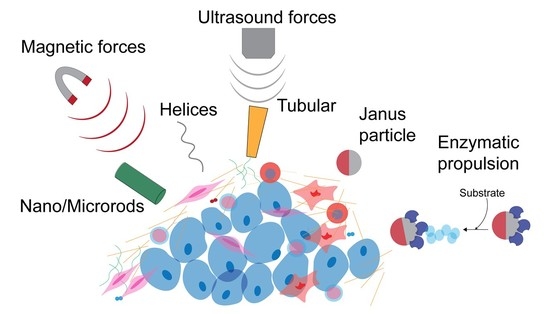
Autonomously Propelled Colloids for Penetration and Payload Delivery in Complex Extracellular Matrices
Abstract
:1. Introduction
2. Biomimetic Models of Extracellular Matrix
2.1. Naturally Derived ECM
2.1.1. Matrigel
2.1.2. ECM Derived from Decellularized Tissues (DT)
2.2. Hydrogels Mimicking ECM
2.2.1. Hyaluronic Acid Hydrogels
2.2.2. Mucin Gels
2.2.3. Collagen Gels
2.2.4. Other Hydrogel ECM Mimics
2.3. Biofilms
3. Self-Propelled Particles Movement in ECM
3.1. Use of Physical Forces for Movement of SPPs in Hydrogels
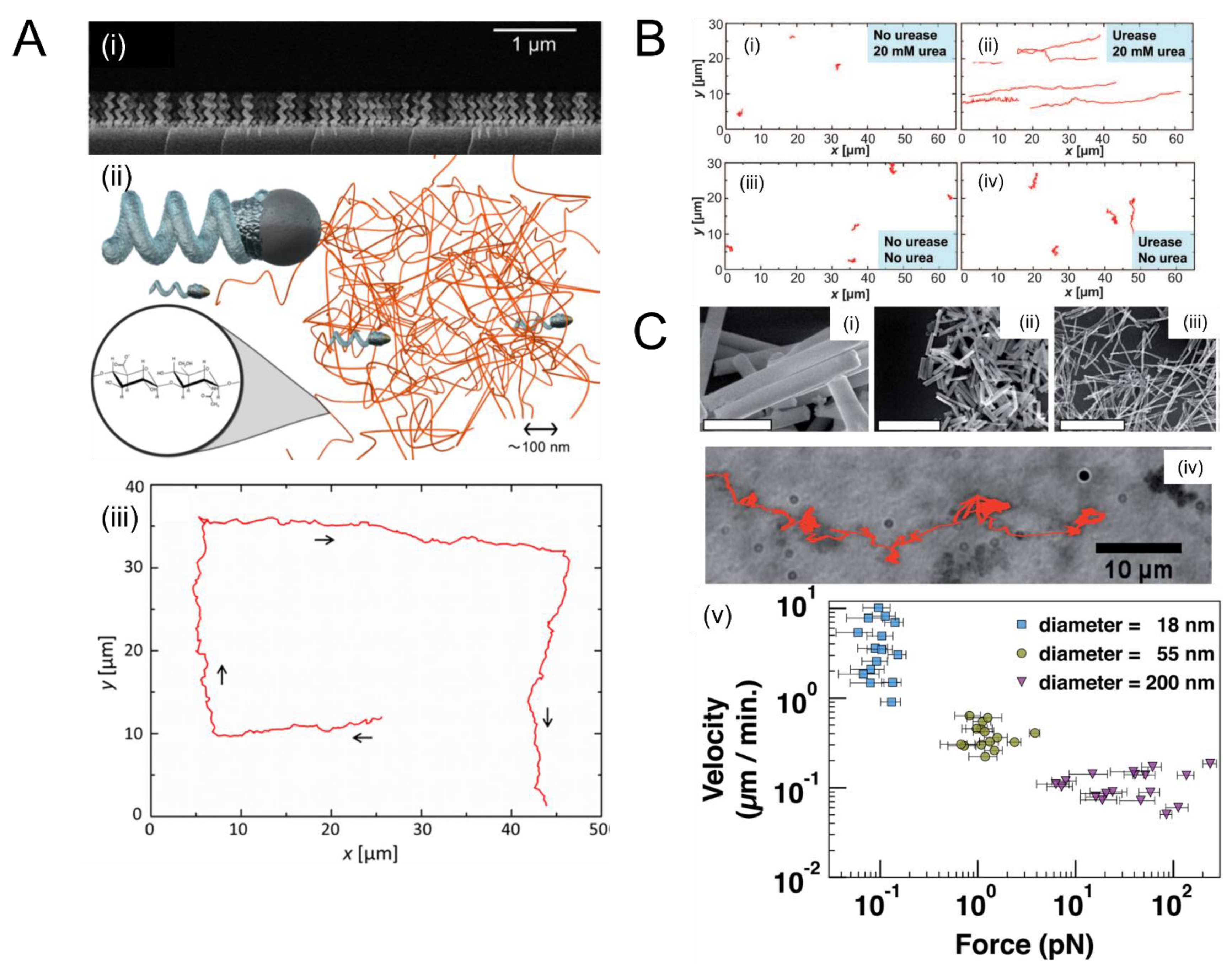
3.1.1. Magnetic Forces
3.1.2. Ultrasound Forces
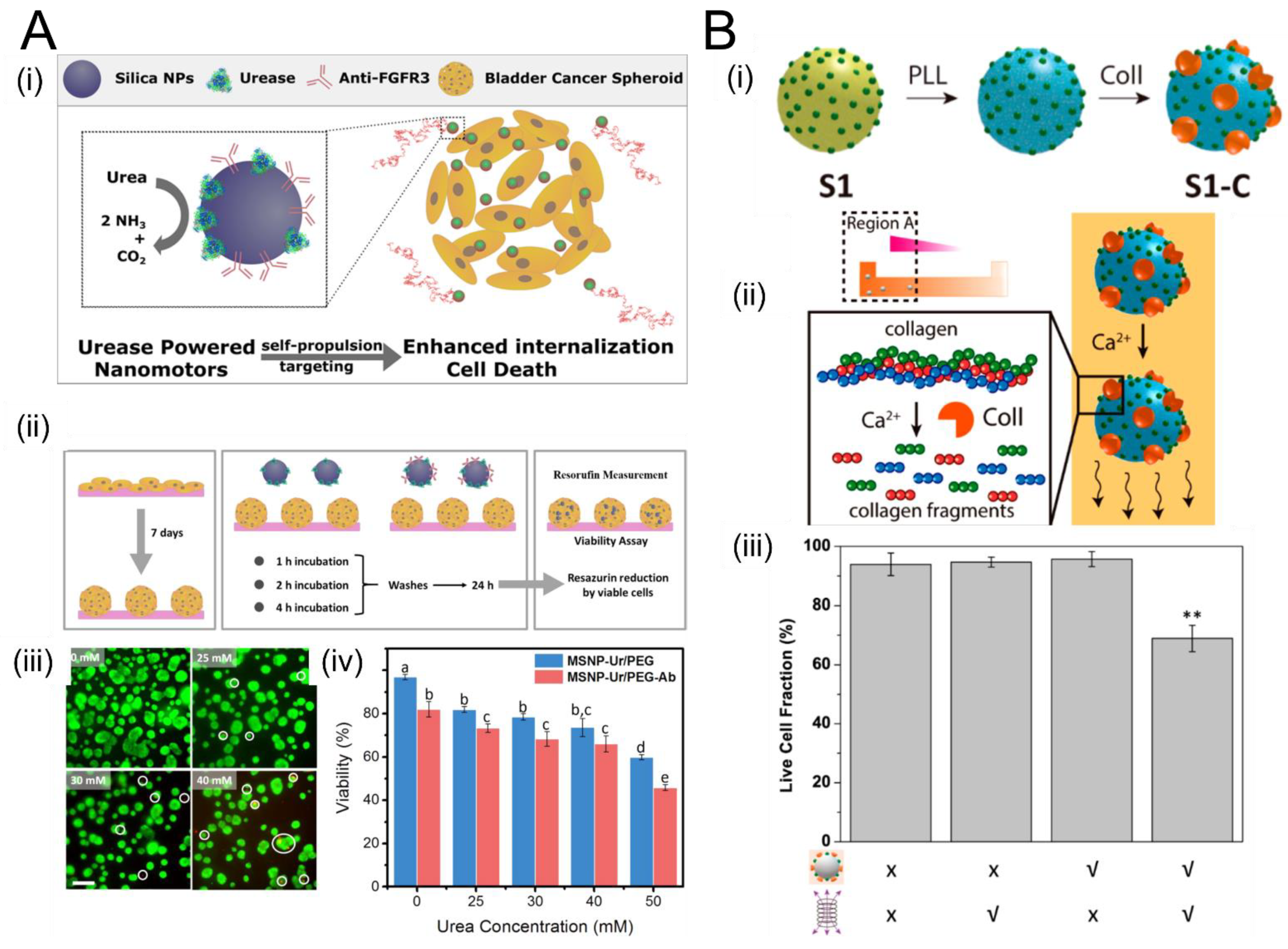
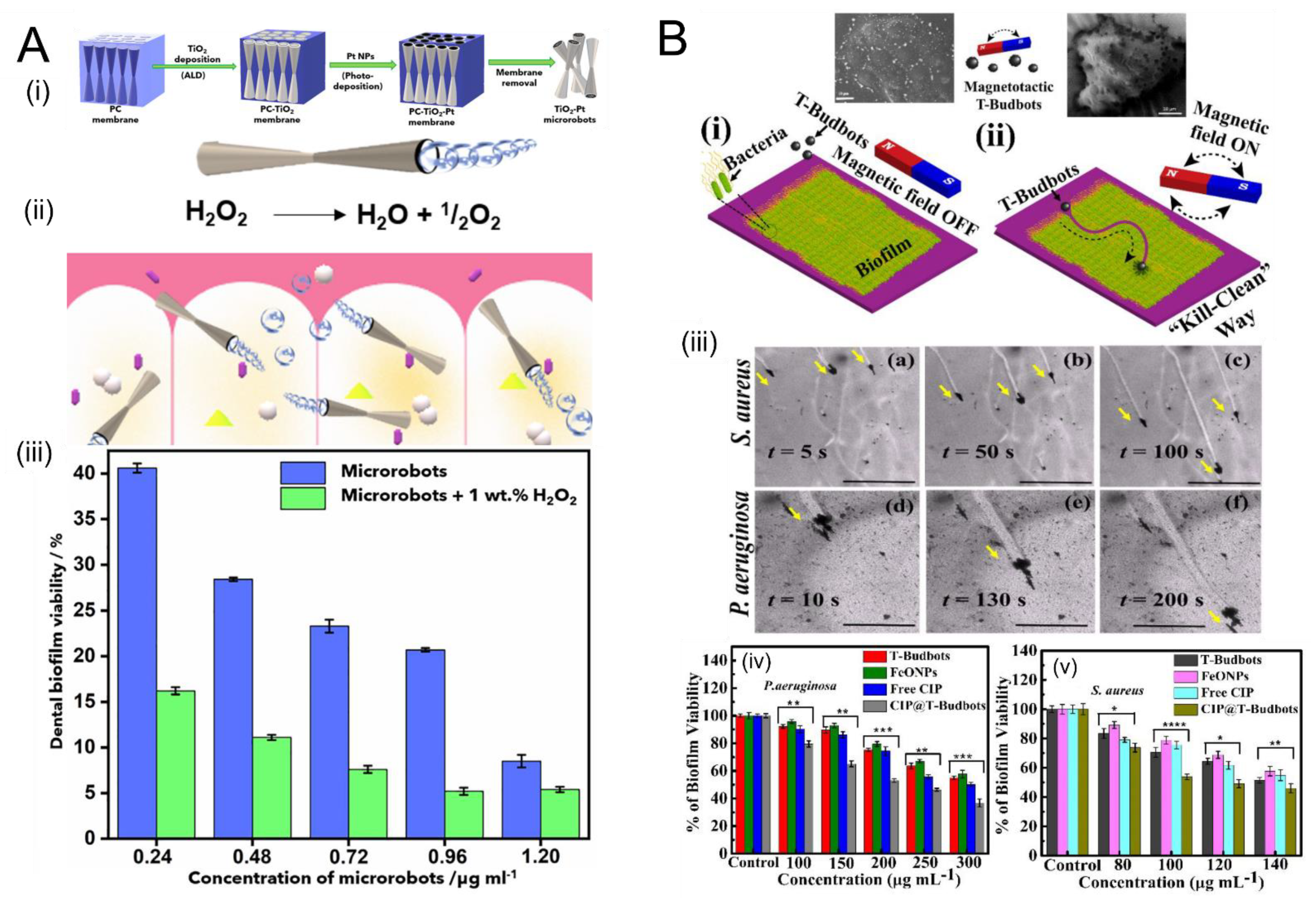
3.2. Remediation of Biofilms Using Chemically-Powered Motion
3.3. Enzymatic Propulsion
| SPP Design | Reference | Type of Propulsion | Advantages | Disadvantages |
|---|---|---|---|---|
| Ferromagnetic nanorods | Mair and Superfine [51] | Magnetophoresis in static magnetic field | Simple fabrication and operation No complex set-up required for propulsion or steering | Slow No data on biocompatibility |
| Nano-/microhelices | Mark et al. [122] Schamel et al. [50] Walker et al. [52] | Rotating magnetic field | Efficient movement in viscoelastic media | Complicated apparatus required to generate rotating magnetic field |
| Thin helical filaments maneuver through narrow ECM mesh | No data on biocompatibility Cargo delivery not trivial | |||
| Rectangular propellers | Ahmed et al. [129] | Ultrasound/Acoustic powered | Synthesized from polymers which are biocompatible Can achieve higher velocities than magnetically propelled SPPs. | Size of the propellers can be a hindrance in delivery |
| Micro-/Nanobullets | Kagan et al. [42] Soto et al. [141] | Ultrasound-induced vaporization of onboard hydrocarbon fuel | Extremely high speeds and strong propulsive forces Demonstrated to penetrate lamb kidney tissue | Challenges controlling initial, final locations, motion direction Biocompatibility of materials and fuels unclear Single-shot implementation |
| Enzymatic Particles | Hortelão et al. [140] Ramos-Docampo et al. [105] | Enzymatic reactions | Use readily-available biological fuels (e.g., urea, glucose) Enzymes undergo chemotaxis, potentially allowing “smart” propelled nanocarriers | Patterning enzymes precisely is challenging Scaffolds used (e.g., silica) are not biocompatible Propulsion mechanism not fully understood |
4. Summary and Outlook
- Effect of ECM mechanics and rheology on propulsion efficiency: The motion efficiency (and thus therapeutic efficacy) of SPPs has been shown recently to depend on the topology, rheological properties, and viscoelastic properties of the medium. Recently, it was reported that shear-thinning effects cause a substantial enhancement in the propulsion of helical microswimmers [114]. On the other hand, it is also known that as ECM becomes stiffer and denser, the velocity is attenuated. Thus, the behavior of a given SPP design in a given ECM is not necessarily trivial to predict a priori.
- Effect of SPP propulsion mechanism, size, and shape on propulsion efficiency: Since the trends are likely to depend strongly on the design of SPP under consideration, systematic analyses with multidimensional parameter spaces (e.g., ECM stiffness, ECM viscoelasticity, porosity, etc.) may be necessary for individual SPP designs.
- Testing in biomimetic materials: As alluded to in #1, the evidence increasingly shows that SPP performance depends strongly on the mechanics, viscoelastic properties, and topology of the ECM (as does cell migration through these same matrices). As a result, while they may be an important first step in verifying propulsion, drug delivery experiments conducted in a simple Petri dish are unlikely to be indicative of future therapeutic potential. Instead, it will be critical that future in vitro studies of SPP performance be conducted in media that accurately recapitulate the mechanical and rheological properties of the tissues in which they are to be used. Vigorous research is underway toward the development of novel, tunable biomaterials that closely resemble those of in vivo tissues. Interdisciplinary collaborations between these groups will be crucial to this stage of development.
- Advanced SPP design methodologies: The size, shape, and propulsion mechanism of SPPs clearly affect their performance in ECMs. With different ECM biochemistry, material properties, porosity, and mechanical and rheological properties, new designs for SPPs may need to be invented. With the advent of advanced manufacturing techniques such as micro- and nanoscale 3D printing, as well as the continued growth of nanofabrication techniques, it may soon become possible for creative researchers to design SPPs with bespoke shapes for a given ECM application.
- Tracking: Although it was not the focus of this review, a crucial component of successful translation of SPP-based therapies to the clinic will require the development of clinically-compatible tracking methodologies. Exciting progress has been made in this area recently with the use of photoacoustic computed tomography (PACT) that enables tracking of magnesium-based SPPs in the digestive tract of animal models [142]. However, the performance of many of these tracking methods within tissues has yet to be demonstrated quantitatively.
- Theoretical and simulation studies: Although this review has focused on experimental demonstrations of SPPs moving in ECM models (and, in some limited cases, in vivo ECM), we wish to emphasize the importance of theoretical investigations to the design of SPPs with optimal properties to maximize the efficiency of motion through ECM. A recent study exemplifies the promise of this approach. Aceves-Sanchez et al. [143] theoretically studied the collective motion in an environment filled with spheres tethered to fixed points in space via linear springs, which play the role of obstacles (such as ECM fibers). They showed that this obstacle-based environment can induce aggregation of SPPs. As they and others have noted [144], aggregation is known to correlate with the ability of metastasizing cancer cells to migrate; by the same token, aggregation should be taken into account when designing future SPP-based therapies, in which it could serve as both a hindrance (e.g., if it stops the motion entirely through steric interactions) or a help (if it allows more cargo to be transported while still permitting motion). Going forward, a close coupling between theory and experiments will be crucial to converge on the most efficacious designs.
Author Contributions
Funding
Conflicts of Interest
References
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Sonbol, H.S. Extracellular Matrix Remodeling in Human Disease. J. Microsc. Ultrastruct. 2018, 6, 123–128. [Google Scholar] [CrossRef]
- Kim, S.-H.; Turnbull, J.; Guimond, S. Extracellular Matrix and Cell Signalling: The Dynamic Cooperation of Integrin, Proteoglycan and Growth Factor Receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muncie, J.M.; Weaver, V.M. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Curr. Top. Dev. Biol. 2018, 130, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Weaver, V.M.; Werb, Z. The Extracellular Matrix: A Dynamic Niche in Cancer Progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Fibrotic Disease and the T H 1/T H 2 Paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Au, J.L.-S.; Yeung, B.Z.; Wientjes, M.G.; Lu, Z.; Wientjes, M.G. Delivery of Cancer Therapeutics to Extracellular and Intracellular Targets: Determinants, Barriers, Challenges and Opportunities. Adv. Drug Deliv. Rev. 2016, 97, 280–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular Matrix and Its Therapeutic Potential for Cancer Treatment. Sig. Transduct. Target. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Estany, S.; Vicens, V.; Llatjόs, R.; Penín, R.; Escobar, I.; Xaubet, A.; Manresa, F.; Dorca, J.; Molina-Molina, M. Extracellular Matrix Profile of Lung in Idiopathic Pulmonary Fibrosis. Eur. Respir. J. 2011, 38, 4775. [Google Scholar]
- Upagupta, C.; Shimbori, C.; Alsilmi, R.; Kolb, M. Matrix Abnormalities in Pulmonary Fibrosis. Eur. Respir. Rev. 2018, 27, 180033. [Google Scholar] [CrossRef] [Green Version]
- Subrahmanyam, N.; Ghandehari, H. Harnessing Extracellular Matrix Biology for Tumor Drug Delivery. J. Pers. Med. 2021, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, L.; Breunig, M. Preventing Obstructions of Nanosized Drug Delivery Systems by the Extracellular Matrix. Adv. Healthc. Mater. 2018, 7, 1700739. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Jiang, T.; Gu, Z. Recent Progress in Multidrug Delivery to Cancer Cells by Liposomes. Nanomedicine 2014, 9, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharm. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol Polymers for PH-Responsive, Targeted Drug Delivery to Cancer Cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, S.; Senapati, S.; Singh, A.P.; Ray, B.; Maiti, P. Controlled Drug Release through Regulated Biodegradation of Poly(Lactic Acid) Using Inorganic Salts. Int. J. Biol. Macromol. 2017, 104, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic Nanoparticles: Technology Overview & Drug Delivery Applications in Oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942. [Google Scholar] [CrossRef]
- Mohapatra, A.; Uthaman, S.; Park, I.-K. External and Internal Stimuli-Responsive Metallic Nanotherapeutics for Enhanced Anticancer Therapy. Front. Mol. Biosci. 2020, 7, 597634. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.X.; Karnik, R.; Wang, A.Z.; Alexis, F.; Levy-Nissenbaum, E.; Hong, S.; Langer, R.S.; Farokhzad, O.C. Targeted Nanoparticles for Cancer Therapy. Nano Today 2007, 2, 14–21. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Jain, R.K.; Langer, R. Engineering and Physical Sciences in Oncology: Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 659–675. [Google Scholar] [CrossRef]
- Torrice, M. Does Nanomedicine Have a Delivery Problem? ACS Cent. Sci. 2016, 2, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Saha, R.N.; Vasanthakumar, S.; Bende, G.; Snehalatha, M. Nanoparticulate Drug Delivery Systems for Cancer Chemotherapy. Mol. Membr. Biol. 2010, 27, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Leserman, L.D.; Barbet, J.; Kourilsky, F.; Weinstein, J.N. Targeting to Cells of Fluorescent Liposomes Covalently Coupled with Monoclonal Antibody or Protein A. Nature 1980, 288, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Ligand-Targeted Therapeutics in Anticancer Therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, Z.; Kamaly, N.; Farokhzad, O.C. Self-Assembled Targeted Nanoparticles: Evolution of Technologies and Bench to Bedside Translation. Acc. Chem. Res. 2011, 44, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted Polymeric Therapeutic Nanoparticles: Design, Development and Clinical Translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef] [PubMed]
- Hrkach, J.; Von Hoff, D.; Mukkaram Ali, M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer Nanotechnology: The Impact of Passive and Active Targeting in the Era of Modern Cancer Biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Florence, A.T. “Targeting” Nanoparticles: The Constraints of Physical Laws and Physical Barriers. J. Control. Release 2012, 164, 115–124. [Google Scholar] [CrossRef]
- Paxton, W.F.; Kistler, K.C.; Olmeda, C.C.; Sen, A.; St. Angelo, S.K.; Cao, Y.; Mallouk, T.E.; Lammert, P.E.; Crespi, V.H. Catalytic Nanomotors: Autonomous Movement of Striped Nanorods. J. Am. Chem. Soc. 2004, 126, 13424–13431. [Google Scholar] [CrossRef]
- Bricard, A.; Caussin, J.-B.; Desreumaux, N.; Dauchot, O.; Bartolo, D. Emergence of Macroscopic Directed Motion in Populations of Motile Colloids. Nature 2013, 503, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, Z.; Li, J.; Tian, C.; Wang, Y. Active Colloidal Molecules Assembled via Selective and Directional Bonds. Nat. Commun. 2020, 11, 2670. [Google Scholar] [CrossRef] [PubMed]
- Peyer, K.E.; Tottori, S.; Qiu, F.; Zhang, L.; Nelson, B.J. Magnetic Helical Micromachines. Chem. Eur. J. 2013, 19, 28–38. [Google Scholar] [CrossRef]
- Chen, X.-Z.; Liu, J.-H.; Dong, M.; Müller, L.; Chatzipirpiridis, G.; Hu, C.; Terzopoulou, A.; Torlakcik, H.; Wang, X.; Mushtaq, F.; et al. Magnetically Driven Piezoelectric Soft Microswimmers for Neuron-like Cell Delivery and Neuronal Differentiation. Mater. Horiz. 2019, 6, 1512–1516. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.T.; Paunov, V.N.; Petsev, D.N.; Velev, O.D. Remotely Powered Self-Propelling Particles and Micropumps Based on Miniature Diodes. Nat. Mater. 2007, 6, 235–240. [Google Scholar] [CrossRef]
- Calvo-Marzal, P.; Sattayasamitsathit, S.; Balasubramanian, S.; Windmiller, J.R.; Dao, C.; Wang, J. Propulsion of Nanowire Diodes. Chem. Commun. 2010, 46, 1623–1624. [Google Scholar] [CrossRef] [PubMed]
- Kagan, D.; Benchimol, M.J.; Claussen, J.C.; Chuluun-Erdene, E.; Esener, S.; Wang, J. Acoustic Droplet Vaporization and Propulsion of Perfluorocarbon-Loaded Microbullets for Targeted Tissue Penetration and Deformation. Angew. Chem. Int. Ed. 2012, 51, 7519–7522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertin, N.; Spelman, T.A.; Stephan, O.; Gredy, L.; Bouriau, M.; Lauga, E.; Marmottant, P. Propulsion of Bubble-Based Acoustic Microswimmers. Phys. Rev. Appl. 2015, 4, 064012. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Nama, N.; McNeill, J.M.; Soto, F.; Yan, Z.; Liu, W.; Wang, W.; Wang, J.; Mallouk, T.E. 3D Steerable, Acoustically Powered Microswimmers for Single-Particle Manipulation. Sci. Adv. 2019, 5, eaax3084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Bai, T.; Chi, Q.; Wang, Z.; Xu, S.; Liu, Q.; Wang, Q. How to Make a Fast, Efficient Bubble-Driven Micromotor: A Mechanical View. Micromachines 2017, 8, 267. [Google Scholar] [CrossRef]
- Chi, Q.; Wang, Z.; Tian, F.; You, J.; Xu, S. A Review of Fast Bubble-Driven Micromotors Powered by Biocompatible Fuel: Low-Concentration Fuel, Bioactive Fluid and Enzyme. Micromachines 2018, 9, 537. [Google Scholar] [CrossRef] [Green Version]
- Burdick, J.; Laocharoensuk, R.; Wheat, P.M.; Posner, J.D.; Wang, J. Synthetic Nanomotors in Microchannel Networks: Directional Microchip Motion and Controlled Manipulation of Cargo. J. Am. Chem. Soc. 2008, 130, 8164–8165. [Google Scholar] [CrossRef]
- Kagan, D.; Laocharoensuk, R.; Zimmerman, M.; Clawson, C.; Balasubramanian, S.; Kang, D.; Bishop, D.; Sattayasamitsathit, S.; Zhang, L.; Wang, J. Rapid Delivery of Drug Carriers Propelled and Navigated by Catalytic Nanoshuttles. Small 2010, 6, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Lammert, P.E.; Zudans, A.W.; Crespi, V.H.; Sen, A. Catalytic Motors for Transport of Colloidal Cargo. Nano Lett. 2008, 8, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of Extracellular Matrix Viscoelasticity on Cellular Behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Schamel, D.; Mark, A.G.; Gibbs, J.G.; Miksch, C.; Morozov, K.I.; Leshansky, A.M.; Fischer, P. Nanopropellers and Their Actuation in Complex Viscoelastic Media. ACS Nano 2014, 8, 8794–8801. [Google Scholar] [CrossRef]
- Mair, L.; Superfine, R. Single Particle Tracking Reveals Biphasic Transport during Nanorod Magnetophoresis through Extracellular Matrix. Soft Matter 2014, 10, 4118–4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, D.; Käsdorf, B.T.; Jeong, H.-H.; Lieleg, O.; Fischer, P. Enzymatically Active Biomimetic Micropropellers for the Penetration of Mucin Gels. Sci. Adv. 2015, 1, e1500501. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Thamphiwatana, S.; Liu, W.; Esteban-Fernández de Ávila, B.; Angsantikul, P.; Sandraz, E.; Wang, J.; Xu, T.; Soto, F.; Ramez, V.; et al. Enteric Micromotor Can Selectively Position and Spontaneously Propel in the Gastrointestinal Tract. ACS Nano 2016, 10, 9536–9542. [Google Scholar] [CrossRef]
- Matera, D.L.; Wang, W.Y.; Baker, B.M. New Directions and Dimensions for Bioengineered Models of Fibrosis. Nat. Rev. Mater. 2021, 6, 192–195. [Google Scholar] [CrossRef]
- Orkin, R.W.; Gehron, P.; McGoodwin, E.B.; Martin, G.R.; Valentine, T.; Swarm, R. A Murine Tumor Producing a Matrix of Basement Membrane. J. Exp. Med. 1977, 145, 204–220. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement Membrane Matrix with Biological Activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Corning Incorporated Life Sciences. Corning Matrigel Matrix Frequently Asked Questions. Available online: https://www.corning.com/catalog/cls/documents/faqs/CLS-DL-CC-026.pdf (accessed on 25 July 2021).
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A Complex Protein Mixture Required for Optimal Growth of Cell Culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Timpl, R.; Rohde, H.; Robey, P.G.; Rennard, S.I.; Foidart, J.M.; Martin, G.R. Laminin—A Glycoprotein from Basement Membranes. J. Biol. Chem. 1979, 254, 9933–9937. [Google Scholar] [CrossRef]
- Kai, W.; Lina, J.; Zichun, H. Functional Peptides from Laminin-1 Improve the Cell Adhesion Capacity of Recombinant Mussel Adhesive Protein. Protein Pept. Lett. 2017, 24, 348–352. [Google Scholar]
- Engbring, J.A.; Kleinman, H.K. The Basement Membrane Matrix in Malignancy. J. Pathol. 2003, 200, 465–470. [Google Scholar] [CrossRef]
- Kikkawa, Y.; Hozumi, K.; Katagiri, F.; Nomizu, M.; Kleinman, H.K.; Koblinski, J.E. Laminin-111-Derived Peptides and Cancer. Cell Adh. Migr. 2013, 7, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Vukicevic, S.; Kleinman, H.K.; Luyten, F.P.; Roberts, A.B.; Roche, N.S.; Reddi, A.H. Identification of Multiple Active Growth Factors in Basement Membrane Matrigel Suggests Caution in Interpretation of Cellular Activity Related to Extracellular Matrix Components. Exp. Cell Res. 1992, 202, 1–8. [Google Scholar] [CrossRef]
- Talbot, N.C.; Caperna, T.J. Proteome Array Identification of Bioactive Soluble Proteins/Peptides in Matrigel: Relevance to Stem Cell Responses. Cytotechnology 2015, 67, 873–883. [Google Scholar] [CrossRef] [Green Version]
- Gillette, K.M.; Forbes, K.; Sehgal, I. Detection of Matrix Metalloproteinases (MMP), Tissue Inhibitor of Metalloproteinase-2, Urokinase and Plasminogen Activator Inhibitor-1 within Matrigel and Growth Factor-Reduced Matrigel Basement Membrane. Tumori 2003, 89, 421–425. [Google Scholar] [CrossRef]
- Borries, M.; Barooji, Y.; Yennek, S.; Grapin-Botton, A.; Berg-Sørensen, K.; Oddershede, L. Quantification of Visco-Elastic Properties of a Matrigel for Organoid Development as a Function of Polymer Concentration. Front. Phys. 2020, 8, 579168. [Google Scholar] [CrossRef]
- Bakunts, K.; Gillum, N.; Karabekian, Z.; Sarvazyan, N. Formation of Cardiac Fibers in Matrigel Matrix. Biotechniques 2008, 44, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Cadar, A.G.; Feaster, T.K.; Durbin, M.D.; Hong, C.C. Production of Single Contracting Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Matrigel Mattress Technique. Curr. Protoc. Stem Cell Biol. 2017, 42, 4A.14.1–4A.14.7. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-Free Growth of Undifferentiated Human Embryonic Stem Cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Mondrinos, M.J.; Koutzaki, S.; Jiwanmall, E.; Li, M.; Dechadarevian, J.-P.; Lelkes, P.I.; Finck, C.M. Engineering Three-Dimensional Pulmonary Tissue Constructs. Tissue Eng. 2006, 12, 717–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Torres, S.; Peza-Chavez, O.; Kuasne, H.; Munguia-Lopez, J.G.; Kort-Mascort, J.; Ferri, L.; Jiang, T.; Rajadurai, C.V.; Park, M.; Sangwan, V.; et al. Alginate–Gelatin–Matrigel Hydrogels Enable the Development and Multigenerational Passaging of Patient-Derived 3D Bioprinted Cancer Spheroid Models. Biofabrication 2021, 13, 025001. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Panek, M.; Grabacka, M.; Pierzchalska, M. The Formation of Intestinal Organoids in a Hanging Drop Culture. Cytotechnology 2018, 70, 1085–1095. [Google Scholar] [CrossRef] [Green Version]
- Zaman, M.H.; Trapani, L.M.; Sieminski, A.; MacKellar, D.; Gong, H.; Kamm, R.D.; Wells, A.; Lauffenburger, D.A.; Matsudaira, P. Migration of Tumor Cells in 3D Matrices Is Governed by Matrix Stiffness along with Cell-Matrix Adhesion and Proteolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 10889–10894. [Google Scholar] [CrossRef] [Green Version]
- Semler, E.J.; Ranucci, C.S.; Moghe, P.V. Mechanochemical Manipulation of Hepatocyte Aggregation Can Selectively Induce or Repress Liver-Specific Function. Biotechnol. Bioeng. 2000, 69, 359–369. [Google Scholar] [CrossRef]
- Soofi, S.S.; Last, J.A.; Liliensiek, S.J.; Nealey, P.F.; Murphy, C.J. The Elastic Modulus of MatrigelTM as Determined by Atomic Force Microscopy. J. Struct. Biol. 2009, 167, 216–219. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.; Walczak, W.J.; Petzold, O.N.; Gimzewski, J.K. In Situ Mechanical Interferometry of Matrigel Films. Langmuir 2009, 25, 36–39. [Google Scholar] [CrossRef] [Green Version]
- Miura, K. Histological Imaging of Gastric Tumors by Scanning Acoustic Microscope. Br. J. Appl. Sci. Technol. 2014, 4, 1–17. [Google Scholar] [CrossRef]
- Hrebikova, H.; Diaz, D.; Mokry, J. Chemical Decellularization: A Promising Approach for Preparation of Extracellular Matrix. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2015, 159, 012–017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed. Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, R.A.J.; Hoehn, J.G. Use of Commercial Porcine Skin for Wound Dressings. Plast. Reconstr. Surg. 1973, 52, 401–405. [Google Scholar] [CrossRef]
- Sackett, S.D.; Tremmel, D.M.; Ma, F.; Feeney, A.K.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’Brien, C.; Li, L.; et al. Extracellular Matrix Scaffold and Hydrogel Derived from Decellularized and Delipidized Human Pancreas. Sci. Rep. 2018, 8, 10452. [Google Scholar] [CrossRef]
- Rosario, D.J.; Reilly, G.C.; Ali Salah, E.; Glover, M.; Bullock, A.J.; Macneil, S. Decellularization and Sterilization of Porcine Urinary Bladder Matrix for Tissue Engineering in the Lower Urinary Tract. Regen. Med. 2008, 3, 145–156. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Nguyen, H.-Q.-D.; Weng, Y.-C. Characterization of Porcine Urinary Bladder Matrix Hydrogels from Sodium Dodecyl Sulfate Decellularization Method. Polymers 2020, 12, 3007. [Google Scholar] [CrossRef]
- O’Neill, J.D.; Anfang, R.; Anandappa, A.; Costa, J.; Javidfar, J.; Wobma, H.M.; Singh, G.; Freytes, D.O.; Bacchetta, M.D.; Sonett, J.R.; et al. Decellularization of Human and Porcine Lung Tissues for Pulmonary Tissue Engineering. Ann. Thorac. Surg. 2013, 96, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Merna, N.; Robertson, C.; La, A.; George, S.C. Optical Imaging Predicts Mechanical Properties During Decellularization of Cardiac Tissue. Tissue Eng. Part. C Methods 2013, 19, 802–809. [Google Scholar] [CrossRef]
- Ijima, H.; Nakamura, S.; Bual, R.; Shirakigawa, N.; Tanoue, S. Physical Properties of the Extracellular Matrix of Decellularized Porcine Liver. Gels 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Hilster, R.H.J.; Sharma, P.K.; Jonker, M.R.; White, E.S.; Gercama, E.A.; Roobeek, M.; Timens, W.; Harmsen, M.C.; Hylkema, M.N.; Burgess, J.K. Human Lung Extracellular Matrix Hydrogels Resemble the Stiffness and Viscoelasticity of Native Lung Tissue. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L698–L704. [Google Scholar] [CrossRef]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering Hydrogels as Extracellular Matrix Mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatej, I.; Popa, M.; Rinaudo, M. Role of the PH on Hyaluronan Behavior in Aqueous Solution. Biomacromolecules 2005, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Krahulec, J.; Krahulcová, J. Increase in Hyaluronic Acid Production by Streptococcus Equi Subsp. Zooepidemicus Strain Deficient in β-Glucuronidase in Laboratory Conditions. Appl. Microbiol. Biotechnol. 2006, 71, 415–422. [Google Scholar] [CrossRef]
- Nimmo, C.M.; Owen, S.C.; Shoichet, M.S. Diels−Alder Click Cross-Linked Hyaluronic Acid Hydrogels for Tissue Engineering. Biomacromolecules 2011, 12, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, S.C.; Fisher, S.A.; Tam, R.Y.; Nimmo, C.M.; Shoichet, M.S. Hyaluronic Acid Click Hydrogels Emulate the Extracellular Matrix. Langmuir 2013, 29, 7393–7400. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, A.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Ambrosio, L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. Biomed. Res. Int. 2015, 2015, 871218. [Google Scholar] [CrossRef] [PubMed]
- Petrou, G.; Crouzier, T. Mucins as Multifunctional Building Blocks of Biomaterials. Biomater. Sci. 2018, 6, 2282–2297. [Google Scholar] [CrossRef] [Green Version]
- Celli, J.; Gregor, B.; Turner, B.; Afdhal, N.H.; Bansil, R.; Erramilli, S. Viscoelastic Properties and Dynamics of Porcine Gastric Mucin. Biomacromolecules 2005, 6, 1329–1333. [Google Scholar] [CrossRef]
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Ewoldt, R.H.; McKinley, G.H.; Bansil, R.; Erramilli, S. Rheology of Gastric Mucin Exhibits a PH-Dependent Sol−Gel Transition. Biomacromolecules 2007, 8, 1580–1586. [Google Scholar] [CrossRef] [Green Version]
- Kočevar-Nared, J.; Kristl, J.; Šmid-Korbar, J. Comparative Rheological Investigation of Crude Gastric Mucin and Natural Gastric Mucus. Biomaterials 1997, 18, 677–681. [Google Scholar] [CrossRef]
- Vicic, M. Rheology and Microstructure of Complex Fluids: Dispersions, Emulsions and Polymer Solutions. Ph.D. Thesis, California Institute of Technology, Pasadena, California, USA, 1999. [Google Scholar]
- McCullagh, C.M.; Jamieson, A.M.; Blackwell, J.; Gupta, R. Viscoelastic Properties of Human Tracheobronchial Mucin in Aqueous Solution. Biopolymers 1995, 35, 149–159. [Google Scholar] [CrossRef]
- Hamed, R.; Fiegel, J. Synthetic Tracheal Mucus with Native Rheological and Surface Tension Properties. J. Biomed. Mater. Res. Part. A 2014, 102, 1788–1798. [Google Scholar] [CrossRef] [Green Version]
- Jeanneret-Grosjean, A.; King, M.; Michoud, M.C.; Liote, H.; Amyot, R. Sampling Technique and Rheology of Human Tracheobronchial Mucus. Am. Rev. Respir. Dis. 1988, 137, 707–710. [Google Scholar] [CrossRef]
- Rubin, B.K.; Ramirez, O.; Zayas, J.G.; Finegan, B.; King, M. Collection and Analysis of Respiratory Mucus from Subjects without Lung Disease. Am. Rev. Respir. Dis. 1990, 141, 1040–1043. [Google Scholar] [CrossRef]
- Bastholm, S.K.; Becher, N.; Stubbe, P.R.; Chronakis, I.S.; Uldbjerg, N. The Viscoelastic Properties of the Cervical Mucus Plug. Acta Obstet. Et Gynecol. Scand. 2014, 93, 201–208. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjugate Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Ramos-Docampo, M.A.; Fernández-Medina, M.; Taipaleenmäki, E.; Hovorka, O.; Salgueiriño, V.; Städler, B. Microswimmers with Heat Delivery Capacity for 3D Cell Spheroid Penetration. ACS Nano 2019, 13, 12192–12205. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Curley, C.J.; Dolan, E.B.; Otten, M.; Hinderer, S.; Duffy, G.P.; Murphy, B.P. An Injectable Alginate/Extra Cellular Matrix (ECM) Hydrogel towards Acellular Treatment of Heart Failure. Drug Deliv. Transl. Res. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Horton, E.R.; Vallmajo-Martin, Q.; Martin, I.; Snedeker, J.G.; Ehrbar, M.; Blache, U. Extracellular Matrix Production by Mesenchymal Stromal Cells in Hydrogels Facilitates Cell Spreading and Is Inhibited by FGF-2. Adv. Healthc. Mater. 2020, 9, 1901669. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, K.; Kumar, P.; van Vuuren, S.F.; Pillay, V.; Choonara, Y.E. Three-Dimensional Printability of an ECM-Based Gelatin Methacryloyl (GelMA) Biomaterial for Potential Neuroregeneration. ACS Omega 2021, 6, 21368–21383. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Sherman-Baust, C.A.; Weeraratna, A.T.; Rangel, L.B.A.; Pizer, E.S.; Cho, K.R.; Schwartz, D.R.; Shock, T.; Morin, P.J. Remodeling of the Extracellular Matrix through Overexpression of Collagen VI Contributes to Cisplatin Resistance in Ovarian Cancer Cells. Cancer Cell 2003, 3, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Nganguia, H.; Zheng, K.; Chen, Y.; Pak, O.S.; Zhu, L. A Note on a Swirling Squirmer in a Shear-Thinning Fluid. Phys. Fluids 2020, 32, 111906. [Google Scholar] [CrossRef]
- Demir, E.; Lordi, N.; Ding, Y.; Pak, O.S. Nonlocal Shear-Thinning Effects Substantially Enhance Helical Propulsion. Phys. Rev. Fluids 2020, 5, 111301. [Google Scholar] [CrossRef]
- Qin, K.; Peng, Z.; Chen, Y.; Nganguia, H.; Zhu, L.; Pak, O.S. Propulsion of an Elastic Filament in a Shear-Thinning Fluid. Soft Matter 2021, 17, 3829–3839. [Google Scholar] [CrossRef]
- Riley, E.E.; Lauga, E. Small-Amplitude Swimmers Can Self-Propel Faster in Viscoelastic Fluids. J. Theor. Biol. 2015, 382, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Riley, E.E.; Lauga, E. Enhanced Active Swimming in Viscoelastic Fluids. EPL Europhys. Lett. 2014, 108, 34003. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Kuhn, S.J.; Hallahan, D.E.; Giorgio, T.D. Characterization of Superparamagnetic Nanoparticle Interactions with Extracellular Matrix in an In Vitro System. Ann. Biomed. Eng. 2006, 34, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.J.; Finch, S.K.; Hallahan, D.E.; Giorgio, T.D. Proteolytic Surface Functionalization Enhances In Vitro Magnetic Nanoparticle Mobility through Extracellular Matrix. Nano Lett. 2006, 6, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Gunzer, M.; Friedl, P.; Niggemann, B.; Bröcker, E.-B.; Kämpgen, E.; Zänker, K.S. Migration of Dendritic Cells within 3-D Collagen Lattices Is Dependent on Tissue Origin, State of Maturation, and Matrix Structure and Is Maintained by Proinflammatory Cytokines. J. Leukoc. Biol. 2000, 67, 622–629. [Google Scholar] [CrossRef]
- Mark, A.G.; Gibbs, J.G.; Lee, T.-C.; Fischer, P. Hybrid Nanocolloids with Programmed Three-Dimensional Shape and Material Composition. Nat. Mater. 2013, 12, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gradilla, V.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Yardımcı, C.; Wiitala, D.; Galarnyk, M.; Wang, J. Ultrasound-Propelled Nanoporous Gold Wire for Efficient Drug Loading and Release. Small 2014, 10, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández de Ávila, B.; Angell, C.; Soto, F.; Lopez-Ramirez, M.A.; Báez, D.F.; Xie, S.; Wang, J.; Chen, Y. Acoustically Propelled Nanomotors for Intracellular SiRNA Delivery. ACS Nano 2016, 10, 4997–5005. [Google Scholar] [CrossRef] [PubMed]
- Soto, F.; Wagner, G.L.; Garcia-Gradilla, V.; Gillespie, K.T.; Lakshmipathy, D.R.; Karshalev, E.; Angell, C.; Chen, Y.; Wang, J. Acoustically Propelled Nanoshells. Nanoscale 2016, 8, 17788–17793. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gradilla, V.; Orozco, J.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Pourazary, A.; Katzenberg, A.; Gao, W.; Shen, Y.; Wang, J. Functionalized Ultrasound-Propelled Magnetically Guided Nanomotors: Toward Practical Biomedical Applications. ACS Nano 2013, 7, 9232–9240. [Google Scholar] [CrossRef]
- Ahmed, D.; Lu, M.; Nourhani, A.; Lammert, P.E.; Stratton, Z.; Muddana, H.S.; Crespi, V.H.; Huang, T.J. Selectively Manipulable Acoustic-Powered Microswimmers. Sci. Rep. 2015, 5, 9744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa, K.; Viktorova, J.; Plutnar, J.; Ruml, T.; Hoang, L.; Pumera, M. Chemical Microrobots as Self-Propelled Microbrushes against Dental Biofilm. Cell Rep. Phys. Sci. 2020, 1, 100181. [Google Scholar] [CrossRef]
- Zhao, X.; Gentile, K.; Mohajerani, F.; Sen, A. Powering Motion with Enzymes. Acc. Chem. Res. 2018, 51, 2373–2381. [Google Scholar] [CrossRef]
- Ghosh, S.; Somasundar, A.; Sen, A. Enzymes as Active Matter. Annu. Rev. Condens. Matter Phys. 2021, 12, 177–200. [Google Scholar] [CrossRef]
- Mano, N.; Heller, A. Bioelectrochemical Propulsion. J. Am. Chem. Soc. 2005, 127, 11574–11575. [Google Scholar] [CrossRef]
- Pantarotto, D.; Browne, W.R.; Feringa, B.L. Autonomous Propulsion of Carbon Nanotubes Powered by a Multienzyme Ensemble. Chem. Commun. 2008, 1533–1535. [Google Scholar] [CrossRef] [Green Version]
- Arqué, X.; Romero-Rivera, A.; Feixas, F.; Patiño, T.; Osuna, S.; Sánchez, S. Intrinsic Enzymatic Properties Modulate the Self-Propulsion of Micromotors. Nat. Commun. 2019, 10, 2826. [Google Scholar] [CrossRef] [Green Version]
- Golestanian, R. Anomalous Diffusion of Symmetric and Asymmetric Active Colloids. Phys. Rev. Lett. 2009, 102, 188305. [Google Scholar] [CrossRef] [Green Version]
- Illien, P.; Zhao, X.; Dey, K.K.; Butler, P.J.; Sen, A.; Golestanian, R. Exothermicity Is Not a Necessary Condition for Enhanced Diffusion of Enzymes. Nano Lett. 2017, 17, 4415–4420. [Google Scholar] [CrossRef] [Green Version]
- Mikhailov, A.S.; Kapral, R. Hydrodynamic Collective Effects of Active Protein Machines in Solution and Lipid Bilayers. Proc. Natl. Acad. Sci. USA 2015, 112, E3639–E3644. [Google Scholar] [CrossRef] [Green Version]
- Dennison, M.; Kapral, R.; Stark, H. Diffusion in Systems Crowded by Active Force-Dipole Molecules. Soft Matter 2017, 13, 3741–3749. [Google Scholar] [CrossRef] [Green Version]
- Somasundar, A.; Ghosh, S.; Mohajerani, F.; Massenburg, L.N.; Yang, T.; Cremer, P.S.; Velegol, D.; Sen, A. Positive and Negative Chemotaxis of Enzyme-Coated Liposome Motors. Nat. Nanotechnol. 2019, 14, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Hortelão, A.C.; Carrascosa, R.; Murillo-Cremaes, N.; Patiño, T.; Sánchez, S. Targeting 3D Bladder Cancer Spheroids with Urease-Powered Nanomotors. ACS Nano 2019, 13, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Soto, F.; Martin, A.; Ibsen, S.; Vaidyanathan, M.; Garcia-Gradilla, V.; Levin, Y.; Escarpa, A.; Esener, S.C.; Wang, J. Acoustic Microcannons: Toward Advanced Microballistics. ACS Nano 2016, 10, 1522–1528. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Yang, Y.; Hu, P.; Li, Y.; Yang, S.-Y.; Wang, L.V.; Gao, W. A Microrobotic System Guided by Photoacoustic Computed Tomography for Targeted Navigation in Intestines in Vivo. Sci. Robot. 2019, 4, eaax0613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aceves-Sanchez, P.; Degond, P.; Keaveny, E.E.; Manhart, A.; Merino-Aceituno, S.; Peurichard, D. Large-Scale Dynamics of Self-Propelled Particles Moving Through Obstacles: Model Derivation and Pattern Formation. Bull. Math. Biol. 2020, 82, 129. [Google Scholar] [CrossRef]
- Cheung, K.J.; Ewald, A.J. A Collective Route to Metastasis: Seeding by Tumor Cell Clusters. Science 2016, 352, 167–169. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Moran, J.L. Autonomously Propelled Colloids for Penetration and Payload Delivery in Complex Extracellular Matrices. Micromachines 2021, 12, 1216. https://doi.org/10.3390/mi12101216
Singh S, Moran JL. Autonomously Propelled Colloids for Penetration and Payload Delivery in Complex Extracellular Matrices. Micromachines. 2021; 12(10):1216. https://doi.org/10.3390/mi12101216
Chicago/Turabian StyleSingh, Shrishti, and Jeffrey L. Moran. 2021. "Autonomously Propelled Colloids for Penetration and Payload Delivery in Complex Extracellular Matrices" Micromachines 12, no. 10: 1216. https://doi.org/10.3390/mi12101216
APA StyleSingh, S., & Moran, J. L. (2021). Autonomously Propelled Colloids for Penetration and Payload Delivery in Complex Extracellular Matrices. Micromachines, 12(10), 1216. https://doi.org/10.3390/mi12101216