Efficiency Enhancement of Electro-Adsorption Desalination Using Iron Oxide Nanoparticle-Incorporated Activated Carbon Nanocomposite
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Methods
2.2. Synthesis of Activated Carbon/Fe2O3 Nanocomposite
2.3. Characterization
2.4. Electrochemical Properties of the Synthesized Electrode
2.5. Electrosorptive Capacity Measurement
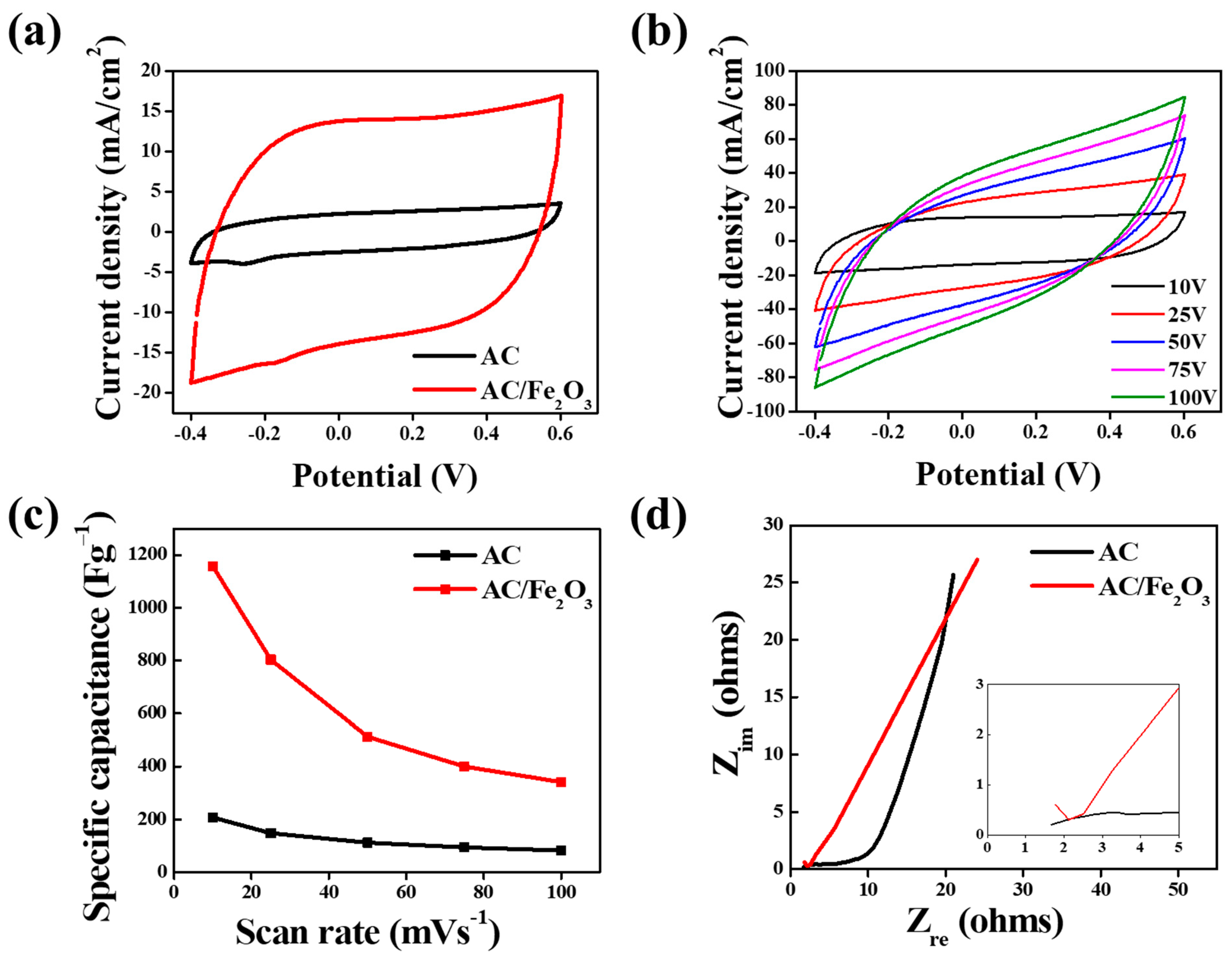
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pekel, J.-F.; Cottam, A.; Gorelick, N.; Belward, A.S. High-resolution mapping of global surface water and its long-term changes. Nature 2016, 540, 418–422. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Chang, B.; Kim, S.; Lee, J.; Yoon, J.; Choi, J.W. Battery Electrode Materials with Omnivalent Cation Storage for Fast and Charge-Efficient Ion Removal of Asymmetric Capacitive Deionization. Adv. Funct. Mater. 2018, 28, 1802665. [Google Scholar] [CrossRef]
- He, F.; Biesheuvel, P.; Bazant, M.; Hatton, T.A. Theory of water treatment by capacitive deionization with redox active porous electrodes. Water Res. 2018, 132, 282–291. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Wan, K.; Huang, L.; Yan, J.; Ma, B.; Huang, X.; Luo, Z.; Zhang, H.; Xiao, T. Removal of fluoride from industrial wastewater by using different adsorbents: A review. Sci. Total Environ. 2021, 773, 145535. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Morris, G.; Qi, D. Using activated carbon electrode in electrosorptive deionisation of brackish water. Desalination 2008, 225, 329–340. [Google Scholar] [CrossRef]
- Tang, W.; Kovalsky, P.; Cao, B.; Waite, T.D. Investigation of fluoride removal from low-salinity groundwater by single-pass constant-voltage capacitive deionization. Water Res. 2016, 99, 112–121. [Google Scholar] [CrossRef]
- Wang, S.; Wang, G.; Wu, T.; Li, C.; Wang, Y.; Pan, X.; Zhan, F.; Zhang, Y.; Wang, S.; Qiu, J. Membrane-Free Hybrid Capacitive Deionization System Based on Redox Reaction for High-Efficiency NaCl Removal. Environ. Sci. Technol. 2019, 53, 6292–6301. [Google Scholar] [CrossRef]
- Younes, H.; Zou, L. Asymmetric configuration of pseudocapacitive composite and rGO electrodes for enhanced capacitive deionization. Environ. Sci. Water Res. Technol. 2019, 6, 392–403. [Google Scholar] [CrossRef]
- Oladunni, J.; Zain, J.H.; Hai, A.; Banat, F.; Bharath, G.; Alhseinat, E. A comprehensive review on recently developed carbon based nanocomposites for capacitive deionization: From theory to practice. Sep. Purif. Technol. 2018, 207, 291–320. [Google Scholar] [CrossRef]
- He, C.; Ma, J.; Zhang, C.; Song, J.; Waite, T.D. Short-circuited closed-cycle operation of flow-electrode CDI for brackish water softening. Environ. Sci. Technol. 2018, 52, 9350–9360. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Bin Wu, H.; Madhavi, S.; Lou, X.W. Controlled Growth of NiMoO4Nanosheet and Nanorod Arrays on Various Conductive Substrates as Advanced Electrodes for Asymmetric Supercapacitors. Adv. Energy Mater. 2014, 5, 1401172. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Yang, Z.; Kang, F.; Inagaki, M. Carbon electrodes for capacitive deionization. J. Mater. Chem. A 2016, 5, 470–496. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, C.; Liu, X.; Xu, X.; Sun, Z.; Pan, L. Review on carbon-based composite materials for capacitive deionization. RSC Adv. 2015, 5, 15205–15225. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Z.; Chua, D.H.C.; Pan, L. Novel nitrogen doped graphene sponge with ultrahigh capacitive deionization performance. Sci. Rep. 2015, 5, 11225. [Google Scholar] [CrossRef]
- Lu, P.-J.; Lin, H.-C.; Yu, W.-T.; Chern, J.-M. Chemical regeneration of activated carbon used for dye adsorption. J. Taiwan Inst. Chem. Eng. 2011, 42, 305–311. [Google Scholar] [CrossRef]
- Yan, C.; Zou, L.; Short, R. Polyaniline-modified activated carbon electrodes for capacitive deionisation. Desalination 2014, 333, 101–106. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B. A short review of activated carbon assisted electrosorption process: An overview, current stage and future prospects. J. Hazard. Mater. 2009, 170, 552–559. [Google Scholar] [CrossRef]
- Ban, A.; Schäfer, A.; Wendt, H. Fundamentals of electrosorption on activated carbon for wastewater treatment of industrial effluents. J. Appl. Electrochem. 1998, 28, 227–236. [Google Scholar] [CrossRef]
- Oda, H.; Nakagawa, Y. Removal of ionic substances from dilute solution using activated carbon electrodes. Carbon 2003, 41, 1037–1047. [Google Scholar] [CrossRef]
- Hou, C.-H.; Huang, J.-F.; Lin, H.-R.; Wang, B.-Y. Preparation of activated carbon sheet electrode assisted electrosorption process. J. Taiwan Inst. Chem. Eng. 2012, 43, 473–479. [Google Scholar] [CrossRef]
- Park, K.-K.; Lee, J.-B.; Park, P.-Y.; Yoon, S.-W.; Moon, J.-S.; Eum, H.-M.; Lee, C.-W. Development of a carbon sheet electrode for electrosorption desalination. Desalination 2007, 206, 86–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Pan, L.; Li, H.; Sun, Z.; Sun, C.; Tay, B.K. Carbon nanotube–ZnO nanocomposite electrodes for supercapacitors. Solid State Ion. 2009, 180, 1525–1528. [Google Scholar] [CrossRef]
- Li, H.; Leong, Z.Y.; Shi, W.; Zhang, J.; Chen, T.; Yang, H.Y. Hydrothermally synthesized graphene and Fe3O4 nanocomposites for high performance capacitive deionization. RSC Adv. 2016, 6, 11967–11972. [Google Scholar] [CrossRef]
- Gu, X.; Hu, M.; Du, Z.; Huang, J.; Wang, C. Fabrication of mesoporous graphene electrodes with enhanced capacitive deionization. Electrochim. Acta 2015, 182, 183–191. [Google Scholar] [CrossRef]
- Al Suwaidi, F.; Younes, H.; Sreepal, V.; Nair, R.R.; Aubry, C.; Zou, L. Strategies for tuning hierarchical porosity of 3D rGO to optimize ion electrosorption. 2D Mater. 2019, 6, 045010. [Google Scholar] [CrossRef]
- Zafra, M.; Lavela, P.; Rasines, G.; Macías, C.; Tirado, J.; Ania, C. A novel method for metal oxide deposition on carbon aerogels with potential application in capacitive deionization of saline water. Electrochim. Acta 2014, 135, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Belaustegui, Y.; Rincón, I.; Fernández-Carretero, F.; Azpiroz, P.; García-Luís, A.; Tanaka, D.A.P. Three-dimensional reduced graphene oxide decorated with iron oxide nanoparticles as efficient active material for high performance capacitive deionization electrodes. Chem. Eng. J. Adv. 2021, 6, 100094. [Google Scholar] [CrossRef]
- Trinh, N.T.; Chung, S.; Lee, J.K.; Lee, J. Development of high quality Fe3O4/rGO composited electrode for low energy water treatment. J. Energy Chem. 2016, 25, 354–360. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, Y.C. Fe3O4-decorated hollow graphene balls prepared by spray pyrolysis process for ultrafast and long cycle-life lithium ion batteries. Carbon 2014, 79, 58–66. [Google Scholar] [CrossRef]
- Yasin, A.S.; Mohamed, A.; Mohamed, I.; Cho, D.-Y.; Park, C.H.; Kim, C.S. Theoretical insight into the structure-property relationship of mixed transition metal oxides nanofibers doped in activated carbon and 3D graphene for capacitive deionization. Chem. Eng. J. 2019, 371, 166–181. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, M.; Liu, P.; Wang, S.; Liu, S.; Feng, H.; Zheng, H.; Cheng, F. Advanced negative electrode of Fe2O3/graphene oxide paper for high energy supercapacitors. Mater. Res. Bull. 2017, 96, 413–418. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, H.-B.; Xu, J.; Wu, X.; Yang, D.; Qu, J.; Yu, Z.-Z. Highly efficient high-pressure homogenization approach for scalable production of high-quality graphene sheets and sandwich-structured α-Fe2O3/graphene hybrids for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 11025–11034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhai, X.; Yan, D.; Ding, C.; Wu, N.; Su, D.; Zhao, Y.; Zhou, H.; Zhao, X.; Li, J.B.; et al. Rational construction the composite of graphene and hierarchical structure assembled by Fe2O3 nanosheets for lithium storage. Electrochim. Acta 2017, 243, 18–25. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, D.; Ding, C.; Su, D.; Ge, Y.; Zhao, Y.; Zhou, H.; Li, J.B.; Jin, H. Fe2O3 nanocubes exposed (012) active facets combination with graphene rendering enhanced lithium storage capability. J. Power Sources 2016, 327, 658–665. [Google Scholar] [CrossRef]
- Yasin, A.S.; Mohamed, H.O.; Mohamed, I.; Mousa, H.M.; Barakat, N.A. Enhanced desalination performance of capacitive deionization using zirconium oxide nanoparticles-doped graphene oxide as a novel and effective electrode. Sep. Purif. Technol. 2016, 171, 34–43. [Google Scholar] [CrossRef]
- Qu, D. Studies of the activated carbons used in double-layer supercapacitors. J. Power Sources 2002, 109, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Hatzell, K.B.; Hatzell, M.C.; Cook, K.M.; Boota, M.; Housel, G.M.; McBride, A.; Kumbur, E.C.; Gogotsi, Y. Effect of Oxidation of Carbon Material on Suspension Electrodes for Flow Electrode Capacitive Deionization. Environ. Sci. Technol. 2015, 49, 3040–3047. [Google Scholar] [CrossRef]
- Jana, M.; Saha, S.; Khanra, P.; Samanta, P.; Koo, H.; Murmu, N.C.; Kuila, T. Non-covalent functionalization of reduced graphene oxide using sulfanilic acid azocromotrop and its application as a supercapacitor electrode material. J. Mater. Chem. A 2015, 3, 7323–7331. [Google Scholar] [CrossRef]
- Zou, L.; Li, L.; Song, H.; Morris, G. Using mesoporous carbon electrodes for brackish water desalination. Water Res. 2008, 42, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhang, D.; Shi, L.; Yan, T. High performance ordered mesoporous carbon/carbon nanotube composite electrodes for capacitive deionization. J. Mater. Chem. 2012, 22, 6603–6612. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, T.; Shi, L.; Peng, Z.; Wen, X.; Zhang, J. Enhanced capacitive deionization performance of graphene/carbon nanotube composites. J. Mater. Chem. 2012, 22, 14696–14704. [Google Scholar] [CrossRef]
- Li, H.; Pan, L.; Nie, C.; Liu, Y.; Sun, Z. Reduced graphene oxide and activated carbon composites for capacitive deionization. J. Mater. Chem. 2012, 22, 15556–15561. [Google Scholar] [CrossRef]
- Yang, J.; Zou, L.; Song, H. Preparing MnO2/PSS/CNTs composite electrodes by layer-by-layer deposition of MnO2 in the membrane capacitive deionisation. Desalination 2012, 286, 108–114. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, S.; Wan, J.; Tang, H.; Chang, L.; He, L.; Zhao, H.; Gao, Y.; Tang, Z. Three-Dimensional Graphene/Metal Oxide Nanoparticle Hybrids for High-Performance Capacitive Deionization of Saline Water. Adv. Mater. 2013, 25, 6270–6276. [Google Scholar] [CrossRef]
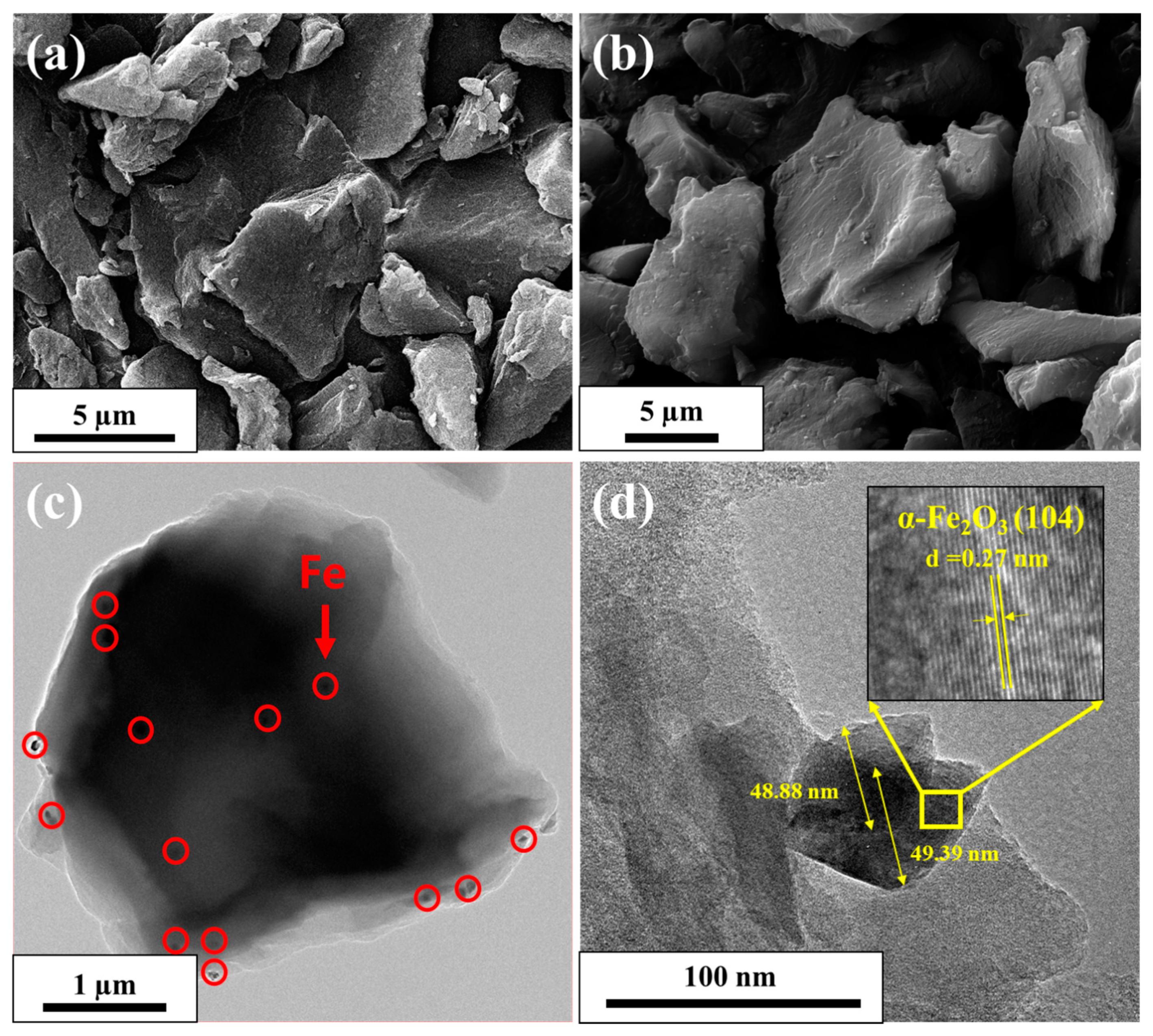
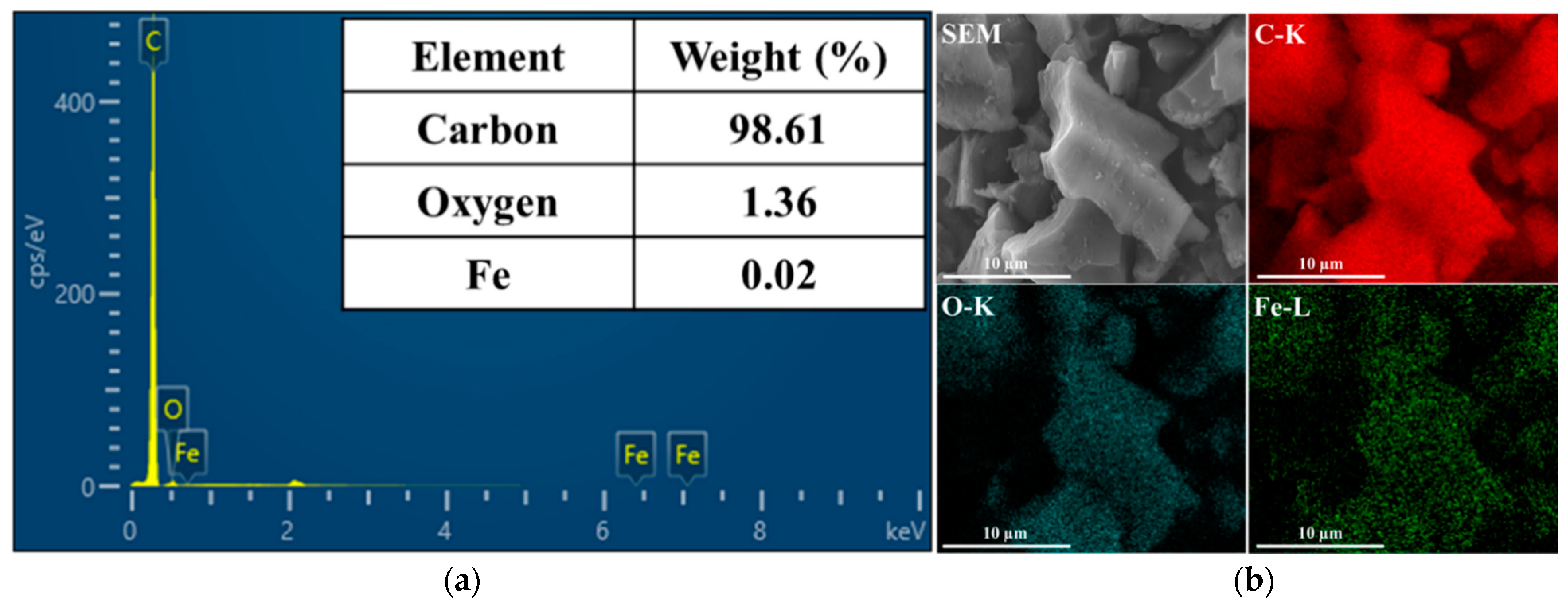
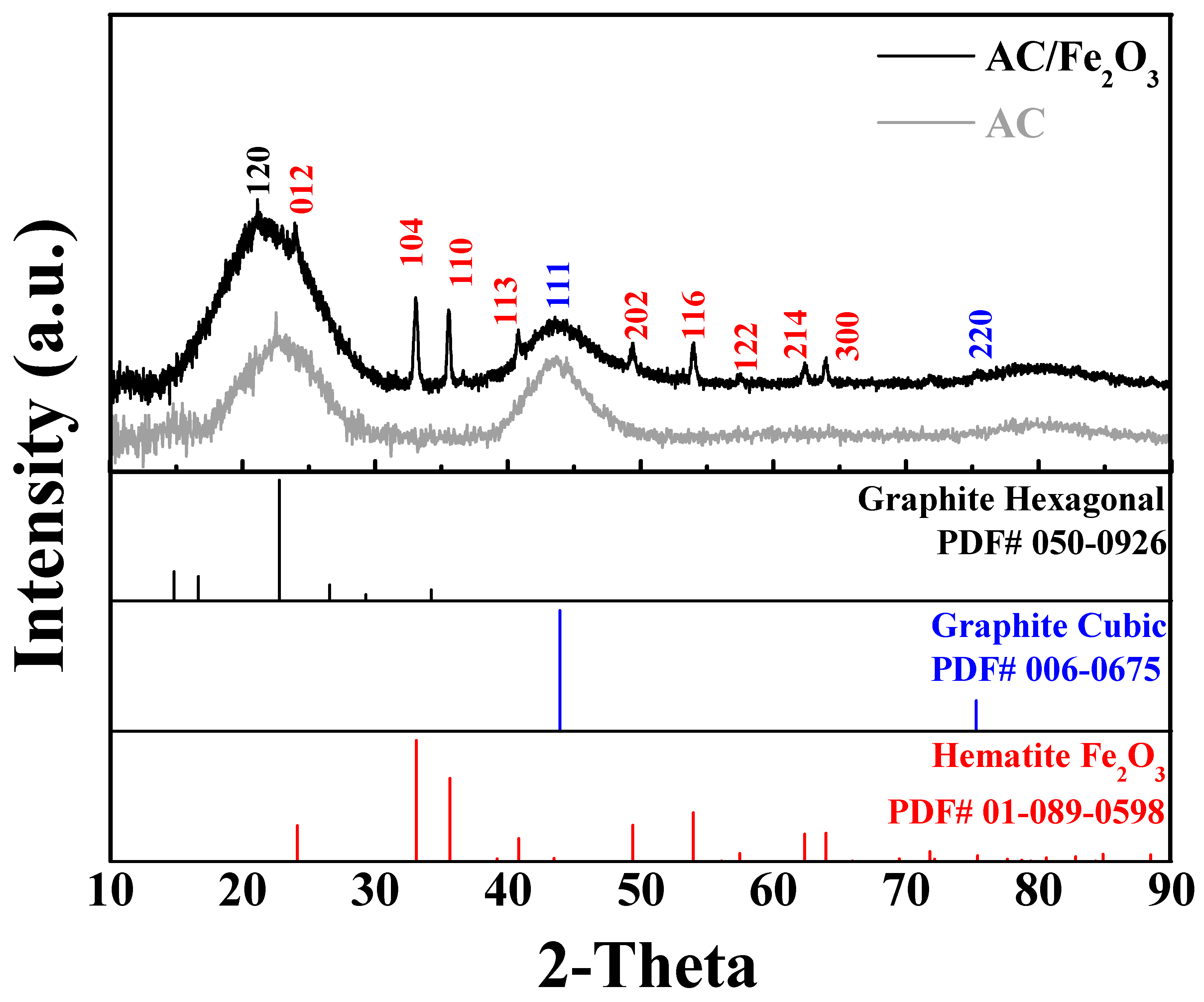
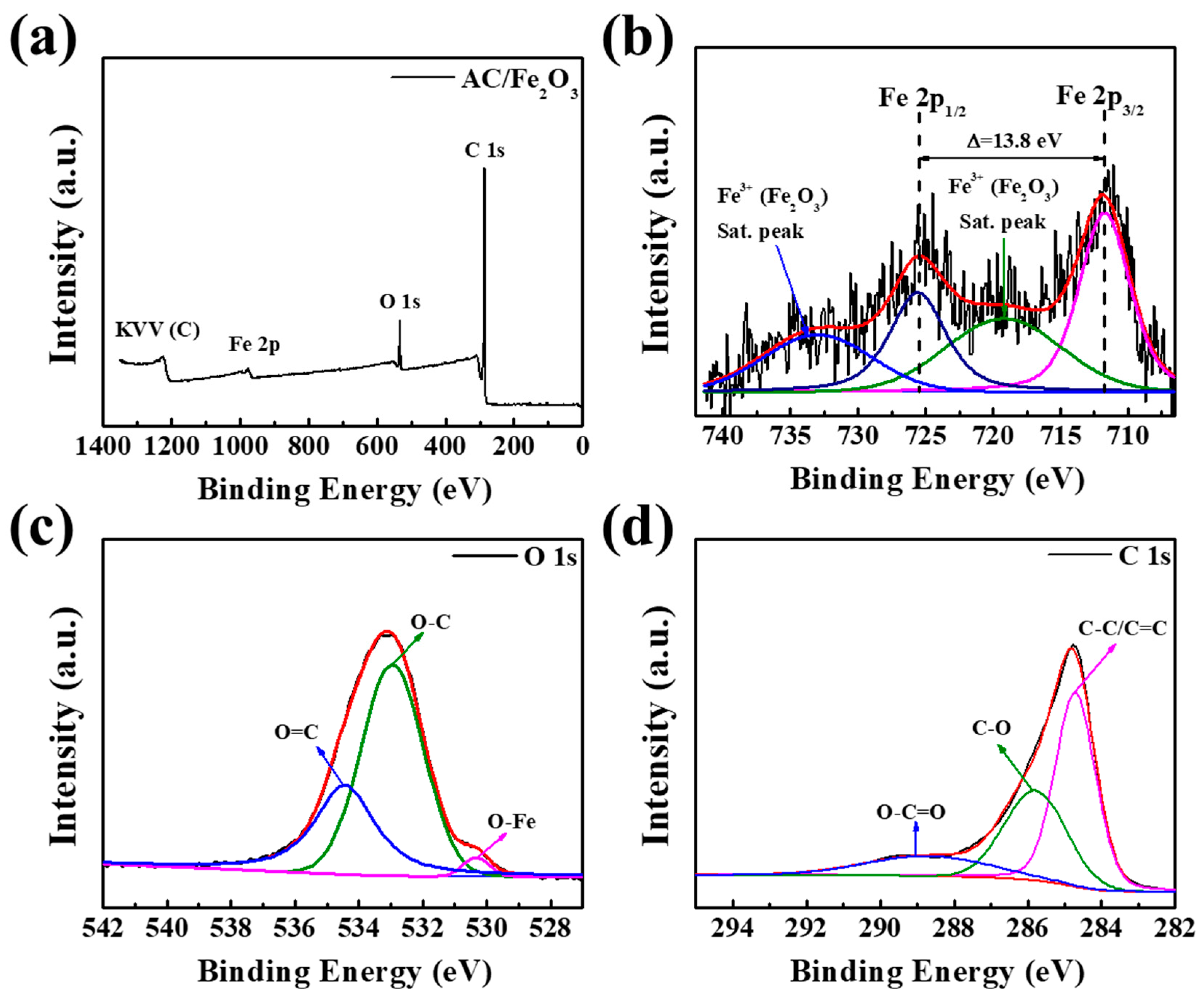


| Lattice Parameters | Hematite, Fe2O3 (Ref.) | AC/Fe2O3 |
|---|---|---|
| Crystal system | Rhombohedral | Rhombohedral |
| Space group | R3c | R3c |
| a | 5.038 (Å) | 5.041 (Å) |
| b | 5.038 (Å) | 5.041 (Å) |
| c | 13.776 (Å) | 13.73 (Å) |
| Name | Peak BE | FWHM (eV) | Atomic % |
|---|---|---|---|
| C 1s | 284.82 | 1.84 | 90.57 |
| Fe 2p | 711.45 | 2.56 | 0.30 |
| O 1s | 533.17 | 2.87 | 8.96 |
| Electrode Material | Specific Capacitance (F g−1)/Scan Rate (mV s−1) | Applied Voltage (V) | Initial Concentration (mg L−1) | Electrosorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|
| MC | 251/1 | 1.2 | 25 | 0.68 | [43] |
| CNTs-MC | 132.6/10 | 1.2 | 40 | 0.69 | [44] |
| RG-CNTs | 175/1 | 2 | ~27 | 1.41 | [45] |
| AC | 169/1 | 1.2 | ~25 | 0.25 | [43] |
| RG-AC | 181/1 | 1.2 | ~500 | 2.94 | [46] |
| AC-MnO2 | 77.2/10 | 1.2 | ~25 | 0.99 | [47] |
| AC/TiO2 NPs | ― | 1.2 | 500 | 2.7 | [48] |
| AC/Fe2O3 | 1157.5/10 | 1.2 | ~50 | 6.76 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasin, A.S.; Mohamed, A.Y.; Kim, D.; Yoon, S.; Ra, H.; Lee, K. Efficiency Enhancement of Electro-Adsorption Desalination Using Iron Oxide Nanoparticle-Incorporated Activated Carbon Nanocomposite. Micromachines 2021, 12, 1148. https://doi.org/10.3390/mi12101148
Yasin AS, Mohamed AY, Kim D, Yoon S, Ra H, Lee K. Efficiency Enhancement of Electro-Adsorption Desalination Using Iron Oxide Nanoparticle-Incorporated Activated Carbon Nanocomposite. Micromachines. 2021; 12(10):1148. https://doi.org/10.3390/mi12101148
Chicago/Turabian StyleYasin, Ahmed S., Ahmed Yousef Mohamed, Donghyun Kim, Sungmin Yoon, Howon Ra, and Kyubock Lee. 2021. "Efficiency Enhancement of Electro-Adsorption Desalination Using Iron Oxide Nanoparticle-Incorporated Activated Carbon Nanocomposite" Micromachines 12, no. 10: 1148. https://doi.org/10.3390/mi12101148
APA StyleYasin, A. S., Mohamed, A. Y., Kim, D., Yoon, S., Ra, H., & Lee, K. (2021). Efficiency Enhancement of Electro-Adsorption Desalination Using Iron Oxide Nanoparticle-Incorporated Activated Carbon Nanocomposite. Micromachines, 12(10), 1148. https://doi.org/10.3390/mi12101148









