Design and Clinical Application of an Integrated Microfluidic Device for Circulating Tumor Cells Isolation and Single-Cell Analysis
Abstract
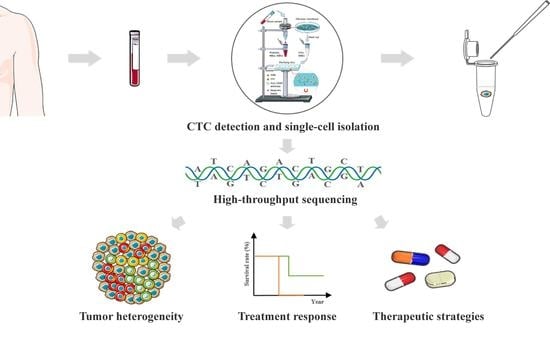
:1. Introduction
2. Materials and Methods
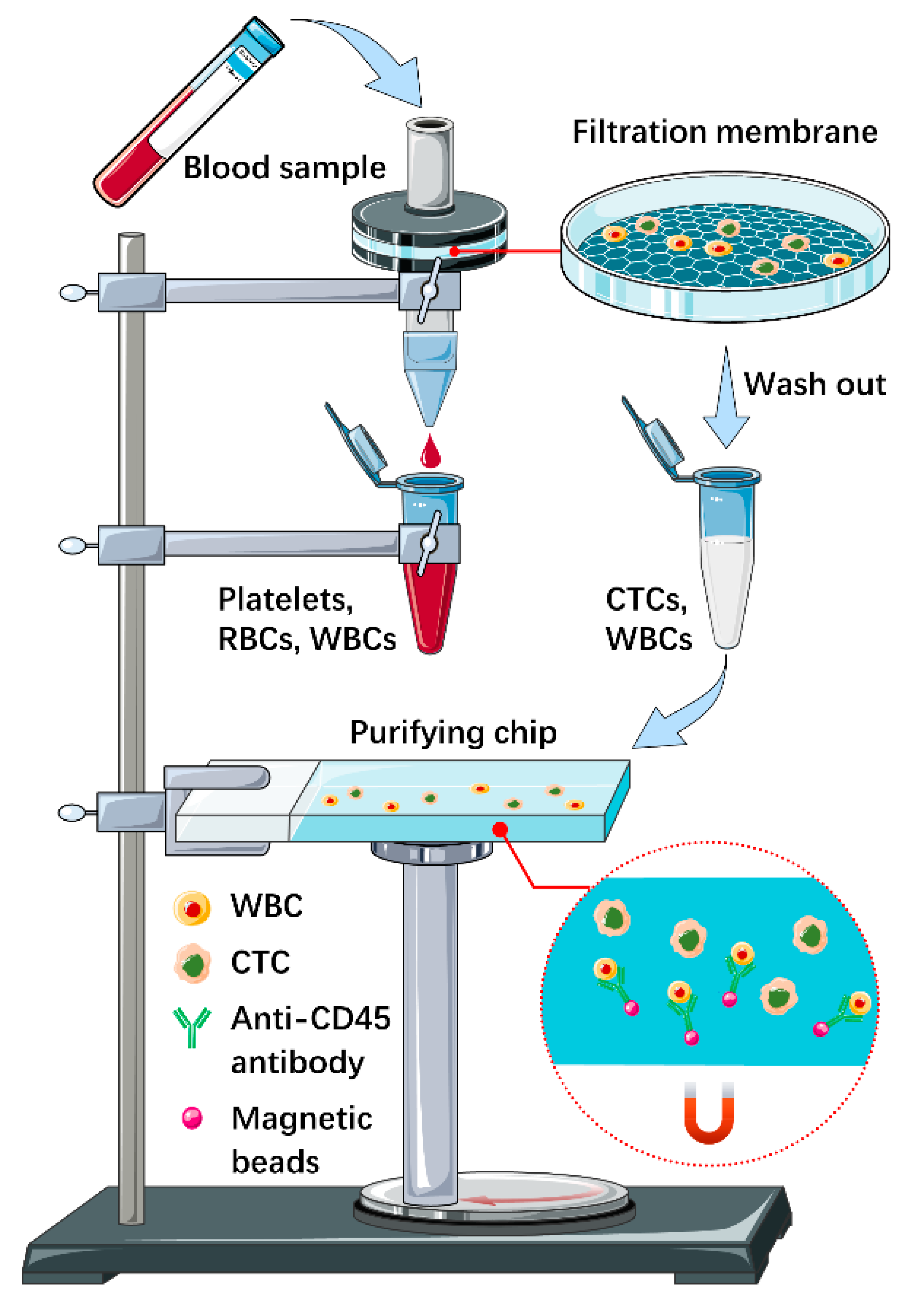
2.1. Microfluidic Chips Fabrication, Filtration Membranes Preparation and System Package
2.2. CTC Detection
2.3. Single-Cell Isolation
2.4. CTCs Identification
2.5. Cell Line Culture and Preparation
2.6. Human Peripheral Blood Samples
2.7. DNA Sequencing and Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
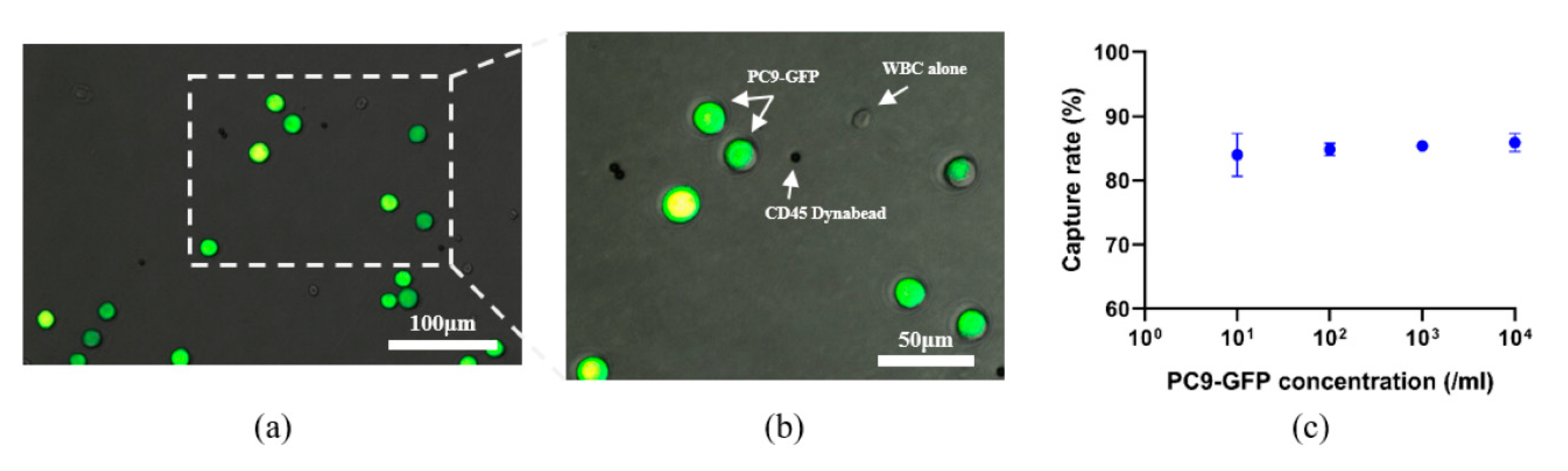
3.1. Design and Performance Verification of the Integrated Microfluidic Device
3.2. Detection of CTCs from Blood Samples of Lung Cancer Patients
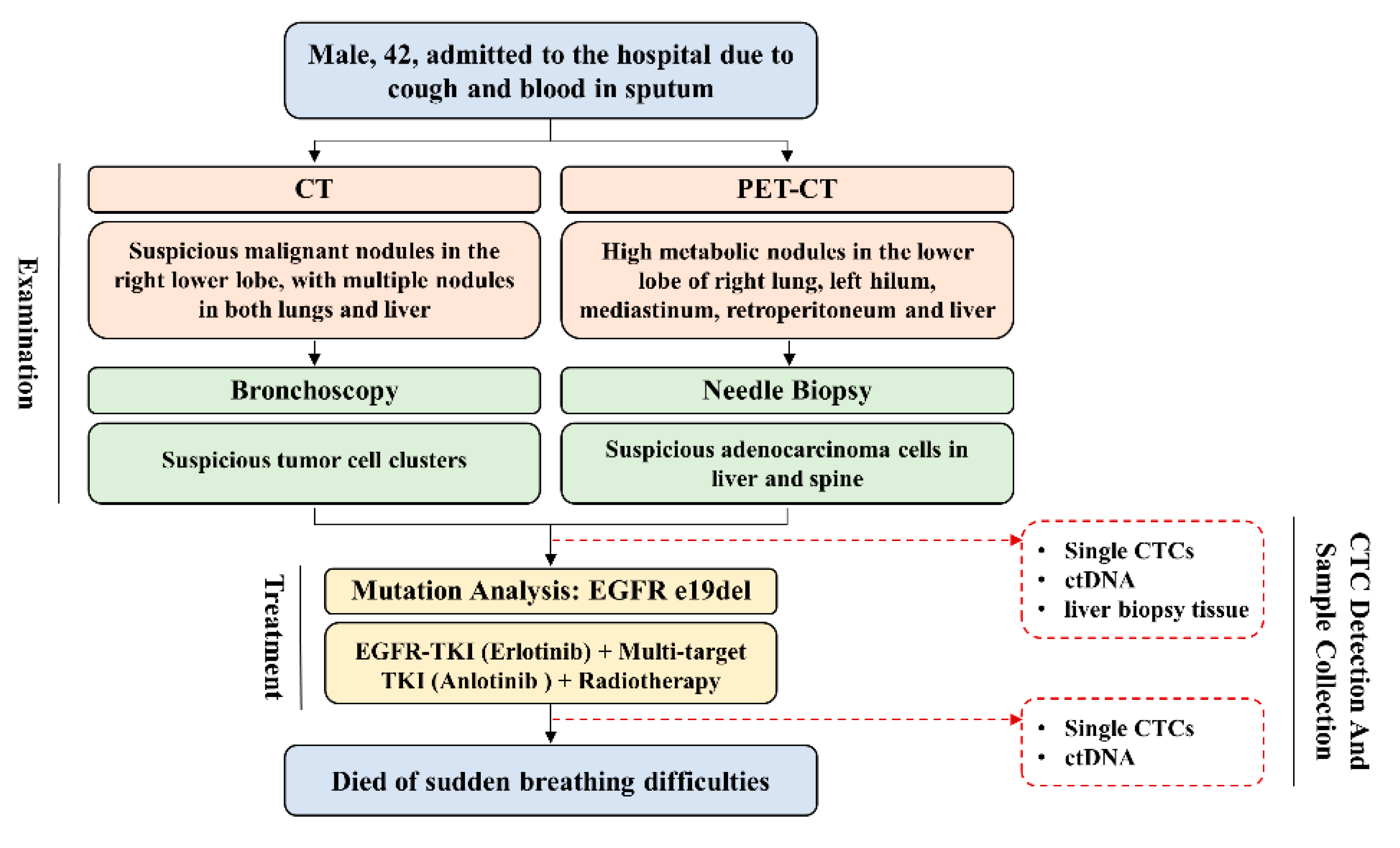
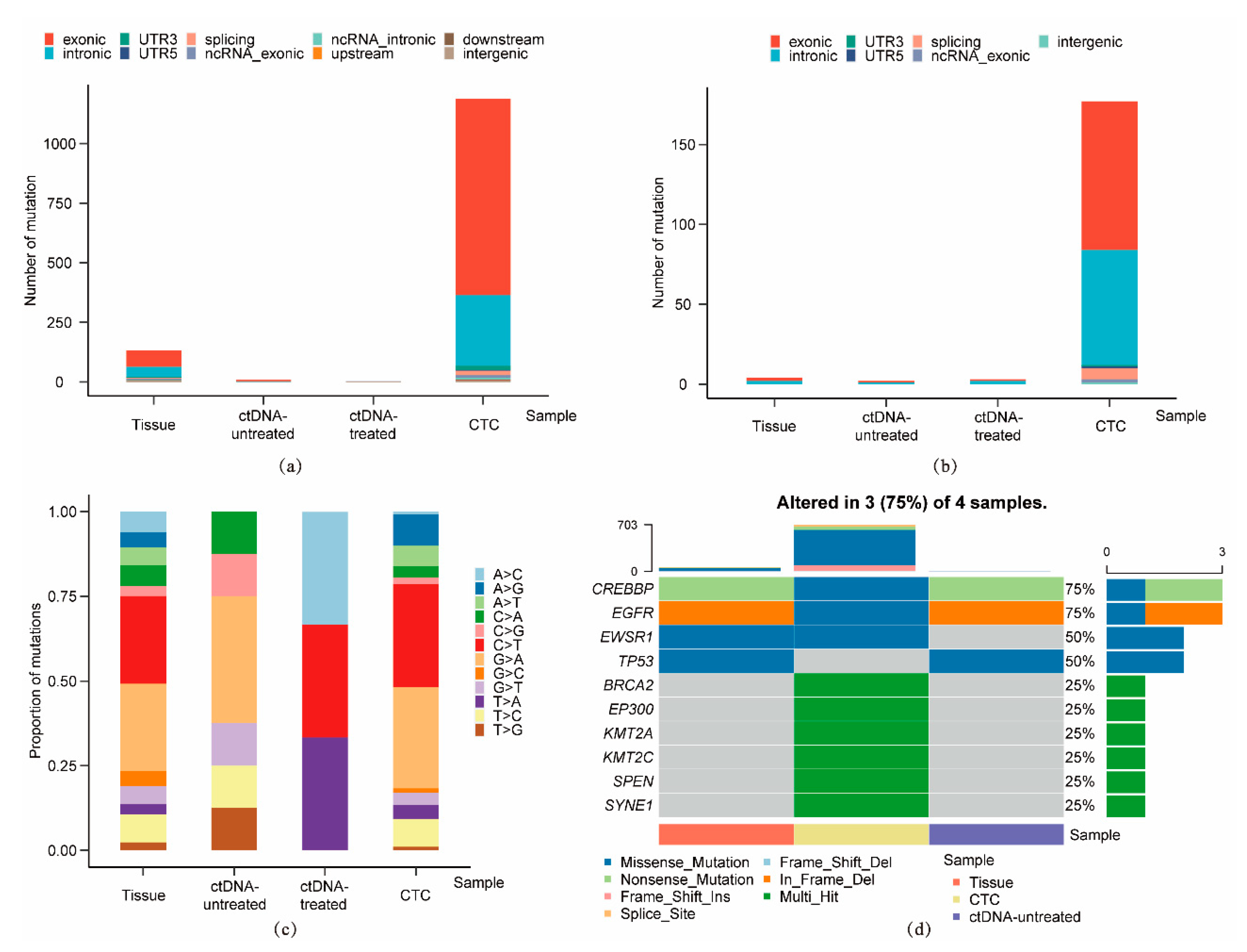
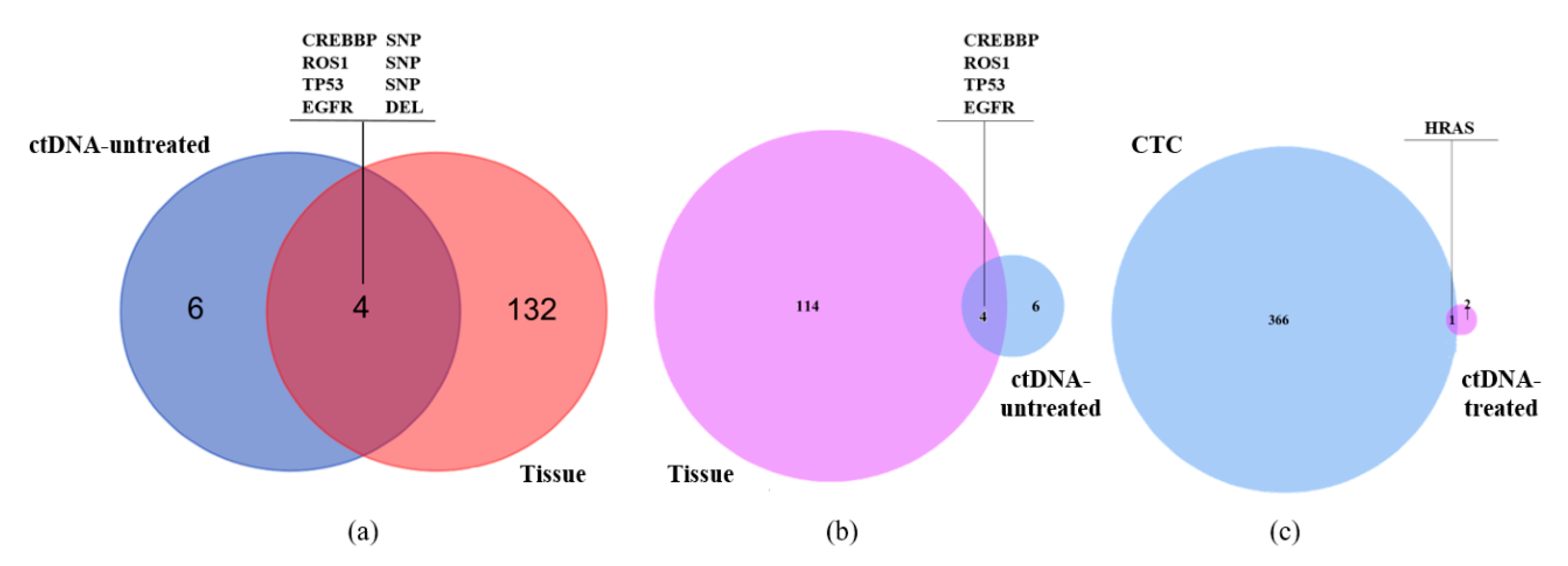
3.3. Single-Cell Analysis of CTC from a Representative Advanced Lung Cancer Patient
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [PubMed]
- Kilgour, E.; Rothwell, D.G.; Brady, G.; Dive, C. Liquid Biopsy-Based Biomarkers of Treatment Response and Resistance. Cancer Cell 2020, 37, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gossett, D.R.; Tse, H.T.; Lee, S.A.; Ying, Y.; Lindgren, A.G.; Yang, O.O.; Rao, J.; Clark, A.T.; Di Carlo, D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl. Acad. Sci. USA 2012, 109, 7630–7635. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, A.C.; Verstreken, C.M.; Fisher, C.L.; Keyser, U.F.; Pagliara, S.; Chalut, K.J. A microfluidic device for characterizing nuclear deformations. Lab Chip 2017, 17, 805–813. [Google Scholar] [CrossRef] [Green Version]
- Bamford, R.A.; Smith, A.; Metz, J.; Glover, G.; Titball, R.W.; Pagliara, S. Investigating the physiology of viable but non-culturable bacteria by microfluidics and time-lapse microscopy. BMC Biol. 2017, 15, 121. [Google Scholar] [CrossRef]
- Vergalli, J.; Dumont, E.; Pajović, J.; Cinquin, B.; Maigre, L.; Masi, M.; Réfrégiers, M.; Pagés, J.M. Spectrofluorimetric quantification of antibiotic drug concentration in bacterial cells for the characterization of translocation across bacterial membranes. Nat. Protoc. 2018, 13, 1348–1361. [Google Scholar] [CrossRef]
- Liu, A.L.; He, F.Y.; Wang, K.; Zhou, T.; Lu, Y.; Xia, X.H. Rapid method for design and fabrication of passive micromixers in microfluidic devices using a direct-printing process. Lab Chip 2005, 5, 974–978. [Google Scholar] [CrossRef]
- Cama, J.; Chimerel, C.; Pagliara, S.; Javer, A.; Keyser, U.F. A label-free microfluidic assay to quantitatively study antibiotic diffusion through lipid membranes. Lab Chip 2014, 14, 2303–2308. [Google Scholar] [CrossRef] [Green Version]
- Mathur, L.; Ballinger, M.; Utharala, R.; Merten, C.A. Microfluidics as an Enabling Technology for Personalized Cancer Therapy. Small 2020, 16, e1904321. [Google Scholar] [CrossRef]
- Lawson, D.A.; Kessenbrock, K.; Davis, R.T.; Pervolarakis, N.; Werb, Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 2018, 20, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, K.; Wang, F.; Jahn, K.; Hu, T.; Tanaka, T.; Sasaki, Y.; Kuipers, J.; Loghavi, S.; Wang, S.A.; Yan, Y.; et al. Clonal evolution of acute myeloid leukemia revealed by high-throughput single-cell genomics. Nat. Commun. 2020, 11, 5327. [Google Scholar] [CrossRef] [PubMed]
- McFarland, J.M.; Paolella, B.R.; Warren, A.; Geiger-Schuller, K.; Shibue, T.; Rothberg, M.; Kuksenko, O.; Colgan, W.N.; Jones, A.; Chambers, E.; et al. Multiplexed single-cell transcriptional response profiling to define cancer vulnerabilities and therapeutic mechanism of action. Nat. Commun. 2020, 11, 4296. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, C.; Soria, E.; Ramírez, N. The identification and isolation of CTCs: A biological Rubik’s cube. Crit. Rev. Oncol. Hematol. 2018, 126, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sa, S.; Wang, L.; Dulmage, K.; Bhagwat, N.; Yee, S.S.; Sen, M.; Pletcher, C.H., Jr.; Moore, J.S.; Saksena, S.; et al. An integrated enrichment system to facilitate isolation and molecular characterization of single cancer cells from whole blood. Cytom. A 2018, 93, 1226–1233. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, T.; Xu, M.; Zhang, W.; Xiong, Y.; Nie, L.; Wang, Q.; Li, H.; Wang, W. A high-throughput liquid biopsy for rapid rare cell separation from large-volume samples. Lab Chip 2018, 19, 68–78. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, H.; Shu, W.; Tian, J.; Huang, Y.; Song, Y.; Wang, R.; Li, E.; Slamon, D.; Hou, D.; et al. An integrated microfluidic device for rapid and high-sensitivity analysis of circulating tumor cells. Sci. Rep. 2017, 7, 42612. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Zhao, H.; Chen, J.; Liu, W.; Li, E.; Wang, Q.; Zhang, L. An Integrated Microfluidic Chip and Its Clinical Application for Circulating Tumor Cell Isolation and Single-Cell Analysis. Cytom. A 2020, 97, 46–53. [Google Scholar] [CrossRef]
- Hao, S.J.; Wan, Y.; Xia, Y.Q.; Zou, X.; Zheng, S.Y. Size-based separation methods of circulating tumor cells. Adv. Drug Deliv. Rev. 2018, 125, 3–20. [Google Scholar] [CrossRef]
- Dianat-Moghadam, H.; Azizi, M.; Eslami-S, Z.; Cortés-Hernández, L.E.; Heidarifard, M.; Nouri, M.; Alix-Panabières, C. The Role of Circulating Tumor Cells in the Metastatic Cascade: Biology, Technical Challenges, and Clinical Relevance. Cancers (Basel) 2020, 12, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franses, J.W.; Philipp, J.; Missios, P.; Bhan, I.; Liu, A.; Yashaswini, C.; Tai, E.; Zhu, H.; Ligorio, M.; Nicholson, B.; et al. Pancreatic circulating tumor cell profiling identifies LIN28B as a metastasis driver and drug target. Nat. Commun. 2020, 11, 3303. [Google Scholar] [CrossRef] [PubMed]
- Klotz, R.; Thomas, A.; Teng, T.; Han, S.M.; Iriondo, O.; Li, L.; Restrepo-Vassalli, S.; Wang, A.; Izadian, N.; MacKay, M.; et al. Circulating Tumor Cells Exhibit Metastatic Tropism and Reveal Brain Metastasis Drivers. Cancer Discov. 2020, 10, 86–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, X.; Zhang, H.; Zhu, Y.; Liang, Y.; Yuan, Z.; Li, J.; Li, J.; Li, X.; Jia, Y.; He, T.; et al. Circulating tumor cells in cancer patients: Developments and clinical applications for immunotherapy. Mol. Cancer 2020, 19, 15. [Google Scholar] [CrossRef] [Green Version]
- Pagliara, S.; Franze, K.; McClain, C.R.; Wylde, G.; Fisher, C.L.; Franklin, R.J.M.; Kabla, A.J.; Keyser, U.F.; Chalut, K.J. Auxetic nuclei in embryonic stem cells exiting pluripotency. Nat. Mater. 2014, 13, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Cama, J.; Voliotis, M.; Metz, J.; Smith, A.; Iannucci, J.; Keyser, U.F.; Tsaneva-Atanasova, K.; Pagliara, S. Single-cell microfluidics facilitates the rapid quantification of antibiotic accumulation in Gram-negative bacteria. Lab Chip 2020, 20, 2765–2775. [Google Scholar] [CrossRef]
- Wang, P.; Robert, L.; Pelletier, J.; Dang, W.L.; Taddei, F.; Wright, A.; Jun, S. Robust growth of Escherichia coli. Curr. Biol. 2010, 20, 1099–1103. [Google Scholar] [CrossRef] [Green Version]
- Łapińska, U.; Glover, G.; Capilla-Lasheras, P.; Young, A.J.; Pagliara, S. Bacterial ageing in the absence of external stressors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180442. [Google Scholar] [CrossRef]
- Brisotto, G.; Biscontin, E.; Rossi, E.; Bulfoni, M.; Piruska, A.; Spazzapan, S.; Poggiana, C.; Vidotto, R.; Steffan, A.; Colombatti, A.; et al. Dysmetabolic Circulating Tumor Cells Are Prognostic in Metastatic Breast Cancer. Cancers (Basel). 2020, 12, 1005. [Google Scholar] [CrossRef] [Green Version]
- Poudineh, M.; Aldridge, P.M.; Ahmed, S.; Green, B.J.; Kermanshah, L.; Nguyen, V.; Tu, C.; Mohamadi, R.M.; Nam, R.K.; Hansen, A.; et al. Tracking the dynamics of circulating tumour cell phenotypes using nanoparticle-mediated magnetic ranking. Nat. Nanotechnol. 2017, 12, 274–281. [Google Scholar] [CrossRef]
- Sinkala, E.; Sollier-Christen, E.; Renier, C.; Rosàs-Canyelles, E.; Che, J.; Heirich, K.; Duncombe, T.A.; Vlassakis, J.; Yamauchi, K.A.; Huang, H.; et al. Profiling protein expression in circulating tumour cells using microfluidic western blotting. Nat. Commun. 2017, 8, 14622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
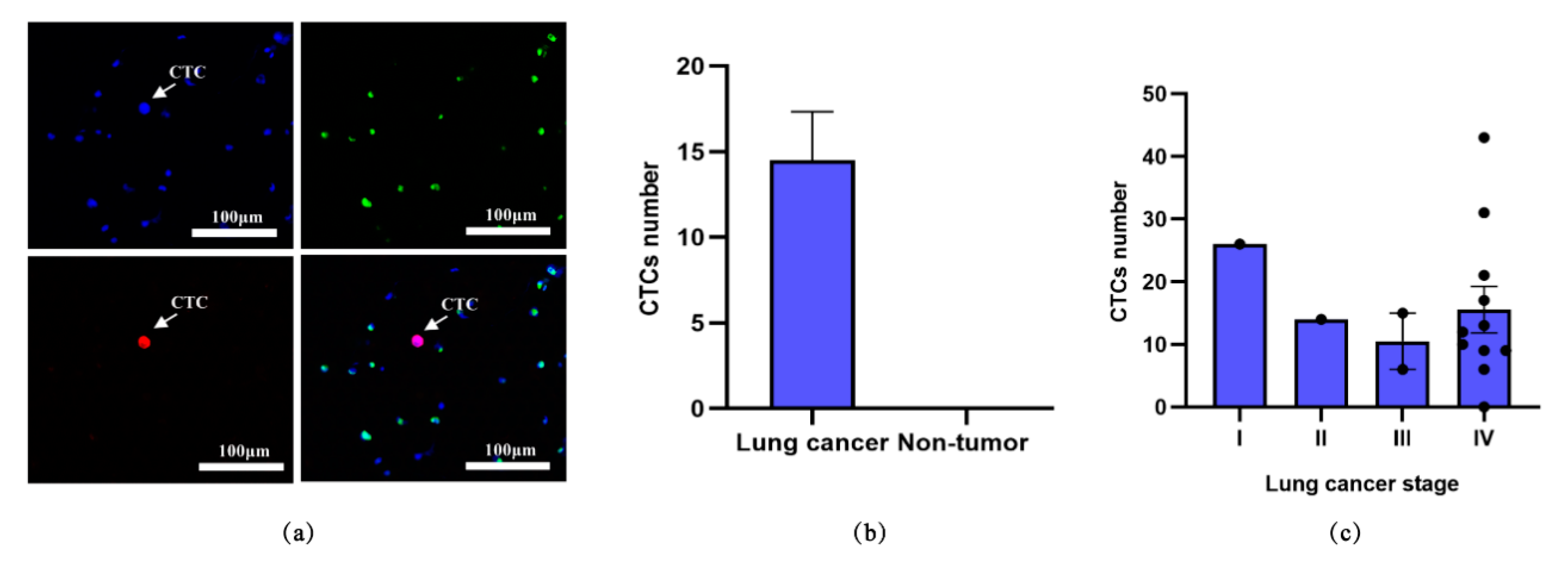
| No. | Gender | Diagnosis | Stage | Histological Type | CTC Count (N) |
|---|---|---|---|---|---|
| 1 | Male | Lung cancer | IV | Adenocarcinoma | 43 |
| 2 | Female | Lung cancer | IV | Adenocarcinoma | 21 |
| 3 | Male | Lung cancer | IV | Small cell carcinoma | 6 |
| 4 | Male | Lung cancer | IV | Large cell carcinoma | 10 |
| 5 | Female | Lung cancer | IV | Adenocarcinoma | 31 |
| 6 | Male | Lung cancer | IV | Adenocarcinoma | 9 |
| 7 | Male | Lung cancer | IV | Adenocarcinoma | 0 |
| 8 | Female | Lung cancer | IIIC | Adenocarcinoma | 15 |
| 9 | Female | Lung cancer | IV | Adenocarcinoma | 13 |
| 10 | Male | Lung cancer | Postoperative | Adenocarcinoma | 0 |
| 11 | Male | Lung cancer | IIA | Adenocarcinoma | 14 |
| 12 | Female | Lung cancer | IV | Adenocarcinoma | 9 |
| 13 | Female | Lung cancer | IV | Adenocarcinoma | 12 |
| 14 | Male | Lung cancer | IV | Squamous cell carcinoma | 17 |
| 15 | Female | Lung cancer | IIIB | Adenocarcinoma | 6 |
| 16 | Male | Lung cancer | IA | Adenocarcinoma | 26 |
| 17 | Male | Pneumonia | — 2 | — 2 | 0 |
| 18 | Male | COPD 1 | — 2 | — 2 | 0 |
| 19 | Male | COPD 1 | — 2 | — 2 | 0 |
| 20 | Male | COPD 1 | — 2 | — 2 | 0 |
| Sample ID | Sample Type | Sequencing Technique | Number of SNVs | Number of InDels |
|---|---|---|---|---|
| S145 | Liver biopsy tissue | Whole exome sequencing | 132 | 4 |
| S124 | ctDNA before treatment | HapOnco-605 gene panel | 8 | 2 |
| S090 | 3 single CTCs before treatment | HapOnco-605 gene panel | – | – |
| S063 | ctDNA after treatment | HapOnco-605 gene panel | 3 | 3 |
| S094 | 1 single CTC after treatment | HapOnco-605 gene panel | 1187 | 177 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liu, W.; Zou, K.; Wei, S.; Zhang, X.; Li, E.; Wang, Q. Design and Clinical Application of an Integrated Microfluidic Device for Circulating Tumor Cells Isolation and Single-Cell Analysis. Micromachines 2021, 12, 49. https://doi.org/10.3390/mi12010049
Xu M, Liu W, Zou K, Wei S, Zhang X, Li E, Wang Q. Design and Clinical Application of an Integrated Microfluidic Device for Circulating Tumor Cells Isolation and Single-Cell Analysis. Micromachines. 2021; 12(1):49. https://doi.org/10.3390/mi12010049
Chicago/Turabian StyleXu, Mingxin, Wenwen Liu, Kun Zou, Song Wei, Xinri Zhang, Encheng Li, and Qi Wang. 2021. "Design and Clinical Application of an Integrated Microfluidic Device for Circulating Tumor Cells Isolation and Single-Cell Analysis" Micromachines 12, no. 1: 49. https://doi.org/10.3390/mi12010049
APA StyleXu, M., Liu, W., Zou, K., Wei, S., Zhang, X., Li, E., & Wang, Q. (2021). Design and Clinical Application of an Integrated Microfluidic Device for Circulating Tumor Cells Isolation and Single-Cell Analysis. Micromachines, 12(1), 49. https://doi.org/10.3390/mi12010049