Influence of Piezoelectric Properties on the Ultrasonic Dispersion of TiO2 Nanoparticles in Aqueous Suspension
Abstract
1. Introduction
2. Experimental Procedure
2.1. PZT Materials and Characterization
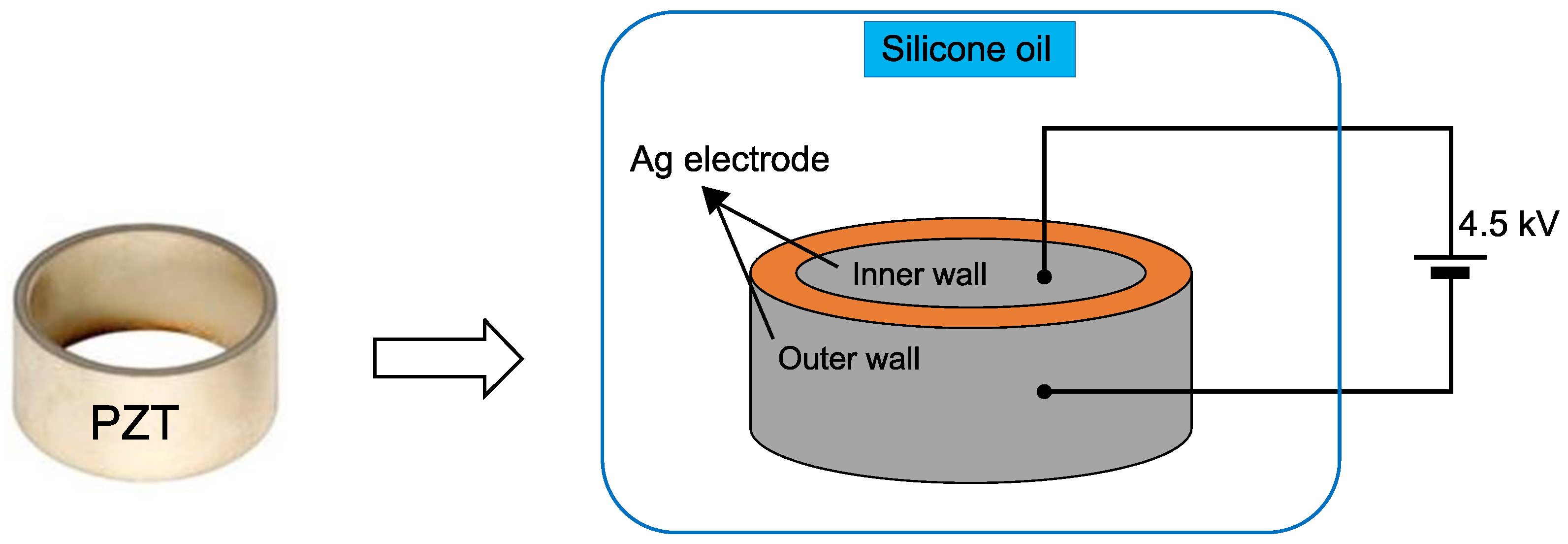
2.2. Polarization and Piezoelectric Properties
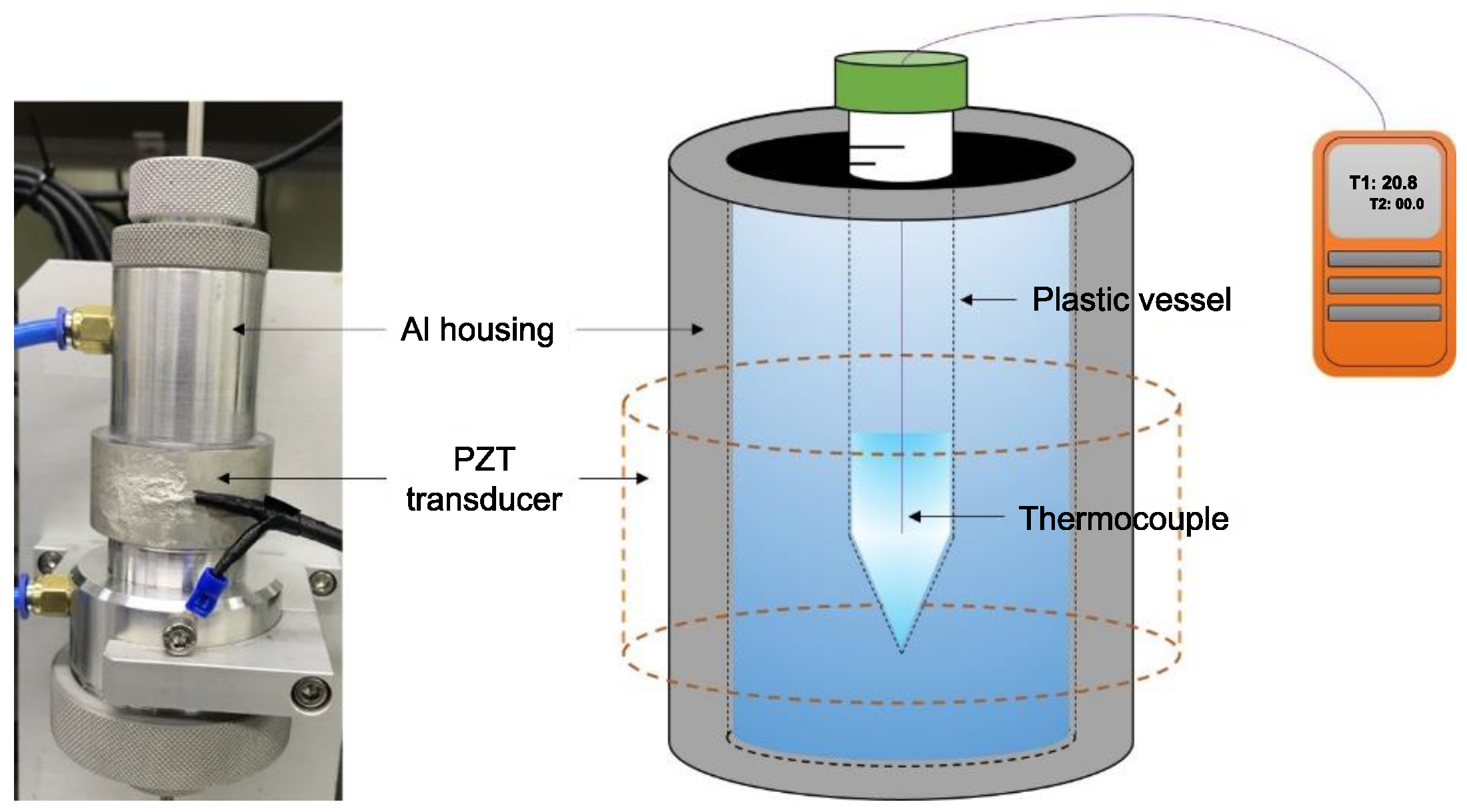
2.3. Experimental Setup
2.3.1. Measurement of Ultrasonic Power
2.3.2. Measurement of Acoustic Pressure
2.3.3. Ultrasonic Dispersion
3. Results and Discussion
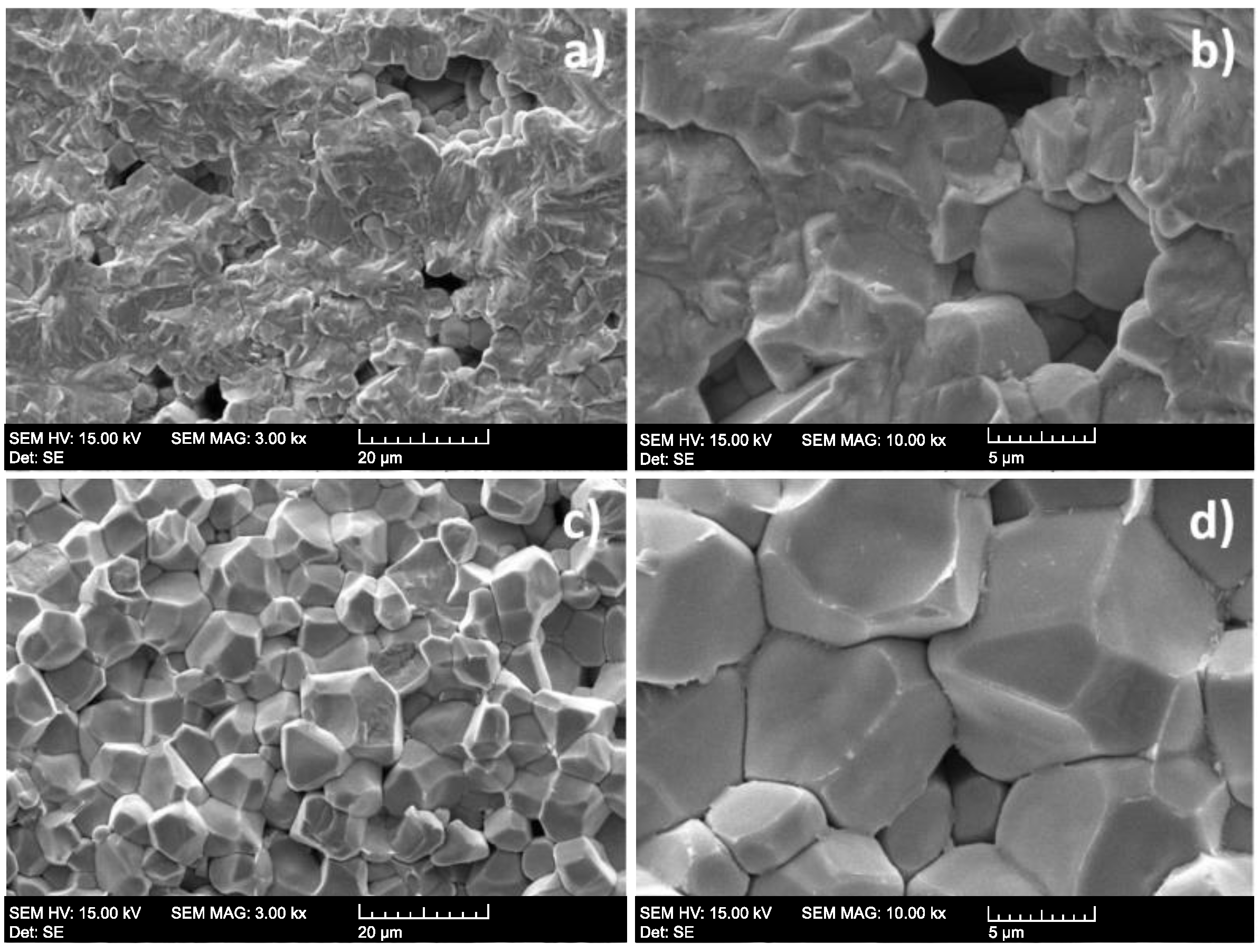
3.1. Characterization of PZT Ceramics
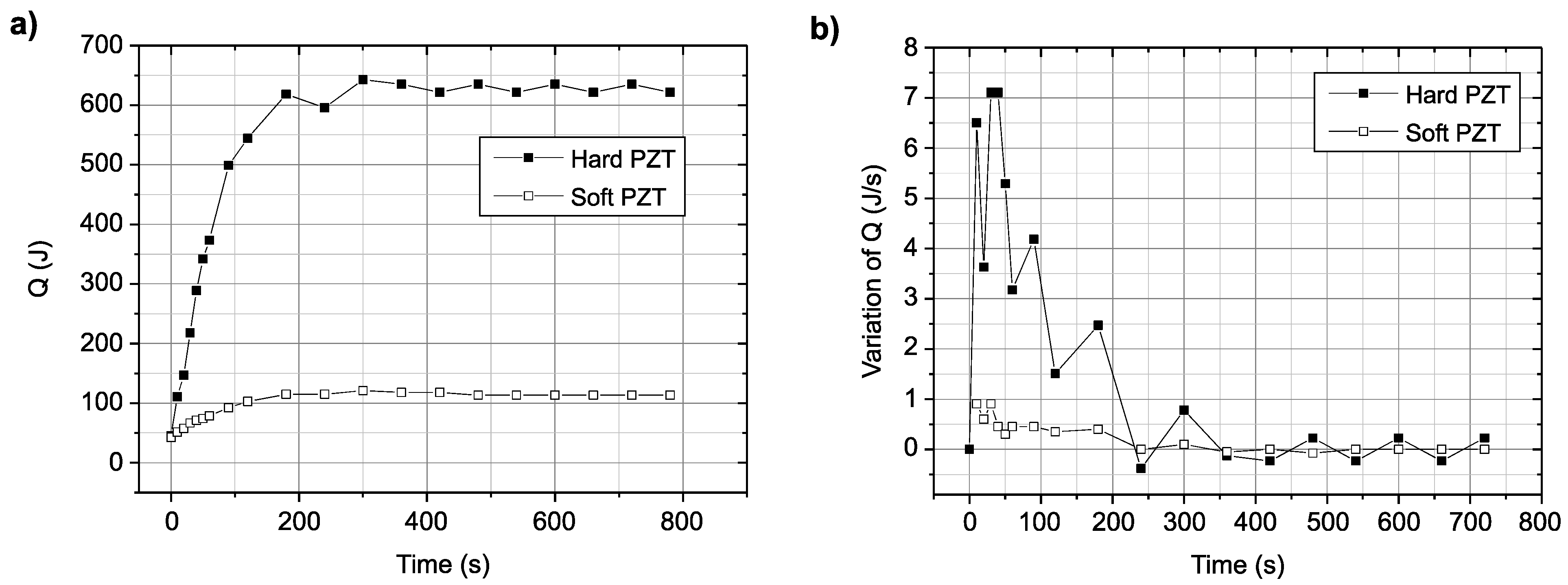
3.2. Calculation of Ultrasonic Power by the Calorimetry Method
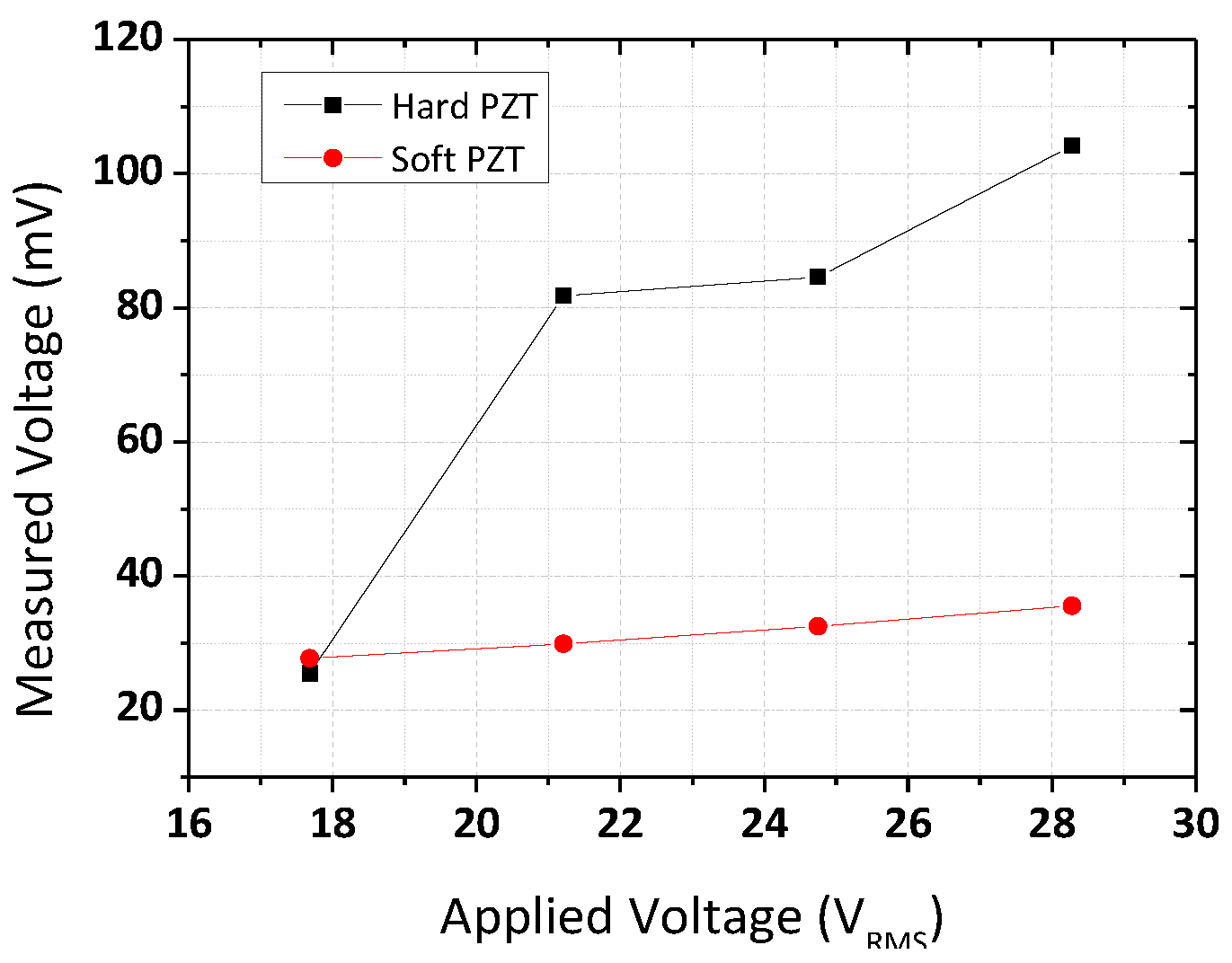
3.3. Measurement of Acoustic Pressure
3.4. Focused Ultrasonic Dispersion of TiO2 Nanoparticles in Water
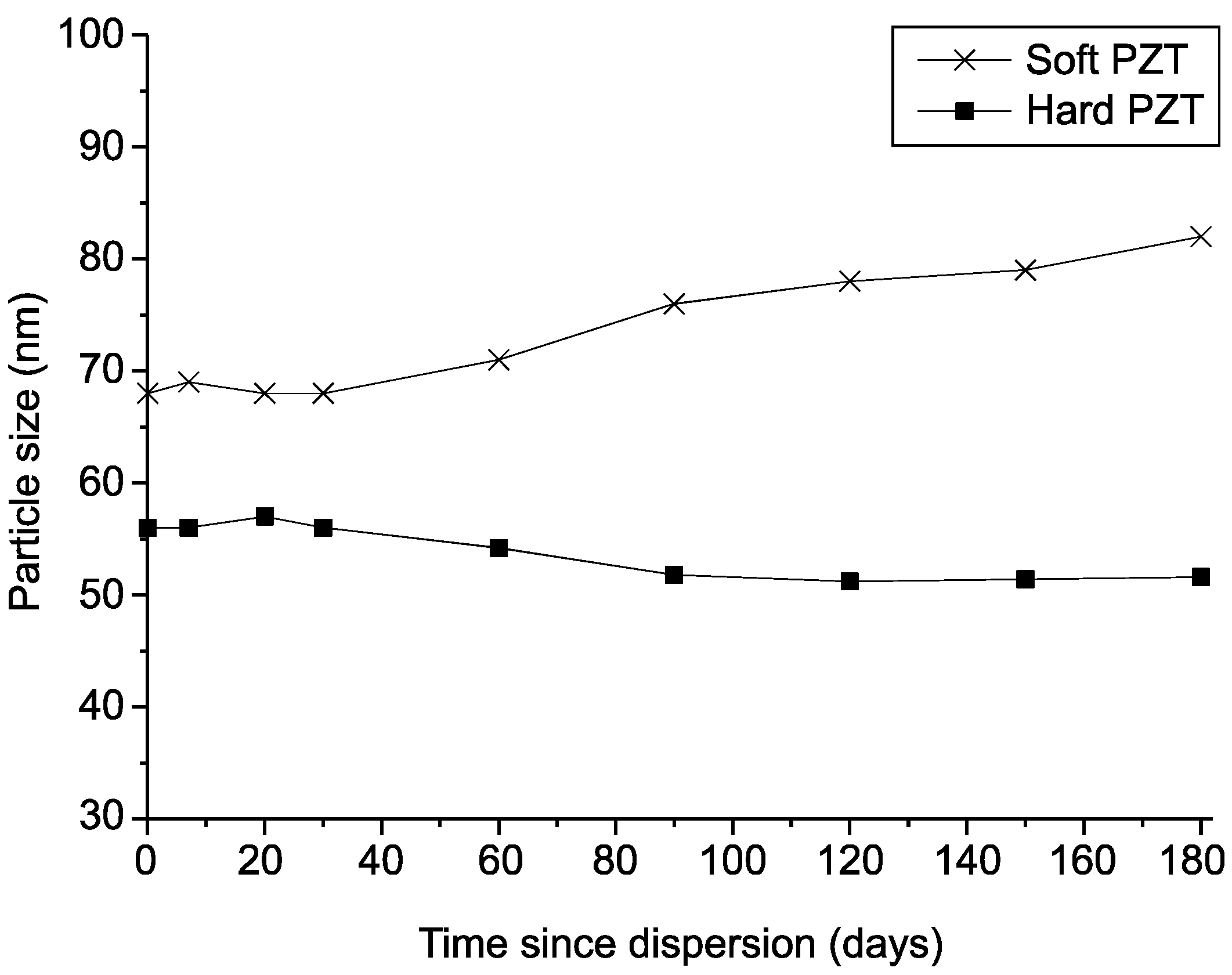
3.4.1. Size Distribution
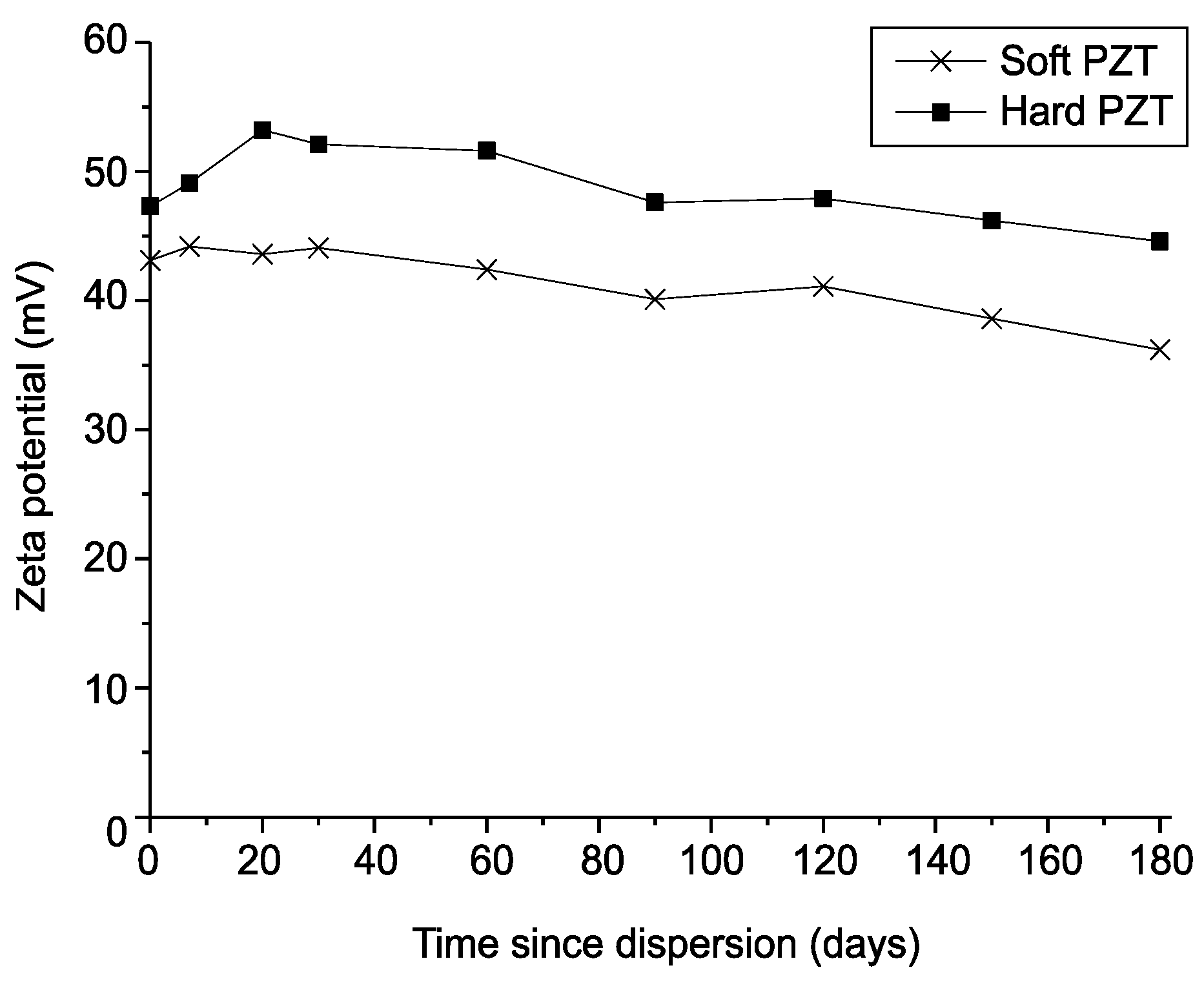
3.4.2. Zeta Potential
3.4.3. Stability of the TiO2 Colloids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sumitomo, S.; Koizumi, H.; Azhar Uddin, M.; Kato, Y. Comparison of dispersion behavior of agglomerated particles in liquid between ultrasonic irradiation and mechanical stirring. Ultrason. Sonochem. 2018, 40, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Inkyo, M.; Tahara, T.; Iwaki, T.; Iskandar, F.; Hogan, C.J., Jr.; Okuyama, K. Experimental investigation of nanoparticle dispersion by beads milling with centrifugal bead separation. J. Colloid Interface Sci. 2006, 304, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Weilin, X.; Jun, D.; Yanwei, Z.; Qi, Z. Experimental study on the impact characteristics of cavitation bubble collapse on awall. Water 2018, 10, 1262. [Google Scholar]
- Schlender, M.; Minke, K.; Schuchmann, P. Sono-chemiluminescence (SCL) in a high-pressure double stage homogenization processes. J. Chem. Eng. Sci. 2016, 142, 1–11. [Google Scholar] [CrossRef]
- Leighton, T.G. The Acoustic Bubble, 1st ed.; Academic Press: London, UK, 1994. [Google Scholar]
- Flannigan, D.J.; Suslick, K.S. Plasma formation and temperature measurement during single-bubble cavitation. Nat. Cell Biol. 2005, 434, 52–55. [Google Scholar] [CrossRef]
- Tzanakis, I.; Eskin, D.; Georgoulas, A.; Fytanidis, D. Incubation pit analysis and calculation of the hydrodynamic impact pressure from the implosion of an acoustic cavitation bubble. Ultrason. Sonochem. 2014, 21, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Manbachi, A.; Cobbold, R.S. Development and application of piezoelectric materials for ultrasound generation and detection. Ultrasound 2011, 19, 187–196. [Google Scholar] [CrossRef]
- Choi, Y.M.; Lee, Y.L.; Lim, E.S.; Trimzi, M.A.; Hwangbo, S.A.; Ham, Y.B. Performance improvement of ring-type PZT ceramics for ultrasonic dispersion system. Micromachines 2020, 11, 144. [Google Scholar] [CrossRef]
- Jaffe, B.; Cook, W.R.; Jaffe, H. Piezoelectric Ceramics, 1st ed.; Academic Press: London, UK, 1971. [Google Scholar]
- Heartling, G.H. Ferroelectric ceramics: History and technology. J. Am. Ceram. Soc. 1999, 82, 797–818. [Google Scholar] [CrossRef]
- Cross, L.E. Ferroelectric materials for electromechanical transducer applications. Mater. Chem. Phys. 1996, 43, 108–115. [Google Scholar] [CrossRef]
- Moulson, A.J.; Herbert, J.M. Electroceramics: Materials, Properties, Applications, 2nd ed.; Wiley–Interscience: New York, NY, USA, 2003. [Google Scholar]
- Uchino, K. Ferroelectric Devices; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Xu, Y.H. Ferroelectric Materials and Their Applications; North Holland Publishing Co.: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Cross, L.E. Relaxor ferroelectrics. Ferroelectronics 1987, 76, 241–267. [Google Scholar] [CrossRef]
- Cao, W.; Cross, L.E. Theoretical model for the morphotropic phase boundary in lead zirconate–lead titanate solid solution. Phys. Rev. B 1993, 47, 4825–4830. [Google Scholar] [CrossRef]
- Takahashi, S. Effects of impurity doping in lead zirconate-titanate ceramics. Ferroelectronics 1982, 41, 143–156. [Google Scholar] [CrossRef]
- Kulcsar, F. Electromechanical properties of lead titanate zirconate ceramics modified with certain three-or five-valent additions. J. Am. Ceram. Soc. 1959, 42, 343–349. [Google Scholar] [CrossRef]
- Boris, S.; Aronov, E.; Aikins, K.; Brown, D.A. Increasing piezoelectric effect in radially polarized soft piezoelectric cylinders by pressure treating and its practical applications. J. Acoust. Soc. Am. 2020, 147, 4145. [Google Scholar]
- Kim, K.M.; Park, S.H.; Kim, S.W.; Son, W.G.; Shin, B.C. Fabrication and characteristic of metal inserted Teflon diaphragm piezoeletric device module using quartz crystal oscillator. J. Kor. Sens. Soc. 2010, 19, 149–154. [Google Scholar]
- North, P.F. Towards an absolute measurement of soil structural stability using ultrasound. Eur. J. Soil Sci. 1976, 27, 451–459. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 2017, 12, 355–371. [Google Scholar] [CrossRef]
- Sazgarnia, A.; Shanei, A.; Eshghi, H.; Hassanzadeh-Khayyat, M.; Esmaily, H.; Shanei, M.M. Detection of sonoluminescence signals in a gel phantom in the presence of Protoporphyrin IX conjugated to gold nanoparticles. Ultrasonics 2013, 53, 29–35. [Google Scholar] [CrossRef]
- Jun, B.-M.; Han, J.; Park, C.M.; Yoon, Y. Ultrasonic degradation of selected dyes using Ti3C2Tx MXene as a sonocatalyst. Ultrason. Sonochem. 2020, 64, 104993. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M.; Mason, T.J. Can sonochemistry take place in the absence of cavitation?—A complementary view of how ultrasound can interact with materials. Ultrason. Sonochem. 2019, 52, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Sokol, M.; Kalabukhov, S.; Shneck, R.Z.; Zaretsky, E.B.; Frage, N. Effect of grain size on the static and dynamic mechanical properties of magnesium aluminate spinel (MgAl 2 O 4 ). J. Eur. Ceram. Soc. 2017, 37, 3417–3424. [Google Scholar] [CrossRef]
- Puchmark, C.; Rujijanagul, G.; Jiansirisomboon, S.; Tunkasiri, T.; Vittayakorn, N.; Comyn, T.; Milne, S. Mechanical property evaluation of PZT/Al2O3 composites prepared by a simple solid-state mixed oxide method. Curr. Appl. Phys. 2006, 6, 323–326. [Google Scholar] [CrossRef]
- Mentler, A.; Schomakers, J.; Kloss, S.; Zechmeister-Boltenstern, S.; Schuller, R.; Mayer, H. Calibration of ultrasonic power output in water, ethanol and sodium polytungstate. Int. Agrophys. 2017, 31, 583–588. [Google Scholar] [CrossRef]
- Koda, S.; Kimur, T.; Kondo, T.; Mitome, H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003, 10, 149–156. [Google Scholar] [CrossRef]
- Tiong, T.J.; Chandesa, T.; Yap, Y.H. Comparison of sonochemiluminescence images using image analysis techniques and identification of acoustic pressure fields via simulation. Ultrason. Sonochem. 2017, 36, 78–87. [Google Scholar] [CrossRef]
- Tzanakis, I.; Darut, G.; Eskin, D.G.; Pericleous, K. Effect of input power and temperature on the cavitation intensity during the ultrasonic treatment of molten aluminium. Trans. Indian Inst. Met. 2015, 68, 1023–1026. [Google Scholar] [CrossRef]
- Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef]
- Greenwood, R.; Kendall, K. Electroacoustic studies of moderately concentrated colloidal suspensions. J. Eur. Ceram. Soc. 1999, 19, 478–488. [Google Scholar]
- Hanaor, D.A.H.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO2. J. Eur. Ceram. Soc. 2012, 32, 235–244. [Google Scholar] [CrossRef]
- O’Brien, R.W.; Midmore, B.R.; Lamb, A.; Hunter, R.J. Electroacoustic studies of moderately concentrated colloidal suspensions. Faraday Discuss. Chem. Soc. 1990, 90, 301–312. [Google Scholar] [CrossRef]
- Young, F.R. Sonoluminescence from water containing dissolved gases. J. Acoust. Soc. Am. 1976, 60, 100–104. [Google Scholar] [CrossRef]
- Bredberg, I.; Keeler, C.; Lysov, V.; Strominger, A. From navier-stokes to einstein. J. High Energy Phys. 2012, 146, 1–18. [Google Scholar] [CrossRef]



| Characteristic | Hard PZT Ceramic (DONGIL TECH. Co., Ltd.) | Soft PZT Ceramic (Piezo Technologies) |
|---|---|---|
| Outer Diameter (mm) | 49.90 | 49.96 |
| Inner Diameter (mm) | 39.90 | 39.82 |
| Length (mm) | 25.00 | 25.00 |
| Frequency Constant (m/s) | 2107.00 | 2035.61 |
| Resonant Frequency (kHz) | 421.40 | 401.50 |
| Resonant Resistance (Ω) | 1.30 | 6.09 |
| Electro-Mechanical Coupling Factor (Keff) | 0.310 | 0.262 |
| Capacitance (nF) | 8.90 | 11.72 |
| Impedance (Ω) | 1.81 | 16.08 |
| Dielectric Constant (ε33T/ε0) | 1439 | 1877 |
| *tan δ | 0.003 | 0.017 |
| Type | Resonant Frequency (kHz) | |
|---|---|---|
| without Housing | with Housing | |
| Hard PZT ceramic | 421.40 | 396.2 |
| Soft PZT ceramic | 401.50 | 396.6 |
| Type | Applied Frequency (kHz) | Applied Power (W) | Exposure Time (min) |
|---|---|---|---|
| Hard PZT transducer | 396.2 | 100 | 120 |
| Soft PZT transducer | 396.6 | 100 | 120 |
| Nanoparticle | Liquid | Suspension |
|---|---|---|
| TiO2 (Degussa (Evonik) P25) | Deionized water | TiO2 colloid Concentration: 1 wt.% Volume: 100 mL Pre-treatment: none pH: 4.9–5.2 |
| Mean diameter: 25 nm | Resistivity: 18.2 MΩ·cm | |
| Density: 3.78 g/cm3 | pH: 7.2–7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwangbo, S.A.; Choi, Y.M.; Lee, T.G. Influence of Piezoelectric Properties on the Ultrasonic Dispersion of TiO2 Nanoparticles in Aqueous Suspension. Micromachines 2021, 12, 52. https://doi.org/10.3390/mi12010052
Hwangbo SA, Choi YM, Lee TG. Influence of Piezoelectric Properties on the Ultrasonic Dispersion of TiO2 Nanoparticles in Aqueous Suspension. Micromachines. 2021; 12(1):52. https://doi.org/10.3390/mi12010052
Chicago/Turabian StyleHwangbo, Seon Ae, Young Min Choi, and Tae Geol Lee. 2021. "Influence of Piezoelectric Properties on the Ultrasonic Dispersion of TiO2 Nanoparticles in Aqueous Suspension" Micromachines 12, no. 1: 52. https://doi.org/10.3390/mi12010052
APA StyleHwangbo, S. A., Choi, Y. M., & Lee, T. G. (2021). Influence of Piezoelectric Properties on the Ultrasonic Dispersion of TiO2 Nanoparticles in Aqueous Suspension. Micromachines, 12(1), 52. https://doi.org/10.3390/mi12010052






