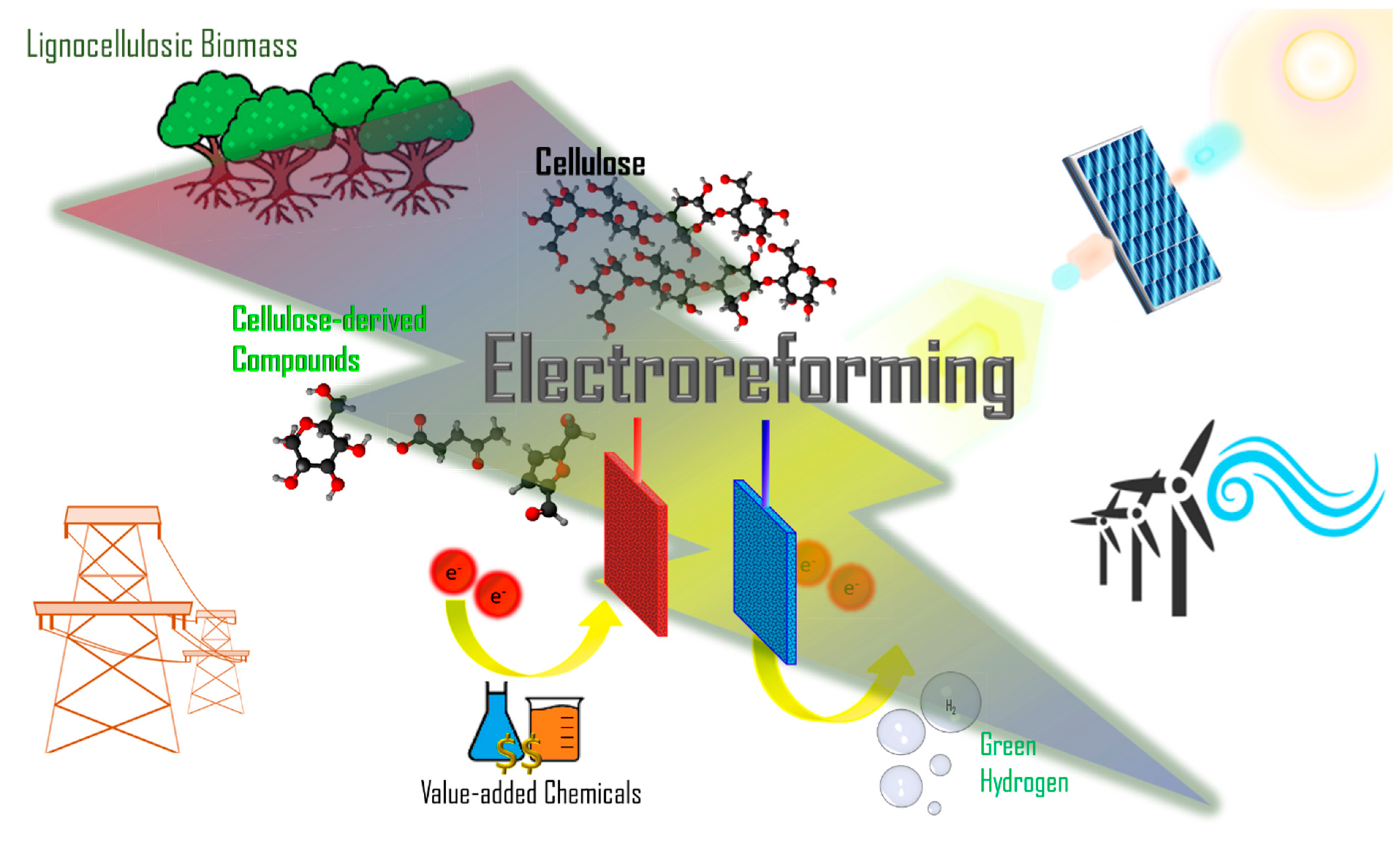
Electroreforming of Biomass for Value-Added Products
Abstract
1. Introduction
2. Electroreforming of Biomass at the Anode
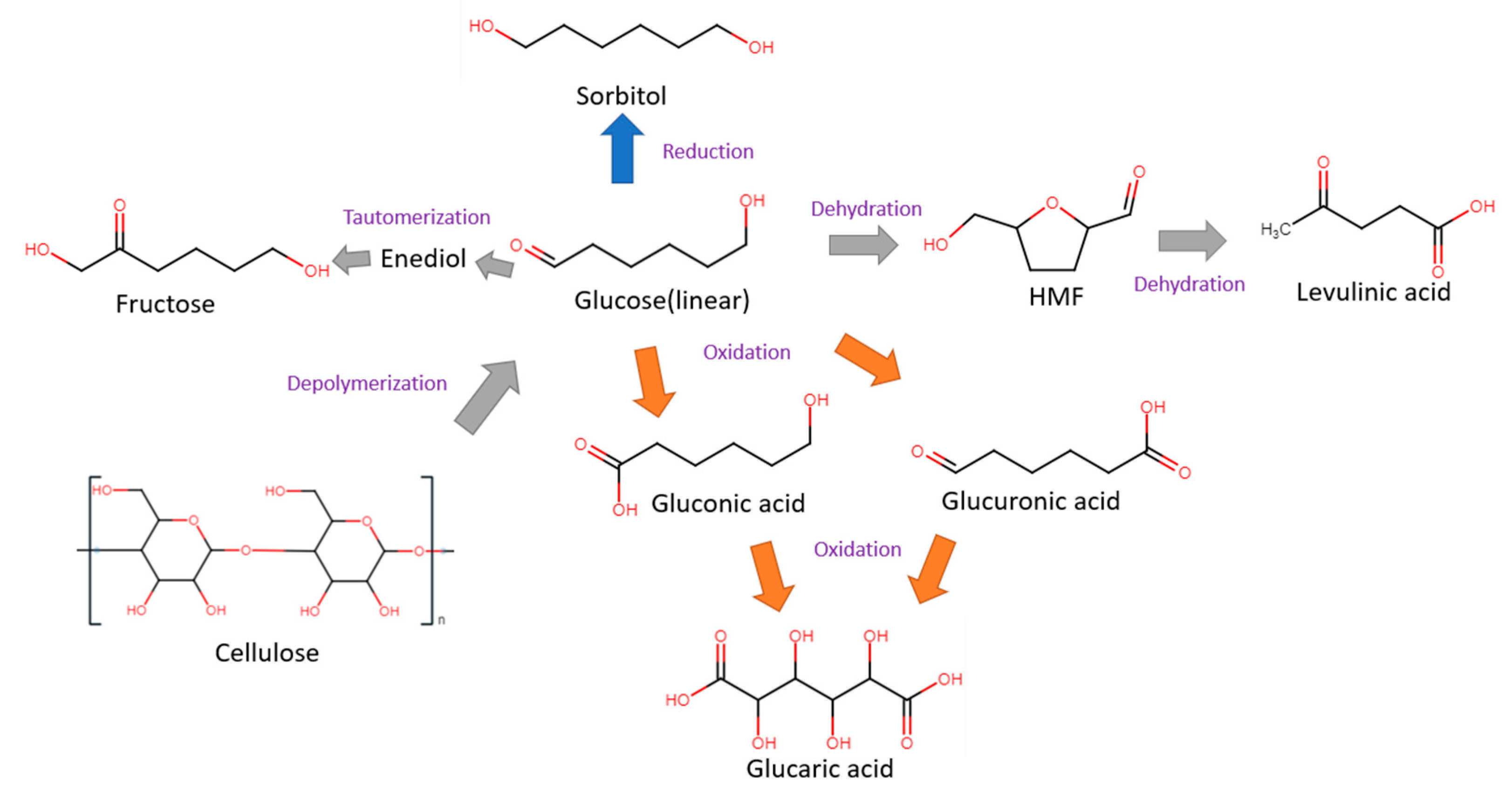
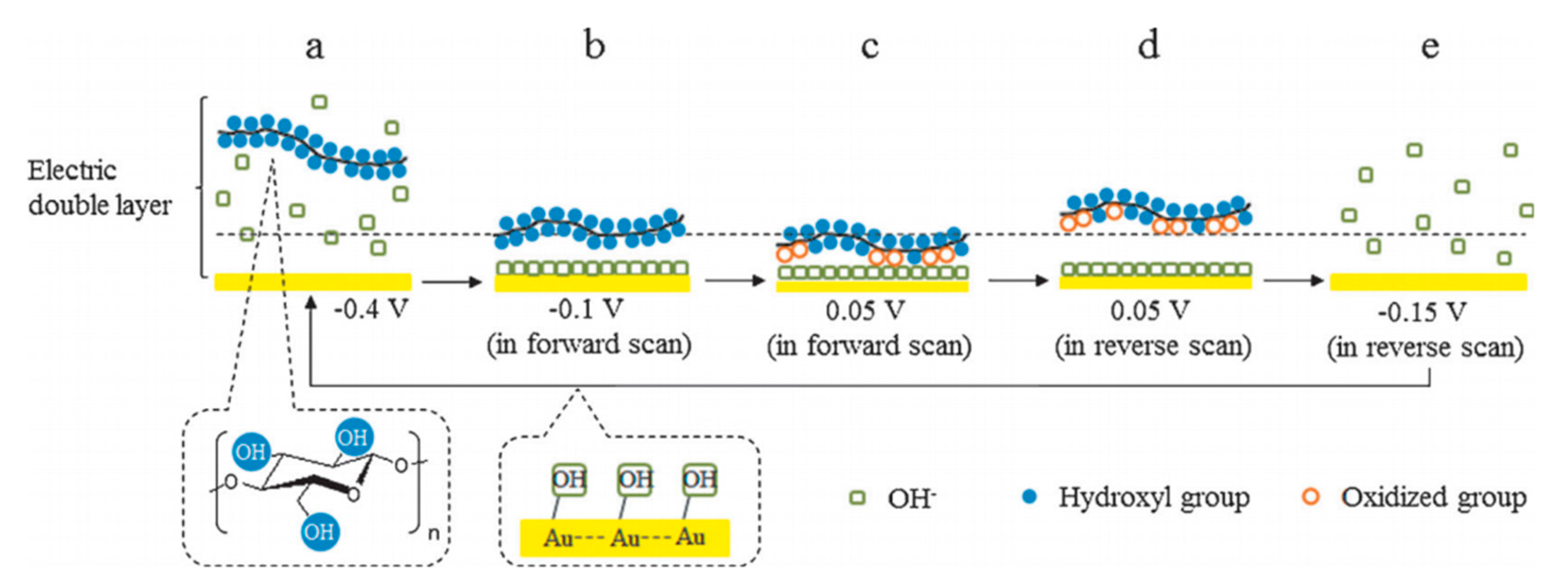
2.1. Cellulose
2.2. Glucose
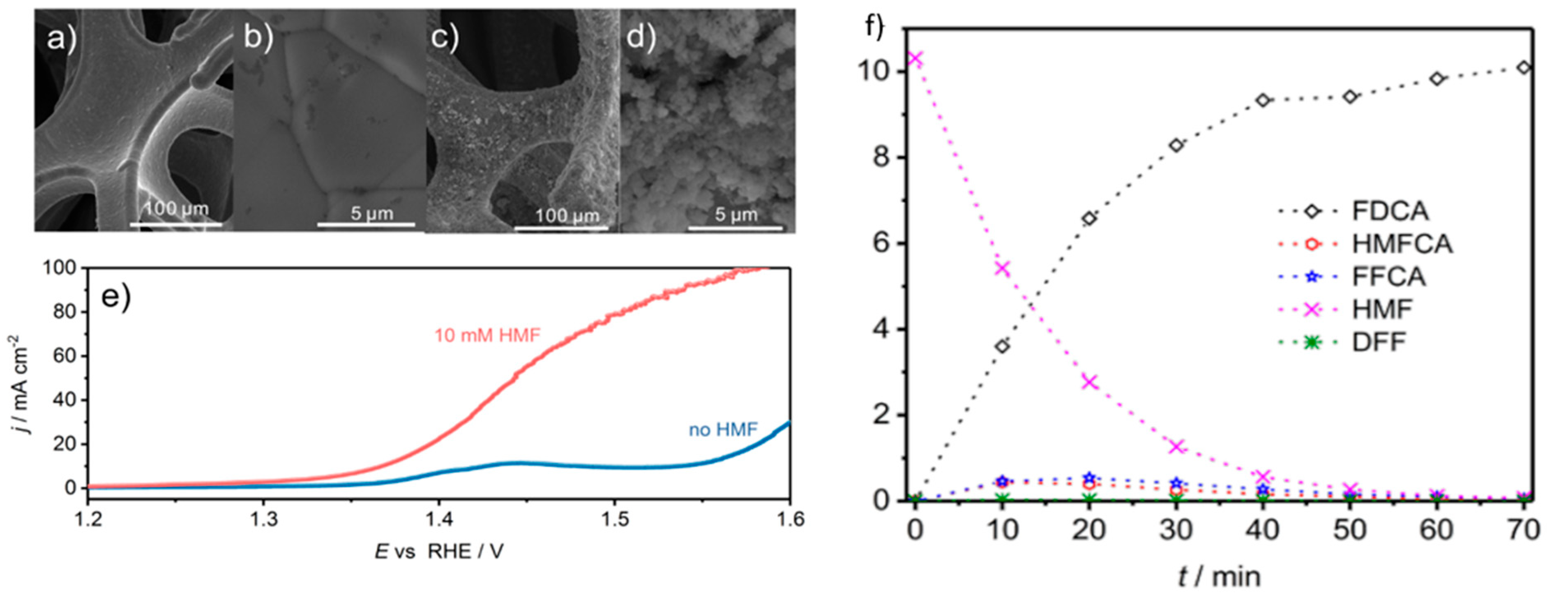
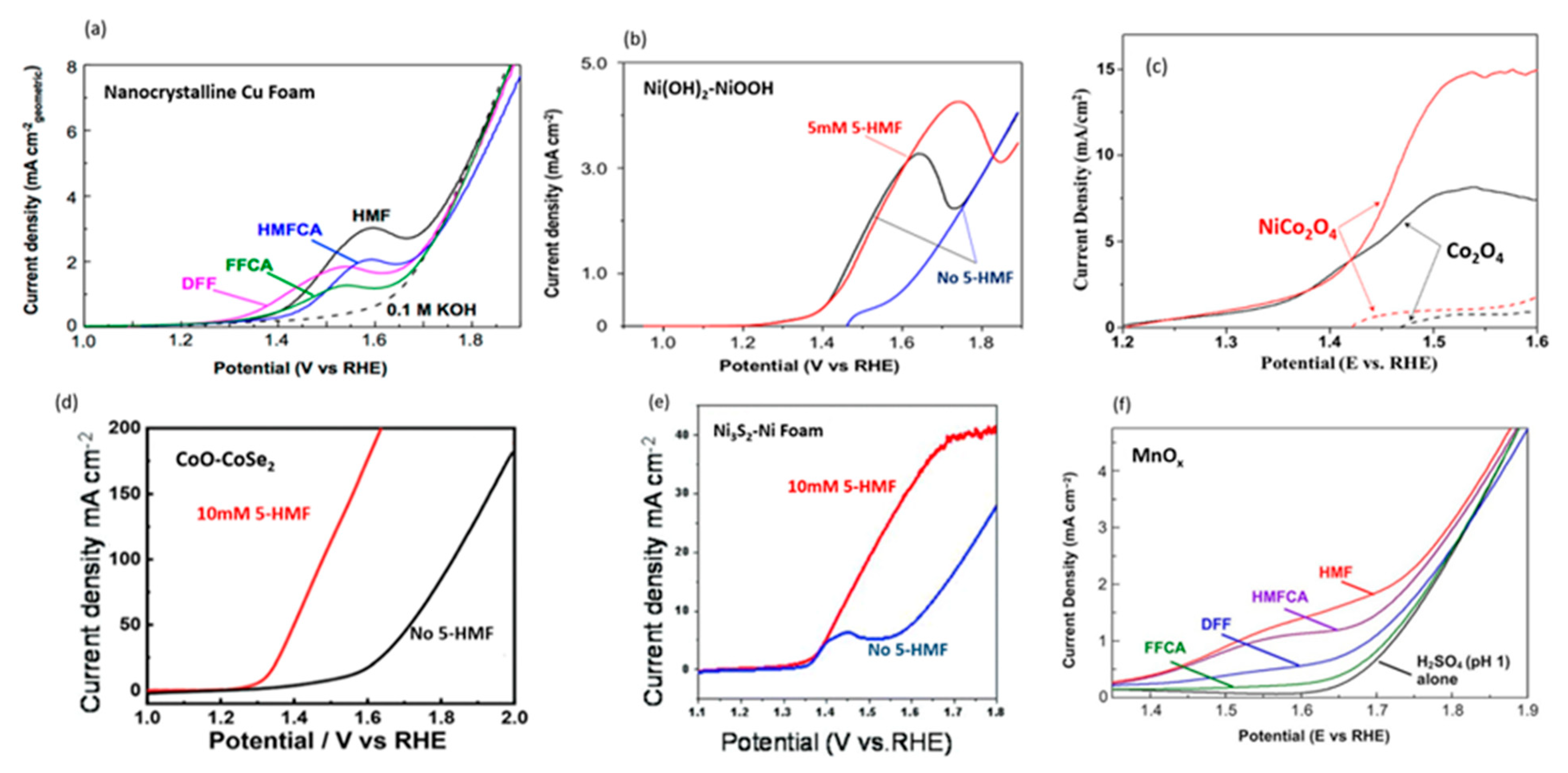
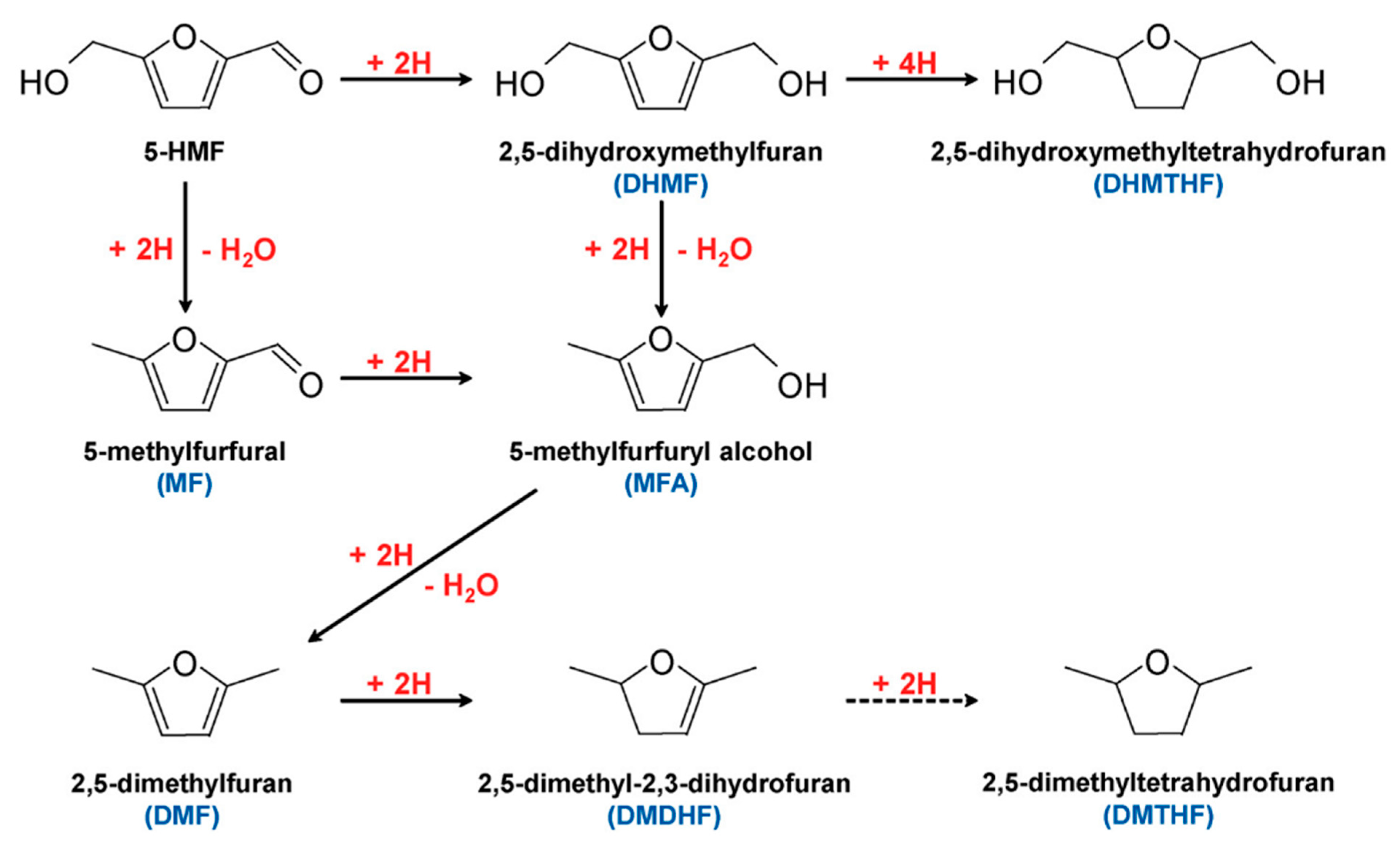
2.3. 5-Hydroxylmethylfurfural
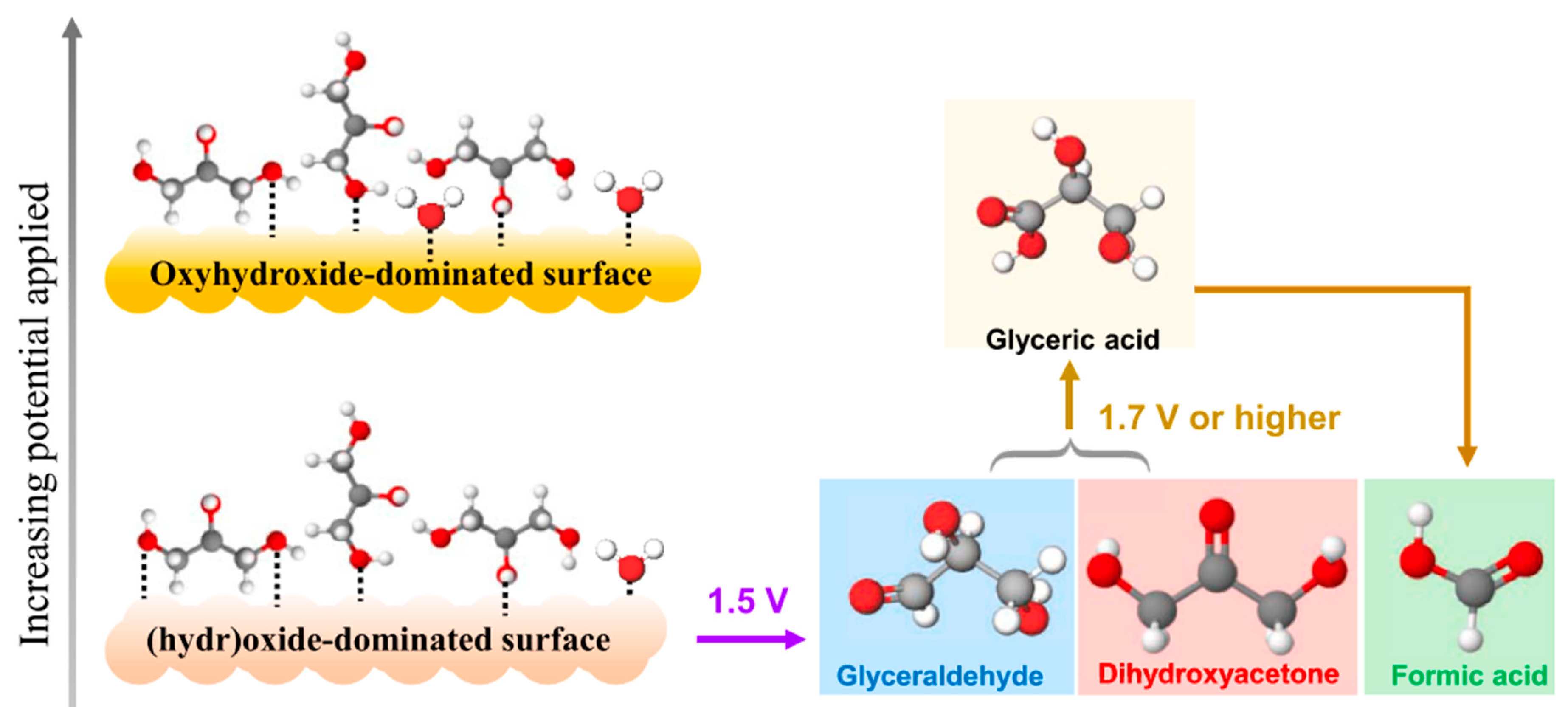
2.4. Other Biomass Derivatives
3. Electroreforming of Biomass at the Cathode
3.1. Cellulose
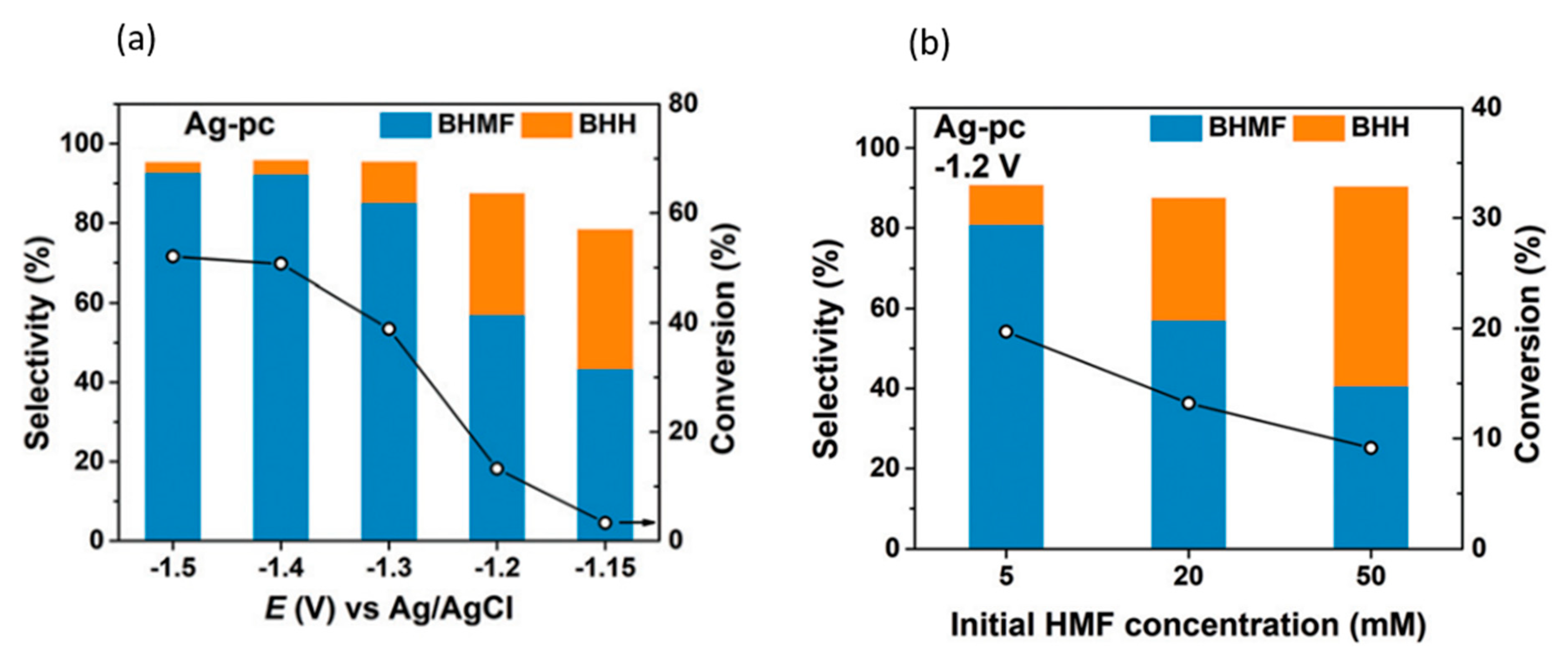
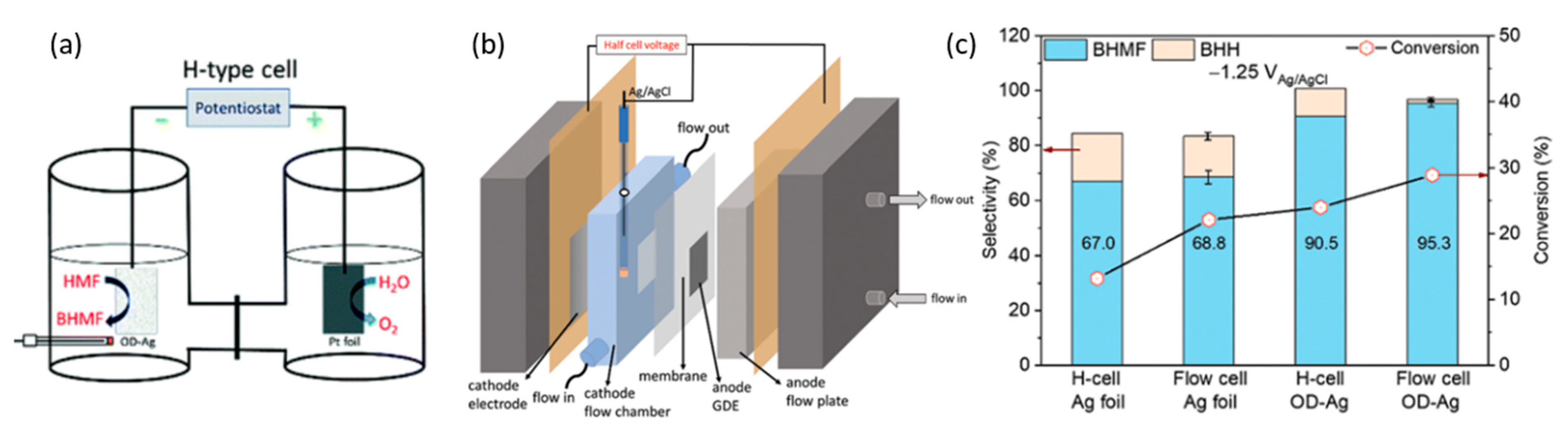
3.2. 5-Hydroxylmethylfurfural
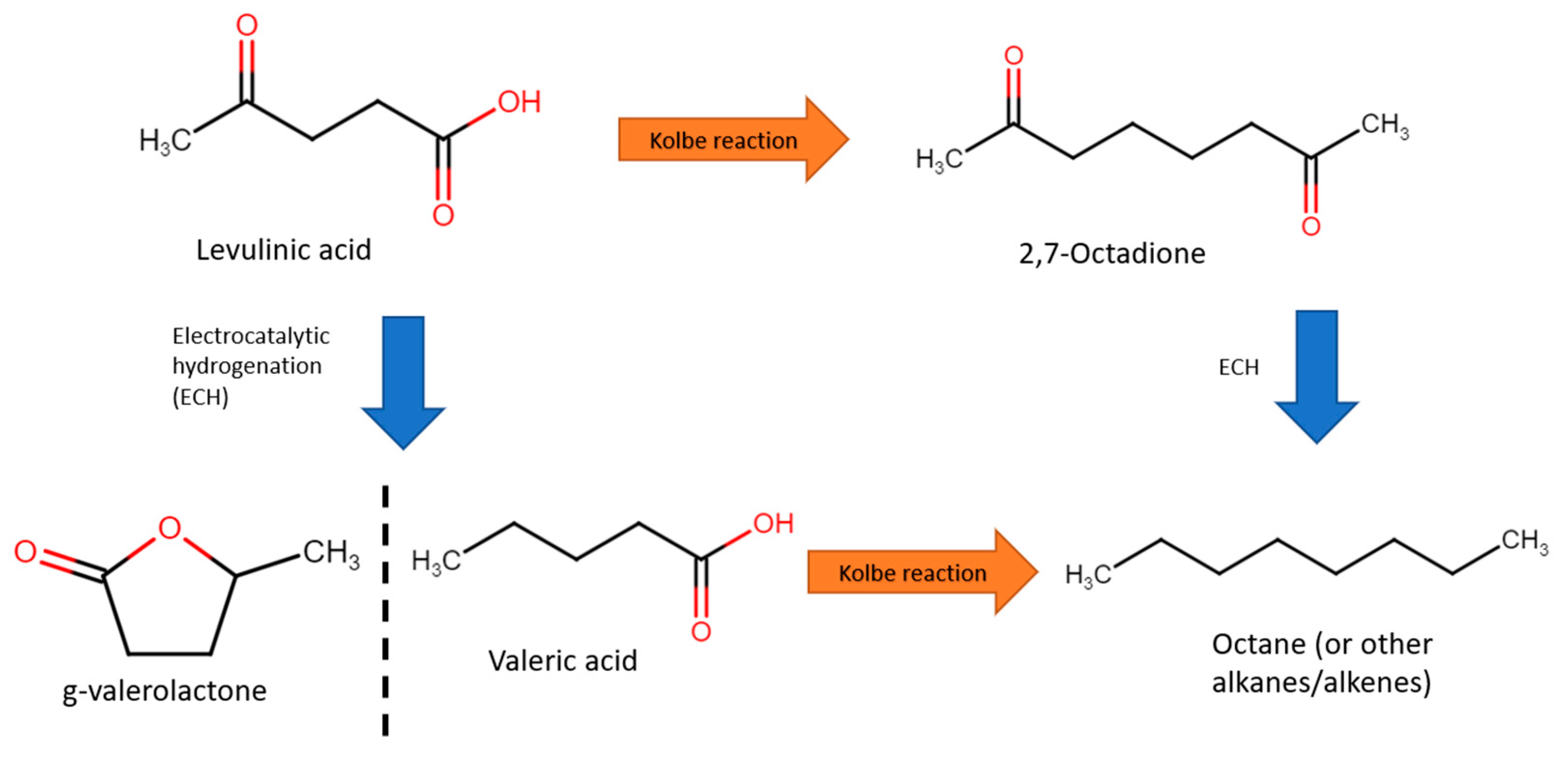
3.3. Levulinic Acid
4. Evolution of Hydrogen Coupled with Biomass Electroreforming
4.1. Glucose Electrooxidation Coupled with Green Hydrogen Generation
4.2. 5-HMF Electrooxidation Coupled with Green Hydrogen Generation
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Global warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change. IPCC Sr15, October 2, pp. 17–20. 2018. Available online: www.environmentalgraphiti.org (accessed on 11 August 2021).
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Jevrejeva, S.; Jackson, L.P.; Grinsted, A.; Lincke, D.; Marzeion, B. Flood damage costs under the sea level rise with warming of 1.5 °C and 2 °C. Environ. Res. Lett. 2018, 13, 074014. [Google Scholar] [CrossRef]
- Gasparrini, A.; Guo, Y.; Sera, F.; Vicedo-Cabrera, A.M.; Huber, V.; Tong, S. Projections of temperature-related excess mortality under climate change scenarios. Lancet Planet. Health 2017, 1, e360–e367. [Google Scholar] [CrossRef]
- Deryng, D.; Conway, D.; Ramankutty, N.; Price, J.; Warren, R. Global crop yield response to extreme heat stress under multiple climate change futures. Environ. Res. Lett. 2014, 9, 034011. [Google Scholar] [CrossRef]
- Mehran, A.; AghaKouchak, A.; Nakhjiri, N.; Stewardson, M.J.; Peel, M.C.; Phillips, T.J.; Wada, Y.; Ravalico, J.K. Compounding Impacts of Human-Induced Water Stress and Climate Change on Water Availability. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Tollefson, J. IPCC says limiting global warming to 1.5 °C will require drastic action. Nature 2018, 562, 172–173. [Google Scholar] [CrossRef]
- De Wit, M.; Faaij, A. European biomass resource potential and costs. Biomass Bioenergy 2010, 34, 188–202. [Google Scholar] [CrossRef]
- Williams, A.; Jones, J.M.; Ma, L.; Pourkashanian, M. Pollutants from the combustion of solid biomass fuels. Prog. Energy Combust. Sci. 2012, 38, 113–137. [Google Scholar] [CrossRef]
- Zhang, Y.H.P. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Pettersen, R.C. The chemical composition of wood. Chem. Solid Wood 1984, 207, 57–126. [Google Scholar]
- Badger, P.C. Trends in New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 2002. [Google Scholar]
- Payen, A. Mémoire sur la composition du tissu propre des plantes et du ligneux. Comptes Rendus 1838, 7, 1052–1056. [Google Scholar]
- Onwukamike, K.N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A. Critical review on sustainable homogeneous cellulose modification: Why renewability is not enough. ACS Sustain. Chem. Eng. 2018, 7, 1826–1840. [Google Scholar] [CrossRef]
- Badger, P. Ethanol from Cellulose: A General Review. Trends new Crop. New Uses 17–21. 2002. Available online: http://large.stanford.edu/publications/coal/references/docs/badger.pdf (accessed on 11 August 2021).
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Shrotri, A.; Fukuoka, A. Synthesis of cello-oligosaccharides by depolymerization of cellulose: A review. Appl. Catal. A Gen. 2021, 621, 118177. [Google Scholar] [CrossRef]
- Pirich, C.L.; Picheth, G.F.; Fontes, A.M.; Delgado-Aguilar, M.; Ramos, L.P. Disruptive enzyme-based strategies to isolate nanocelluloses: A review. Cellulose 2020, 27, 5457–5475. [Google Scholar] [CrossRef]
- Yin, S.; Tan, Z. Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions. Appl. Energy 2012, 92, 234–239. [Google Scholar] [CrossRef]
- Pavasars, I.; Hagberg, J.; Borén, H.; Allard, B. Alkaline degradation of cellulose: Mechanisms and kinetics. J. Polym. Environ. 2003, 11, 39–47. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Orozco, A.; Ahmad, M.; Rooney, D.; Walker, G. Dilute acid hydrolysis of cellulose and cellulosic bio-waste using a microwave reactor system. Process Saf. Environ. Prot. 2007, 85, 446–449. [Google Scholar] [CrossRef]
- Iranmahboob, J.; Nadim, F.; Monemi, S. Optimizing acid-hydrolysis: A critical step for production of ethanol from mixed wood chips. Biomass Bioenergy 2002, 22, 401–404. [Google Scholar] [CrossRef]
- Hou, C.; Liu, H.; Mohammad, F.B. Preparation of ordered mesoporous F–H2Ti3O7 nanosheets using orthorhombic HTiOF3 as a precursor and their highly efficient degradation of tetracycline hydrochloride under simulated sunlight. J. Solid State Chem. 2021, 300, 122288. [Google Scholar] [CrossRef]
- Hou, C.; Liu, H.; Li, Y. The preparation of three-dimensional flower-like TiO2/TiOF2photocatalyst and its efficient degradation of tetracycline hydrochloride. RSC Adv. 2021, 11, 14957–14969. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, D.; Wang, J.; Tu, W.; Wu, D.; Koh, S.W.; Gao, P.; Xu, Z.J.; Deng, S.; Zhou, Y.; et al. Raw biomass electroreforming coupled to green hydrogen generation. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield—A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Isogai, A.; Atalla, R.H. Dissolution of cellulose in aqueous NaOH solutions. Cellulose 1998, 5, 309–319. [Google Scholar] [CrossRef]
- Yan, L.; Qi, X. Degradation of cellulose to organic acids in its homogeneous alkaline aqueous solution. ACS Sustain. Chem. Eng. 2014, 2, 897–901. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Sugano, Y.; Vestergaard, M.; Yoshikawa, H.; Saito, M.; Tamiya, E. Direct Electrochemical Oxidation of Cellulose: A Cellulose-Based Fuel Cell System. Electroanalysis 2010, 22, 1688–1694. [Google Scholar] [CrossRef]
- Sugano, Y.; Latonen, R.-M.; Akieh-Pirkanniemi, M.; Bobacka, J.; Ivaska, A. Electrocatalytic Oxidation of Cellulose at a Gold Electrode. ChemSusChem 2014, 7, 2240–2247. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, M.; Zhao, G. Electrocatalytic oxidation of cellulose to gluconate on carbon aerogel supported gold nanoparticles anode in alkaline medium. Catalysts 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Tan, X.; Deng, W.; Liu, M.; Zhang, Q.; Wang, Y. Carbon nanotube-supported gold nanoparticles as efficient catalysts for selective oxidation of cellobiose into gluconic acid in aqueous medium. Chem. Commun. 2009, 46, 7179–7181. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Ye, A.; Deng, W.; Zhang, Q.; Wang, Y. Selective Conversion of Cellobiose and Cellulose into Gluconic Acid in Water in the Presence of Oxygen, Catalyzed by Polyoxometalate-Supported Gold Nanoparticles. Chem. A Eur. J. 2012, 18, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Jin, Y.; Zhao, G.; Li, M.; Li, D. Electrosorption-promoted photodegradation of opaque wastewater on a novel TiO2/carbon aerogel electrode. Environ. Sci. Technol. 2010, 44, 1780–1785. [Google Scholar] [CrossRef]
- Ma, C.; Xue, W.; Li, J.; Xing, W.; Hao, Z. Mesoporous carbon-confined Au catalysts with superior activity for selective oxidation of glucose to gluconic acid. Green Chem. 2013, 15, 1035–1041. [Google Scholar] [CrossRef]
- Sugano, Y.; Kumar, N.; Peurla, M.; Roine, J.; Aho, A.; Bobacka, J.; Mikkola, J.P. Specific Electrocatalytic Oxidation of Cellulose at Carbon Electrodes Modified by Gold Nanoparticles. ChemCatChem 2016, 8, 2401–2405. [Google Scholar] [CrossRef]
- Meng, D.; Li, G.; Liu, Z.; Yang, F. Study of depolymerization of cotton cellulose by Pb/PbO2 anode electrochemical catalysis in sulfuric acid solution. Polym. Degrad. Stab. 2011, 96, 1173–1178. [Google Scholar] [CrossRef]
- Domb, A.J.; Kost, J.; Wiseman, D. Handbook of Biodegradable Polymers; CRC Press: Boca Raton, FL, USA, 1998; Volume 7. [Google Scholar]
- Brown, H.T. XX—On the electrolysis of sugar solutions. (Preliminary notice). J. Chem. Soc. 1872, 25, 578–579. [Google Scholar] [CrossRef][Green Version]
- Zółtaszek, R.; Hanausek, M.; Kiliańska, Z.M.; Walaszek, Z. The biological role of D-glucaric acid and its derivatives: Potential use in medicine. Postepy Hig. Med. Dosw (Online) 2008, 62, 451–462. [Google Scholar]
- Werpy, T.; Petersen, G. Top Value Added Chemicals From Biomass: Volume I—Results of Screening For Potential Candidates From Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar] [CrossRef]
- Mehtiö, T.; Toivari, M.; Wiebe, M.G.; Harlin, A.; Penttilä, M.; Koivula, A. Production and applications of carbohydrate-derived sugar acids as generic biobased chemicals. Crit. Rev. Biotechnol. 2016, 36, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.N.; Hash, K.; Davey, C.-L.; Mills, H.; Williams, H.; Kiely, D.E. Modifications in the nitric acid oxidation of D-glucose. Carbohydr. Res. 2012, 350, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Bao, J. Evaluation of cement retarding performance of cellulosic sugar acids. Constr. Build. Mater. 2019, 202, 522–527. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic acid: Properties, applications and microbial production. Food Technol. Biotechnol. 2006, 44, 2. [Google Scholar]
- Hayes, G.C.; Becer, C.R. Levulinic acid: A sustainable platform chemical for novel polymer architectures. Polym. Chem. 2020, 11, 4068–4077. [Google Scholar] [CrossRef]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethylfurfural and its derivatives. Arkivoc 2001, 1, 17–54. [Google Scholar] [CrossRef]
- Bin, D.; Wang, H.; Li, J.; Wang, H.; Yin, Z.; Kang, J.; He, B.; Li, Z. Controllable oxidation of glucose to gluconic acid and glucaric acid using an electrocatalytic reactor. Electrochim. Acta 2014, 130, 170–178. [Google Scholar] [CrossRef]
- Solmi, S.; Morreale, C.; Ospitali, F.; Agnoli, S.; Cavani, F. Oxidation of d-Glucose to Glucaric Acid Using Au/C Catalysts. ChemCatChem 2017, 9, 2797–2806. [Google Scholar] [CrossRef]
- Moggia, G.; Kenis, T.; Daems, N.; Breugelmans, T. Electrochemical Oxidation of d-Glucose in Alkaline Medium: Impact of Oxidation Potential and Chemical Side Reactions on the Selectivity to d-Gluconic and d-Glucaric Acid. ChemElectroChem 2020, 7, 86–95. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Pineda, A.; Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Glucose to fructose isomerization in aqueous media over homogeneous and heterogeneous catalysts. ChemCatChem 2016, 8, 1100–1110. [Google Scholar] [CrossRef]
- Moggia, G.; Schalck, J.; Daems, N.; Breugelmans, T. Two-steps synthesis of D-glucaric acid via D-gluconic acid by electrocatalytic oxidation of D-glucose on gold electrode: Influence of operational parameters. Electrochim. Acta 2021, 374, 137852. [Google Scholar] [CrossRef]
- Tominaga, M.; Shimazoe, T.; Nagashima, M.; Taniguchi, I. Electrocatalytic oxidation of glucose at gold nanoparticle-modified carbon electrodes in alkaline and neutral solutions. Electrochem. Commun. 2005, 7, 189–193. [Google Scholar] [CrossRef]
- Liu, W.J.; Xu, Z.; Zhao, D.; Pan, X.Q.; Li, H.C.; Hu, X.; Fan, Z.; Wang, W.; Zhao, G.; Yu, H.Q. Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Neha, N.; Kouamé, B.S.R.; Rafaïdeen, T.; Baranton, S.; Coutanceau, C. Remarkably Efficient Carbon-Supported Nanostructured Platinum-Bismuth Catalysts for the Selective Electrooxidation of Glucose and Methyl-Glucoside. Electrocatalysis 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Rao, J.R.; Richter, G.J.; von Sturm, F.; Weidlich, E. The performance of glucose electrodes and the characteristics of different biofuel cell constructions. Bioelectrochem. Bioenerg. 1976, 3, 139–150. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Smyrnioti, D.; Nikolaidis, G.N.; Tsitsimaka, I.; Christodoulou, E.; Bikiaris, D.N.; Charitopoulou, M.A.; Achilias, D.S.; Kapnisti, M.; Papageorgiou, G.Z. Sustainable plastics from biomass: Blends of polyesters based on 2, 5-furandicarboxylic acid. Polymers 2020, 12, 225. [Google Scholar] [CrossRef]
- Smith, P.B. Bio-based sources for terephthalic acid. In Green Polymer Chemistry: Biobased Materials and Biocatalysis; ACS Publications: Washington, DC, USA, 2015; pp. 453–469. [Google Scholar]
- Rajendran, S.; Raghunathan, R.; Hevus, I.; Krishnan, R.; Ugrinov, A.; Sibi, M.P.; Webster, D.C.; Sivaguru, J. Programmed photodegradation of polymeric/oligomeric materials derived from renewable bioresources. Angew. Chemie 2015, 127, 1175–1179. [Google Scholar] [CrossRef]
- Liu, W.J.; Dang, L.; Xu, Z.; Yu, H.Q.; Jin, S.; Huber, G.W. Electrochemical oxidation of 5-hydroxymethylfurfural with NiFe layered double hydroxide (LDH) nanosheet catalysts. ACS Catal. 2018, 8, 5533–5541. [Google Scholar] [CrossRef]
- Weidner, J.; Barwe, S.; Sliozberg, K.; Piontek, S.; Masa, J.; Apfel, U.P.; Schuhmann, W. Cobalt-metalloid alloys for electrochemical oxidation of 5-hydroxymethylfurfural as an alternative anode reaction in lieu of oxygen evolution during water splitting. Beilstein J. Org. Chem. 2018, 14, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.H.; Taitt, B.J.; Choi, K.S. Copper-Based Catalytic Anodes to Produce 2,5-Furandicarboxylic Acid, a Biomass-Derived Alternative to Terephthalic Acid. ACS Catal. 2018, 8, 1197–1206. [Google Scholar] [CrossRef]
- Taitt, B.J.; Nam, D.H.; Choi, K.S. A Comparative Study of Nickel, Cobalt, and Iron Oxyhydroxide Anodes for the Electrochemical Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. ACS Catal. 2019, 9, 660–670. [Google Scholar] [CrossRef]
- Kang, M.J.; Park, H.; Jegal, J.; Hwang, S.Y.; Kang, Y.S.; Cha, H.G. Electrocatalysis of 5-hydroxymethylfurfural at cobalt based spinel catalysts with filamentous nanoarchitecture in alkaline media. Appl. Catal. B Environ. 2019, 242, 85–91. [Google Scholar] [CrossRef]
- Cai, M.; Ding, S.; Gibbons, B.; Yang, X.; Kessinger, M.C.; Morris, A.J. Nickel(ii)-modified covalent-organic framework film for electrocatalytic oxidation of 5-hydroxymethylfurfural (HMF). Chem. Commun. 2020, 56, 14361–14364. [Google Scholar] [CrossRef]
- Huang, X.; Song, J.; Hua, M.; Xie, Z.; Liu, S.; Wu, T.; Yang, G.; Han, B. Enhancing the electrocatalytic activity of CoO for the oxidation of 5-hydroxymethylfurfural by introducing oxygen vacancies. Green Chem. 2020, 22, 843–849. [Google Scholar] [CrossRef]
- Hu, K.; Zhang, M.; Liu, B.; Yang, Z.; Li, R.; Yan, K. Efficient electrochemical oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid using the facilely synthesized 3D porous WO3/Ni electrode. Mol. Catal. 2021, 504, 111459. [Google Scholar] [CrossRef]
- Wang, W.; Kong, F.; Zhang, Z.; Yang, L.; Wang, M. Sulfidation of nickel foam with enhanced electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Dalt. Trans. 2021, 50, 10922–10927. [Google Scholar] [CrossRef] [PubMed]
- Kubota, S.R.; Choi, K.S. Electrochemical oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid (Fdca) in acidic media enabling spontaneous fdca separation. ChemSusChem 2018, 11, 2138–2145. [Google Scholar] [CrossRef]
- Santos, T.R.D.; Nilges, P.; Sauter, W.; Harnisch, F.; Schröder, U. Electrochemistry for the generation of renewable chemicals: Electrochemical conversion of levulinic acid. RSC Adv. 2015, 5, 26634–26643. [Google Scholar] [CrossRef]
- Nilges, P.; Santos, T.R.D.; Harnisch, F.; Schröder, U. Electrochemistry for biofuel generation: Electrochemical conversion of levulinic acid to octane. Energy Environ. Sci. 2012, 5, 5231–5235. [Google Scholar] [CrossRef]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Baranton, S.; Coutanceau, C. Electrochemical valorisation of glycerol. ChemSusChem 2012, 5, 2106–2124. [Google Scholar] [CrossRef]
- Du, L.; Shao, Y.; Sun, J.; Yin, G.; Du, C.; Wang, Y. Electrocatalytic valorisation of biomass derived chemicals. Catal. Sci. Technol. 2018, 8, 3216–3232. [Google Scholar] [CrossRef]
- Rahim, S.A.N.M.; Lee, C.S.; Abnisa, F.; Aroua, M.K.; Daud, W.A.W.; Cognet, P.; Pérès, Y. A review of recent developments on kinetics parameters for glycerol electrochemical conversion—A by-product of biodiesel. Sci. Total Environ. 2020, 705, 135137. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hirohara, M.; Maekawa, T.; Chang, R.; Hayashi, T.; Chiang, C.Y. Selective electro-oxidation of glycerol to dihydroxyacetone by a non-precious electrocatalyst—CuO. Appl. Catal. B Environ. 2020, 265, 118543. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paul, S.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
- Vo, T.-G.; Ho, P.-Y.; Chiang, C.-Y. Operando mechanistic studies of selective oxidation of glycerol to dihydroxyacetone over amorphous cobalt oxide. Appl. Catal. B Environ. 2021, 300, 120723. [Google Scholar] [CrossRef]
- Kwon, Y.; de Jong, E.; van der Waal, J.K.; Koper, M.T.M. Selective electrocatalytic oxidation of sorbitol to fructose and sorbose. ChemSusChem 2015, 8, 970–973. [Google Scholar] [CrossRef]
- Döbereiner, J.W. Ueber die medicinische und chemische Anwendung und die vortheilhafte Darstellung der Ameisensäure. Ann. Pharm. 1832, 3, 141–146. [Google Scholar] [CrossRef]
- Nilges, P.; Schröder, U. Electrochemistry for biofuel generation: Production of furans by electrocatalytic hydrogenation of furfurals. Energy Environ. Sci. 2013, 6, 2925–2931. [Google Scholar] [CrossRef]
- Kubota, S.R.; Choi, K.S. Electrochemical Valorization of Furfural to Maleic Acid. ACS Sustain. Chem. Eng. 2018, 6, 9596–9600. [Google Scholar] [CrossRef]
- Román, A.M.; Agrawal, N.; Hasse, J.C.; Janik, M.J.; Medlin, J.W.; Holewinski, A. Electro-oxidation of furfural on gold is limited by furoate self-assembly. J. Catal. 2020, 391, 327–335. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.; Fan, H.X.; Li, Y.; Li, G. Electrochemical control of the conversion of cellulose oligosaccharides into glucose. J. Ind. Eng. Chem. 2014, 20, 3487–3492. [Google Scholar] [CrossRef]
- Kwon, Y.; de Jong, E.; Raoufmoghaddam, S.; Koper, M.T.M. Electrocatalytic Hydrogenation of 5-Hydroxymethylfurfural in the Absence and Presence of Glucose. ChemSusChem 2013, 6, 1659–1667. [Google Scholar] [CrossRef]
- Kwon, Y.; Birdja, Y.Y.; Raoufmoghaddam, S.; Koper, M.T.M. Electrocatalytic Hydrogenation of 5-Hydroxymethylfurfural in Acidic Solution. ChemSusChem 2015, 8, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Daniel, R.; Li, H.; Xu, H.; Shuai, S.; Richards, P. Laminar burning velocities of 2, 5-dimethylfuran compared with ethanol and gasoline. Energy Fuels 2010, 24, 3898–3905. [Google Scholar] [CrossRef]
- Moreau, C.; Belgacem, M.N.; Gandini, A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar] [CrossRef]
- Esteves, L.M.; Brijaldo, M.H.; Oliveira, E.G.; Martinez, J.J.; Rojas, H.; Caytuero, A.; Passos, F.B. Effect of support on selective 5-hydroxymethylfurfural hydrogenation towards 2, 5-dimethylfuran over copper catalysts. Fuel 2020, 270, 117524. [Google Scholar] [CrossRef]
- Chadderdon, X.H.; Chadderdon, D.J.; Pfennig, T.; Shanks, B.H.; Li, W. Paired electrocatalytic hydrogenation and oxidation of 5-(hydroxymethyl)furfural for efficient production of biomass-derived monomers. Green Chem. 2019, 21, 6210–6219. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Wang, B.X.; Qin, L.; Li, Q.; Fan, Y.M. A non-noble bimetallic alloy in the highly selective electrochemical synthesis of the biofuel 2,5-dimethylfuran from 5-hydroxymethylfurfural. Green Chem. 2019, 21, 1108–1113. [Google Scholar] [CrossRef]
- Liu, H.; Lee, T.H.; Chen, Y.; Cochran, E.W.; Li, W. Paired electrolysis of 5-(hydroxymethyl)furfural in flow cells with a high-performance oxide-derived silver cathode. Green Chem. 2021, 23, 5056–5063. [Google Scholar] [CrossRef]
- Robertson, G.P.; Hamilton, S.K.; Barham, B.L.; Dale, B.E.; Izaurralde, R.C.; Jackson, R.D.; Landisscott, D.A.; Swinton, M.; Thelen, K.D.; Tiedje, J.M. Cellulosic biofuel contributions to a sustainable energy future: Choices and outcomes. Science 2017, 356, 6345. [Google Scholar] [CrossRef] [PubMed]
- Bessou, C.; Ferchaud, F.; Gabrielle, B.; Mary, B. Biofuels, greenhouse gases and climate change. Sustain. Agric. 2011, 2, 365–468. [Google Scholar]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric biofuels: A platform of cellulosic transportation fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Wang, D.; Dumesic, J.A. Catalytic upgrading of levulinic acid to 5-nonanone. Green Chem. 2010, 12, 574–577. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, L.; Yan, Z. Catalytic hydrogenation and oxidation of biomass-derived levulinic acid. BioResources 2011, 6, 686–699. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, Z.; Qi, J.; Chadderdon, D.J.; Qiu, Y.; Warsko, K.M.; Li, W. Electricity storage in biofuels: Selective electrocatalytic reduction of levulinic acid to valeric acid or γ-valerolactone. ChemSusChem 2013, 6, 674–686. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—a sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Du, Y.; Chen, X.; Qi, J.; Wang, P.; Liang, C. Synthesis of valeric acid by selective electrocatalytic hydrogenation of biomass-derived levulinic acid. Catalysts 2020, 10, 692. [Google Scholar] [CrossRef]
- Kurig, N.; Meyers, J.; Holzhaüser, F.J.; Palkovits, S.; Palkovits, R. (Non-)Kolbe Chemistry Going with the Flow: The Continuous Electrolysis of Biogenic Acids. ACS Sustain. Chem. Eng. 2021, 9, 1229–1234. [Google Scholar] [CrossRef]
- Du, P.; Zhang, J.; Liu, Y.; Huang, M. Hydrogen generation from catalytic glucose oxidation by Fe-based electrocatalysts. Electrochem. Commun. 2017, 83, 11–15. [Google Scholar] [CrossRef]
- Rafaïdeen, T.; Baranton, S.; Coutanceau, C. Highly efficient and selective electrooxidation of glucose and xylose in alkaline medium at carbon supported alloyed PdAu nanocatalysts. Appl. Catal. B Environ. 2019, 243, 641–656. [Google Scholar] [CrossRef]
- Rafaïdeen, T.; Neha, N.; Kouamé, B.R.S.; Baranton, S.; Coutanceau, C. Electroreforming of Glucose and Xylose in Alkaline Medium at Carbon Supported Alloyed Pd3Au7 Nanocatalysts: Effect of Aldose Concentration and Electrolysis Cell Voltage. Clean Technol. 2020, 2, 13. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, P.; Wang, S.; Zhou, Q.; Na, B.; Li, H.; Tian, J.; Zhang, Y.; Deng, C.; Zhang, L. Engineered porous Co–Ni alloy on carbon cloth as an efficient bifunctional electrocatalyst for glucose electrolysis in alkaline environment. J. Alloys Compd. 2020, 823, 153784. [Google Scholar] [CrossRef]
- Lin, C.; Li, H.; Zhang, P.; Deng, C.; Meng, L.; Zhou, Q.; Wang, S.; Wu, J.; Liu, C.; Qian, Y. Boosting water electrolysis with anodic glucose oxidation reaction over engineered cobalt nickel hydroxide nanosheet on carbon cloth. J. Electroanal. Chem. 2020, 861, 113946. [Google Scholar] [CrossRef]
- Liu, X.; Cai, P.; Wang, G.; Wen, Z. Nickel doped MoS2 nanoparticles as precious-metal free bifunctional electrocatalysts for glucose assisted electrolytic H2 generation. Int. J. Hydrogen Energy 2020, 45, 32940–32948. [Google Scholar] [CrossRef]
- Zheng, D.; Li, J.; Ci, S.; Cai, P.; Ding, Y.; Zhang, M.; Wen, Z. Three-birds-with-one-stone electrolysis for energy-efficiency production of gluconate and hydrogen. Appl. Catal. B Environ. 2020, 277, 119178. [Google Scholar] [CrossRef]
- Ding, Y.; Greiner, M.; Schlögl, R.; Heumann, S. A Metal-Free Electrode: From Biomass-Derived Carbon to Hydrogen. ChemSusChem 2020, 4064–4068. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, C.; Zhou, L.; Duan, W.; Song, Y.; Zhang, F.; Zhang, J.; Bao, W.; Yuxuan, L.u.; Fu, F. Refining d-band center in Ni0.85Se by Mo doping: A strategy for boosting hydrogen generation via coupling electrocatalytic oxidation 5-hydroxymethylfurfural. Chem. Eng. J. 2021, 422, 130125. [Google Scholar] [CrossRef]
- Jiang, N.; You, B.; Boonstra, R.; Rodriguez, I.M.T.; Sun, Y. Integrating Electrocatalytic 5-Hydroxymethylfurfural Oxidation and Hydrogen Production via Co-P-Derived Electrocatalysts. ACS Energy Lett. 2016, 1, 386–390. [Google Scholar] [CrossRef]
- Fan, K.; Chen, H.; Ji, Y.; Huang, H.; Claesson, P.M.; Daniel, Q.; Philippe, B.; Rensmo, H.; Li, F.; Sun, L.; et al. Nickel-vanadium monolayer double hydroxide for efficient electrochemical water oxidation. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
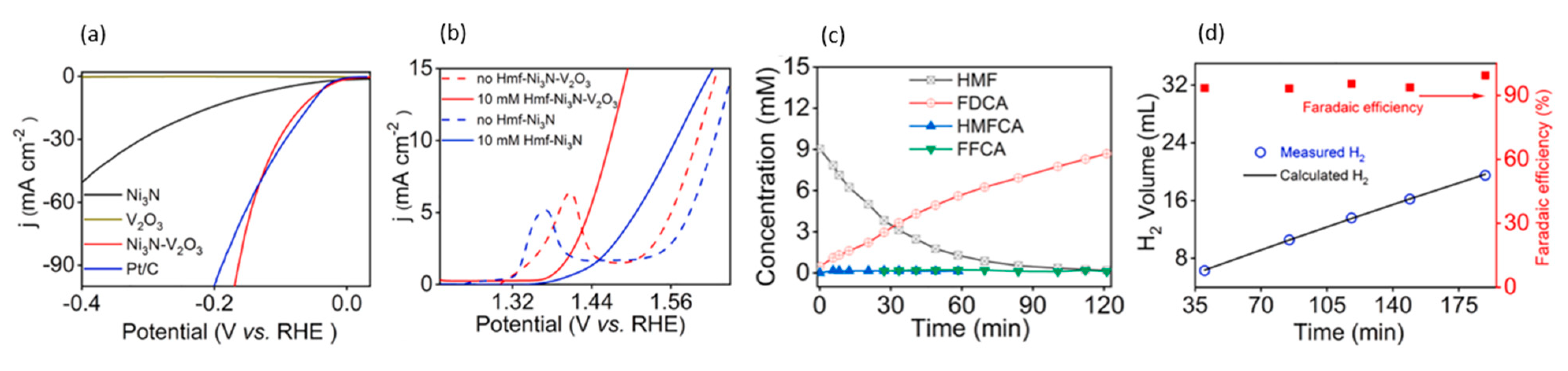
- Liang, S.; Pan, L.; Thomas, T.; Zhu, B.; Chen, C.; Zhang, J.; Shen, H.; Liu, J.; Yang, M. Ni3N-V2O3 enables highly efficient 5-(Hydroxymethyl) furfural oxidation enabling membrane free hydrogen production. Chem. Eng. J. 2021, 415, 128864. [Google Scholar] [CrossRef]
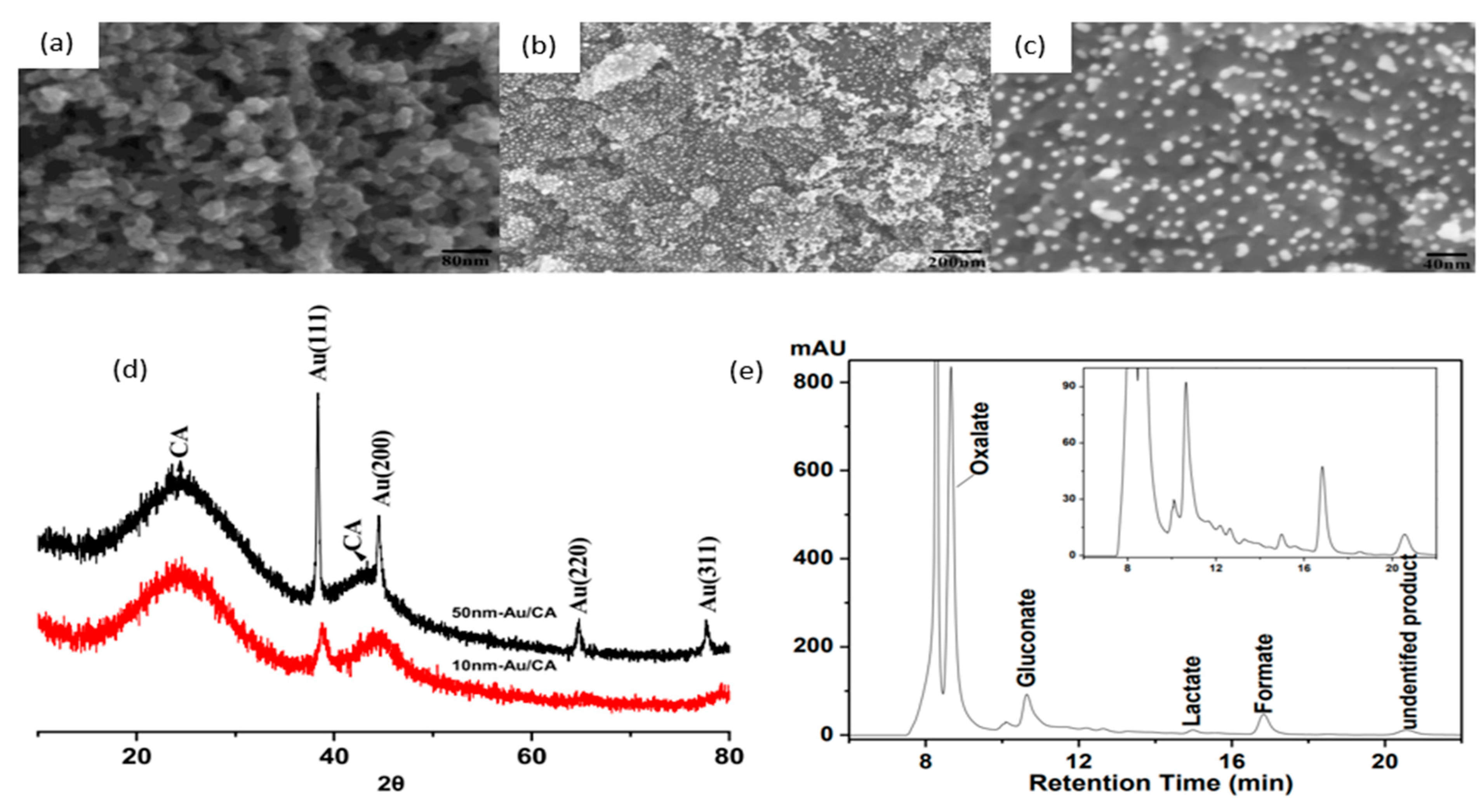
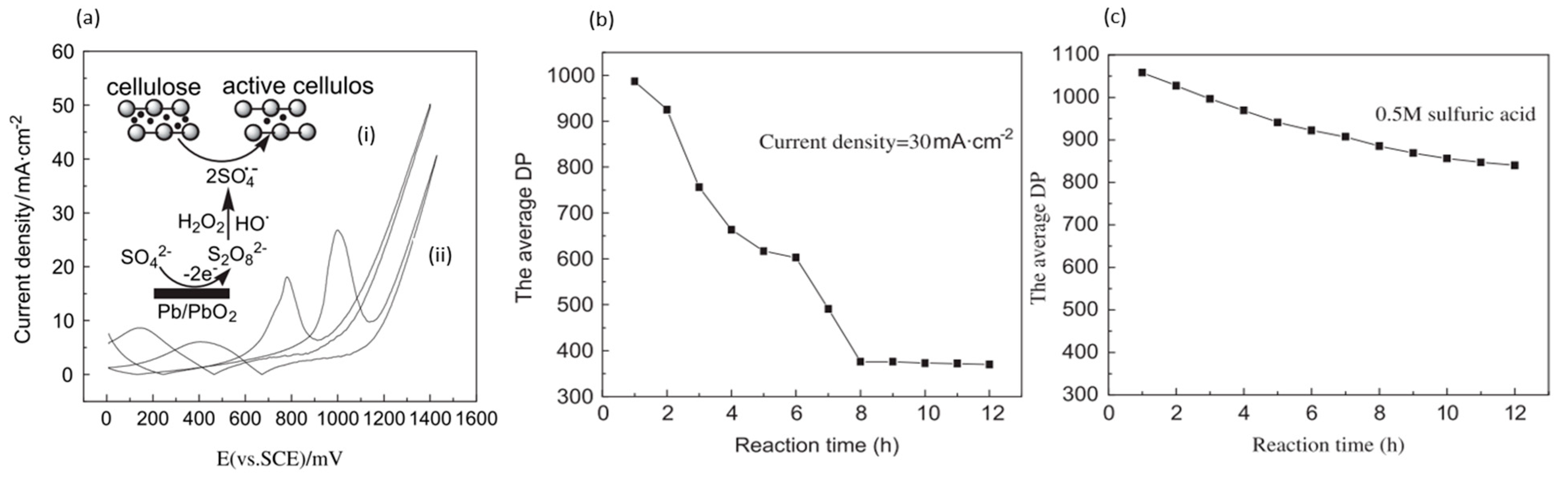
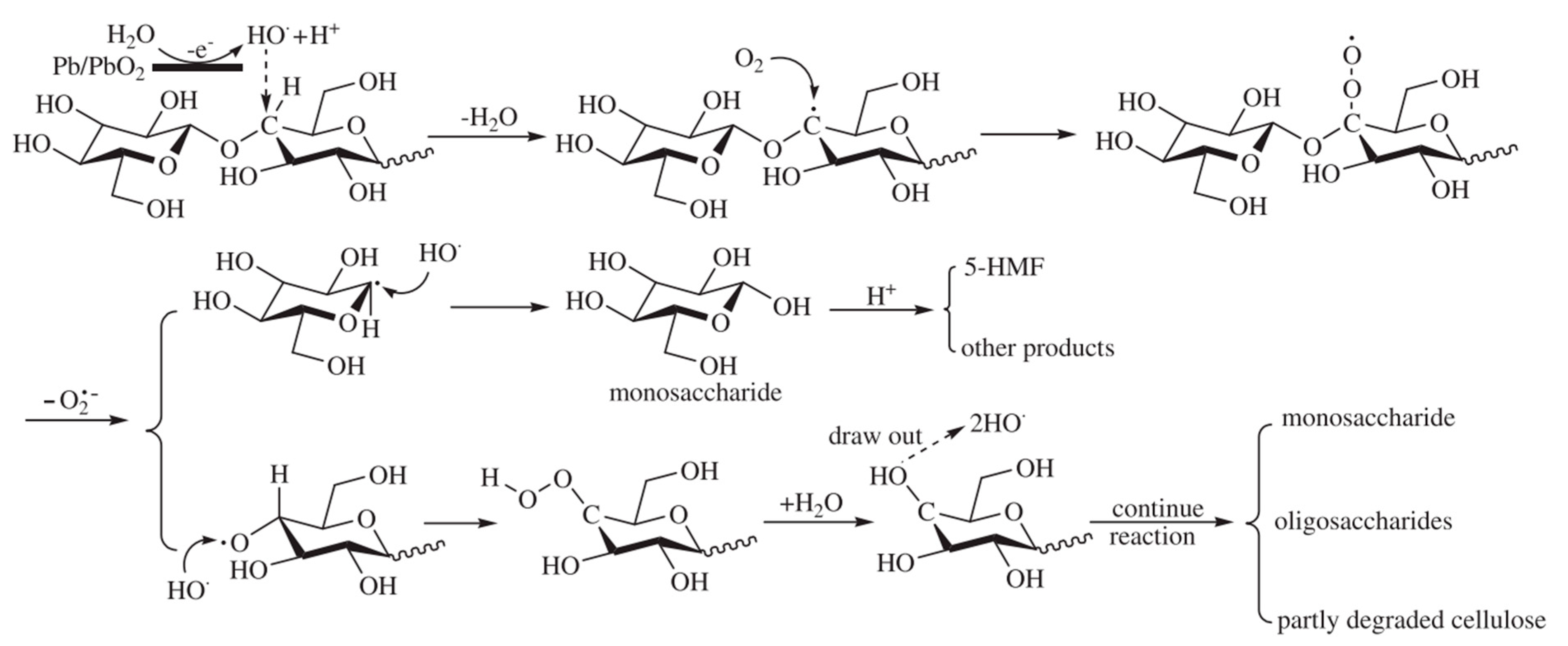

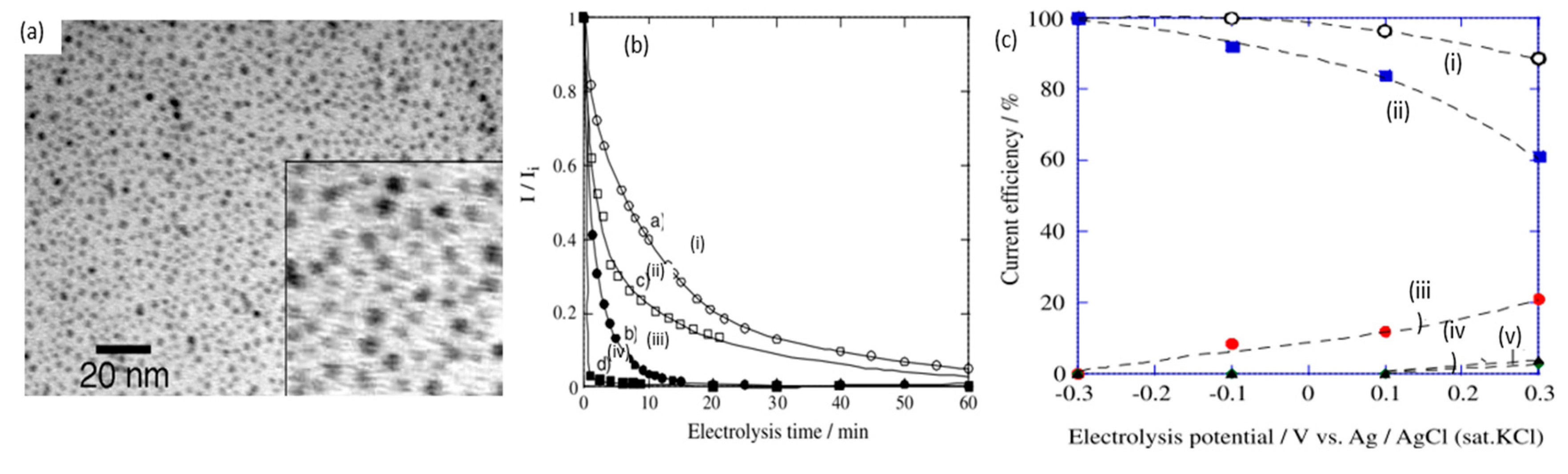



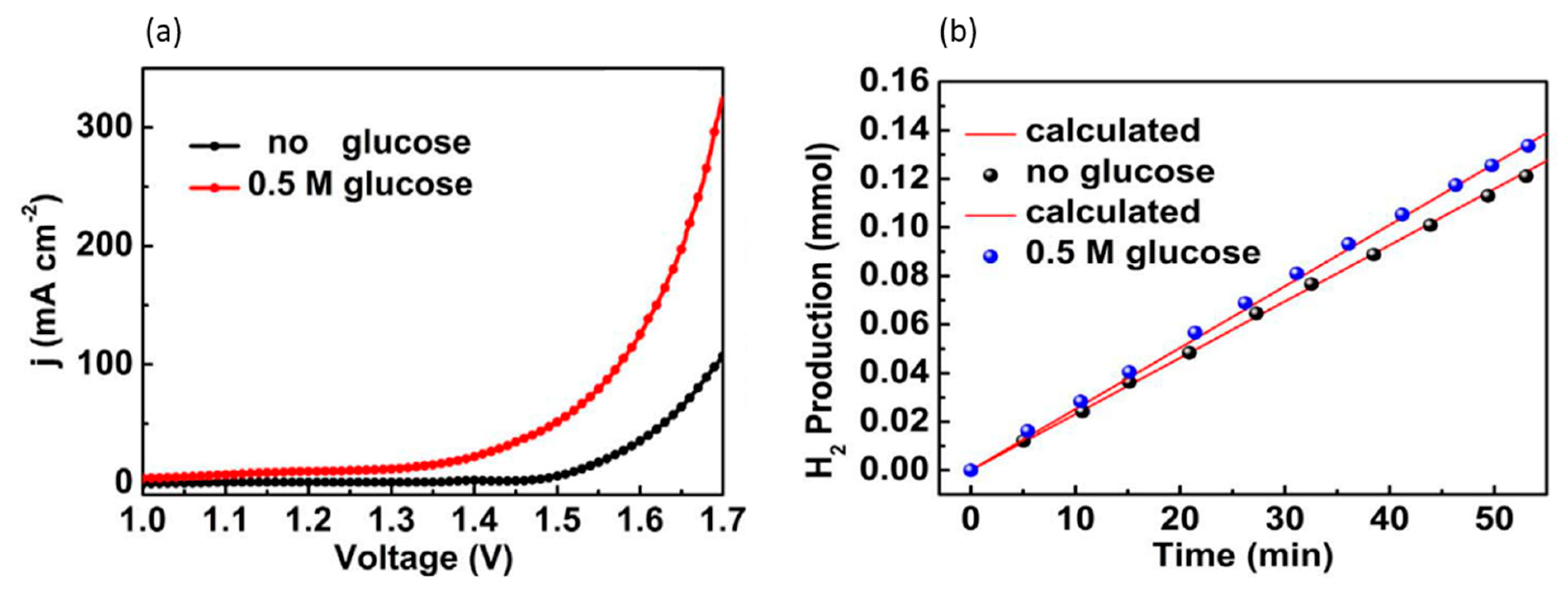

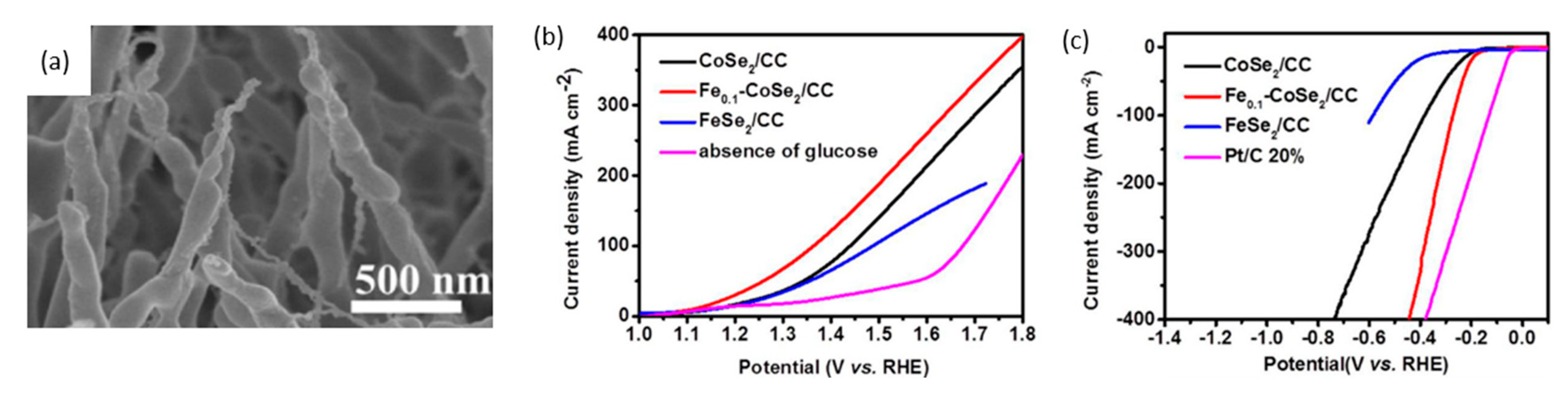
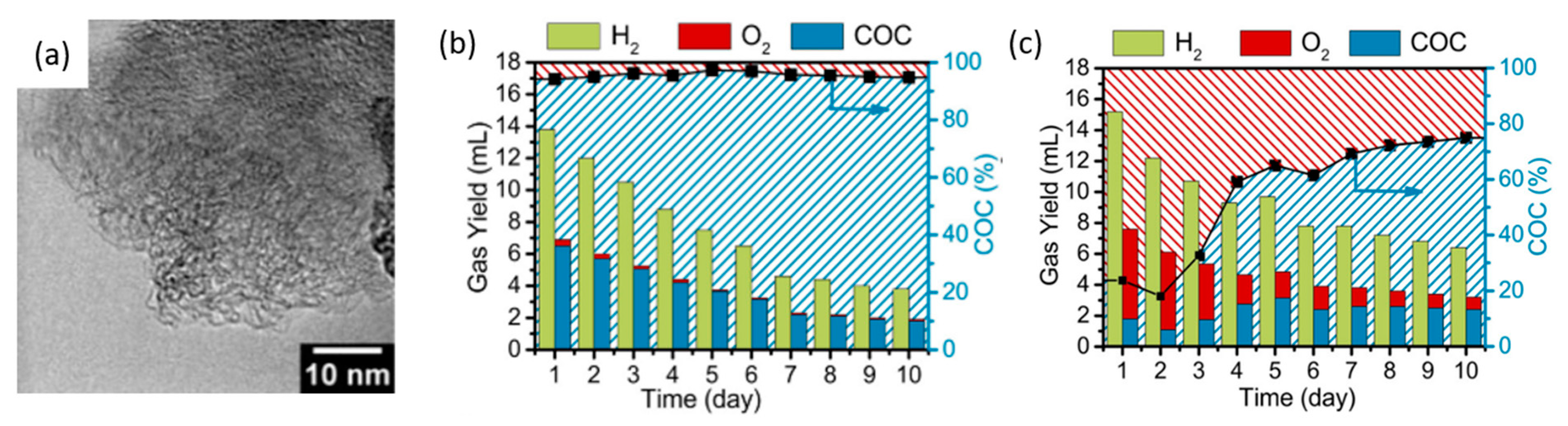
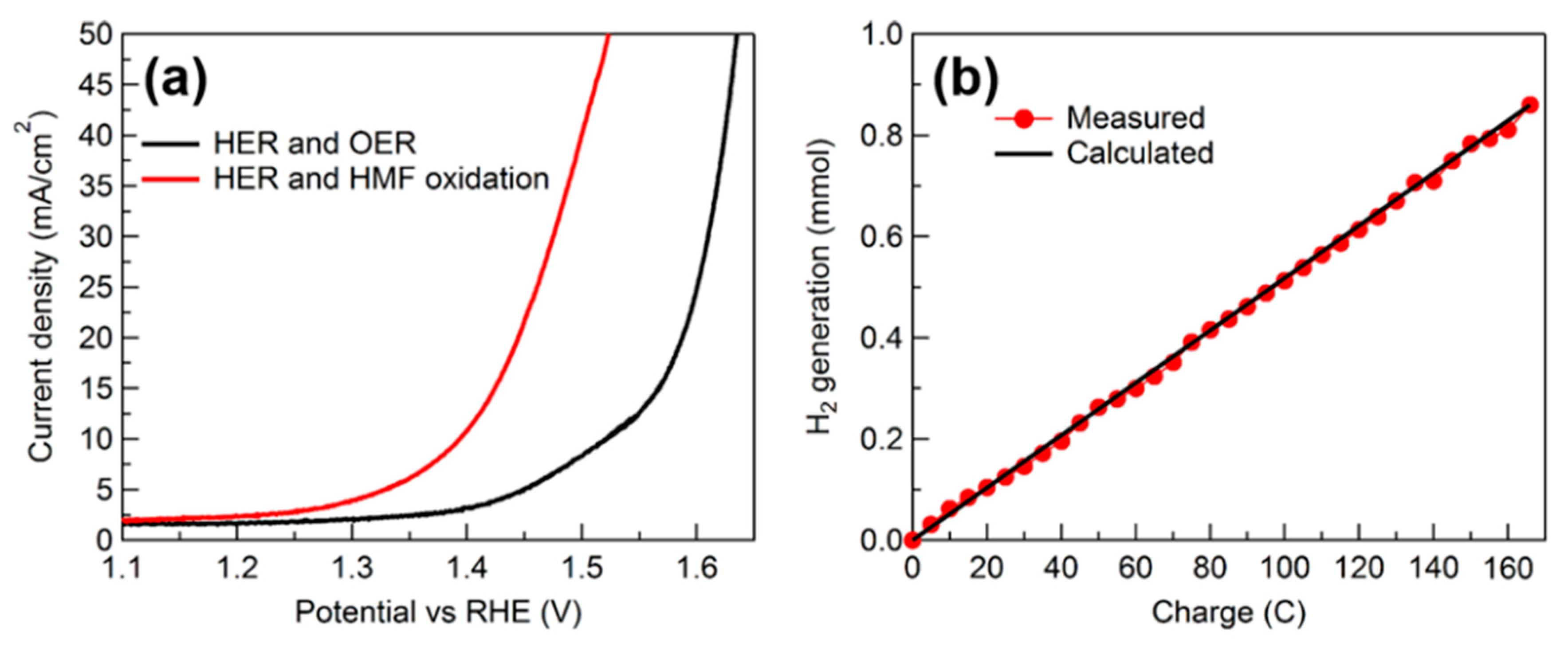
| Year | Electrocatalyst | Feedstock | Desired Products | Electrolyte | Electrolytic Tests and Conditions (Potentials Respect to RHE) | Yield/Output | Faradaic Efficiency | Reference |
|---|---|---|---|---|---|---|---|---|
| 2010 | polycrystalline Au | NaOH dissolved ball milled cellulose | Carboxyl groups | 1.33 M NaOH | CV characterization (0.90 V to 1.40 V) | - | - | [34] |
| 2015 | Au nanoparticles/carbon aerogel | NaOH dissolved cellulose | Gluconate | 0.125 M NaOH | 10 mA/cm2 at 18 h | 67.80% | - | [36] |
| 2016 | Au nanoparticles/carbon paper | NaOH dissolved cellulose | - | 1.33 M NaOH | CV characterization (0.60 V to 1.40 V) | - | - | [41] |
| 2011 | Pb/PbO2 | Cotton cellulose | Soluble sugars, 5-HMF | 0.5 M H2SO4 | 30 mA/cm2 at 12 h | Soluble sugar: 2.5%, 5-HMF: 1.8% | - | [42] |
| 2014 | MnO2/Ti | Glucose | Glucaric and gluconic acids | 0.07 M Na2SO4 (pH 7) | 1.41 V at 19 min | 99% (both glucaric and gluconic acids) | 37.00% | [54] |
| 2020 | Polycrystalline Cu, Au, Pt | Glucose | Glucaric and gluconic acids | 0.1 M NaOH | 0.70 V at 65 h | selectivities up to 86.8% for gluconic acid, 13.5% for glucaric acid | - | [56] |
| 2020 | NiFeOx/NF, NiFeNx/NF, NiFe(OH)x/NF, NF, RuO2/NF, Pt-C/NF | Glucose | Glucaric and gluconic acids | 1 M KOH | 1.40 V at 18 h | 11.6% (glucaric acid), 4.7% (gluconic acid) | 87% (both) | [60] |
| 2021 | Pt9-Bi1/C, Pt/C | Glucose | Gluconic acid | 0.1 M NaOH | 0.30 V at 6 h | 40% yield | ~100.0% | [61] |
| 2018 | NiFe, NiAl, NiGa, NI(OH)2 | 5-hydroxymethylfurfural (5-HMF) | 2,5-furandicarboxylic acid (FDCA) | 1 M KOH | 1.33 V at 10 h | 98.00% | 98.60% | [66] |
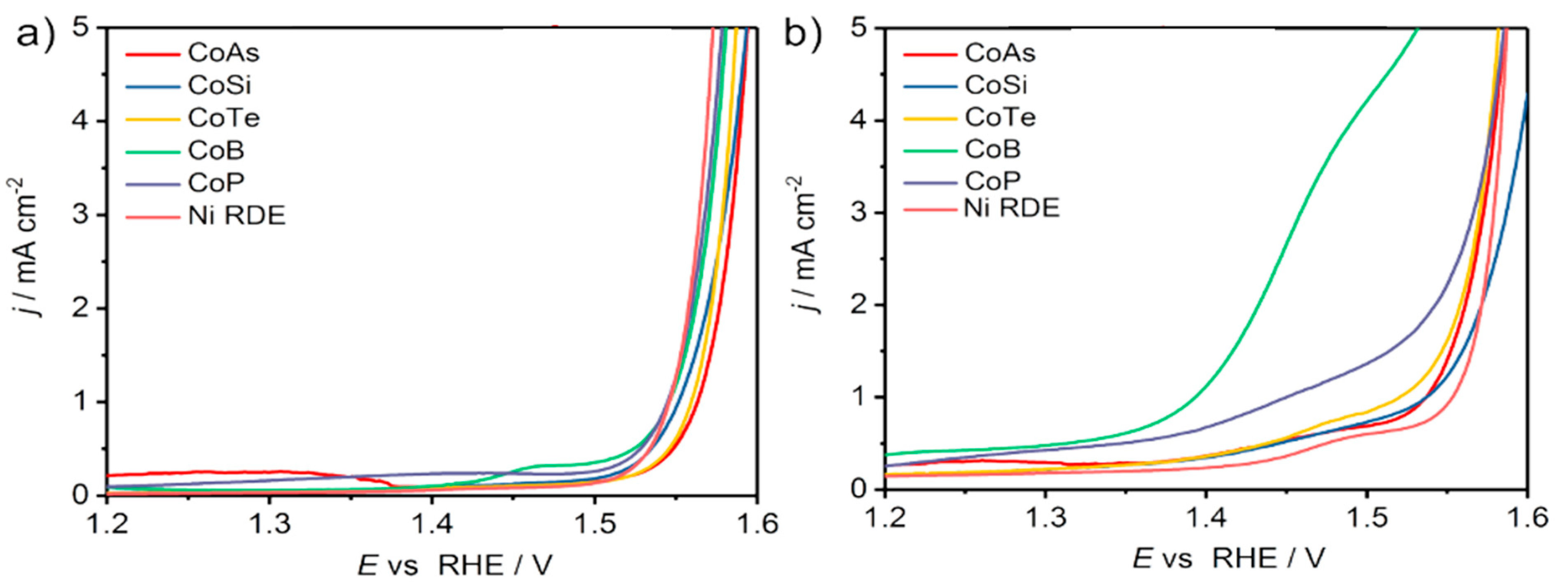
| 2018 | CoP, CoB, CoTe, Co2Si, CoAs on Ni foam | 5-HMF | FDCA | 1 M KOH | 1.45 V at 70 min | 94.00% | 98.00% | [67] |
| 2018 | electrodeposited Cu/Cu foam | 5-HMF | FDCA | 0.1 M KOH | 1.62 V at 8 h | 96.40% | 95.30% | [68] |
| 2019 | NiOOH, CoOOH, FeOOH/flouride doped tin oxide (FTO) glass | 5-HMF | FDCA | 0.1 M KOH | 1.47 V at 4.7 h | 96.00% | 96.00% | [69] |
| 2019 | NiCo2O4, Co3O4/Ni foam | 5-HMF | FDCA | 1 M KOH | 1.50 V at 53 min | 90.40% | 87.50% | [70] |
| 2020 | TpBpy-Ni@FTO | 5-HMF | FDCA | 0.1 M LiClO4 | 1.55 V at 40 min | 58.00% | - | [71] |
| 2020 | CoO-CoSe2 | 5-HMF | FDCA | 1 M KOH | 1.43 V at 1 h | 99.00% | 97.90% | [72] |
| 2021 | WO3 on Ni foam | 5-HMF | FDCA | 1 M KOH | 1.57 V at 351 min | 81.50% | 79.50% | [73] |
| 2021 | Ni3S2/NF, NiS, NiO, NiC, NF | 5-HMF | FDCA | 1 M KOH | 1.50 V at 2 h | 98.30% | 93.50% | [74] |
| 2018 | MnOx/FTO glass | 5-HMF | FDCA and maleic acid | 0.05 H2SO4 | 1.60 V (duration not reported) | 53.8% FDCA, 21.9% maleic acid | 33.8% (FDCA) | [75] |
| 2015 | Pt, C, Cu, Fe, Ni, Pb | Levulinic acid | Valeric acid, g-valerolactone, 4-hydroxy-2-butanone and other hydrocarbons | 0.5 M H2SO4 (C for g-valerolactone and Pb for valeric acid), 0.2 M NaOH (C for 4-hydroxy-2-butanone) | −1.80 V (g-valerolactone and valeric acid), 6.00 V (4-hydroxy-2-butanone), at 4 to 8 h | 27.2% for g-valerolactone, ~56% for valeric acid, 6% selectivity for 4-hydroxy-2-butanone | 60.0% for valeric acid, 18.0% for g-valerolactone, 5.0% for 4-hydroxy-2-butanone | [76] |
| 2020 | CuO | Glycerol | 1,3-dihydroxyacetone | 0.1 M Na2B4O7 | 3 mA/cm2 at 3 h | selectivity of 60.0% | - | [82] |
| 2021 | CoOx | Glycerol | 1,3-dihydroxyacetone | 0.1 M Na2B4O7 | 1.50 V at 3 h | selectivity of 60.0% | 49.40% | [84] |
| 2015 | Bi-Pt/C, Sb-Pt/C | Sorbitol | Varied | 0.5 M H2SO4 | CV characterization (0 to 1.60 V) | - | - | [85] |
| 2018 | PbO2, MnO2, Pt | Furfural | Maleic acid | H2SO4 (pH of 1) | 2.00 V (duration not reported) | 65.10% | - | [88] |
| 2020 | Au/Carbon cloth | Furfural | Furoic acid | 0.25 M HClO4 | CV characterization (0 to 1.50 V) | - | - | [89] |
| Year | Electrocatalyst | Feedstock | Products | Electrolyte | Electrolytic Tests and Conditions (Potentials Respect to RHE) | Yield | Faradaic Efficiency | Reference |
|---|---|---|---|---|---|---|---|---|
| 2014 | MnO2/graphite/PTFE | short chain cellulose oligosaccharides | Glucose | 0.1 M Na2SO4 (pH of 3) | −0.58 V at 8 h | 72.40% | - | [90] |
| 2013 | Cu, Ni, Pt, Pb, C, Al | 5-HMF | 2,5-dimethylfuran (DMF) | 0.5 M H2SO4 | 10 mA/cm2, 2 to 4 h | selectivity of 35.6% | up to 67% | [87] |
| 2013 | Fe, Ni, Ag, Cd, In, Au, Sn, Sb, Co, Bi, Pd, Pb | 5-HMF | 1,5-dihydroxymethylfuran (DHMF) | 0.1 M Na2SO4 (pH of 7) | CV characterization for different electrodes | - | - | [91] |
| 2015 | Pd, Pt, Al, Zn, In, Sb, Co, Ag, Cd, Bi | 5-HMF | DHMF | 0.5 M H2SO4 | CV characterization for different electrodes | - | - | [92] |
| 2019 | Ag/C | 5-HMF | 2,5-bis(hydroxymethylfuran) (BHMF) | 0.5 M borate buffer (pH of 9) | −0.46 at 30 min, paired with TEMPO mediated 5-HMF oxidation | 15.9% | 89.3% (cathode) | [96] |
| 2019 | CuNi, Cu, Ni | 5-HMF | DMF | 0.2 M Sulfate buffer (pH of 2) | −0.48 V (duration not reported) | 34.00% | 84.60% | [97] |
| 2021 | Oxide Derived (OD) Ag | 5-HMF | BHMF | 0.5 M borate buffer (pH of 9) | −0.51 V at 3 h, paired with TEMPO 5-HMF | 28.60% | ~80% (cathode) | [98] |
| 2012 | Pb | Levulinic acid | Valeric acid (ECH), octane (Kolbe) | 0.5 M H2SO4 (ECH), water or methanol at pH of 5.5 (Kolbe) | −1.41 V at 4 h (ECH), 3.895 V (duration unspecified) (Kolbe) | Valeric acid selectivity 97.2%, octane selectivity 51.6% | 27% (ECH), 66.5% (Kolbe) | [77] |
| 2013 | Cu, Pb | Levulinic acid | Valeric acid, g-valerolactone | 0.5 M H2SO4 (pH of 0), K2HPO4 buffer (pH of 7.5) | −1.10 V to −1.50 V, 4 h | Valeric acid maximum yield ~12%, g-valerolactone max yield ~1% | - | [104] |
| 2015 | C, Cu, Ni, Pb, Fe | Levulinic acid | g-valerolactone | Varied (acidic, neutral, alkaline) | −1.80 V, 4 to 8 h | valeric acid 56.0% (Pb acidic), g-valerolactone 28.0% (Fe alkaline) | ~20% | [76] |
| 2020 | Pb, Pt, Zn, Cu, Co, Ti | Levulinic acid | Valeric acid | 0.5 M H2SO4 | −1.40 V at 4 h | 43.20% | 94.00% | [107] |
| 2021 | Pt | Levulinic acid | 1,2-octanedione | 0.1 M KOH | - | 75.00% | 67.00% | [108] |
| Year | Electrocatalyst | Feedstock | Desired Product(s) | Electrolyte (Catholyte) | Electrolytic Conditions | Coupled Product Yield (If Any) | OER Potential | Reactant Oxidation Potential | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 2017 | Fe2P/Stainless steel mesh | 0.5 M glucose | Hydrogen | 10 M KOH | CV characterization | - | 1.52 V for 10 mA/cm2 | 1.22 V for 10 mA/cm2 | [109] |
| 2019 | Pd-Au/C | 0.1 M glucose | Hydrogen, gluconic acid | 0.1 M NaOH | CV characterization, applied 0.40 V at 6 h | Gluconate yield 58.3% | - | 0.2 V for 1 mA/cm2, 0.5 V for max 4 mA/cm2 | [110] |
| 2020 | Co, Ni, Co-Ni/Carbon cloth | 0.1 M glucose | Hydrogen | 1 M KOH | CV characterization | - | 1.39 V for 10 mA/cm2 | 1.096 V for 10 mA/cm2 | [112] |
| 2020 | Co0.5 Ni0.5(OH)2 Nanosheet | 0.1 M glucose | Hydrogen | 1 M KOH | CV characterization | - | 1.47 V for 10 mA/cm2 | 1.22 V for 10 mA/cm2 | [113] |
| 2020 | Ni-MoS2/carbon paper | 0.3 M glucose | Hydrogen | 1 M KOH | CV characterization | - | 1.64 V for 10 mA/cm2 | 1.46 V for 10 mA/cm2 | [114] |
| 2020 | Fe0.1-CoSe2/Carbon cloth | 0.5 M glucose | Hydrogen, gluconic acid | 0.5 M H2SO4 | CV characterization | - | 1.34 V for 10 mA/cm2 | 0.72 V for 10 mA/cm2 | [115] |
| 2020 | glucose derived carbon/glassy carbon | sacrificial carbon at cathode | Hydrogen | 0.1 M KOH | CV characterization | - | OER onset 1.52 V | Carbon oxidation onset 1.02 V | [116] |
| 2021 | Mo-Ni0.85Se/NF | 10 mM 5-HMF | Hydrogen, FDCA | 1 M KOH | 1.40 V at 2 h | FDCA yield 95.0%, FE 95.0% | Overall 1.68 V for 50 mA/cm2 | Overall 1.5 V for 50 mA/cm2 | [117] |
| 2016 | Co-P/Cu foam | 50 mM 5-HMF | Hydrogen, FDCA | 1 M KOH | 1.42 V at 6 h | FDCA yield 90.0% | Anodic 1.53 V, overall 1.59 V for 20 mA/cm2 | anodic 1.38 V, overall 1.44 V for 20 mA/cm2 | [118] |
| 2021 | Ni3N-V2O3 | 10 mM 5-HMF | Hydrogen, FDCA | 1 M KOH | 10 mA/cm2 at 112 min | FDCA yield 96.1% | - | 1.4 V for 10 mA/cm2 | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Z.I.; Lee, L.Q.; Li, H. Electroreforming of Biomass for Value-Added Products. Micromachines 2021, 12, 1405. https://doi.org/10.3390/mi12111405
Lai ZI, Lee LQ, Li H. Electroreforming of Biomass for Value-Added Products. Micromachines. 2021; 12(11):1405. https://doi.org/10.3390/mi12111405
Chicago/Turabian StyleLai, Zi Iun, Li Quan Lee, and Hong Li. 2021. "Electroreforming of Biomass for Value-Added Products" Micromachines 12, no. 11: 1405. https://doi.org/10.3390/mi12111405
APA StyleLai, Z. I., Lee, L. Q., & Li, H. (2021). Electroreforming of Biomass for Value-Added Products. Micromachines, 12(11), 1405. https://doi.org/10.3390/mi12111405






