Biomimetic Composite Coatings for Activation of Titanium Implant Surfaces: Methodological Approach and In Vivo Enhanced Osseointegration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
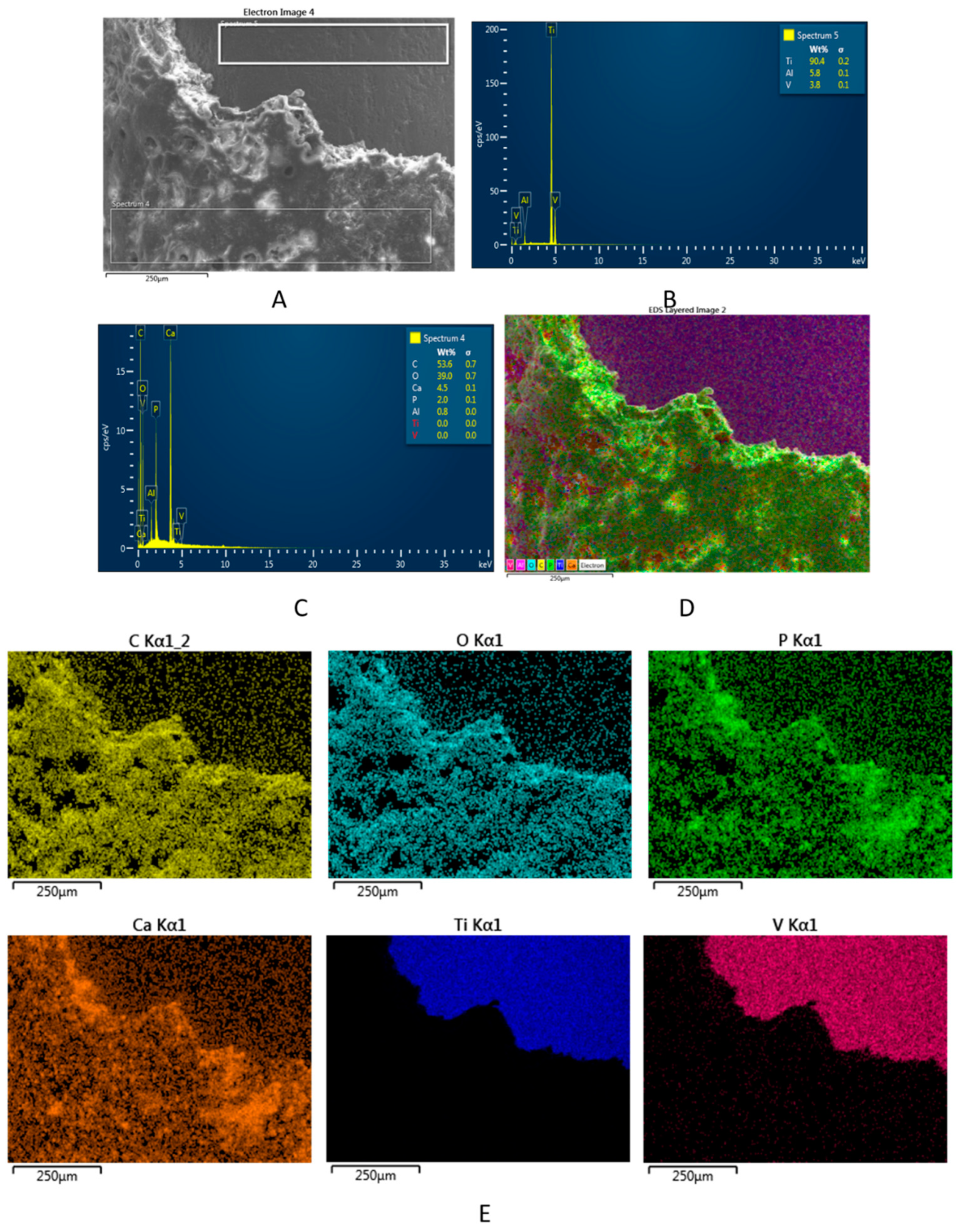
2.2. Characterization Methods of the Ti Implants and Composite Coatings
2.3. Surgical Protocol and Procedures
2.4. Bone Markers: Alkaline Phosphatase and Osteocalcin
2.5. Advanced Micro-CT Approach
2.6. Hematoxylin and Eosin (H&E) Staining Procedure: Histological Assessment of the Osseointegration
2.7. Bone–Implant Interface
2.8. Statistical Analysis
3. Results
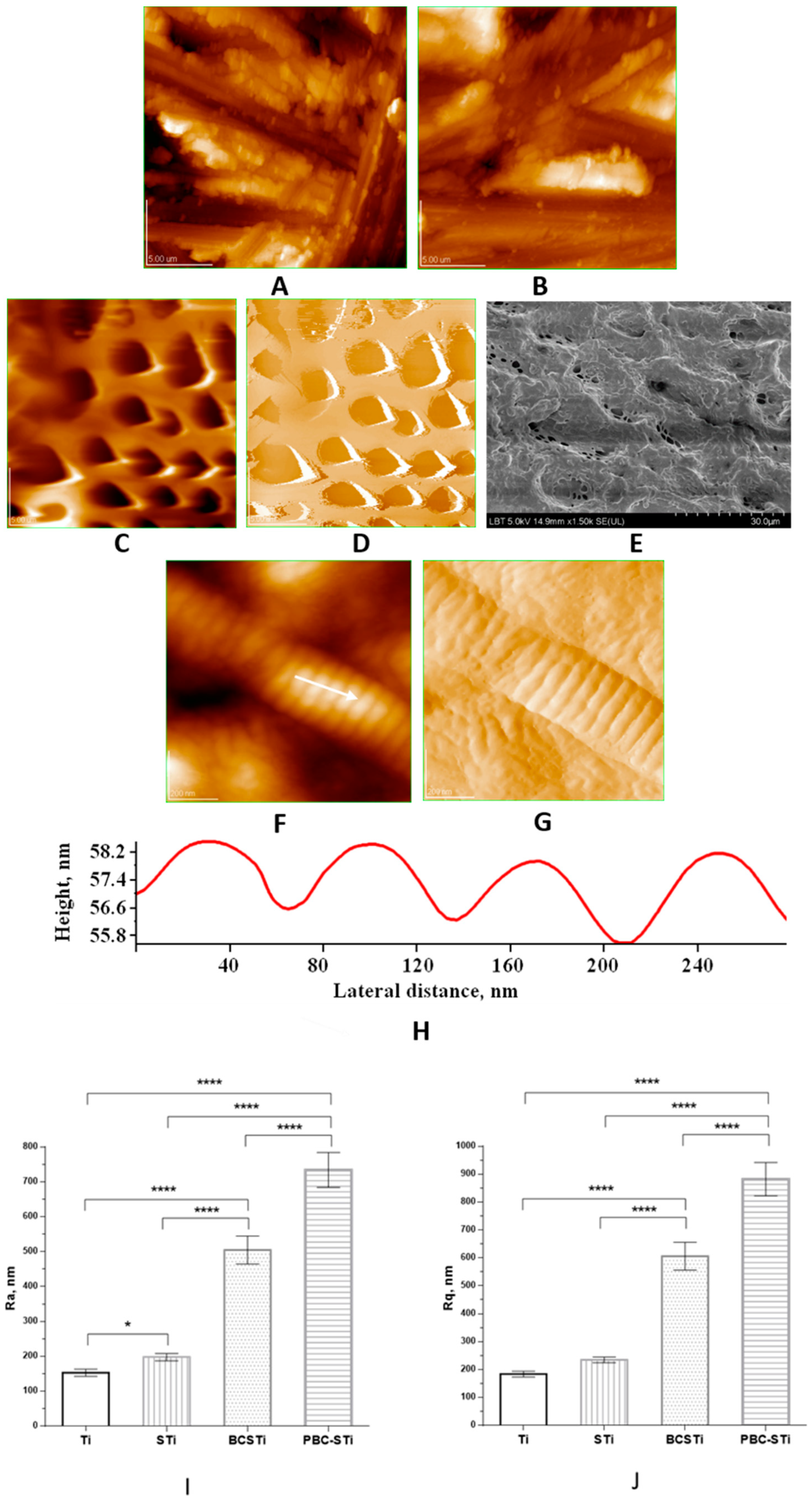
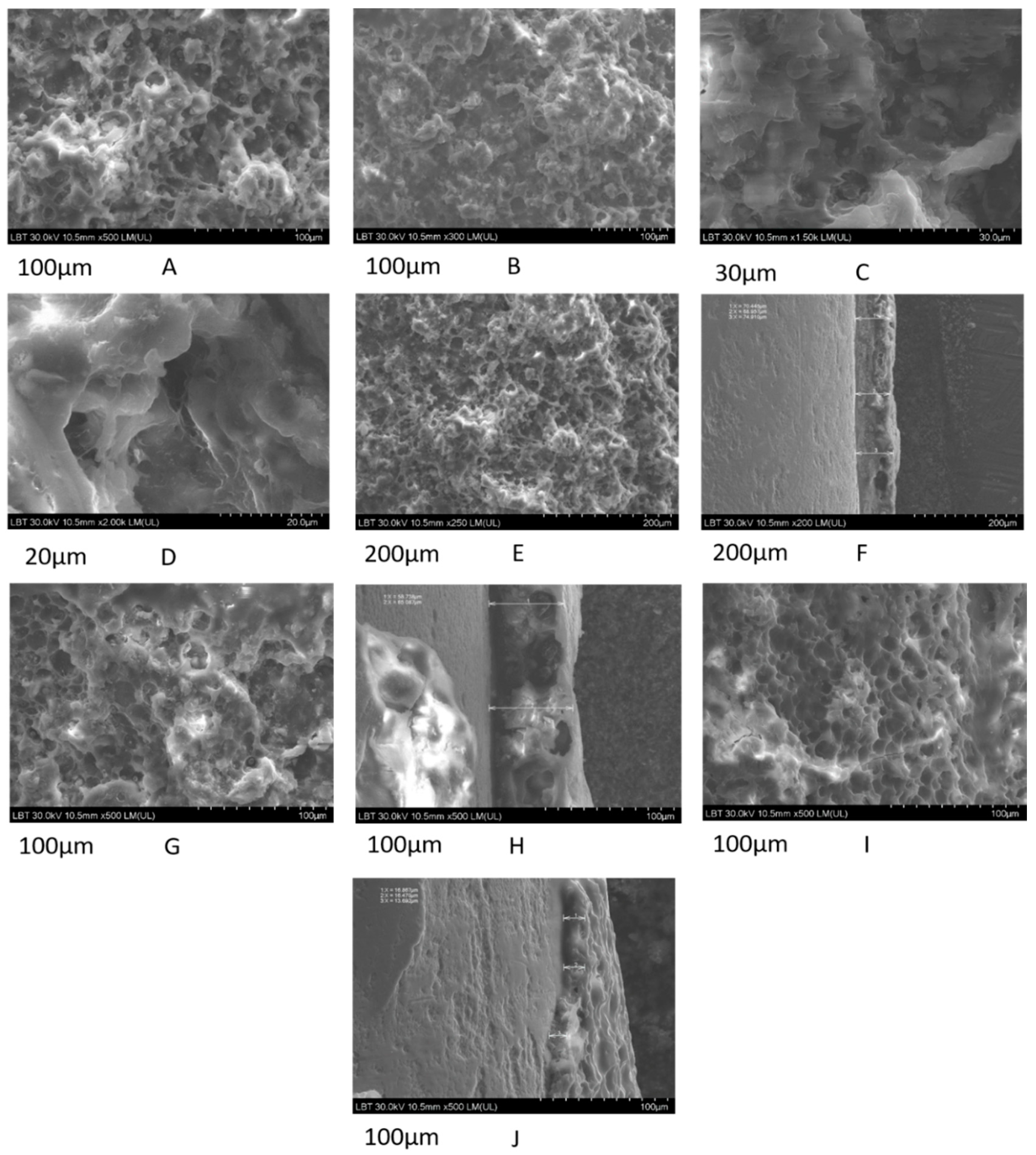
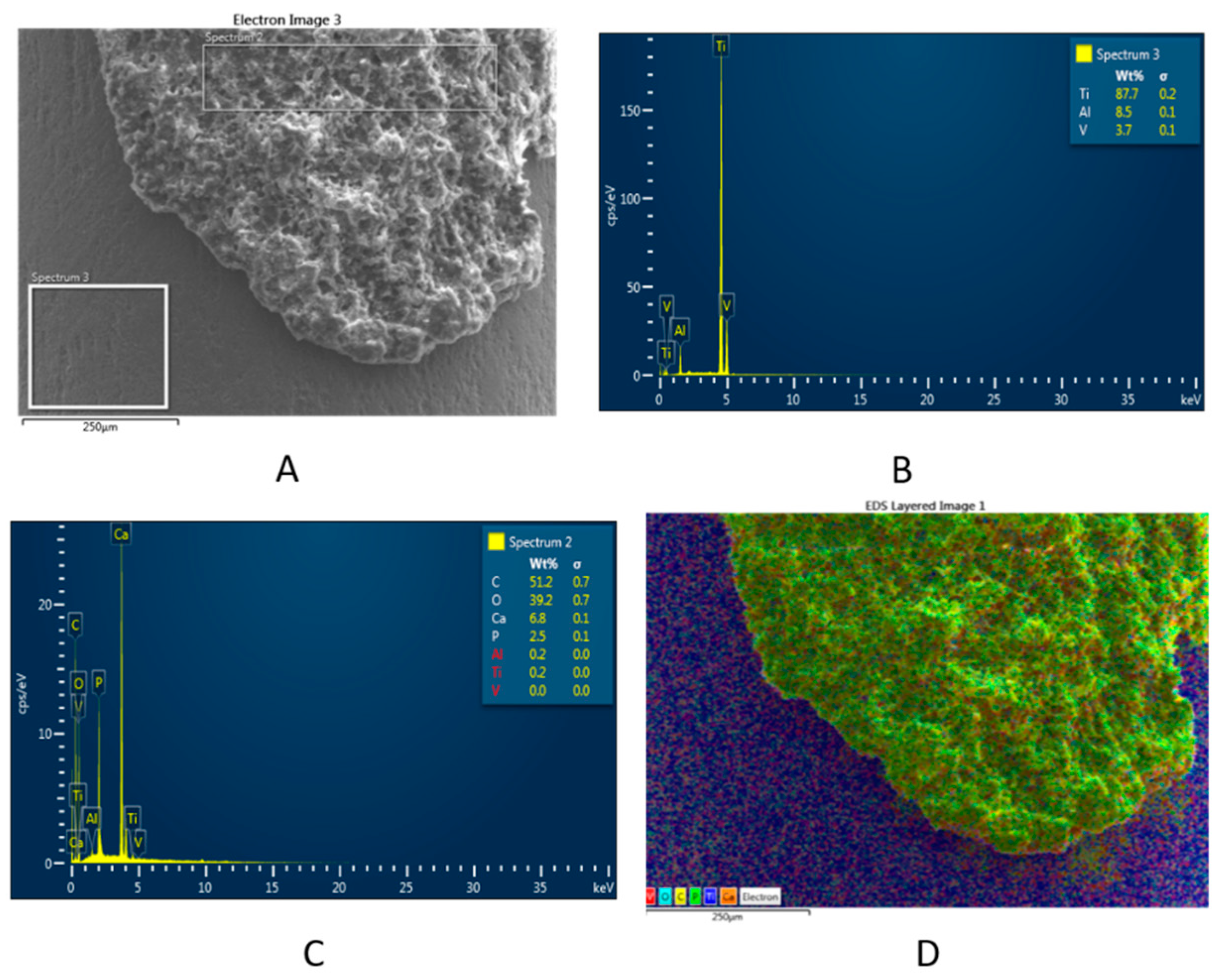
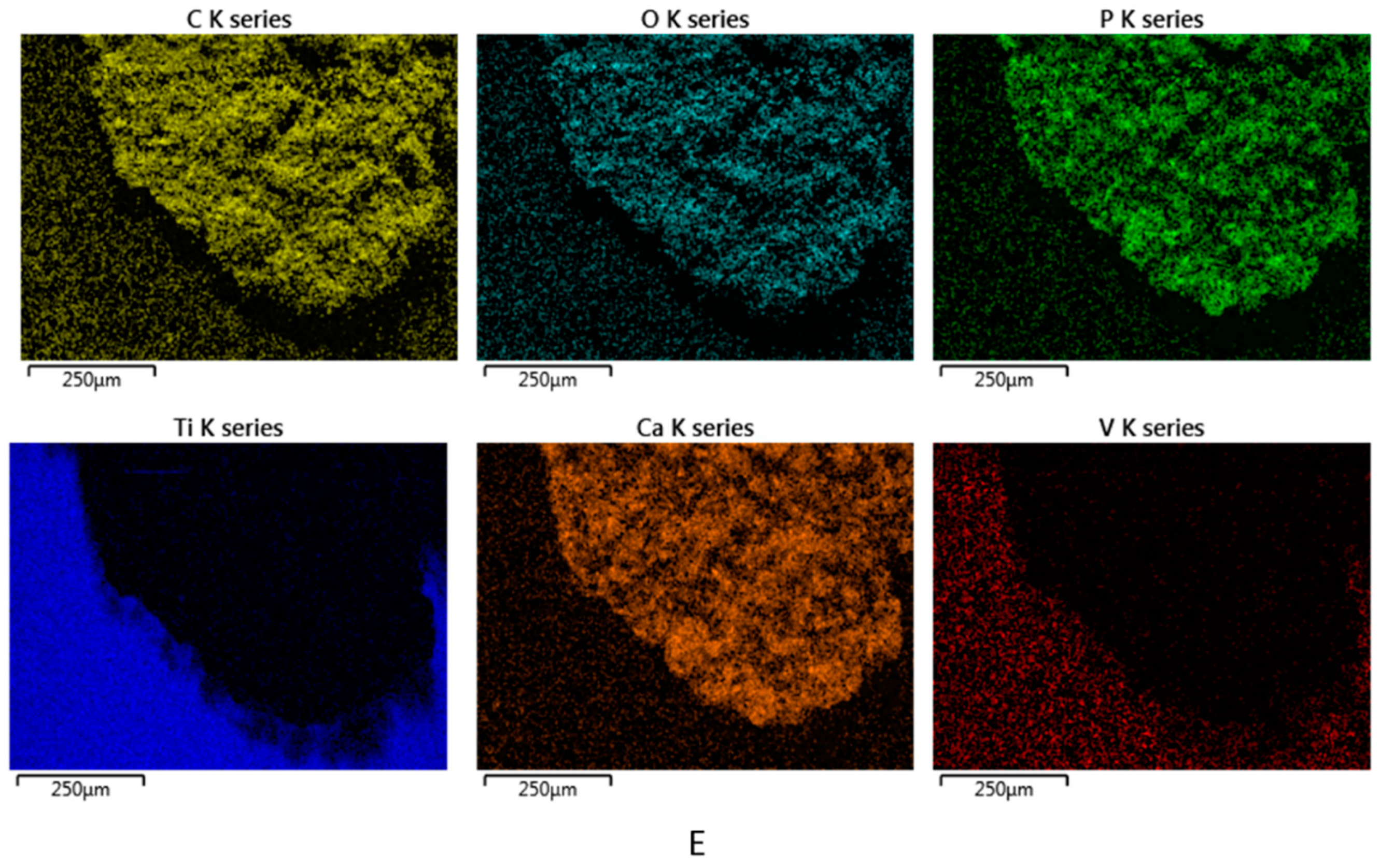
3.1. Topography and Surface Structure of Modified Ti Implants
3.2. Alkaline Phosphatase and Osteocalcin vs. Animal Groups at Various Time Points
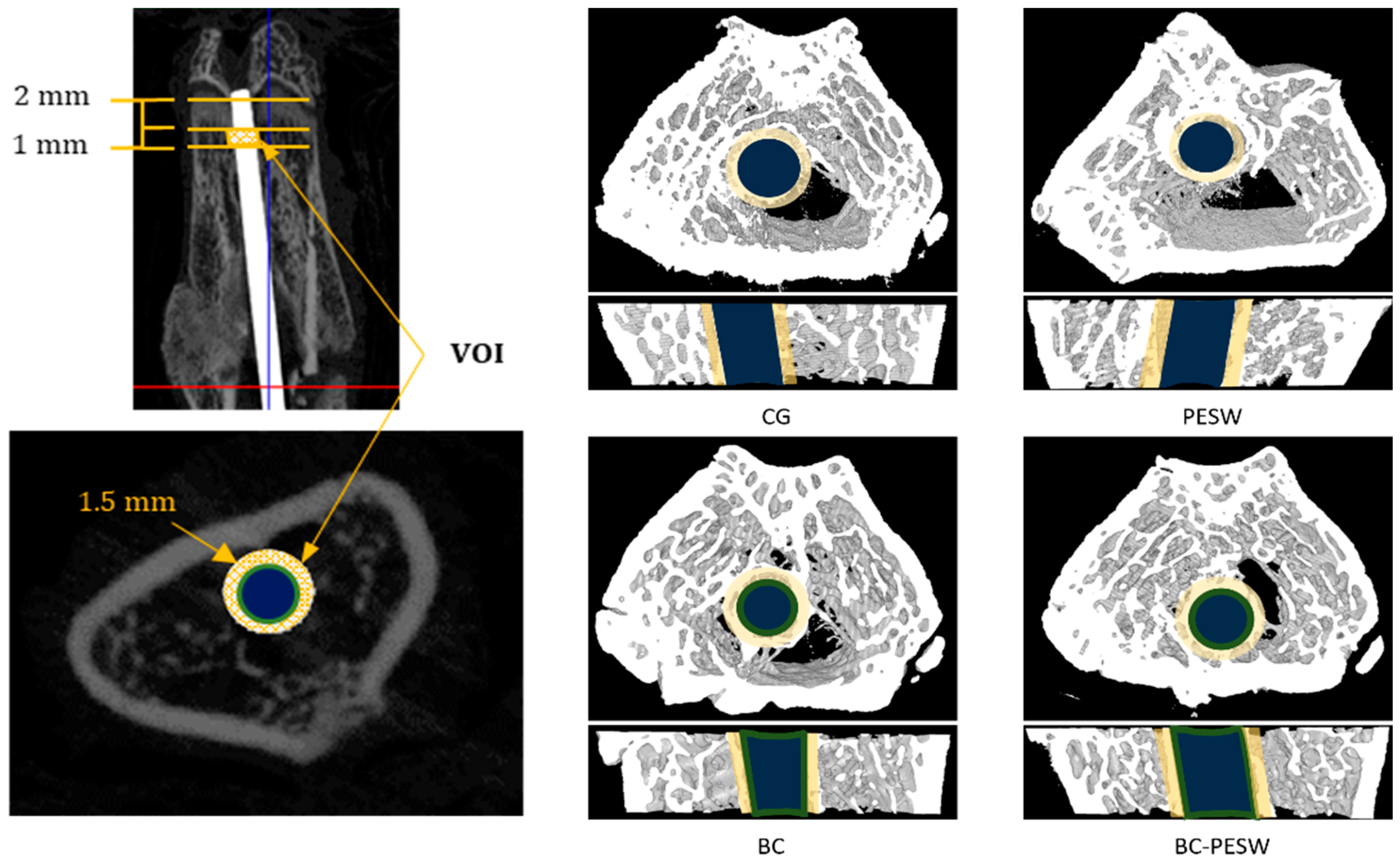
3.3. Micro-CT Examination
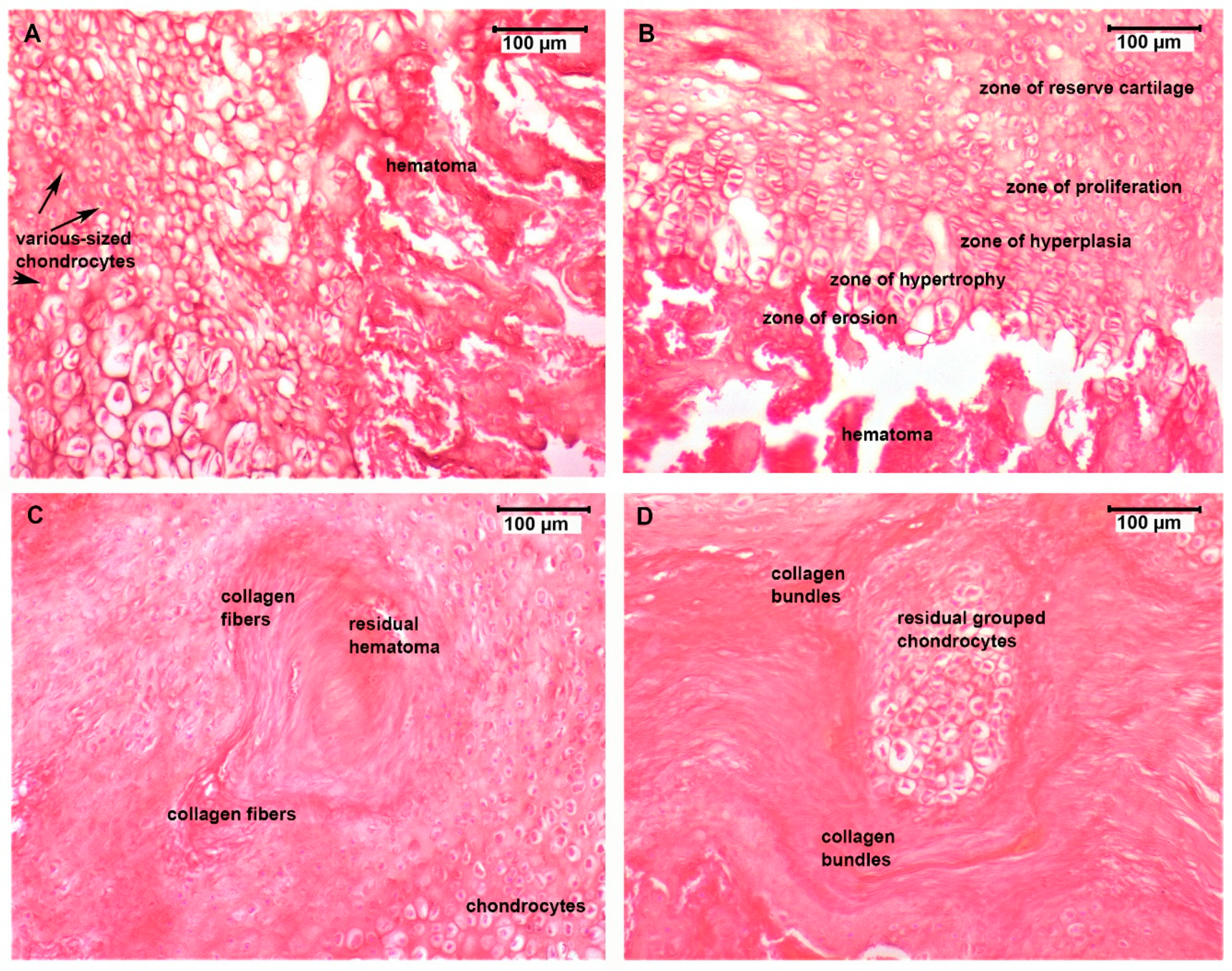
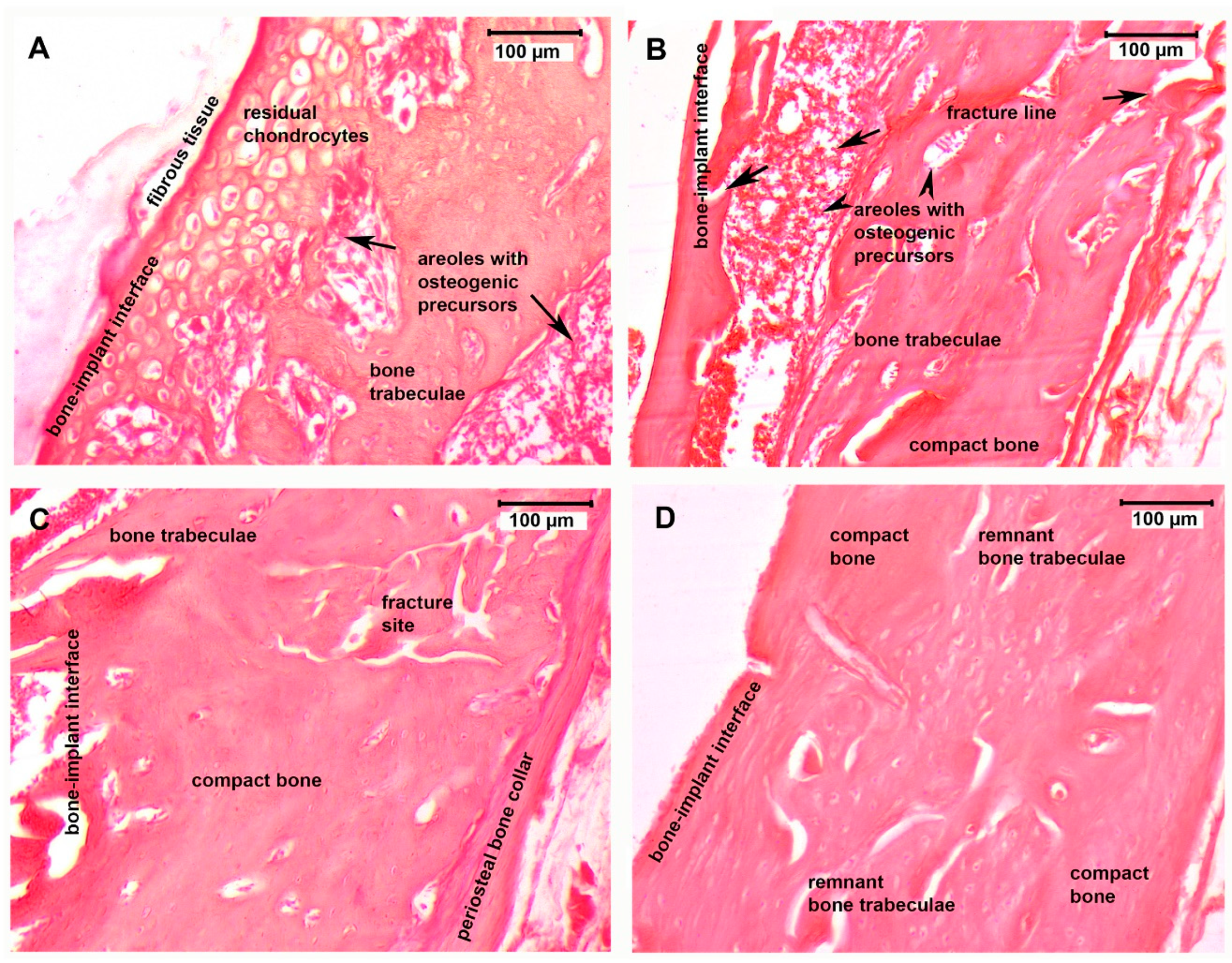
3.4. Histological Assessment
3.5. Fine Structure of the Bone–Implant Interface
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar] [PubMed]
- Isaacson, B.M.; Jeyapalina, S. Osseointegration: A review of the fundamentals for assuring cementless skeletal fixation. Orthop. Res. Rev. 2014, 6, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.-P.; Kiattavorncharoen, S.; Lauer, H.C.; Meyer, U.; Kubler, N.R.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An In Vivo study. Head. Face Med. 2008, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.H.; Yan, L.; Hao, D.J.; Liu, S.; Yang, M.; He, B.R.; Liu, Z.K. Calcium alginate template-mineral substituted hydroxyapatite hydrogel coated titanium implant for tibia bone regeneration. Int. J. Pharm. 2020, 582, 119303. [Google Scholar] [CrossRef]
- Novaes, A.B., Jr.; Scombatti de Souz, S.L.; Rezende Matins de Barros, R.; Yamashira Pereira, K.K.; Iezzi, G.; Piatelli, A. Influence of implant surfaces on osseointegration. Braz. Dent. J. 2010, 21, 471–481. [Google Scholar] [CrossRef]
- Wang, W.; Poh, C.K. Titanium Alloys in Orthopaedics. In Titanium Alloys—Advances in Properties Control; Sieniawski, J., Ziaja, W., Eds.; Intech Open: London, UK, 2013; pp. 1–20. [Google Scholar]
- Hermida, J.C.; Bergula, A.; Dimaano, F.; Hawkins, M.; Colwell, C.W., Jr.; D’Lima, D.D. An In Vivo evaluation of bone response to three implant surfaces using a rabbit intramedullary rod model. J. Orthop. Surg. Res. 2010, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- D’Antonio, J.A.; Capello, W.N.; Crothers, O.D.; Laffe, W.L.; Manley, M.T. Early clinical experience with hydroxyapatite-coated femoral implants. J. Bone Jt. Surg. Am. 1992, 74, 995–1008. [Google Scholar] [CrossRef]
- D’Antonio, J.A.; Capello, W.N.; Manley, M.T. Remodeling of bone around hydroxyapatite-coated femoral stems. J. Bone Jt. Surg. Am 1996, 78, 1226–1234. [Google Scholar] [CrossRef]
- Kroon, P.O.; Freeman, M.A. Hydroxyapatite coating of hip prostheses. Effect on migration into the femur. J. Bone Jt. Surg. Br. 1992, 74, 518–522. [Google Scholar] [CrossRef]
- Beck, G.R., Jr. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J. Cell Biochem. 2003, 90, 234–243. [Google Scholar] [CrossRef]
- Stanford, C.M.; Keller, J.C.; Solursh, M. Bone cell expression on titanium surfaces is altered by sterilization treatments. J. Dent. Res. 1994, 73, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.S.G.; Clark, P. The effects of topographic and mechanical properties of materials on cell behavior. CRC Crit. Rev. Biocompat. 1990, 5, 343–362. [Google Scholar]
- Feighan, J.E.; Goldberg, V.M.; Davy, D.; Parr, J.A.; Stevenson, S. The influence of surface-blasting on the incorporation of titanium-alloy implants in a rabbit intramedullary model. J. Bone Jt. Surg. Am. 1995, 77, 1380–1395. [Google Scholar] [CrossRef]
- Wong, M.; Eulenberger, J.; Schenk, R.; Hunziker, E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J. Biomed. Mater. Res. 1995, 29, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Hacking, S.A.; Tanzer, M.; Harvey, E.J.; Krygier, J.J.; Bobyn, J.D. Relative contributions of chemistry and topography to the osseointegration of hydroxyapatite coatings. Clin. Orthop. Relat. Res. 2002, 405, 24–38. [Google Scholar] [CrossRef]
- Daugaard, H.; Elmengaard, B.; Bechtold, J.E.; Jensen, T.; Soballe, K. The effect on bone growth enhancement of implant coatings with hydroxyapatite and collagen deposited electrochemically and by plasma spray. J. Biomed. Mater. Res. A 2010, 92, 913–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Darimont, G.L.; Cloots, R.; Heinen, E.; Seidel, L.; Legrand, R. In Vivo behaviour of hydroxyapatite coatings on titanium implants: A quantitative study in the rabbit. Biomaterials 2002, 23, 2569–2575. [Google Scholar] [CrossRef]
- Tomoaia, G.; Soritau, O.; Tomoaia-Cotisel, M.; Pop, L.B.; Pop, A.; Mocanu, A.; Horovitz, O.; Bobos, L.D. Scaffolds made of nanostructured phosphates, collagen and chitosan for cell culture. Powder Technol. 2013, 238, 99–107. [Google Scholar] [CrossRef]
- Mocanu, A.; Furtos, G.; Rapuntean, S.; Horovitz, O.; Flore, C.; Garbo, C.; Danisteanu, A.; Rapuntean, G.; Prejmerean, C.; Tomoaia-Cotisel, M. Synthesis; characterization and antimicrobial effects of composites based on multi-substituted hydroxyapatite and silver nanoparticles. App. Surf. Sci. 2014, 298, 225–235. [Google Scholar] [CrossRef]
- Tomoaia, G.; Mocanu, A.; Vida-Simiti, I.; Jumate, N.; Bobos, L.D.; Soritau, O.; Tomoaia-Cotisel, M. Silicon effect on the composition and structure of nanocalcium phosphates: In Vitro biocompatibility to human osteoblasts. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 37, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.; Cadar, O.; Frangopol, P.T.; Petean, I.; Tomoaia, G.; Paltinean, G.A.; Racz, C.P.; Horovitz, O.; Tomoaia-Cotisel, M. Ions release from hydroxyapatite and substituted hydroxyapatites in different immersion liquids: In Vitro experiments and theoretical modelling study. R. Soc. Open Sci. 2021, 8, 201785. [Google Scholar] [CrossRef]
- Rapuntean, S.; Frangopol, P.T.; Hodisan, I.; Tomoaia, G.; Oltean-Dan, D.; Mocanu, A.; Prejmerean, C.; Soritau, O.; Racz, L.Z.; Tomoaia-Cotisel, M. In Vitro response of human osteoblasts cultured on strontium substituted hydroxyapatites. Rev. Chim. 2018, 69, 3537–3544. [Google Scholar] [CrossRef]
- Garbo, C.; Locs, J.; D’Este, M.; Demazeau, G.; Mocanu, A.; Roman, C.; Horovitz, O.; Tomoaia-Cotisel, M. Advanced Mg, Zn, Sr, Si multi-substituted hydroxyapatites for bone regeneration. Int. J. Nanomed. 2020, 15, 1037–1058. [Google Scholar] [CrossRef] [Green Version]
- Oltean-Dan, D.; Dogaru, G.B.; Tomoaia-Cotisel, M.; Apostu, D.; Mester, A.; Benea, H.R.C.; Paiusan, M.G.; Jianu, E.M.; Mocanu, A.; Balint, R.; et al. Enhancement of bone consolidation using high-frequency pulsed electromagnetic short-waves and titanium implants coated with biomimetic composite embedded into PLA matrix: In Vivo evaluation. Int. J. Nanomed. 2019, 14, 5799–5816. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed Mater. Res. B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Y.; Ding, W.; Zhang, C. Filling behavior, morphology evolution and crystallization behaviour of microinjection molded poly (lactic acid)/hydroxyapatite nanocomposites. Compos. Part A Appl. Sci. Manuf. 2015, 72, 85–95. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly (lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Zhou, H.; Touny, A.H.; Bhaduri, S.B. Fabrication of novel PLA/CDHA bionanocomposite fibers for tissue engineering applications via electrospinning. J. Mater. Sci. Mater. Med. 2011, 22, 1183–1193. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Nowicki, M.; Vukovic-Kwiatkowska, I.; Nowakowska, S. Crosslinked blends of poly (lactic acid) and polyacrylates: AFM, DSC and XRD studies. J. Polym. Res. 2013, 20, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Surya, S.; Ray, S.S. Polylactide based nanostructured biomaterials and their applications. J. Nanosci. Nanotechnol. 2007, 7, 2596–2615. [Google Scholar]
- McManus, A.J.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Evaluation of cytocompatibility and bending modulus of nanoceramic/polymer composites. J. Biomed. Mater. Res. A 2005, 72, 98–106. [Google Scholar] [CrossRef]
- Loo, S.C.J.; Moore, T.; Banik, B.; Alexis, F. Biomedical applications of hydroxyapatite nanopaticles. Curr. Pharm. Biotechnol. 2010, 11, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Neo, M.; Tamura, J.; Fujibayashi, S.; Takemoto, M.; Shikinami, Y.; Okazaki, K.; Nakamura, T. In Vivo evaluation of a porous hydroxyapatite/ poly-DL-lactide composite for bone tissue engineering. J. Biomed. Mater. Res. A 2007, 81, 930–938. [Google Scholar] [CrossRef]
- Tomoaia, G.; Tomoaia-Cotisel, M.; Pop, L.B.; Pop, A.; Horovitz, O.; Mocanu, A.; Jumate, N.; Bobos, L.D. Synthesis and characterization of some composites based on nanostructured phosphates, collagen and chitosan. Rev. Roum. Chim. 2011, 56, 1039–1046. [Google Scholar]
- Tomoaia, G.; Pop, L.B.; Petean, I.; Tomoaia-Cotisel, M. Significance of surface structure on orthopedic materials. Mater. Plast. 2012, 49, 48–54. [Google Scholar]
- Tomoaia-Cotisel, M.; Tomoaia-Cotisel, A.; Yupsanis, T.; Tomoaia, G.; Balea, I.; Racz, C. Coating layers of major storage protein from aleurone cells of barley studied by atomic force microscopy. Rev. Roum. Chim. 2006, 51, 1181–1185. [Google Scholar]
- Zdrenghea, U.V.; Tomoaia, G.; Pop-Toader, D.-V.; Mocanu, A.; Horovitz, O.; Tomoaia-Cotisel, M. Procaine effect on human erythrocyte membrane explored by atomic force microscopy. Comb. Chem. High Throughput Screen. 2011, 14, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Tomoaia, G.; Horovitz, O.; Mocanu, A.; Nita, A.; Avram, A.; Racz, C.P.; Soritau, O.; Ceneriu, M.; Tomoaia-Cotisel, M. Effects of doxorubicin mediated by gold nanoparticles and resveratrol in two human cervical tumor cell lines. Colloids Surf. B Biointerfaces 2015, 135, 726–734. [Google Scholar] [CrossRef]
- Choi, J.Y.C.; Choi, C.A.; Yeo, I.S.L. Spiral scanning imaging and quantitative calculation of the 3-dimensional screw-shaped bone-implant interface on micro-computed tomography. J. Periodontal Implant. Sci. 2018, 48, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, O.; Probst, F.A.; Wolff, K.D.; Jeschke, A.; Weitz, J.; Deppe, H.; Kolk, A. Comparative 3D micro-CT and 2D histomorphometry analysis of dental implant osseointegration in the maxilla of minipigs. J. Clin. Periodontol. 2017, 44, 418–427. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Huang, Y.J.; Wu, H.H.; Liu, Y.A.; Liu, Y.S.; Lee, O.K. Osteocalcin mediates biomineralization during osteogenic maturation in human mesenchymal stromal cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Pedrazzi, G.; Mattioli-Belmonte, M.; Guizzardi, S. The use of pulsed electromagnetic fields to promote bone responses to biomaterials In Vitro and In Vivo. Int. J. Biomater. 2018, 2018, 8935750. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, V.; Palmieri, A.; Pezzetti, F.; Massari, L.; Carinci, F. Effects of pulsed electromagnetic fields on human osteoblastlike cells (MG-63): A pilot study. Clin. Orthop. Relat. Res. 2018, 468, 2260–2277. [Google Scholar] [CrossRef] [Green Version]
- Barnaba, S.; Papalia, R.; Ruzzini, L.; Sgambato, A.; Maffulli, N.; Denaro, V. Effect of pulsed electromagnetic fields on human osteoblast cultures. Physiother. Res. Int. 2013, 18, 109–114. [Google Scholar] [CrossRef]
- Ongaro, A.; Pellati, A.; Bagheri, L.; Fortini, C.; Setti, S.; De Mattei, S. Pulsed electromagnetic fields stimulate osteogenic differentiation in human bone marrow and adipose tissue derived mesenchymal stem cells. Bioelecromagnetics 2014, 35, 426–436. [Google Scholar] [CrossRef]
- Marom, R.; Shur, I.; Solomon, R.; Benayahu, D. Characterization of adhesion and differentiation markers of osteogenic marrow stromal cells. J. Cell Physiol. 2005, 202, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Neumann, M.; Hanisch, U.; Reinstorf, A.; Pompe, W.; Zwipp, H.; Biewener, A. Osteocalcin enhances bone remodeling around hydroxyapatite/collagen composites. J. Biomed. Mater. Res. A 2005, 73, 284–294. [Google Scholar] [CrossRef]
- Castellani, C.; Lindtner, R.A.; Hausbrandt, P.; Tschegg, E.; Stanzl-Tschegg, S.E.; Zanoni, G.; Beck, S.; Weinberg, A.-M. Bone-implant interface strength and osseointegration: Biodegradable magnesium alloy versus standard titanium control. Acta Biomater. 2010, 7, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Lim, D.; Choi, B.H.; Kim, H.S. Suggestion of new methodology for evaluation of osseointegration between implant and bone based on μ-CT images. Int. J. Precis. Eng. Manuf. 2010, 11, 785–790. [Google Scholar] [CrossRef]
- Vertesich, K.; Sosa, B.R.; Niu, Y.; Ji, G.; Suhardi, V.; Turajane, K.; Mun, S.; Xu, R.; Windhager, R.; Park-Min, K.H.; et al. Alendronate enhances osseointegration in a murine implant model. J. Orthop. Res. 2021, 39, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, H.; Xu, J.; Li, X.; Qi, M.; Hu, M. Morphology, composition, and bioactivity of strontium-doped brushite coatings deposited on titanium implants via electrochemical deposition. Int. J. Mol. Sci. 2014, 15, 9952–9962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Z.S.; Bai, B.L.; He, X.W.; Liu, W.; Li, H.; Zhou, Q.; Sun, T.; Huang, Z.L.; Tu, K.K.; Lv, Y.X.; et al. A comparative study of strontium-substituted hydroxyapatite coating on implant’s osseointegration for osteopenic rats. Med. Biol. Eng. Comput. 2016, 54, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Scannell, B.; Frick, S.L. Skeletal growth, development, and healing as related to pediatric trauma. In Green’s Skeletal Trauma in Children, 6th ed.; Mencio, G.A., Frick, S.L., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 1–17. [Google Scholar]
- Bumbu, B.A.; Bumbu, A.; Rus, V.; Gal, A.F.; Miclaus, V. Histological evidence concerning the osseointegration of titanium implants in the fractured rabbit femur. J. Histotechnol. 2016, 39, 47–52. [Google Scholar] [CrossRef]
- Lyu, L.; Yang, S.; Jing, Y.; Wang, J. Examining trabecular morphology and chemical composition of peri-scaffold osseointegrated bone. J. Orthop. Surg. Res. 2020, 15, 406. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, S.; Ashukina, N.; Maltseva, V.; Ivanov, G.; Badnaoui, A.A.; Schearzkopf, R. Evaluation of the bone morphology around four types of porous metal implants placed in distal femur of ovariectomized rats. J. Orthop. Surg. Res. 2020, 15, 296. [Google Scholar] [CrossRef]
- Ma, R.; Guo, D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J. Orthop. Surg. Res. 2019, 14, 32. [Google Scholar] [CrossRef]
- Walsh, W.R.; Pelletier, M.H.; Bertollo, N.; Lovric, V.; Wang, T.; Morberg, P.; Harrington Parr, W.C.; Bergadano, D. Bone ongrowth and mechanical fixation of implants in cortical and cancellous bone. J. Orthop. Surg. Res. 2020, 15, 177. [Google Scholar] [CrossRef]
- Baier, M.; Staudt, P.; Klein, R.; Sommer, U.; Wenz, R.; Grafe, I.; Meeder, P.J.; Nawroth, P.P.; Kasperk, C. Strontium enhances osseointegration of calcium phosphate cement: A histomorphometric pilot study in ovariectomized rats. J. Orthop. Surg. Res. 2013, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Bertollo, N.; Lau, A.; Taki, N.; Nishino, T.; Mishima, H.; Kawamura, H.; Walsh, W.R. Osseointegration of porous titanium implants with and without electrochemically deposited DCPD coating in an ovine model. J. Orthop. Surg. Res. 2011, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.Y.; Park, J.I.; Chae, J.S.; Yeo, I.S.L. Comparison of micro-computed tomography and histomorphometry in the measurement of bone-implant contact ratios. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Liu, Y.C.; Lin, G.S.; Chang, H.H.; Li, Y.T.; Yang, Y.C.; Matsuyama, H.; Lee, B.S.; Chen, Y.W.; Tung, K.L. Flame-sprayed strontium- and magnesium-doped hydroxyapatite on titanium implants for osseointegration enhancement. Surf. Coat. Techol. 2020, 386, 125452. [Google Scholar] [CrossRef]
- Galli, S.; Stocchero, M.; Andersson, M.; Karlsson, J.; He, W.; Lilin, T.; Wennerberg, A.; Jimbo, R. The effect of magnesium on early osseointegration in osteoporotic bone: A histological and gene expression investigation. Osteoporos. Int. 2017, 28, 2195–2205. [Google Scholar] [CrossRef] [Green Version]
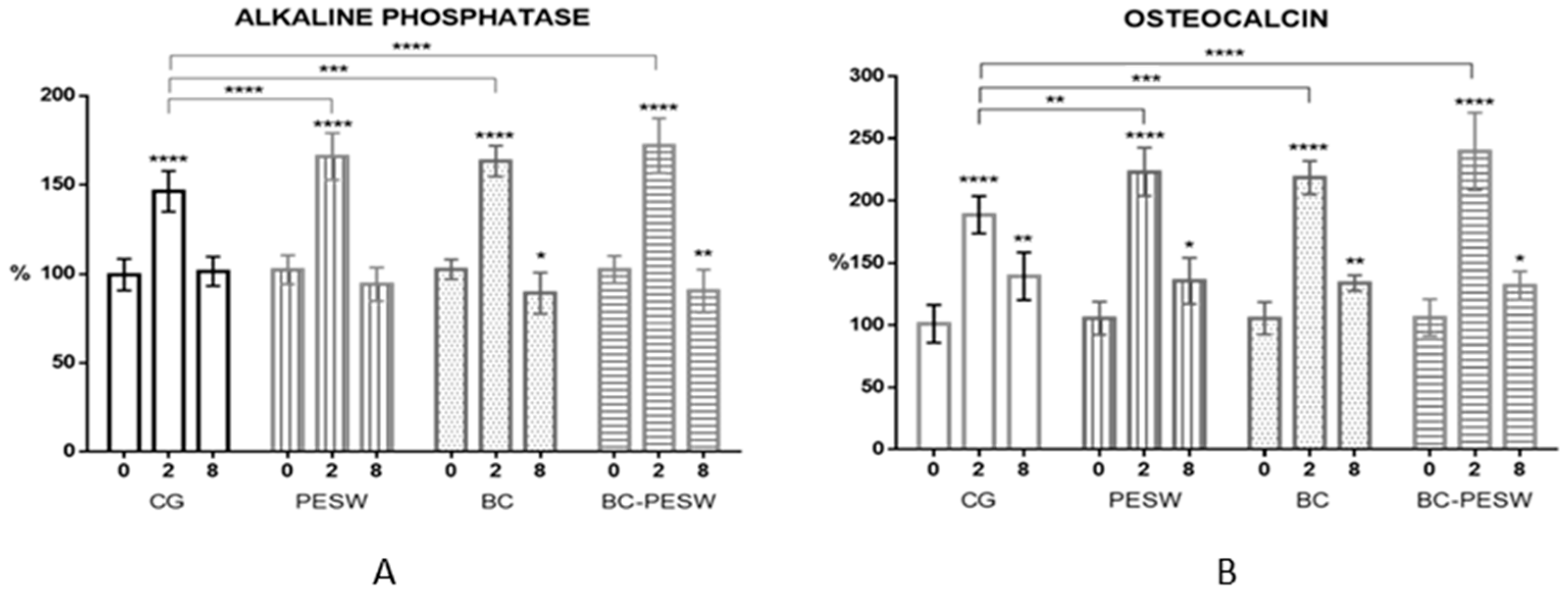
| Bone Marker | Time after Surgery | Animal Group | |||
|---|---|---|---|---|---|
| CG | PESW | BC | BC-PESW | ||
| ALP (%) | 0 weeks | 100 ± 9 | 103 ± 8 | 103 ±6 | 103 ± 7 |
| 2 weeks | 144 ± 11 **** | 172 ± 7 **** | 168 ± 8 **** | 181 ± 11 **** | |
| 8 weeks | 102 ± 8 | 92 ± 8 | 90 ± 12 * | 86 ± 9 ** | |
| OCN (%) | 0 weeks | 100 ± 15 | 105 ± 13 | 105 ± 13 | 106 ± 15 |
| 2 weeks | 189 ± 15 **** | 223 ± 20 **** | 222 ± 12 **** | 242 ± 20 **** | |
| 8 weeks | 140 ± 19 ** | 136 ± 19 * | 134 ± 12 ** | 132 ± 12 * | |
| Osseointegration Marker | CG | PESW | BC | BC-PESW |
|---|---|---|---|---|
| BV/TV (%) | 24.4 ± 3.8 | 28.9 ± 4 | 36.8 ± 4.6 *,& | 42.7 ± 6 *,& |
| Tb.N (1/mm) | 154 ± 18 | 166 ± 20 | 180 ± 18 * | 189 ± 20 * |
| Tb.Th (µm) | 153 ± 13 | 174 ± 13 * | 176 ± 17 * | 195 ± 14 *,& |
| Tb.Sp (µm) | 386 ± 44 | 353 ± 47 | 319 ± 39 * | 287 ± 58 *,& |
| BIC (%) | 21 ± 5 | 27 ± 7 | 48 ± 8 *,& | 54 ± 11 *,& |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oltean-Dan, D.; Dogaru, G.-B.; Jianu, E.-M.; Riga, S.; Tomoaia-Cotisel, M.; Mocanu, A.; Barbu-Tudoran, L.; Tomoaia, G. Biomimetic Composite Coatings for Activation of Titanium Implant Surfaces: Methodological Approach and In Vivo Enhanced Osseointegration. Micromachines 2021, 12, 1352. https://doi.org/10.3390/mi12111352
Oltean-Dan D, Dogaru G-B, Jianu E-M, Riga S, Tomoaia-Cotisel M, Mocanu A, Barbu-Tudoran L, Tomoaia G. Biomimetic Composite Coatings for Activation of Titanium Implant Surfaces: Methodological Approach and In Vivo Enhanced Osseointegration. Micromachines. 2021; 12(11):1352. https://doi.org/10.3390/mi12111352
Chicago/Turabian StyleOltean-Dan, Daniel, Gabriela-Bombonica Dogaru, Elena-Mihaela Jianu, Sorin Riga, Maria Tomoaia-Cotisel, Aurora Mocanu, Lucian Barbu-Tudoran, and Gheorghe Tomoaia. 2021. "Biomimetic Composite Coatings for Activation of Titanium Implant Surfaces: Methodological Approach and In Vivo Enhanced Osseointegration" Micromachines 12, no. 11: 1352. https://doi.org/10.3390/mi12111352
APA StyleOltean-Dan, D., Dogaru, G.-B., Jianu, E.-M., Riga, S., Tomoaia-Cotisel, M., Mocanu, A., Barbu-Tudoran, L., & Tomoaia, G. (2021). Biomimetic Composite Coatings for Activation of Titanium Implant Surfaces: Methodological Approach and In Vivo Enhanced Osseointegration. Micromachines, 12(11), 1352. https://doi.org/10.3390/mi12111352








