Fused Deposition Modeling of Microfluidic Chips in Transparent Polystyrene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Printing Materials
2.2. Filament Preparation
2.3. Fused Deposition Modelling
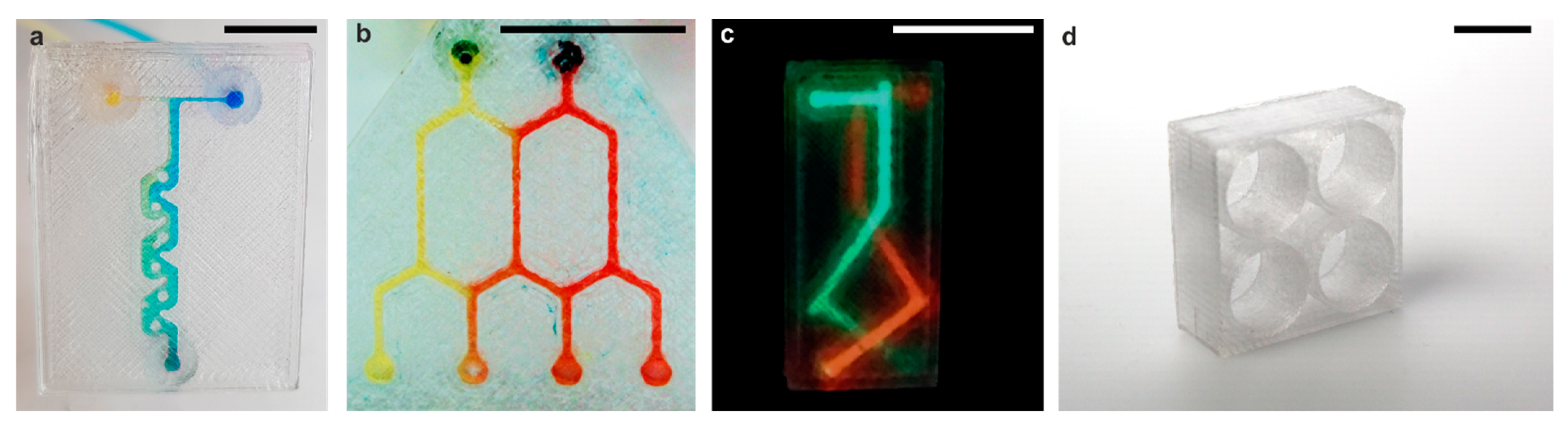
2.4. Microfluidic Experiments
2.5. Contact Angle Measurements
2.6. UV/Vis Measurements
2.7. Differential Scanning Calorimetry
2.8. Cell Culture
2.9. Live/Dead Staining
3. Results and Discussion
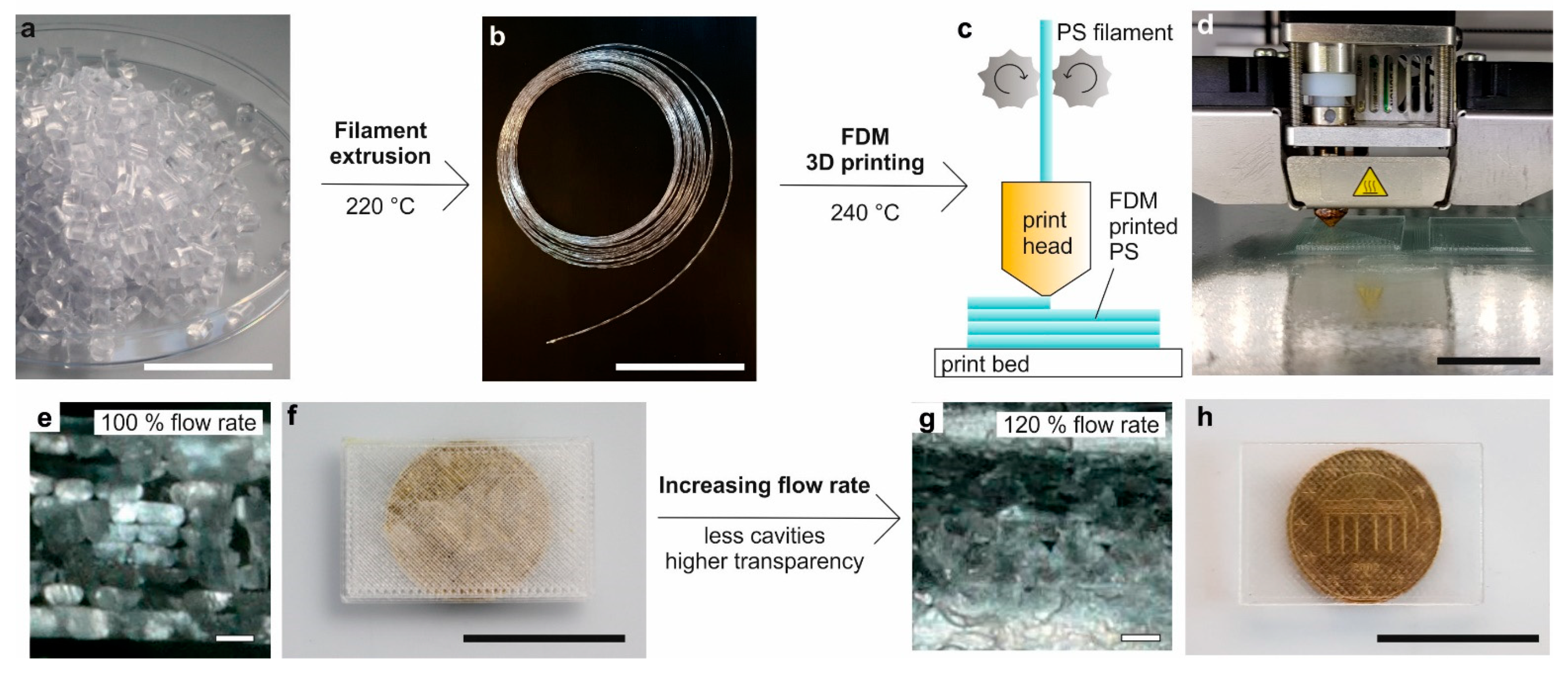
3.1. Extrusion of PS-Filament for FDM Printing
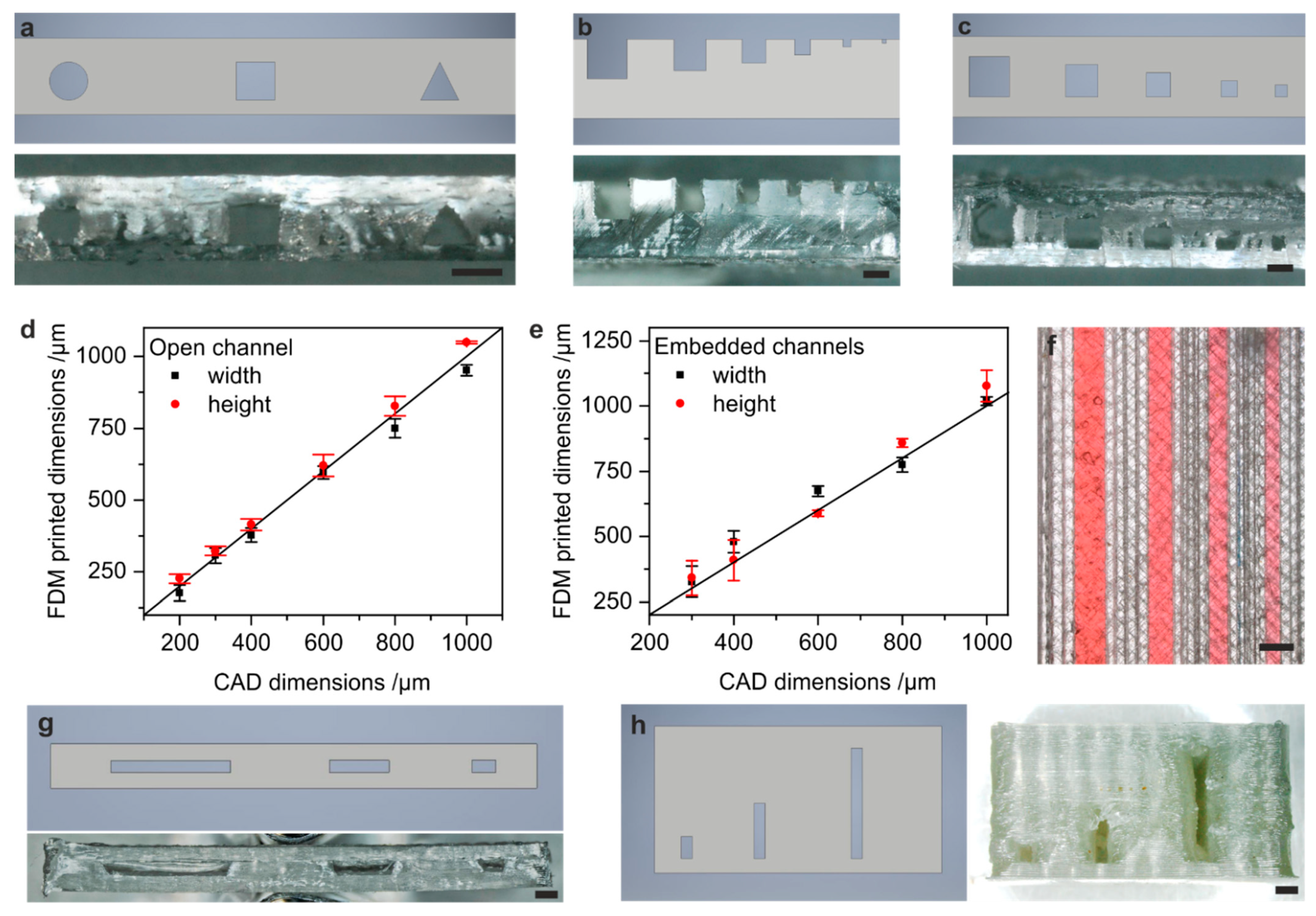
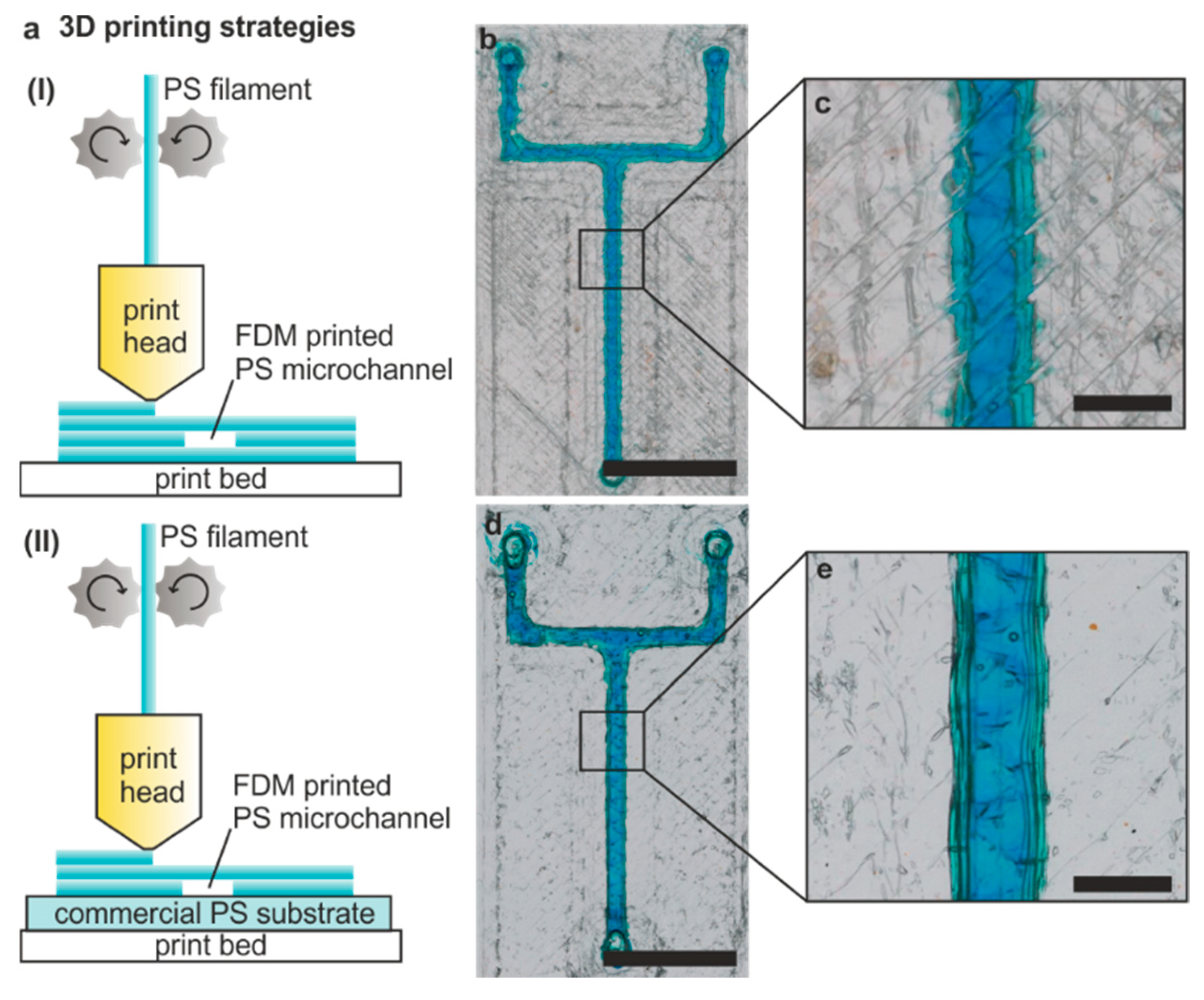
3.2. FDM of PS Microfluidic Devices
3.3. Surface Contact Angle
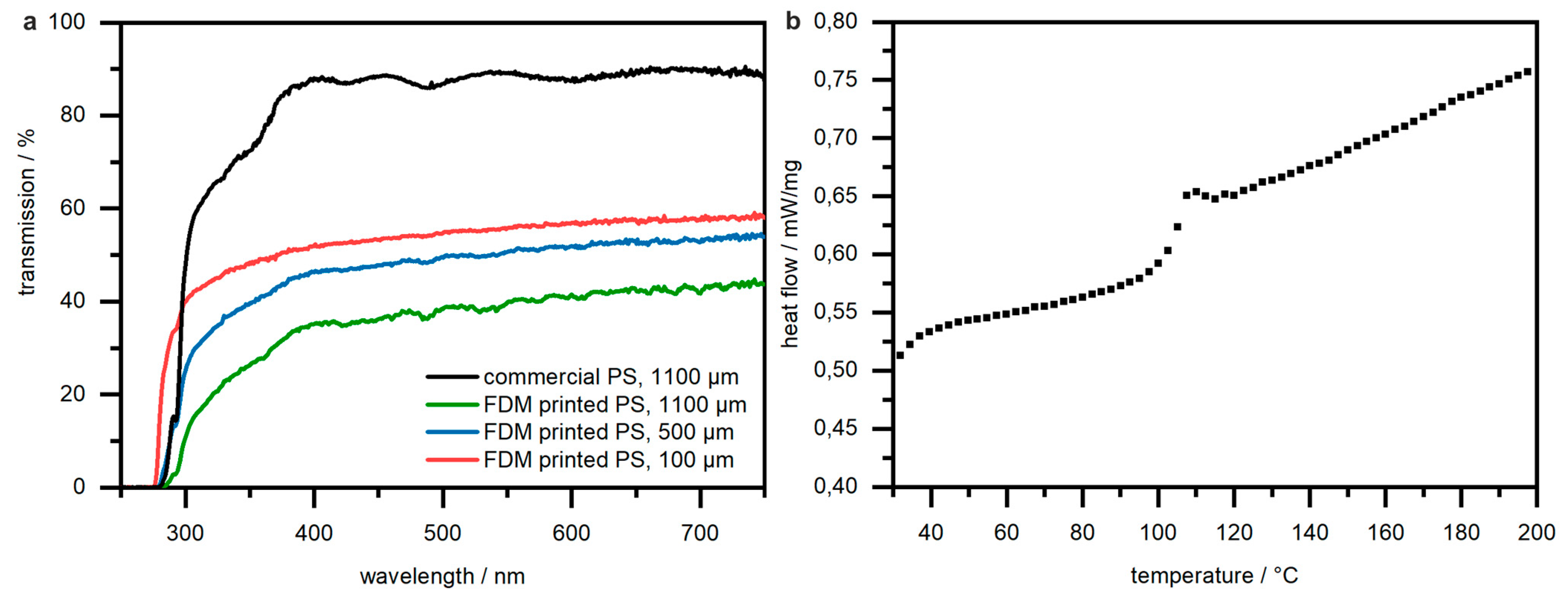
3.4. UV/Vis Spectroscopy and DSC Measurements
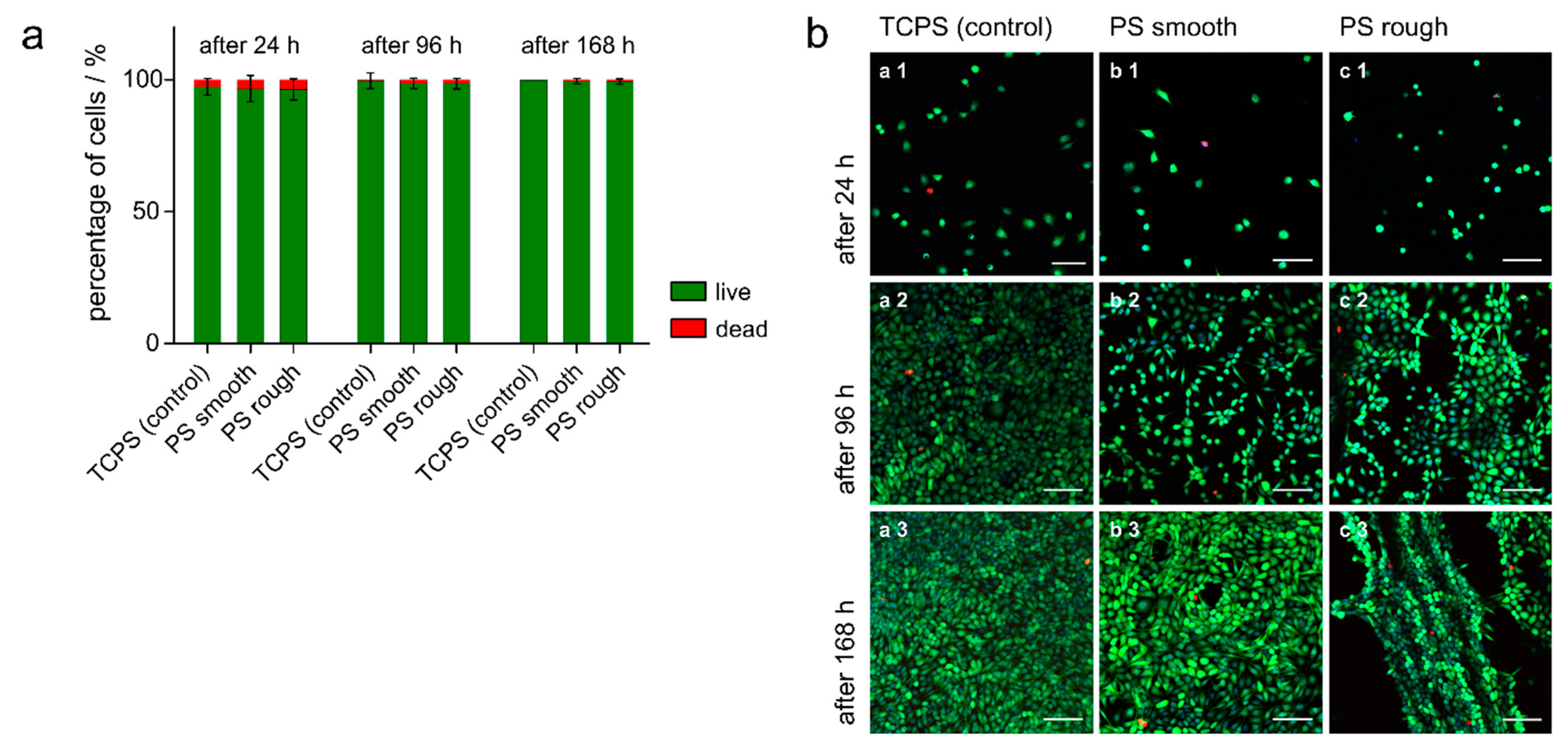
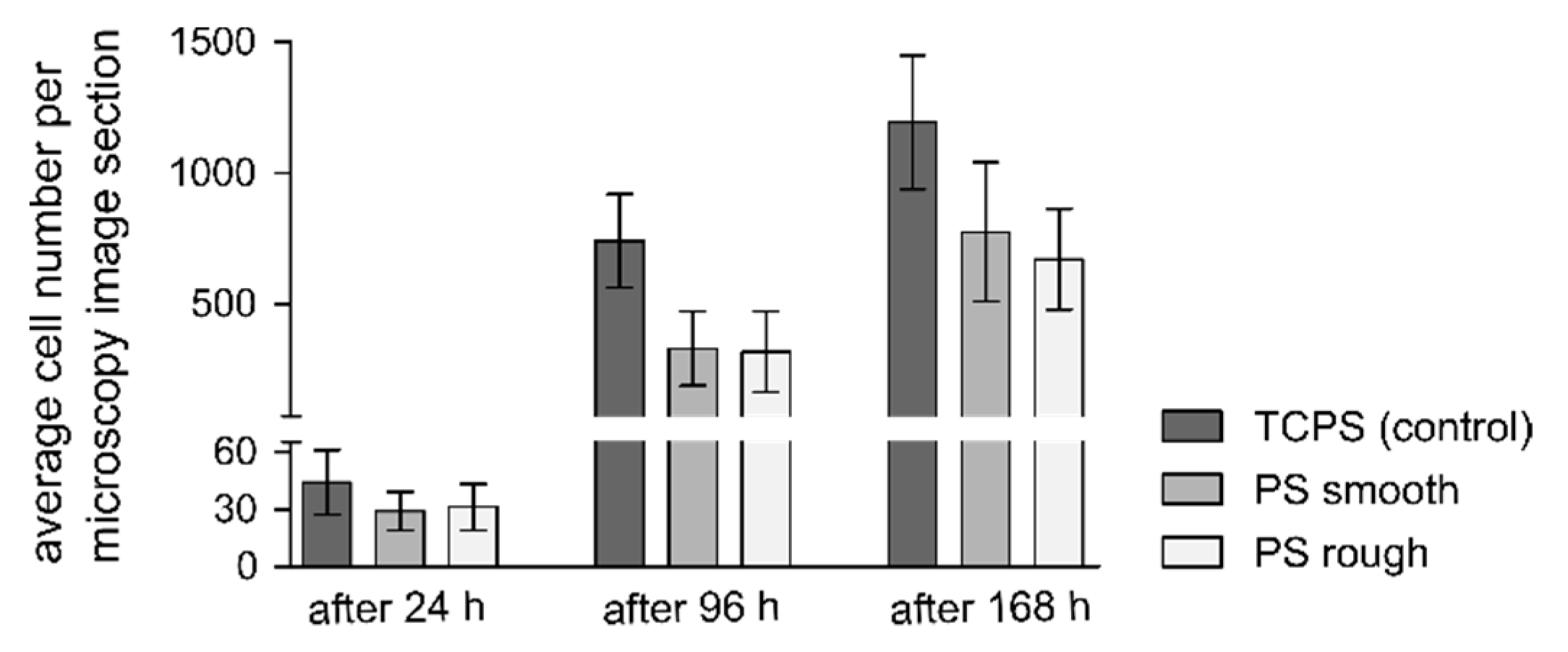
3.5. Live/dead Staining
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Křenková, J.; Foret, F. Immobilized Microfluidic Enzymatic Reactors. Electrophoresis 2004, 25, 3550–3563. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Li, B.; Su, X.; Qin, J.; Lin, B. Microvalve-Actuated Precise Control of Individual Droplets in Microfluidic Devices. Lab Chip 2009, 9, 1340–1343. [Google Scholar] [CrossRef]
- Puchberger-Enengl, D.; van den Driesche, S.; Krutzler, C.; Keplinger, F.; Vellekoop, M.J. Hydrogel-Based Microfluidic Incubator for Microorganism Cultivation and Analyses. Biomicrofluidics 2015, 9, 014127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchison, J.R.; Erikson, R.L.; Sheen, A.M.; Ozanich, R.M.; Kelly, R.T. Reagent-Free and Portable Detection of Bacillus Anthracis Spores Using a Microfluidic Incubator and Smartphone Microscope. Analyst 2015, 140, 6269–6276. [Google Scholar] [CrossRef]
- Neuži, P.; Giselbrecht, S.; Länge, K.; Huang, T.J.; Manz, A. Revisiting Lab-on-a-Chip Technology for Drug Discovery. Nat. Rev. Drug Discov. 2012, 11, 620–632. [Google Scholar] [CrossRef]
- Lan, F.; Demaree, B.; Ahmed, N.; Abate, A.R. Single-Cell Genome Sequencing at Ultra-High-Throughput with Microfluidic Droplet Barcoding. Nat. Biotechnol. 2017, 35, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Furlan, C.; Dirks, R.A.M.; Thomas, P.C.; Jones, R.C.; Wang, J.; Lynch, M.; Marks, H.; Vermeulen, M. Miniaturised Interaction Proteomics on a Microfluidic Platform with Ultra-Low Input Requirements. Nat. Commun. 2019, 10, 1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic Diagnostic Technologies for Global Public Health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef]
- Choi, N.W.; Cabodi, M.; Held, B.; Gleghorn, J.P.; Bonassar, L.J.; Stroock, A.D. Microfluidic Scaffolds for Tissue Engineering. Nat. Mater. 2007, 6, 908–915. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Effenhauser, C.; Manz, A.; Widmer, H. Glass Chips for High-Speed Capillary Electrophoresis Separations with Submicrometer Plate Heights. Anal. Chem. 1993, 65, 2637–2642. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(Dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of Microfluidic Systems in Poly(Dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Fujii, T. PDMS-Based Microfluidic Devices for Biomedical Applications. Microelectron. Eng. 2002, 61–62, 907–914. [Google Scholar] [CrossRef]
- Kotz, F.; Mader, M.; Dellen, N.; Risch, P.; Kick, A.; Helmer, D.; Rapp, B.E. Fused Deposition Modeling of Microfluidic Chips in Polymethylmethacrylate. Micromachines 2020, 11, 873. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The Upcoming 3D-Printing Revolution in Microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [Green Version]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D Printed Microfluidic Devices: Enablers and Barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [Green Version]
- Gaal, G.; Gaal, V.; Braunger, M.L.; Riul, A.; Rodrigues, V. FDM 3D Printing in Biomedical and Microfluidic Applications. In 3D Printing in Biomedical Engineering; Singh, S., Prakash, C., Singh, R., Eds.; Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2020; pp. 127–145. ISBN 9789811554247. [Google Scholar]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef]
- Naderi, A.; Bhattacharjee, N.; Folch, A. Digital Manufacturing for Microfluidics. Annu. Rev. Biomed. Eng. 2019, 21, 325–364. [Google Scholar] [CrossRef]
- Pranzo, D.; Larizza, P.; Filippini, D.; Percoco, G. Extrusion-Based 3D Printing of Microfluidic Devices for Chemical and Biomedical Applications: A Topical Review. Micromachines 2018, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.D.; Ramkumar, N.; Gale, B.K. Flexible, Transparent, Sub-100 Μm Microfluidic Channels with Fused Deposition Modeling 3D-Printed Thermoplastic Polyurethane. J. Micromech. Microeng. 2019, 29, 095010. [Google Scholar] [CrossRef]
- Bressan, L.P.; Adamo, C.B.; Quero, R.F.; de Jesus, D.P.; da Silva, J.A.F. A Simple Procedure to Produce FDM-Based 3D-Printed Microfluidic Devices with an Integrated PMMA Optical Window. Anal. Methods 2019, 11, 1014–1020. [Google Scholar] [CrossRef]
- Romanov, V.; Samuel, R.; Chaharlang, M.; Jafek, A.R.; Frost, A.; Gale, B.K. FDM 3D Printing of High-Pressure, Heat-Resistant, Transparent Microfluidic Devices. Anal. Chem. 2018, 90, 10450–10456. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Macdonald, N.P.; Guijt, R.M.; Breadmore, M.C. Using Printing Orientation for Tuning Fluidic Behavior in Microfluidic Chips Made by Fused Deposition Modeling 3D Printing. Anal. Chem. 2017, 89, 12805–12811. [Google Scholar] [CrossRef]
- Rubin, H. Altering Bacteriological Plastic Petri Dishes for Tissue Culture Use. Public Health Rep. 1966, 81, 843–844. [Google Scholar]
- Zeiger, A.S.; Hinton, B.; Van Vliet, K.J. Why the Dish Makes a Difference: Quantitative Comparison of Polystyrene Culture Surfaces. Acta Biomater. 2013, 9, 7354–7361. [Google Scholar] [CrossRef]
- Barker, S.L.; LaRocca, P.J. Method of Production and Control of a Commercial Tissue Culture Surface. J. Tissue Cult. Methods 1994, 16, 151–153. [Google Scholar] [CrossRef]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers Are from PDMS-Land, Biologists Are from Polystyrenia. Lab Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Becker, H. Mind the Gap! Lab Chip 2010, 10, 271–273. [Google Scholar] [CrossRef]
- Becker, H.; Gärtner, C. Polymer Microfabrication Technologies for Microfluidic Systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Nargang, T.M.; Brockmann, L.; Nikolov, P.M.; Schild, D.; Helmer, D.; Keller, N.; Sachsenheimer, K.; Wilhelm, E.; Pires, L.; Dirschka, M.; et al. Liquid Polystyrene: A Room-Temperature Photocurable Soft Lithography Compatible Pour-and-Cure-Type Polystyrene. Lab Chip 2014, 14, 2698–2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goral, V.N.; Hsieh, Y.-C.; Petzold, O.N.; Faris, R.A.; Yuen, P.K. Hot Embossing of Plastic Microfluidic Devices Using Poly(Dimethylsiloxane) Molds. J. Micromech. Microeng. 2010, 21, 17002–17010. [Google Scholar] [CrossRef]
- Russo, A.P.; Apoga, D.; Dowell, N.; Shain, W.; Turner, A.M.P.; Hoch, H.C.; Turner, J.N. Microfabricated Plastic Devices from Silicon Using Soft Intermediates. Biomed. Microdevices 2002, 4, 277–283. [Google Scholar] [CrossRef]
- Young, E.W.K.; Berthier, E.; Guckenberger, D.J.; Sackmann, E.; Lamers, C.; Meyvantsson, I.; Huttenlocher, A.; Beebe, D.J. Rapid Prototyping of Arrayed Microfluidic Systems in Polystyrene for Cell-Based Assays. Anal. Chem. 2011, 83, 1408–1417. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Fan, Y.; Kodzius, R.; Foulds, I.G. Fabrication of Polystyrene Microfluidic Devices Using a Pulsed CO2 Laser System. Microsyst. Technol. 2012, 18, 373–379. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; de Groot, T.E.; Wan, A.M.D.; Beebe, D.J.; Young, E.W.K. Micromilling: A Method for Ultra-Rapid Prototyping of Plastic Microfluidic Devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [Green Version]
- Grimes, A.; Breslauer, D.N.; Long, M.; Pegan, J.; Lee, L.P.; Khine, M. Shrinky-Dink Microfluidics: Rapid Generation of Deep and Rounded Patterns. Lab Chip 2007, 8, 170–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-S.; Breslauer, D.N.; Luna, J.I.; Grimes, A.; Chin, W.; Lee, L.P.; Khine, M. Shrinky-Dink Microfluidics: 3D Polystyrene Chips. Lab Chip 2008, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Feng, X.; Xu, Y.; Liu, B.-F. A Microsecond Microfluidic Mixer for Characterizing Fast Biochemical Reactions. Talanta 2012, 88, 175–180. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji—An Open Source Platform for Biological Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Kwok, D.Y.; Lam, C.N.C.; Li, A.; Zhu, K.; Wu, R.; Neumann, A.W. Low-Rate Dynamic Contact Angles on Polystyrene and the Determination of Solid Surface Tensions. Polym. Eng. Sci. 1998, 38, 1675–1684. [Google Scholar] [CrossRef]
- Ellison, A.H.; Zisman, W.A. Wettability Studies on Nylon, Polyethylene Terephthalate and Polystyrene. J. Phys. Chem. 1954, 58, 503–506. [Google Scholar] [CrossRef]
- Rieger, J. The Glass Transition Temperature of Polystyrene: Results of a Round Robin Test. J. Therm. Anal. 1996, 46, 965–972. [Google Scholar] [CrossRef]
- Ahrberg, C.D.; Manz, A.; Chung, B.G. Polymerase Chain Reaction in Microfluidic Devices. Lab Chip 2016, 16, 3866–3884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The Evolution of Polystyrene as a Cell Culture Material. Tissue Eng. B Rev. 2018, 24, 359–372. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mader, M.; Rein, C.; Konrat, E.; Meermeyer, S.L.; Lee-Thedieck, C.; Kotz-Helmer, F.; Rapp, B.E. Fused Deposition Modeling of Microfluidic Chips in Transparent Polystyrene. Micromachines 2021, 12, 1348. https://doi.org/10.3390/mi12111348
Mader M, Rein C, Konrat E, Meermeyer SL, Lee-Thedieck C, Kotz-Helmer F, Rapp BE. Fused Deposition Modeling of Microfluidic Chips in Transparent Polystyrene. Micromachines. 2021; 12(11):1348. https://doi.org/10.3390/mi12111348
Chicago/Turabian StyleMader, Markus, Christof Rein, Eveline Konrat, Sophia Lena Meermeyer, Cornelia Lee-Thedieck, Frederik Kotz-Helmer, and Bastian E. Rapp. 2021. "Fused Deposition Modeling of Microfluidic Chips in Transparent Polystyrene" Micromachines 12, no. 11: 1348. https://doi.org/10.3390/mi12111348
APA StyleMader, M., Rein, C., Konrat, E., Meermeyer, S. L., Lee-Thedieck, C., Kotz-Helmer, F., & Rapp, B. E. (2021). Fused Deposition Modeling of Microfluidic Chips in Transparent Polystyrene. Micromachines, 12(11), 1348. https://doi.org/10.3390/mi12111348





