Preliminary Study on Fluidized Bed Chemical Mechanical Polishing (FB-CMP) Process for Stainless Steel 304 (SS304)
Abstract
1. Introduction
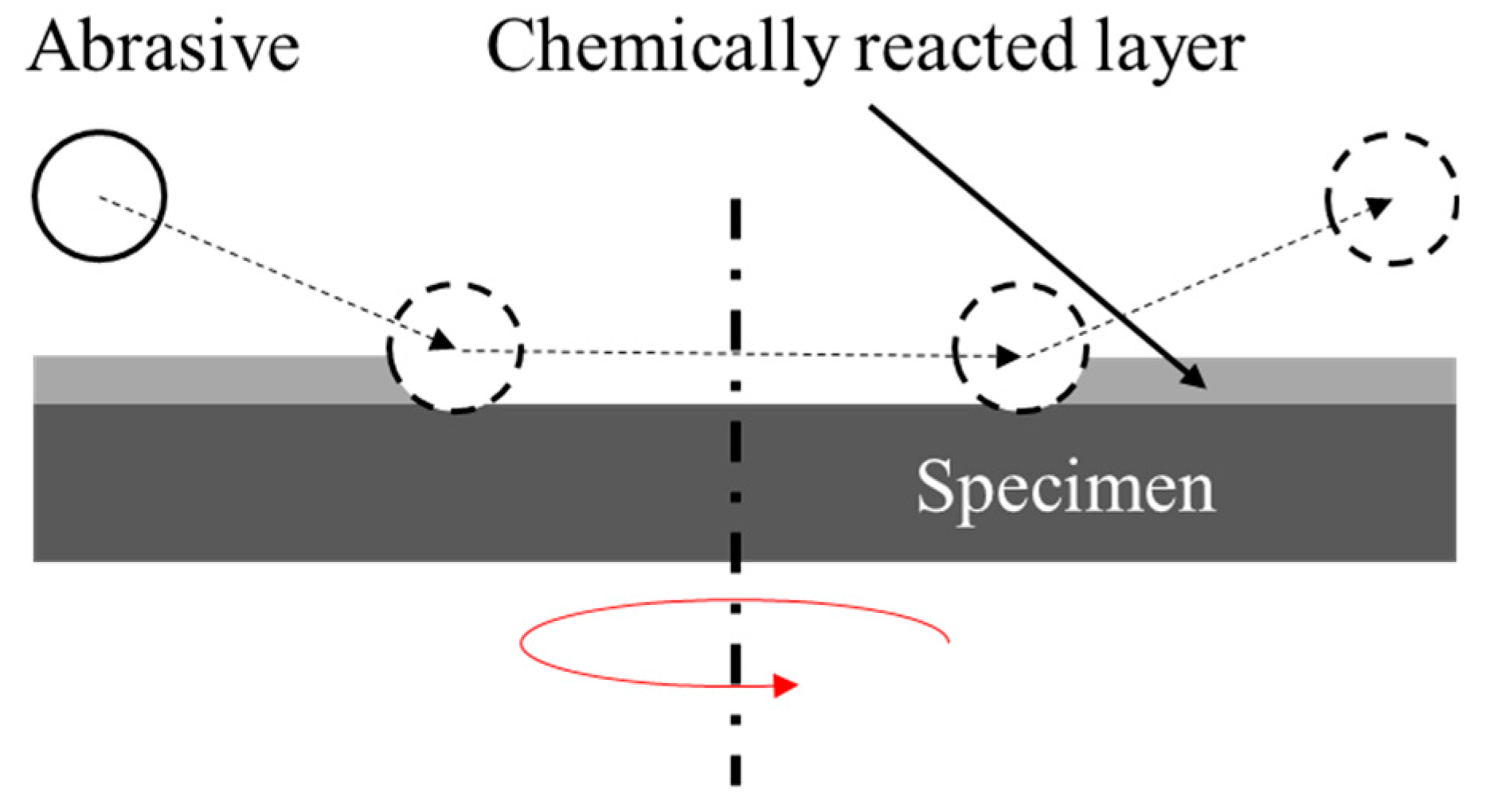
2. Fluidized Bed Chemical Mechanical Polishing
- The surface chemical reaction between the chemical solution and specimen creates a chemically reacted layer that facilitates material removal on the surface of the specimen;
- The chemically reacted layer on the surface of the specimen is removed by the shear force of the rotating specimen and fluidized abrasive particles;
- The newly revealed surface of the specimen is repeatedly removed via the process from 1 to 2.
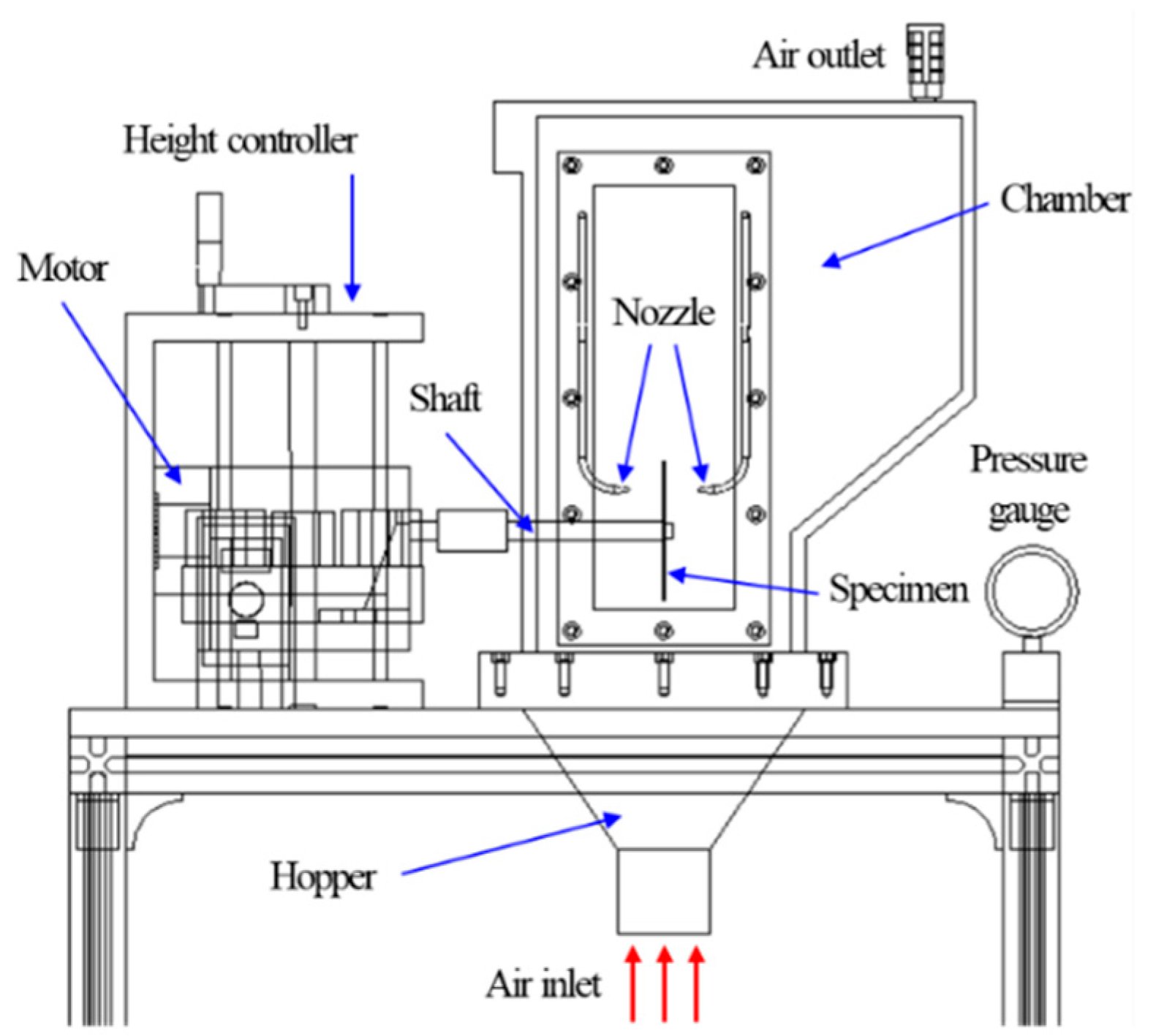
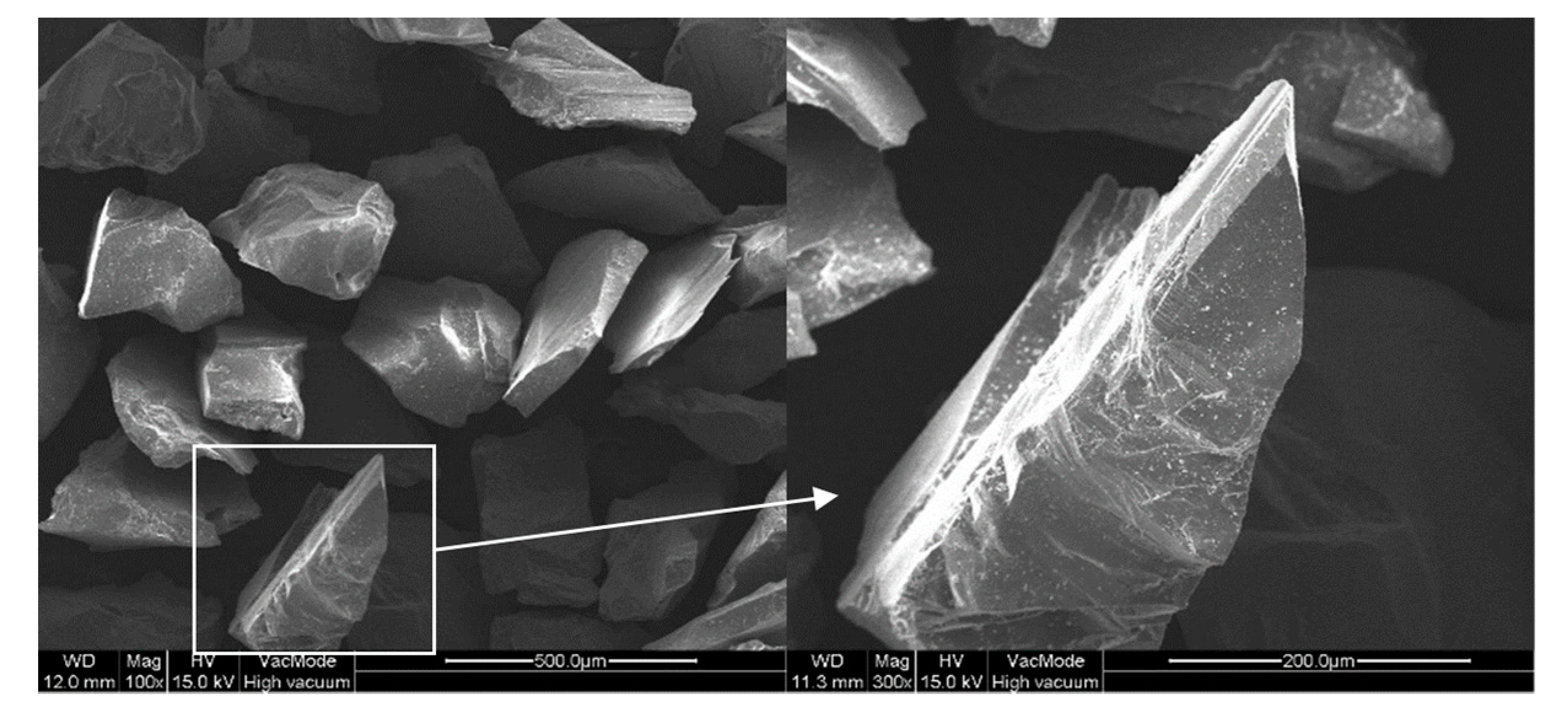
3. Experimental
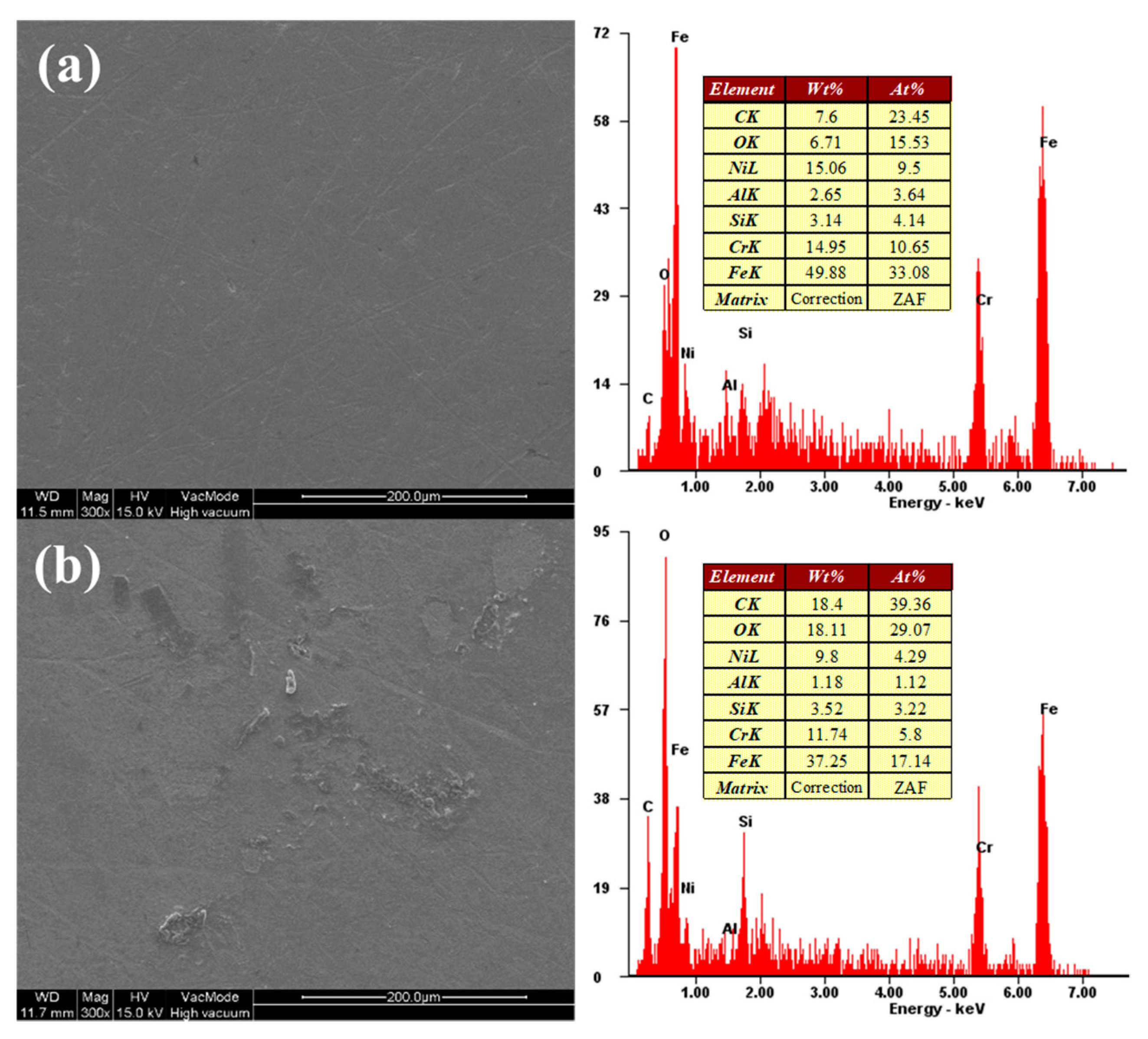
4. Results and Discussion
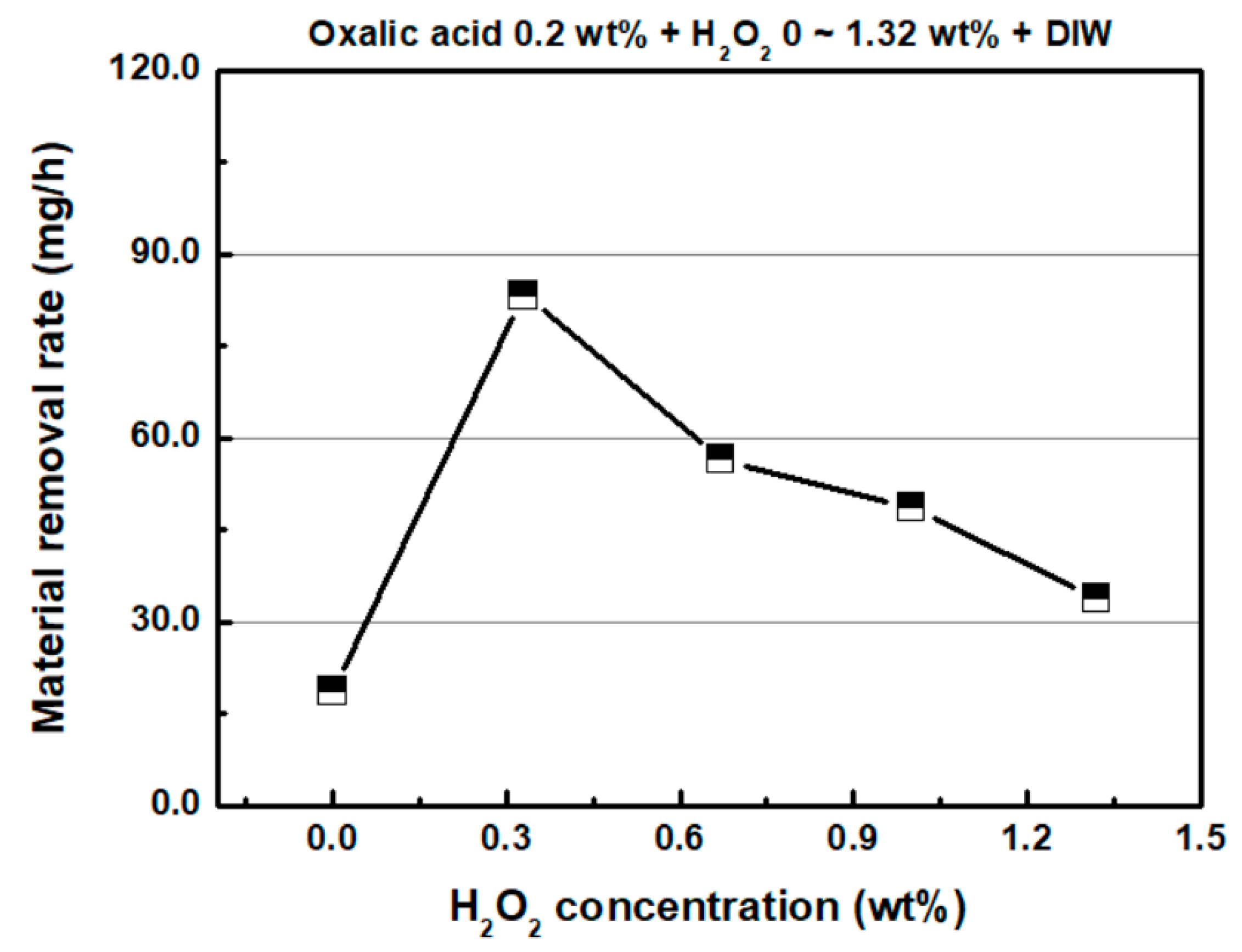
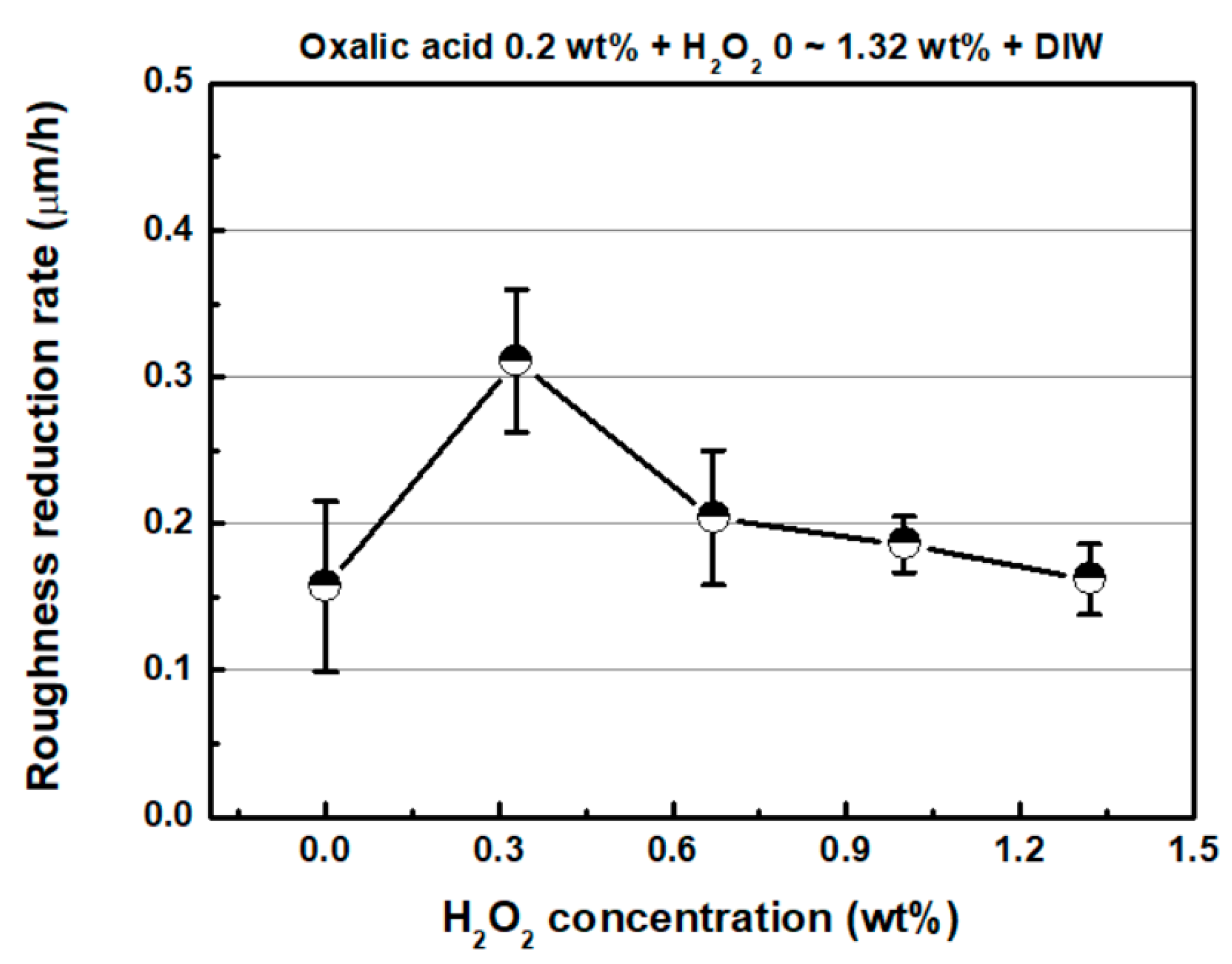
4.1. Effect of Hydrogen Peroxide (H2O2)
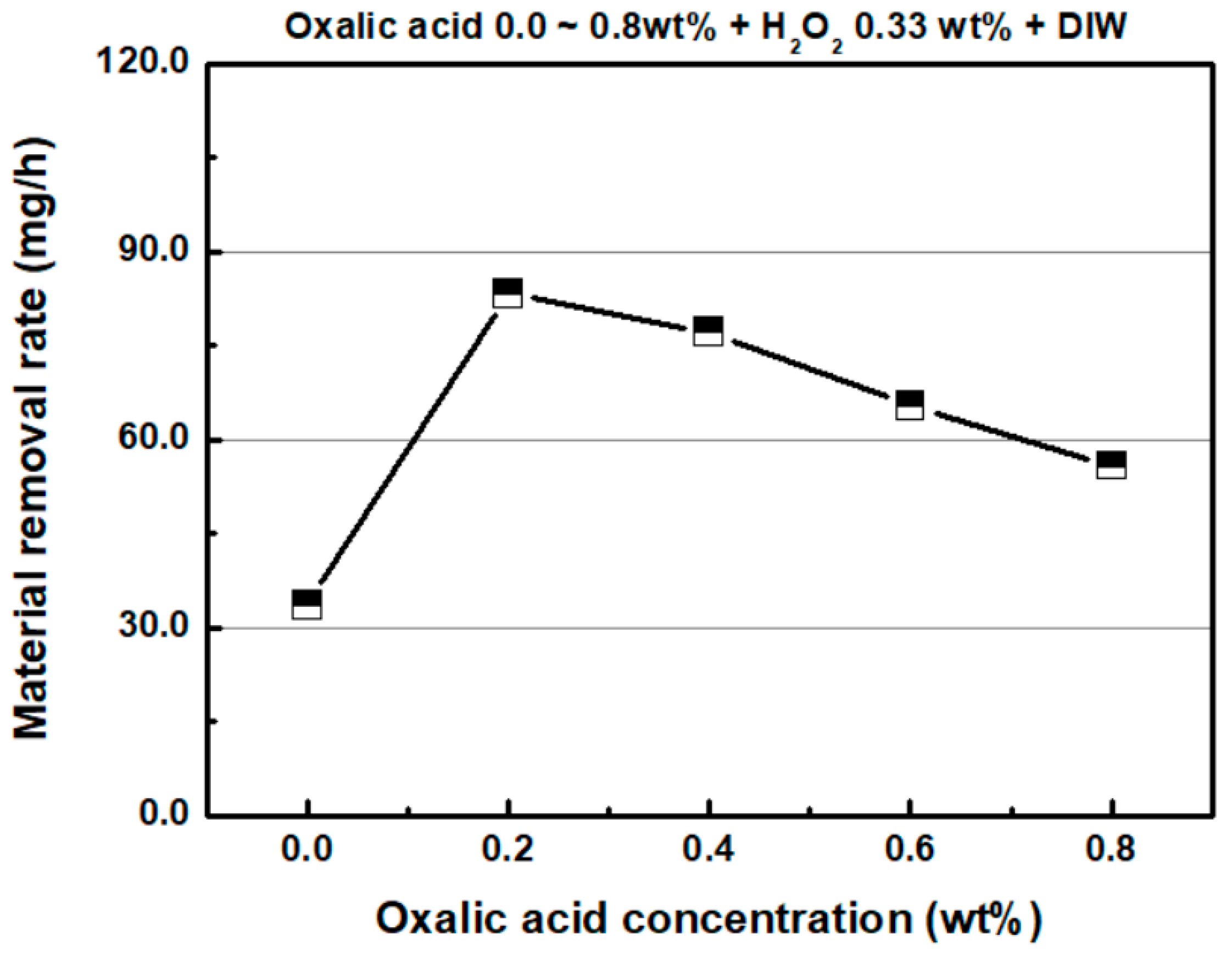
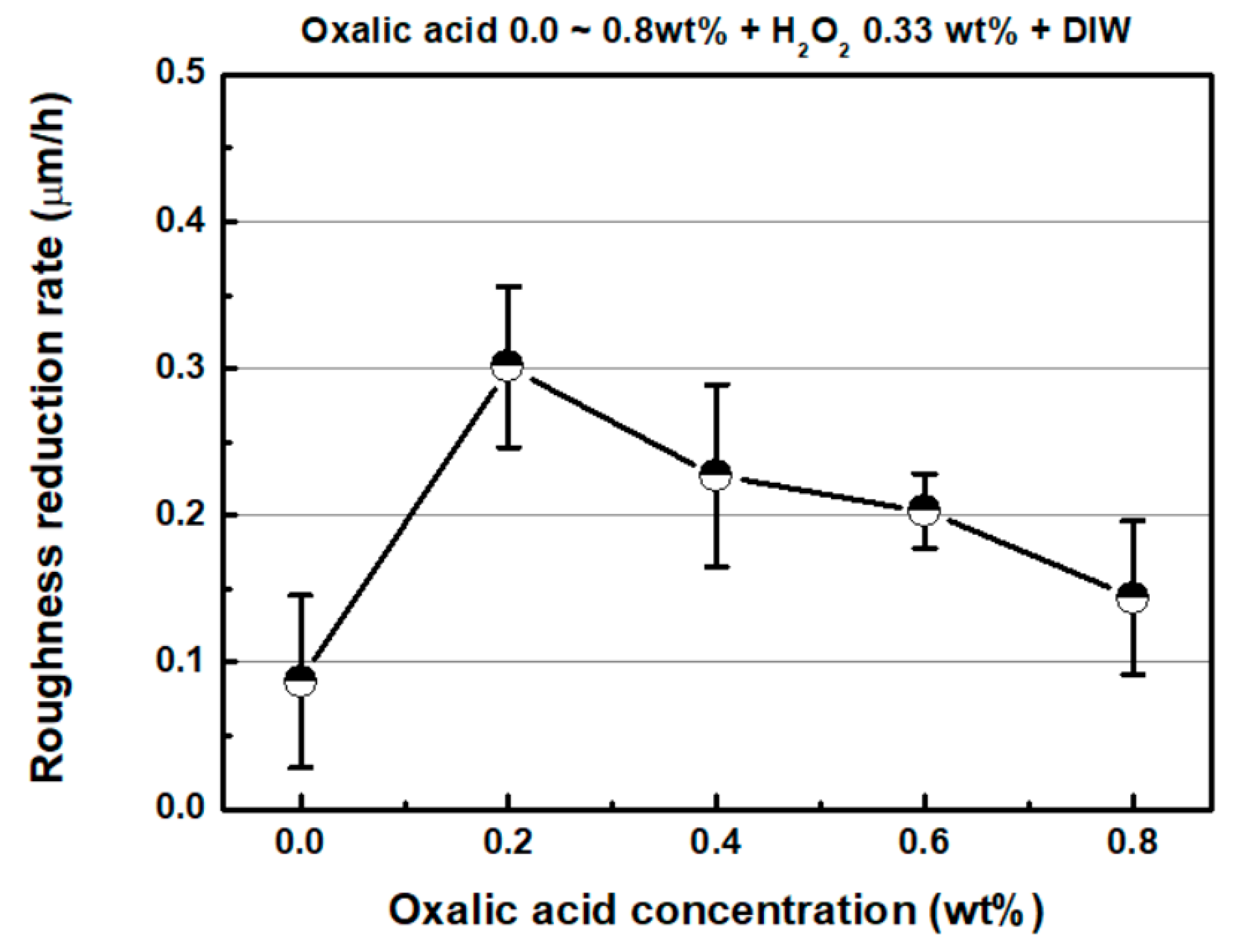
4.2. Effect of Oxalic Acid
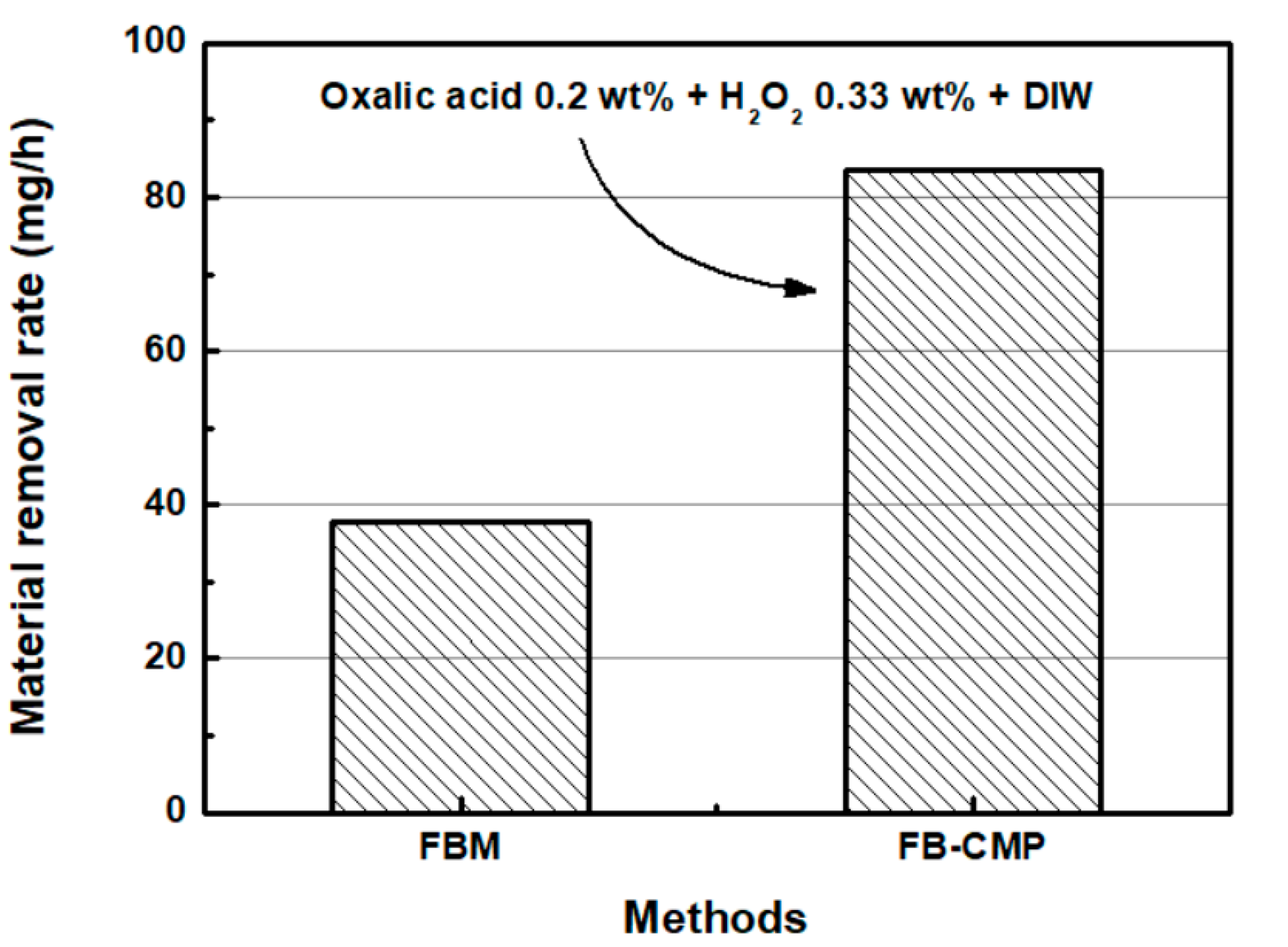
4.3. Comparison of FBM and FB-CMP
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barletta, M. Progress in abrasive fluidized bed machining. J. Mater. Proc. Technol. 2009, 209, 6087–6102. [Google Scholar] [CrossRef]
- Massarsky, M.; Davidson, D.A. Turbo-Abrasive Machining and Finishing. Met. Finish. 1997, 95, 29–31. [Google Scholar] [CrossRef]
- Barletta, M.; Gisario, A.; Venettacci, S.; Bubino, G. A comparative evaluation of fluidized bed assisted drag finishing and centrifugal disk dry finishing. Eng. Sci. Technol. Int. J. 2014, 17, 63–72. [Google Scholar] [CrossRef][Green Version]
- Barletta, M. A new technology in surface finishing: Fluidized bed machining (FBM) of aluminium alloys. J. Mater. Proc. Technol. 2006, 173, 157–165. [Google Scholar] [CrossRef]
- Francis, N.K.; Viswanadhan, K.G.; Paulose, M.M. Swirling Abrasive Fluidized Bed Machining: Effect of Process Parameters on Machining Performance. Mater. Manufact. Proc. 2015, 30, 852–857. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.; Hwang, H.; Seo, J.; Lee, D.; Lee, H. Effect of Rotating Speed and Air Flow Rate on Material Removal Characteristics in Abrasive Fluidized Bed Machining of Polyacetal. J. Korean Soc. Tribol. Lubr. Eng. 2017, 33, 214–219. [Google Scholar]
- Kim, T.; Lee, H. Simulation and experimental analysis of abrasive fluidized bed machining process. J. Mech. Sci. Technol. 2020, 34, 2153–2160. [Google Scholar] [CrossRef]
- Lee, H. Tribology Research Trends in Chemical Mechanical Polishing (CMP) Process. Tribol. Lubr. 2018, 34, 115–122. [Google Scholar]
- Lee, H.; Sung, I.-H. Chemical Mechanical Polishing: A Selective Review of R&D Trends in abrasive Particle Behaviors and Wafer Materials. Tribol. Lubr. 2019, 35, 274–285. [Google Scholar]
- Lee, D.; Lee, H.; Jeong, H. Slurry components in metal mechanical planarization (CMP) process: A review. Int. J. Precis. Eng. Manufact. 2016, 17, 1751–1762. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, H.D. Chemical and mechanical balance in polishing of electronic materials for defect-free surfaces. CIRP-Ann. 2009, 58, 485–490. [Google Scholar] [CrossRef]
- Lee, H.; Park, Y.; Lee, S.; Jeong, H. Effect of wafer size on material removal rate and its distribution in chemical mechanical polishing of silicon dioxide film. J. Mech. Sci. Technol. 2013, 27, 2911–2916. [Google Scholar] [CrossRef]
- Ein-Eli, Y.; Starosvetsky, D. Review on copper chemical-mechanical polishing (CMP) and post-CMP cleaning in ultra large system integrated (ULSI)-An electrochemical perspective. Electrochim. Acta 2007, 52, 1825–1838. [Google Scholar] [CrossRef]
- Krishnan, M.; Nalaskowski, J.W.; Cook, L.M. Chemical Mechanical Planarization: Slurry Chemistry, Materials, and Mechanisms. Chem. Rev. 2010, 110, 178–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, H. Estimating the mechanical properties of polyurethane-impregnated felt pads. J. Mech. Sci. Technol. 2017, 31, 5705–5710. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Jeong, H. Mechanical Aspect of the Chemical Mechanical Polishing Process: A Review. Int. J. Precis. Eng. Manufact. 2016, 17, 525–536. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S. Investigation of pad wear in CMP with swing-arm conditioning and uniformity of material removal. Precis. Eng. 2017, 49, 85–91. [Google Scholar] [CrossRef]
- Zhao, G.; Wei, Z.; Wang, W.; Feng, D.; Xu, A.; Liu, W.; Song, Z. Review on modeling and application of chemical mechanical polishing. Nanotechnol. Rev. 2020, 9, 182–189. [Google Scholar] [CrossRef]
- Terrell, E.J.; Higgs III, C.F. Hydrodynamics of Slurry Flow in Chemical Mechanical Polishing: A Review. J. Electrochem. Soc. 2006, 153, K15–K22. [Google Scholar] [CrossRef]
- Hu, X.; Song, Z.; Liu, W.; Qin, F.; Zhang, Z.; Wang, H. Chemical mechanical polishing of stainless steel foil as flexible substrate. Appl. Surf. Sci. 2012, 258, 5798–5802. [Google Scholar] [CrossRef]
- Jiang, L.; He, Y.; Yang, Y.; Luo, J. Chemical Mechanical Polishing of Stainless Steel as Solar Cell Substrate. ECS J. Solid State Sci. Technol. 2015, 4, P162–P170. [Google Scholar] [CrossRef]
- Jiang, L.; He, Y.; Luo, J. Chemical mechanical polishing of steel substrate using colloidal silica-based slurries. Appl. Surf. Sci. 2015, 330, 487–495. [Google Scholar] [CrossRef]
- Chen, J.; Yao, J.; Ma, L.; Fu, S.; Su, J. Study of the slurry in CMP 304 Ultra-Thin Stainless Steel Surface. Adv. Mater. Res. 2014, 1027, 235–239. [Google Scholar] [CrossRef]
- Cheng, H.; Ma, L.; Yao, J.; Su, J. Study on Process Parameters in CMP Ultra-Thin Stainless Steel Sheet. Adv. Mater. Res. 2014, 1027, 58–62. [Google Scholar] [CrossRef]
- Lee, D.; Kim, H.; Park, B.; Kim, D.; Jeong, H.; Lee, H. Electrochemical Analysis of the Slurry Composition for Chemical Mechanical Polishing of Flexible Stainless-Steel Substrates. J. Fric. Wear 2017, 38, 482–489. [Google Scholar] [CrossRef]
- Pohjanne, P.; Vepsäläinen, M.; Saario, T.; Sipilä, K.; Romu, J.; Saukkonen, T.; Hänninen, H.; Heikkilä, M.; Koskiniemi, J.; Berg, C.-G. Effect of electro-chemical potential on stress corrosion cracking susceptibility of EN 1.4301(AISI 304) austenitic stainless steels in simulated hot black liquor. Corrosion 2015, 71, 887–894. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.; Xie, X.; Hu, W. A material removal rate model-based chemical action of ultra-thin SUS304 substrate in chemical mechanical polishing. Int. J. Adv. Manuf. Technol. 2016, 85, 287–290. [Google Scholar] [CrossRef]
- Baah, C.A.; Baah, J.I. Inhibition effect of oxalic acid on copper corrosion. Anti-Corros. Meth. Mater. 2000, 47, 105–108. [Google Scholar] [CrossRef]
- Gorantla, V.R.K.; Babel, A.; Pandija, S.; Babu, S.V. Oxalic Acid as a Complexing Agent in CMP Slurries for Copper. Electrochem. Solid-State Lett. 2005, 8, G131–G134. [Google Scholar] [CrossRef]
- Giacomelli, G.; Giacomelli, F.C.; Baptista, J.A.A.; Spinelli, A. The effect of oxalic acid on the corrosion of carbon steel. Anti-Corros. Meth. Mater. 2004, 51, 105–111. [Google Scholar] [CrossRef]







| Parameters | Condition | |
|---|---|---|
| Specimen | Material | Stainless steel (SS304) |
| Diameter (mm) | 100 | |
| Thickness (mm) | 0.5 | |
| Abrasive | Material | Alumina (Al2O3) |
| Diameter (μm) | 250 | |
| Air pressure (kPa) | 40 | |
| Shaft rotation speed (rpm) | 1600 | |
| Chemical solution | Base chemical | DIW |
| Oxidizer | Hydrogen peroxide (0.0–1.32 wt %) | |
| Complexing agent | Oxalic acid (0.0–0.8 wt %) | |
| Flow rate (mL/min) | 60 | |
| Injection interval (min) | 10 (for 5 s) | |
| Processing time (min) | 60 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Lee, H. Preliminary Study on Fluidized Bed Chemical Mechanical Polishing (FB-CMP) Process for Stainless Steel 304 (SS304). Micromachines 2020, 11, 705. https://doi.org/10.3390/mi11070705
Kim T, Lee H. Preliminary Study on Fluidized Bed Chemical Mechanical Polishing (FB-CMP) Process for Stainless Steel 304 (SS304). Micromachines. 2020; 11(7):705. https://doi.org/10.3390/mi11070705
Chicago/Turabian StyleKim, Taekyoung, and Hyunseop Lee. 2020. "Preliminary Study on Fluidized Bed Chemical Mechanical Polishing (FB-CMP) Process for Stainless Steel 304 (SS304)" Micromachines 11, no. 7: 705. https://doi.org/10.3390/mi11070705
APA StyleKim, T., & Lee, H. (2020). Preliminary Study on Fluidized Bed Chemical Mechanical Polishing (FB-CMP) Process for Stainless Steel 304 (SS304). Micromachines, 11(7), 705. https://doi.org/10.3390/mi11070705






