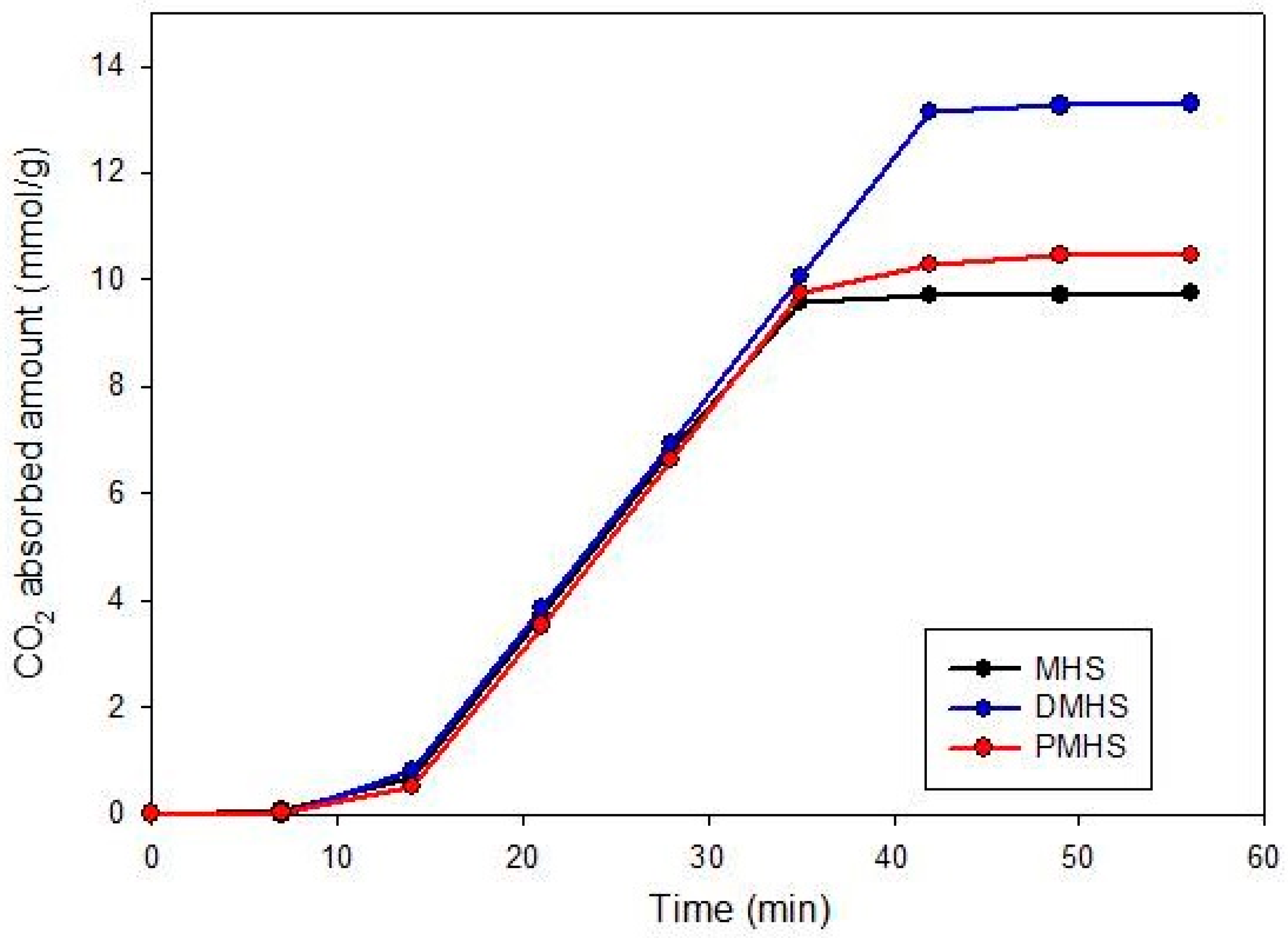
| Sample Name | Pore Volume (cm3/g) | Amine Modification Percent(%) | CO2 Capacity (mmol/g) |
|---|---|---|---|
| MHS | 0.321 | 77.94 | 9.757 |
| PHMS | 0.933 | 81.94 | 10.450 |
| DMHS | 1.012 | 99.68 | 13.296 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-t.; Bae, J.-Y. Synthesis and Characteristics of Double-Shell Mesoporous Hollow Silica Nanomaterials to Improve CO2 Adsorption Performance. Micromachines 2021, 12, 1424. https://doi.org/10.3390/mi12111424
Lee J-t, Bae J-Y. Synthesis and Characteristics of Double-Shell Mesoporous Hollow Silica Nanomaterials to Improve CO2 Adsorption Performance. Micromachines. 2021; 12(11):1424. https://doi.org/10.3390/mi12111424
Chicago/Turabian StyleLee, Jong-tak, and Jae-Young Bae. 2021. "Synthesis and Characteristics of Double-Shell Mesoporous Hollow Silica Nanomaterials to Improve CO2 Adsorption Performance" Micromachines 12, no. 11: 1424. https://doi.org/10.3390/mi12111424
APA StyleLee, J.-t., & Bae, J.-Y. (2021). Synthesis and Characteristics of Double-Shell Mesoporous Hollow Silica Nanomaterials to Improve CO2 Adsorption Performance. Micromachines, 12(11), 1424. https://doi.org/10.3390/mi12111424






