Development of Artificial Cell Models Using Microfluidic Technology and Synthetic Biology
Abstract
1. Introduction
2. Conventional Methods of Giant Lipid Vesicle Formation
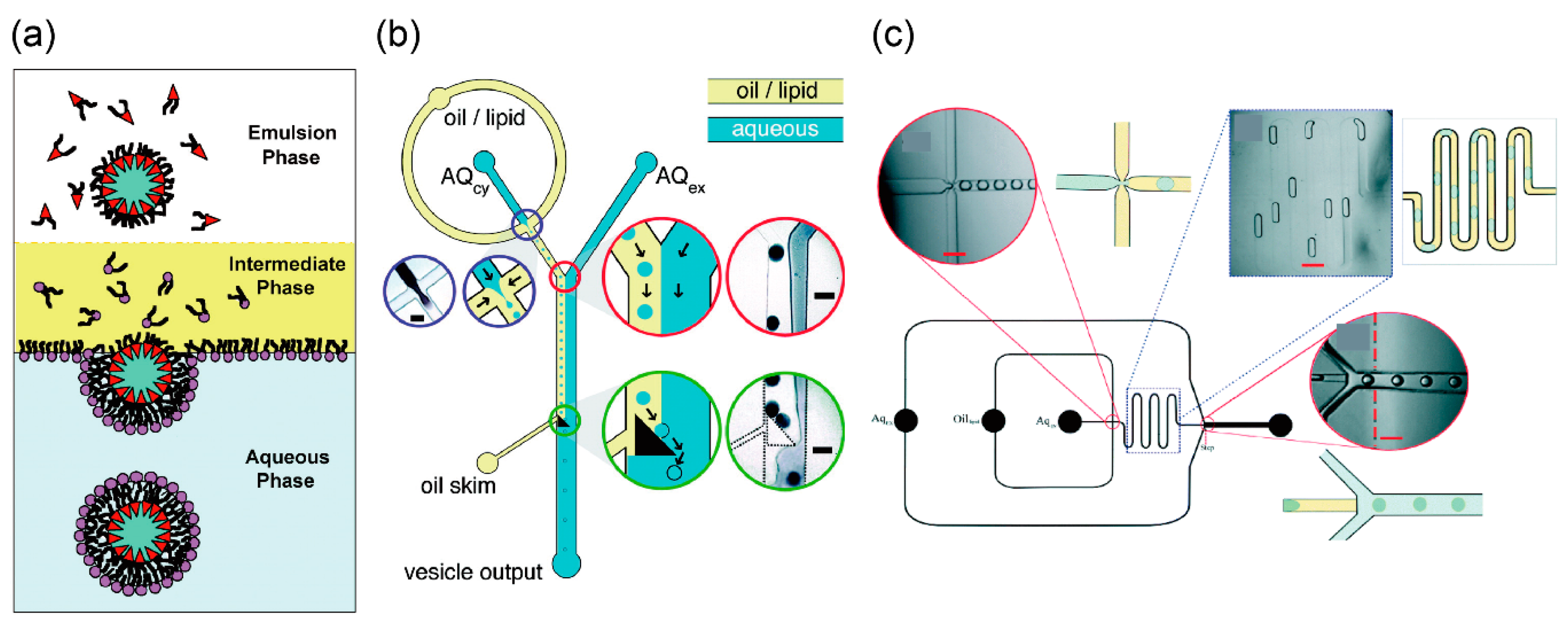
3. Formation of Giant Lipid Vesicles Using Microfluidic Technologies
3.1. Droplet Transfer Method
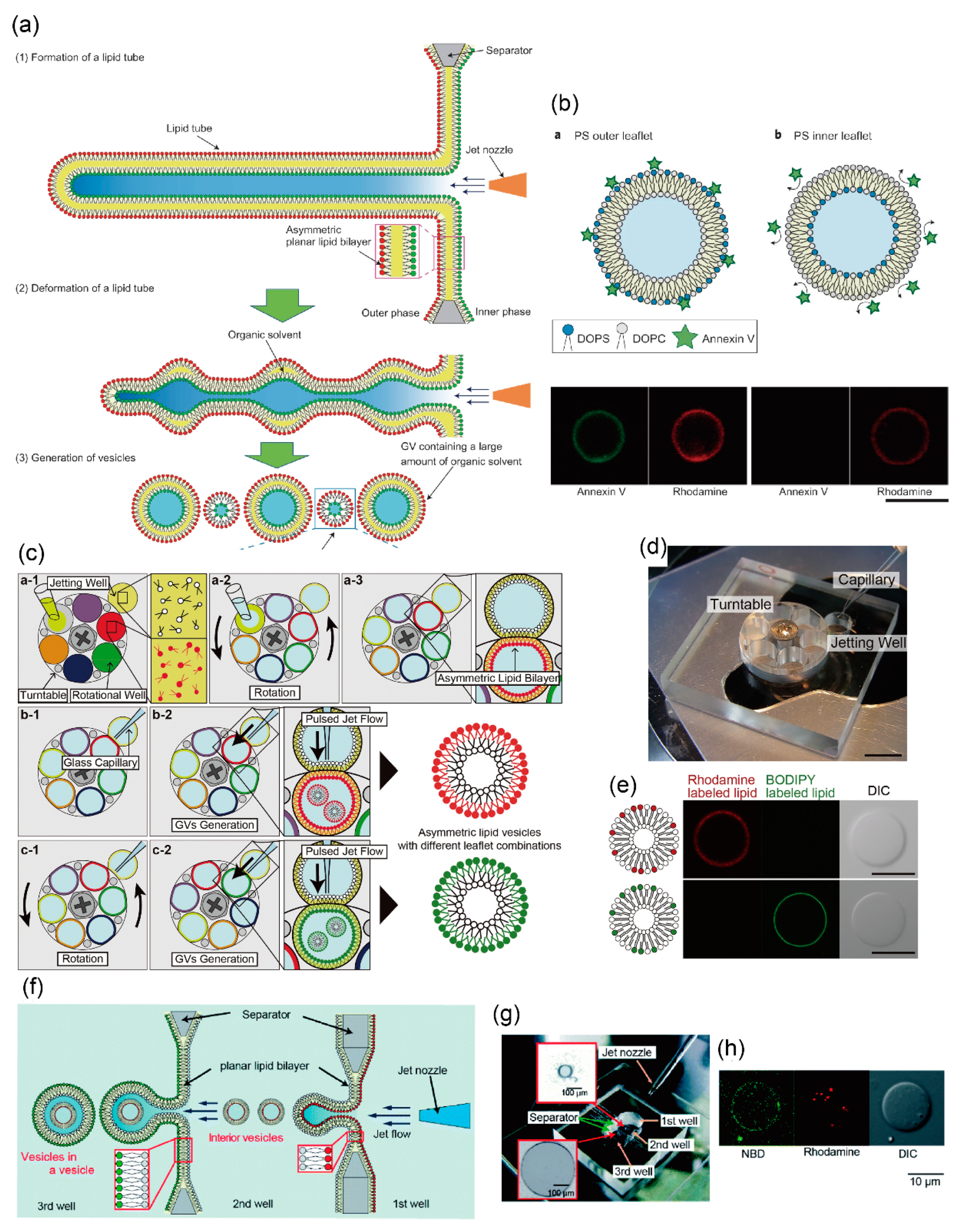
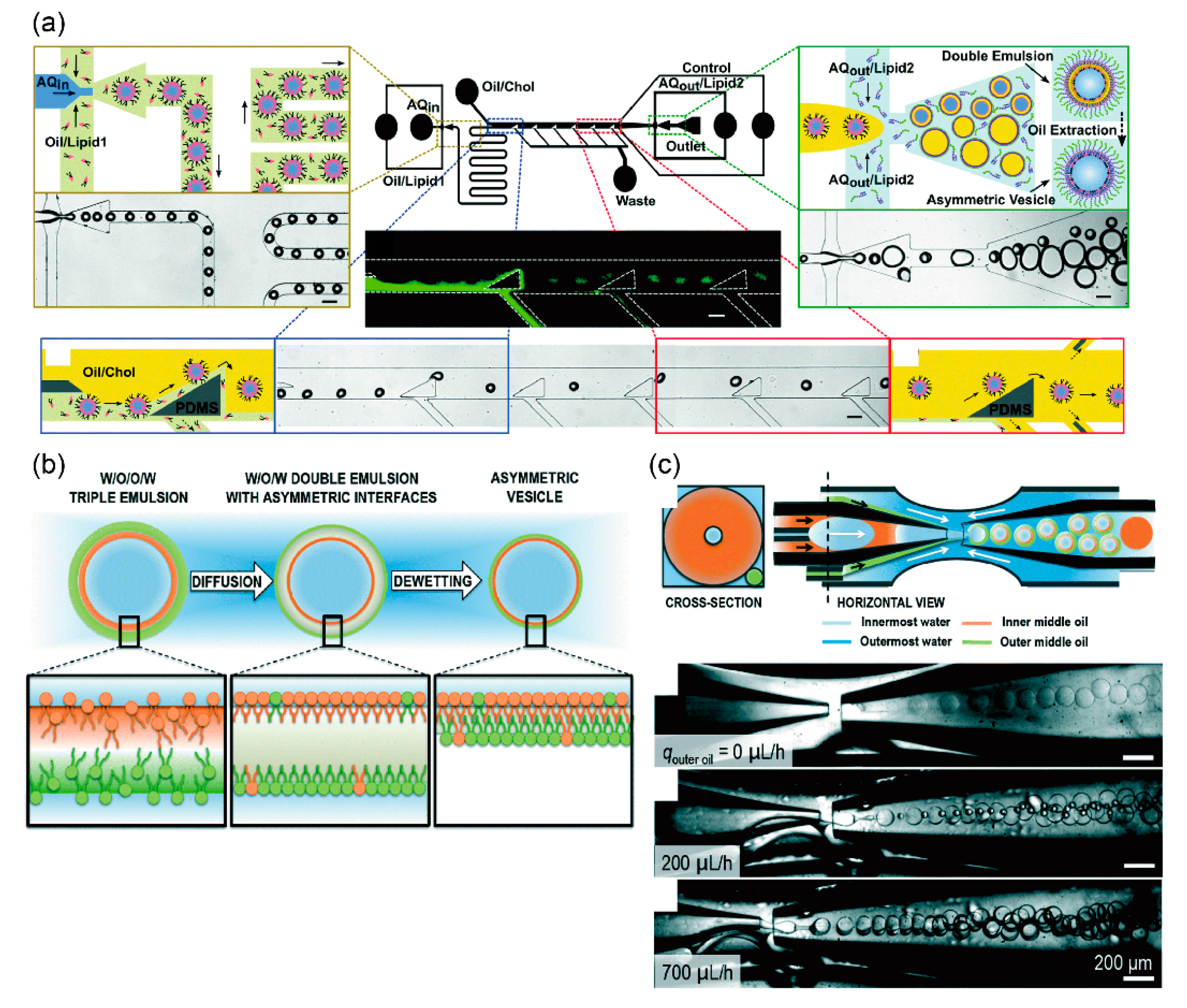
3.2. Asymmetric Lipid Vesicles Formation Using Microfluidic Technology
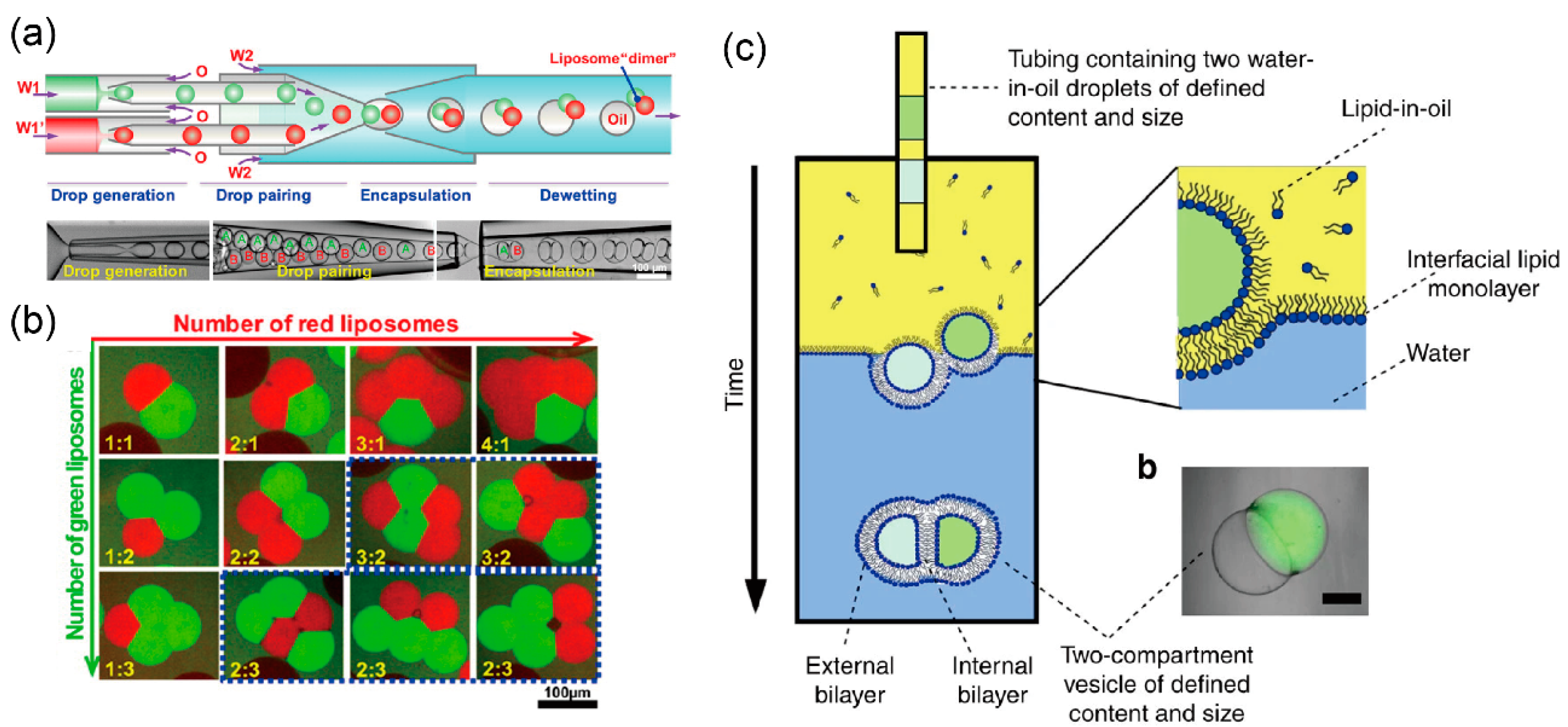
4. Development of Giant Vesicles with Complex Shapes Using Microfluidic Technology
5. Artificial Cell Models Using Giant Lipid Vesicles
5.1. Biological Compartments for Producing Artificial Cell Models
5.1.1. Pore-Forming Protein
5.1.2. Other Membrane Proteins for Maintaining and Stimulating Reactions
5.2. Protein Expression in Giant Vesicles Using Cell-Free Protein Synthesis Systems
5.3. Giant Lipid Vesicles Containing Multiple Components for Sequential Reactions
6. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Szoka, F.; Papahadjopoulos, D. Comparative Properties and Methods of Preparation of Lipid Vesicles (Liposomes). Annu. Rev. Biophys. Bioeng. 1980, 9, 467–508. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant Vesicles: Preparations and Applications. Chembiochem 2010, 11, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Payandeh, J.; Scheuer, T.; Zheng, N.; Catterall, W.A. The Crystal Structure of a Voltage-Gated Sodium Channel. Nature 2011, 475, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA Receptor Subunit Diversity: Impact on Receptor Properties, Synaptic Plasticity and Disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting New Molecular Targets for Known Drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane Transporters in Drug Development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar]
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the Third Wave of Biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef]
- Jeffrey Conn, P.; Christopoulos, A.; Lindsley, C.W. Allosteric Modulators of GPCRs: A Novel Approach for the Treatment of CNS Disorders. Nat. Rev. Drug Discov. 2009, 8, 41–54. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.F.; Kobilka, B.K. The Structure and Function of G-Protein-Coupled Receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial Reactive Oxygen Species Regulate Cellular Signaling and Dictate Biological Outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Velvizhi, G.; Annie Modestra, J.; Srikanth, S. Microbial Fuel Cell: Critical Factors Regulating Bio-Catalyzed Electrochemical Process and Recent Advancements. Renew. Sustain. Energy Rev. 2014, 40, 779–797. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-Kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug Targeting to Tumors: Principles, Pitfalls and (Pre-) Clinical Progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Akashi, K.; Miyata, H.; Itoh, H.; Kinosita, K. Preparation of Giant Liposomes in Physiological Conditions and Their Characterization under an Optical Microscope. Biophys. J. 1996, 71, 3242–3250. [Google Scholar] [CrossRef]
- Veatch, S.L.; Polozov, I.V.; Gawrisch, K.; Keller, S.L. Liquid Domains in Vesicles Investigated by NMR and Fluorescence Microscopy. Biophys. J. 2004, 86, 2910–2922. [Google Scholar] [CrossRef]
- Gokhale, N.A.; Abraham, A.; Digman, M.A.; Gratton, E.; Cho, W. Phosphoinositide Specificity of and Mechanism of Lipid Domain Formation by Annexin A2-P11 Heterotetramer. J. Biol. Chem. 2005, 280, 42831–42840. [Google Scholar] [CrossRef]
- Wollert, T.; Hurley, J.H. Molecular Mechanism of Multivesicular Body Biogenesis by ESCRT Complexes. Nature 2010, 464, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.M.; Yamazaki, M. Spontaneous Insertion of Lipopolysaccharide into Lipid Membranes from Aqueous Solution. Chem. Phys. Lipids 2011, 164, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.; Pécréaux, J.; Lenoir, G.; Falson, P.; Rigaud, J.-L.; Bassereau, P. A New Method for the Reconstitution of Membrane Proteins into Giant Unilamellar Vesicles. Biophys. J. 2004, 87, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Tsumoto, K.; Arakawa, S.; Shimizu, S.; Morita, I.; Yoshimura, T.; Akiyoshi, K. Preparation of Connexin43-Integrated Giant Liposomes by a Baculovirus Expression-Liposome Fusion Method. Biotechnol. Bioeng. 2010, 107, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Tsumoto, K.; Yoshimura, T.; Akiyoshi, K. Cadherin-Integrated Liposomes with Potential Application in a Drug Delivery System. Biomaterials 2011, 32, 9899–9907. [Google Scholar] [CrossRef] [PubMed]
- Romanov, J.; Walczak, M.; Ibiricu, I.; Schüchner, S.; Ogris, E.; Kraft, C.; Martens, S. Mechanism and Functions of Membrane Binding by the Atg5-Atg12/Atg16 Complex during Autophagosome Formation. EMBO J. 2012, 31, 4304–4317. [Google Scholar] [CrossRef]
- Drücker, P.; Iacovache, I.; Bachler, S.; Zuber, B.; Babiychuk, E.B.; Dittrich, P.S.; Draeger, A. Membrane Deformation and Layer-by-Layer Peeling of Giant Vesicles Induced by the Pore-Forming Toxin Pneumolysin. Biomater. Sci. 2019, 7, 3693–3705. [Google Scholar] [CrossRef]
- Walde, P.; Ichikawa, S. Enzymes inside Lipid Vesicles: Preparation, Reactivity and Applications. Biomol. Eng. 2001, 18, 143–177. [Google Scholar] [CrossRef]
- Luisi, P.L.; Walde, P.; Oberholzer, T. Lipid Vesicles as Possible Intermediates in the Origin of Life. Curr. Opin. Colloid Interface Sci. 1999, 4, 33–39. [Google Scholar] [CrossRef]
- Stano, P. Minimal Cells: Relevance and Interplay of Physical and Biochemical Factors. Biotechnol. J. 2011, 6, 850–859. [Google Scholar] [CrossRef]
- Kurihara, K.; Tamura, M.; Shohda, K.; Toyota, T.; Suzuki, K.; Sugawara, T. Self-Reproduction of Supramolecular Giant Vesicles Combined with the Amplification of Encapsulated DNA. Nat. Chem. 2011, 3, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Caschera, F.; Noireaux, V. Integration of Biological Parts toward the Synthesis of a Minimal Cell. Curr. Opin. Chem. Biol. 2014, 22, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Hu, S.; Chen, X. Artificial Cells: From Basic Science to Applications. Mater. Today 2016, 19, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Schwille, P.; Diez, S. Synthetic Biology of Minimal Systems. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 223–242. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and Polymersomes: A Comparative Review towards Cell Mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef]
- Schwille, P.; Spatz, J.; Landfester, K.; Bodenschatz, E.; Herminghaus, S.; Sourjik, V.; Erb, T.J.; Bastiaens, P.; Lipowsky, R.; Hyman, A.; et al. MaxSynBio: Avenues Towards Creating Cells from the Bottom Up. Angew. Chem. 2018, 57, 13382–13392. [Google Scholar] [CrossRef]
- Jeong, S.; Nguyen, H.T.; Kim, C.H.; Ly, M.N.; Shin, K. Toward Artificial Cells: Novel Advances in Energy Conversion and Cellular Motility. Adv. Funct. Mater. 2020, 30, 1907182. [Google Scholar] [CrossRef]
- Matosevic, S. Synthesizing Artificial Cells from Giant Unilamellar Vesicles: State-of-the Art in the Development of Microfluidic Technology. BioEssays 2012, 34, 992–1001. [Google Scholar] [CrossRef]
- van Swaay, D.; deMello, A. Microfluidic Methods for Forming Liposomes. Lab Chip 2013, 13, 752–767. [Google Scholar] [CrossRef]
- Kamiya, K.; Takeuchi, S. Giant Liposome Formation toward the Synthesis of Well-Defined Artificial Cells. J. Mater. Chem. B 2017, 5, 5911–5923. [Google Scholar] [CrossRef]
- Huang, Y.; Kim, S.H.; Arriaga, L.R. Emulsion Templated Vesicles with Symmetric or Asymmetric Membranes. Adv. Colloid Interface Sci. 2017, 247, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Trantidou, T.; Friddin, M.S.; Salehi-Reyhani, A.; Ces, O.; Elani, Y. Droplet Microfluidics for the Construction of Compartmentalised Model Membranes. Lab Chip 2018, 18, 2488–2509. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Wang, P.; DeMello, A.; Feng, L.; Zhu, X.; Wen, W.; Kodzius, R.; Gong, X. Synthesis of Biomaterials Utilizing Microfluidic Technology. Genes 2018, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Supramaniam, P.; Ces, O.; Salehi-Reyhani, A. Microfluidics for Artificial Life: Techniques for Bottom-Up Synthetic Biology. Micromachines 2019, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Xie, R.; Xiong, J.; Liang, Q. Microfluidics for Biosynthesizing: From Droplets and Vesicles to Artificial Cells. Small 2020, 16, 1903940. [Google Scholar] [CrossRef] [PubMed]
- Sawasaki, T.; Ogasawara, T.; Morishita, R.; Endo, Y. A Cell-Free Protein Synthesis System for High-Throughput Proteomics. Proc. Natl. Acad. Sci. USA 2002, 99, 14652–14657. [Google Scholar] [CrossRef]
- Katzen, F.; Chang, G.; Kudlicki, W. The Past, Present and Future of Cell-Free Protein Synthesis. Trends Biotechnol. 2005, 23, 150–156. [Google Scholar] [CrossRef]
- Villarreal, F.; Tan, C. Cell-Free Systems in the New Age of Synthetic Biology. Front. Chem. Sci. Eng. 2017, 11, 58–65. [Google Scholar] [CrossRef]
- Dubuc, E.; Pieters, P.A.; van der Linden, A.J.; van Hest, J.C.; Huck, W.T.; de Greef, T.F. Cell-Free Microcompartmentalised Transcription–translation for the Prototyping of Synthetic Communication Networks. Curr. Opin. Biotechnol. 2019, 58, 72–80. [Google Scholar] [CrossRef]
- Horne, R.W.; Bangham, A.D.; Whittaker, V.P. Negatively Stained Lipoprotein Membranes. Nature 1963, 200, 1340. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Horger, K.S.; Estes, D.J.; Capone, R.; Mayer, M. Films of Agarose Enable Rapid Formation of Giant Liposomes in Solutions of Physiologic Ionic Strength. J. Am. Chem. Soc. 2009, 131, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Lira, R.B.; Dimova, R.; Riske, K.A. Giant Unilamellar Vesicles Formed by Hybrid Films of Agarose and Lipids Display Altered Mechanical Properties. Biophys. J. 2014, 107, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Sprong, H.; van der Sluijs, P.; van Meer, G. How Proteins Move Lipids and Lipids Move Proteins. Nat. Rev. Mol. Cell Biol. 2001, 2, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, T.; Hrafnsdóttir, S.; Devaux, P.F.; van Meer, G. Lipid Distribution and Transport across Cellular Membranes. Semin. Cell Dev. Biol. 2001, 12, 139–148. [Google Scholar] [CrossRef]
- Muthusamy, B.-P.; Natarajan, P.; Zhou, X.; Graham, T.R. Linking Phospholipid Flippases to Vesicle-Mediated Protein Transport. Biochim. Biophys. Acta 2009, 1791, 612–619. [Google Scholar] [CrossRef]
- Marquardt, D.; Geier, B.; Pabst, G. Asymmetric Lipid Membranes: Towards More Realistic Model Systems. Membranes 2015, 5, 180–196. [Google Scholar] [CrossRef]
- Fujimoto, T.; Parmryd, I. Interleaflet Coupling, Pinning, and Leaflet Asymmetry—Major Players in Plasma Membrane Nanodomain Formation. Front. Cell Dev. Biol. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- London, E. Membrane Structure—Function Insights from Asymmetric Lipid Vesicles. Acc. Chem. Res. 2019, 52, 2382–2391. [Google Scholar] [CrossRef]
- Henderson, J.C.; Zimmerman, S.M.; Crofts, A.A.; Boll, J.M.; Kuhns, L.G.; Herrera, C.M.; Trent, M.S. The Power of Asymmetry: Architecture and Assembly of the Gram-Negative Outer Membrane Lipid Bilayer. Annu. Rev. Microbiol. 2016, 70, 255–278. [Google Scholar] [CrossRef]
- May, K.L.; Silhavy, T.J. Making a Membrane on the Other Side of the Wall. Biochim. Biophys. Acta 2017, 1862, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Production of Unilamellar Vesicles Using an Inverted Emulsion. Langmuir 2003, 19, 2870–2879. [Google Scholar] [CrossRef]
- Pautot, S.; Frisken, B.J.; Weitz, D.A.; Lubensky, T.C. Engineering Asymmetric Vesicles. Proc. Natl. Acad. Sci. USA 2003, 100, 10718–10721. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.C.; Hettiarachchi, K.; Siu, M.; Pan, Y.R.; Lee, A.P. Controlled Microfluidic Encapsulation of Cells, Proteins, and Microbeads in Lipid Vesicles. J. Am. Chem. Soc. 2006, 128, 5656–5658. [Google Scholar] [CrossRef]
- Hu, P.C.; Malmstadt, N. Microfluidic Fabrication of Giant Lipid Vesicles. ACS Appl. Mater. Interfaces 2012, 3, 1434–1440. [Google Scholar] [CrossRef]
- Matosevic, S.; Paegel, B.M. Stepwise Synthesis of Giant Unilamellar Vesicles on a Microfluidic Assembly Line. J. Am. Chem. Soc. 2011, 133, 2798–2800. [Google Scholar] [CrossRef]
- Abkarian, M.; Loiseau, E.; Massiera, G. Continuous Droplet Interface Crossing Encapsulation (CDICE) for High Throughput Monodisperse Vesicle Design. Soft Matter 2011, 7, 4610–4614. [Google Scholar] [CrossRef]
- Morita, M.; Onoe, H.; Yanagisawa, M.; Ito, H.; Ichikawa, M.; Fujiwara, K.; Saito, H.; Takinoue, M. Droplet-Shooting and Size-Filtration (DSSF) Method for Synthesis of Cell-Sized Liposomes with Controlled Lipid Compositions. Chembiochem 2015, 16, 2029–2035. [Google Scholar] [CrossRef]
- Karamdad, K.; Law, R.V.; Seddon, J.M.; Brooks, N.J.; Ces, O. Preparation and Mechanical Characterisation of Giant Unilamellar Vesicles by a Microfluidic Method. Lab Chip 2015, 15, 557–562. [Google Scholar] [CrossRef]
- Karamdad, K.; Law, R.V.; Seddon, J.M.; Brooks, N.J.; Ces, O. Studying the Effects of Asymmetry on the Bending Rigidity of Lipid Membranes Formed by Microfluidics. Chem. Commun. 2016, 52, 5277–5280. [Google Scholar] [CrossRef]
- Blosser, M.C.; Horst, B.G.; Keller, S.L. CDICE Method Produces Giant Lipid Vesicles under Physiological Conditions of Charged Lipids and Ionic Solutions. Soft Matter 2016, 12, 7364–7371. [Google Scholar] [CrossRef] [PubMed]
- Romanov, V.; McCullough, J.; Gale, B.K.; Frost, A. A Tunable Microfluidic Device Enables Cargo Encapsulation by Cell- or Organelle-Sized Lipid Vesicles Comprising Asymmetric Lipid Bilayers. Adv. Biosyst. 2019, 3, 1900010. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, K.; Suzuki, H.; Takeuchi, S. Formation of Giant Lipid Vesiclelike Compartments from a Planar Lipid Membrane by a Pulsed Jet Flow. J. Am. Chem. Soc. 2007, 129, 12608–12609. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, K.; Suzuki, H.; Takeuchi, S. Lipid Bilayer Formation by Contacting Monolayers in a Microfluidic Device for Membrane Protein Analysis. Anal. Chem. 2006, 78, 8169–8174. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Tsuji, Y.; Sato, K.; Osaki, T.; Kamiya, K.; Hirano, M.; Ide, T.; Miki, N.; Takeuchi, S. Automated Parallel Recordings of Topologically Identified Single Ion Channels. Sci. Rep. 2013, 3, 1995. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, Y.; Kawano, R.; Osaki, T.; Kamiya, K.; Miki, N.; Takeuchi, S. Droplet Split-and-Contact Method for High-Throughput Transmembrane Electrical Recording. Anal. Chem. 2013, 85, 10913–10919. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Osaki, T.; Nakao, K.; Kawano, R.; Fujii, S.; Misawa, N.; Hayakawa, M.; Takeuchi, S. Electrophysiological Measurement of Ion Channels on Plasma/Organelle Membranes Using an on-Chip Lipid Bilayer System. Sci. Rep. 2018, 8, 17498. [Google Scholar] [CrossRef]
- Yasuga, H.; Kawano, R.; Takinoue, M.; Tsuji, Y.; Osaki, T.; Kamiya, K.; Miki, N.; Takeuchi, S. Logic Gate Operation by DNA Translocation through Biological Nanopores. PLoS ONE 2016, 11, e0149667. [Google Scholar] [CrossRef]
- Fujii, S.; Nobukawa, A.; Osaki, T.; Morimoto, Y.; Kamiya, K.; Misawa, N.; Takeuchi, S. Pesticide Vapor Sensing Using an Aptamer, Nanopore, and Agarose Gel on a Chip. Lab Chip 2017, 17, 2421–2425. [Google Scholar] [CrossRef]
- Hiratani, M.; Ohara, M.; Kawano, R. Amplification and Quantification of an Antisense Oligonucleotide from Target MicroRNA Using Programmable DNA and a Biological Nanopore. Anal. Chem. 2017, 89, 2312–2317. [Google Scholar] [CrossRef]
- Fujii, S.; Kamiya, K.; Osaki, T.; Misawa, N.; Hayakawa, M.; Takeuchi, S. Purification-Free MicroRNA Detection by Using Magnetically Immobilized Nanopores on Liposome Membrane. Anal. Chem. 2018, 90, 10217–10222. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Fujii, S.; Kamiya, K.; Osaki, T.; Takaku, T.; Takahashi, Y.; Takeuchi, S. Construction of a Biohybrid Odorant Sensor Using Biological Olfactory Receptors Embedded into Bilayer Lipid Membrane on a Chip. ACS Sens. 2019, 4, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, J.C.; Richmond, D.L.; Li, T.H.; Liu, A.P.; Parekh, S.H.; Fletcher, D.A. Unilamellar Vesicle Formation and Encapsulation by Microfluidic Jetting. Proc. Natl. Acad. Sci. USA 2008, 105, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, J.C.; Richmond, D.L.; Li, T.H.; Brochard-Wyart, F.; Fletcher, D.A. Inkjet Formation of Unilamellar Lipid Vesicles for Cell-like Encapsulation. Lab Chip 2009, 9, 2003–2009. [Google Scholar] [CrossRef]
- Richmond, D.L.; Schmid, E.M.; Martens, S.; Stachowiak, J.C.; Liska, N.; Fletcher, D.A. Forming Giant Vesicles with Controlled Membrane Composition, Asymmetry, and Contents. Proc. Natl. Acad. Sci. USA 2011, 108, 9431–9436. [Google Scholar] [CrossRef]
- Belardi, B.; Son, S.; Vahey, M.D.; Wang, J.; Hou, J.; Fletcher, D.A. Claudin-4 Reconstituted in Unilamellar Vesicles Is Sufficient to Form Tight Interfaces That Partition Membrane Proteins. J. Cell Sci. 2019, 132, jcs221556. [Google Scholar] [CrossRef]
- Kamiya, K.; Kawano, R.; Osaki, T.; Akiyoshi, K.; Takeuchi, S. Cell-Sized Asymmetric Lipid Vesicles Facilitate the Investigation of Asymmetric Membranes. Nat. Chem. 2016, 8, 881–889. [Google Scholar] [CrossRef]
- Gotanda, M.; Kamiya, K.; Osaki, T.; Fujii, S.; Misawa, N.; Miki, N.; Takeuchi, S. Sequential Generation of Asymmetric Lipid Vesicles Using a Pulsed-Jetting Method in Rotational Wells. Sens. Actuators B Chem. 2018, 261, 392–397. [Google Scholar] [CrossRef]
- Gotanda, M.; Kamiya, K.; Osaki, T.; Miki, N.; Takeuchi, S. Automatic Generation System of Cell-Sized Liposomes. Sens. Actuators B Chem. 2019, 292, 57–63. [Google Scholar] [CrossRef]
- Kamiya, K.; Osaki, T.; Takeuchi, S. Formation of Vesicles-in-a-Vesicle with Asymmetric Lipid Components Using a Pulsed-Jet Flow Method. RSC Adv. 2019, 9, 30071–30075. [Google Scholar] [CrossRef]
- Shum, H.C.; Lee, D.; Yoon, I.; Kodger, T.; Weitz, D.A. Double Emulsion Templated Monodisperse Phospholipid Vesicles. Langmuir 2008, 24, 7651–7653. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, L.R.; Datta, S.S.; Kim, S.H.; Amstad, E.; Kodger, T.E.; Monroy, F.; Weitz, D.A. Ultrathin Shell Double Emulsion Templated Giant Unilamellar Lipid Vesicles with Controlled Microdomain Formation. Small 2014, 10, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, J.W.; Cho, J.-C.; Weitz, D.A. Double-Emulsion Drops with Ultra-Thin Shells for Capsule Templates. Lab Chip 2011, 11, 3162–3166. [Google Scholar] [CrossRef] [PubMed]
- Caschera, F.; Lee, J.W.; Ho, K.K.Y.; Liu, A.P.; Jewett, M.C. Cell-Free Compartmentalized Protein Synthesis inside Double Emulsion Templated Liposomes with in Vitro Synthesized and Assembled Ribosomes. Chem. Commun. 2016, 52, 5467–5469. [Google Scholar] [CrossRef]
- Deng, N.N.; Huck, W.T.S. Microfluidic Formation of Monodisperse Coacervate Organelles in Liposomes. Angew. Chem. 2017, 56, 9736–9740. [Google Scholar] [CrossRef] [PubMed]
- Michelon, M.; Huang, Y.; de la Torre, L.G.; Weitz, D.A.; Cunha, R.L. Single-Step Microfluidic Production of W/O/W Double Emulsions as Templates for β-Carotene-Loaded Giant Liposomes Formation. Chem. Eng. J. 2019, 366, 27–32. [Google Scholar] [CrossRef]
- Lu, L.; Schertzer, J.W.; Chiarot, P.R. Continuous Microfluidic Fabrication of Synthetic Asymmetric Vesicles. Lab Chip 2015, 15, 3591–3599. [Google Scholar] [CrossRef]
- Maktabi, S.; Schertzer, J.W.; Chiarot, P.R. Dewetting-Induced Formation and Mechanical Properties of Synthetic Bacterial Outer Membrane Models (GUVs) with Controlled Inner-Leaflet Lipid Composition. Soft Matter 2019, 15, 3938–3948. [Google Scholar] [CrossRef]
- Arriaga, L.R.; Huang, Y.; Kim, S.-H.; Aragones, J.L.; Ziblat, R.; Koehler, S.A.; Weitz, D.A. Single-Step Assembly of Asymmetric Vesicles. Lab Chip 2019, 19, 749–756. [Google Scholar] [CrossRef]
- Elani, Y.; Solvas, X.C.I.; Edel, J.B.; Law, R.V.; Ces, O. Microfluidic Generation of Encapsulated Droplet Interface Bilayer Networks (Multisomes) and Their Use as Cell-like Reactors. Chem. Commun. 2016, 52, 5961–5964. [Google Scholar] [CrossRef]
- Baxani, D.K.; Morgan, A.J.L.; Jamieson, W.D.; Allender, C.J.; Barrow, D.A.; Castell, O.K. Bilayer Networks within a Hydrogel Shell: A Robust Chassis for Artificial Cells and a Platform for Membrane Studies. Angew. Chem. 2016, 55, 14240–14245. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S.; Paegel, B.M. Layer-by-Layer Cell Membrane Assembly. Nat. Chem. 2013, 5, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.-N.; Yelleswarapu, M.; Huck, W.T.S. Monodisperse Uni- and Multicompartment Liposomes. J. Am. Chem. Soc. 2016, 138, 7584–7591. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.-N.; Yelleswarapu, M.; Zheng, L.; Huck, W.T.S. Microfluidic Assembly of Monodisperse Vesosomes as Artificial Cell Models. J. Am. Chem. Soc. 2017, 139, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Elani, Y.; Law, R.V.; Ces, O. Vesicle-Based Artificial Cells as Chemical Microreactors with Spatially Segregated Reaction Pathways. Nat. Commun. 2014, 5, 5305. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Frohnmayer, J.P.; Benk, L.T.; Haller, B.; Janiesch, J.-W.; Heitkamp, T.; Börsch, M.; Lira, R.B.; Dimova, R.; Lipowsky, R.; et al. Sequential Bottom-up Assembly of Mechanically Stabilized Synthetic Cells by Microfluidics. Nat. Mater. 2018, 17, 89–96. [Google Scholar] [CrossRef]
- Kawano, R. Synthetic Ion Channels and DNA Logic Gates as Components of Molecular Robots. Chem. Phys. Chem. 2018, 19, 359–366. [Google Scholar] [CrossRef]
- Haque, F.; Li, J.; Wu, H.-C.; Liang, X.-J.; Guo, P. Solid-State and Biological Nanopore for Real-Time Sensing of Single Chemical and Sequencing of DNA. Nano Today 2013, 8, 56–74. [Google Scholar] [CrossRef]
- Branton, D.; Deamer, D.W.; Marziali, A.; Bayley, H.; Benner, S.A.; Butler, T.; Di Ventra, M.; Garaj, S.; Hibbs, A.; Huang, X.; et al. The Potential and Challenges of Nanopore Sequencing. Nat. Biotechnol. 2008, 26, 1146–1153. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, D.; Tan, Q.; Wang, M.X.; Gu, L.-Q. Nanopore-Based Detection of Circulating MicroRNAs in Lung Cancer Patients. Nat. Nanotechnol. 2011, 6, 668–674. [Google Scholar] [CrossRef]
- Noireaux, V.; Libchaber, A. A Vesicle Bioreactor as a Step toward an Artificial Cell Assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 17669–17674. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Bayley, H.; Valeva, A.; Walev, I.; Walker, B.; Weller, U.; Kehoe, M.; Palmer, M. Staphylococcal Alpha-Toxin, Streptolysin-O, and Escherichia Coli Hemolysin: Prototypes of Pore-Forming Bacterial Cytolysins. Arch. Microbiol. 1996, 165, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kunishige, R.; Kano, F.; Murata, M. The Cell Resealing Technique for Manipulating, Visualizing, and Elucidating Molecular Functions in Living Cells. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129329. [Google Scholar] [CrossRef] [PubMed]
- Pressman, B.C. Biological Applications of Ionophores. Annu. Rev. Biochem. 1976, 45, 501–530. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, V.; Yakisich, J.; Kumar, A.; Azad, N.; Iyer, A. Ionophores: Potential Use as Anticancer Drugs and Chemosensitizers. Cancers 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, X.; Mu, W.; Han, X. Chemical Signal Communication between Two Protoorganelles in a Lipid-Based Artificial Cell. Anal. Chem. 2019, 91, 6859–6864. [Google Scholar] [CrossRef]
- Debnath, M.; Chakraborty, S.; Kumar, Y.P.; Chaudhuri, R.; Jana, B.; Dash, J. Ionophore Constructed from Non-Covalent Assembly of a G-Quadruplex and Liponucleoside Transports K+-Ion across Biological Membranes. Nat. Commun. 2020, 11, 469. [Google Scholar] [CrossRef]
- Zhang, F.; Vierock, J.; Yizhar, O.; Fenno, L.E.; Tsunoda, S.; Kianianmomeni, A.; Prigge, M.; Berndt, A.; Cushman, J.; Polle, J.; et al. The Microbial Opsin Family of Optogenetic Tools. Cell 2011, 147, 1446–1457. [Google Scholar] [CrossRef]
- Kahya, N.; Pécheur, E.-I.; de Boeij, W.P.; Wiersma, D.A.; Hoekstra, D. Reconstitution of Membrane Proteins into Giant Unilamellar Vesicles via Peptide-Induced Fusion. Biophys. J. 2001, 81, 1464–1474. [Google Scholar] [CrossRef]
- Dezi, M.; Di Cicco, A.; Bassereau, P.; Levy, D. Detergent-Mediated Incorporation of Transmembrane Proteins in Giant Unilamellar Vesicles with Controlled Physiological Contents. Proc. Natl. Acad. Sci. USA 2013, 110, 7276–7281. [Google Scholar] [CrossRef]
- Almendro-Vedia, V.G.; Natale, P.; Mell, M.; Bonneau, S.; Monroy, F.; Joubert, F.; López-Montero, I. Nonequilibrium Fluctuations of Lipid Membranes by the Rotating Motor Protein F 1 F 0 -ATP Synthase. Proc. Natl. Acad. Sci. USA 2017, 114, 11291–11296. [Google Scholar] [CrossRef] [PubMed]
- Altamura, E.; Milano, F.; Tangorra, R.R.; Trotta, M.; Omar, O.H.; Stano, P.; Mavelli, F. Highly Oriented Photosynthetic Reaction Centers Generate a Proton Gradient in Synthetic Protocells. Proc. Natl. Acad. Sci. USA 2017, 114, 3837–3842. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Majd, S. Reconstitution and Functional Studies of Hamster P-Glycoprotein in Giant Liposomes. PLoS ONE 2018, 13, e0199279. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Schwille, P. Cell-Free Protein Synthesis and Its Perspectives for Assembling Cells from the Bottom-Up. Adv. Biosyst. 2019, 3, 1800322. [Google Scholar] [CrossRef]
- Greenberg, J.R.; Carroll, E. Reconstitution of Functional MRNA-Protein Complexes in a Rabbit Reticulocyte Cell-Free Translation System. Mol. Cell. Biol. 1985, 5, 342–351. [Google Scholar] [CrossRef]
- Madin, K.; Sawasaki, T.; Ogasawara, T.; Endo, Y. A Highly Efficient and Robust Cell-Free Protein Synthesis System Prepared from Wheat Embryos: Plants Apparently Contain a Suicide System Directed at Ribosomes. Proc. Natl. Acad. Sci. USA 2000, 97, 559–564. [Google Scholar] [CrossRef]
- Tarui, H.; Murata, M.; Tani, I.; Imanishi, S.; Nishikawa, S.; Hara, T. Establishment and Characterization of Cell-Free Translation/Glycosylation in Insect Cell ( Spodoptera Frugiperda 21) Extract Prepared with High Pressure Treatment. Appl. Microbiol. Biotechnol. 2001, 55, 446–453. [Google Scholar] [CrossRef]
- Brödel, A.K.; Sonnabend, A.; Kubick, S. Cell-Free Protein Expression Based on Extracts from CHO Cells. Biotechnol. Bioeng. 2014, 111, 25–36. [Google Scholar] [CrossRef]
- Mikami, S.; Masutani, M.; Sonenberg, N.; Yokoyama, S.; Imataka, H. An Efficient Mammalian Cell-Free Translation System Supplemented with Translation Factors. Protein Expr. Purif. 2006, 46, 348–357. [Google Scholar] [CrossRef]
- Shimizu, Y.; Inoue, A.; Tomari, Y.; Suzuki, T.; Yokogawa, T.; Nishikawa, K.; Ueda, T. Cell-Free Translation Reconstituted with Purified Components. Nat. Biotechnol. 2001, 19, 751–755. [Google Scholar] [CrossRef]
- Renauld, S.; Cortes, S.; Bersch, B.; Henry, X.; De Waard, M.; Schaack, B. Functional Reconstitution of Cell-Free Synthesized Purified Kv Channels. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Berrier, C.; Park, K.H.; Abes, S.; Bibonne, A.; Betton, J.M.; Ghazi, A. Cell-Free Synthesis of a Functional Ion Channel in the Absence of a Membrane and in the Presence of Detergent. Biochemistry 2004, 43, 12585–12591. [Google Scholar] [CrossRef] [PubMed]
- Moritani, Y.; Nomura, S.I.M.; Morita, I.; Akiyoshi, K. Direct Integration of Cell-Free-Synthesized Connexin-43 into Liposomes and Hemichannel Formation. FEBS J. 2010, 277, 3343–3352. [Google Scholar] [CrossRef] [PubMed]
- Liguori, L.; Marques, B.; Villegas-Mendez, A.; Rothe, R.; Lenormand, J.-L. Liposomes-Mediated Delivery of pro-Apoptotic Therapeutic Membrane Proteins. J. Control. Release 2008, 126, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Lyford, L.K.; Rosenberg, R.L. Cell-Free Expression and Functional Reconstitution of Homo-Oligomeric A7 Nicotinic Acetylcholine Receptors into Planar Lipid Bilayers. J. Biol. Chem. 1999, 274, 25675–25681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishihara, G.; Goto, M.; Saeki, M.; Ito, K.; Hori, T.; Kigawa, T.; Shirouzu, M.; Yokoyama, S. Expression of G Protein Coupled Receptors in a Cell-Free Translational System Using Detergents and Thioredoxin-Fusion Vectors. Protein Expr. Purif. 2005, 41, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Ogasawara, T.; Ozawa, T.; Muraguchi, A.; Jih, P.-J.; Morishita, R.; Uchigashima, M.; Watanabe, M.; Fujimoto, T.; Iwasaki, T.; et al. Production of Monoclonal Antibodies against GPCR Using Cell-Free Synthesized GPCR Antigen and Biotinylated Liposome-Based Interaction Assay. Sci. Rep. 2015, 5, 11333. [Google Scholar] [CrossRef]
- Matthies, D.; Haberstock, S.; Joos, F.; Dötsch, V.; Vonck, J.; Bernhard, F.; Meier, T. Cell-Free Expression and Assembly of ATP Synthase. J. Mol. Biol. 2011, 413, 593–603. [Google Scholar] [CrossRef]
- Kuruma, Y.; Ueda, T. The PURE System for the Cell-Free Synthesis of Membrane Proteins. Nat. Protoc. 2015, 10, 1328–1344. [Google Scholar] [CrossRef]
- Long, A.R.; O’Brien, C.C.; Alder, N.N. The Cell-Free Integration of a Polytopic Mitochondrial Membrane Protein into Liposomes Occurs Cotranslationally and in a Lipid-Dependent Manner. PLoS ONE 2012, 7, e46332. [Google Scholar] [CrossRef]
- Rampioni, G.; D’Angelo, F.; Messina, M.; Zennaro, A.; Kuruma, Y.; Tofani, D.; Leoni, L.; Stano, P. Synthetic Cells Produce a Quorum Sensing Chemical Signal Perceived by Pseudomonas Aeruginosa. Chem. Commun. 2018, 54, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Adamala, K.P.; Martin-Alarcon, D.A.; Guthrie-Honea, K.R.; Boyden, E.S. Engineering Genetic Circuit Interactions within and between Synthetic Minimal Cells. Nat. Chem. 2016, 9, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Park, S.-J.; Lee, K.A.; Kim, S.-H.; Kim, H.; Meroz, Y.; Mahadevan, L.; Jung, K.-H.; Ahn, T.K.; Parker, K.K.; et al. Photosynthetic Artificial Organelles Sustain and Control ATP-Dependent Reactions in a Protocellular System. Nat. Biotechnol. 2018, 36, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Hindley, J.W.; Elani, Y.; McGilvery, C.M.; Ali, S.; Bevan, C.L.; Law, R.V.; Ces, O. Light-Triggered Enzymatic Reactions in Nested Vesicle Reactors. Nat. Commun. 2018, 9, 1093. [Google Scholar] [CrossRef] [PubMed]
- Hindley, J.W.; Zheleva, D.G.; Elani, Y.; Charalambous, K.; Barter, L.M.C.; Booth, P.J.; Bevan, C.L.; Law, R.V.; Ces, O. Building a Synthetic Mechanosensitive Signaling Pathway in Compartmentalized Artificial Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 16711–16716. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, S.; Ueda, T.; Kuruma, Y. Artificial Photosynthetic Cell Producing Energy for Protein Synthesis. Nat. Commun. 2019, 10, 1325. [Google Scholar] [CrossRef]
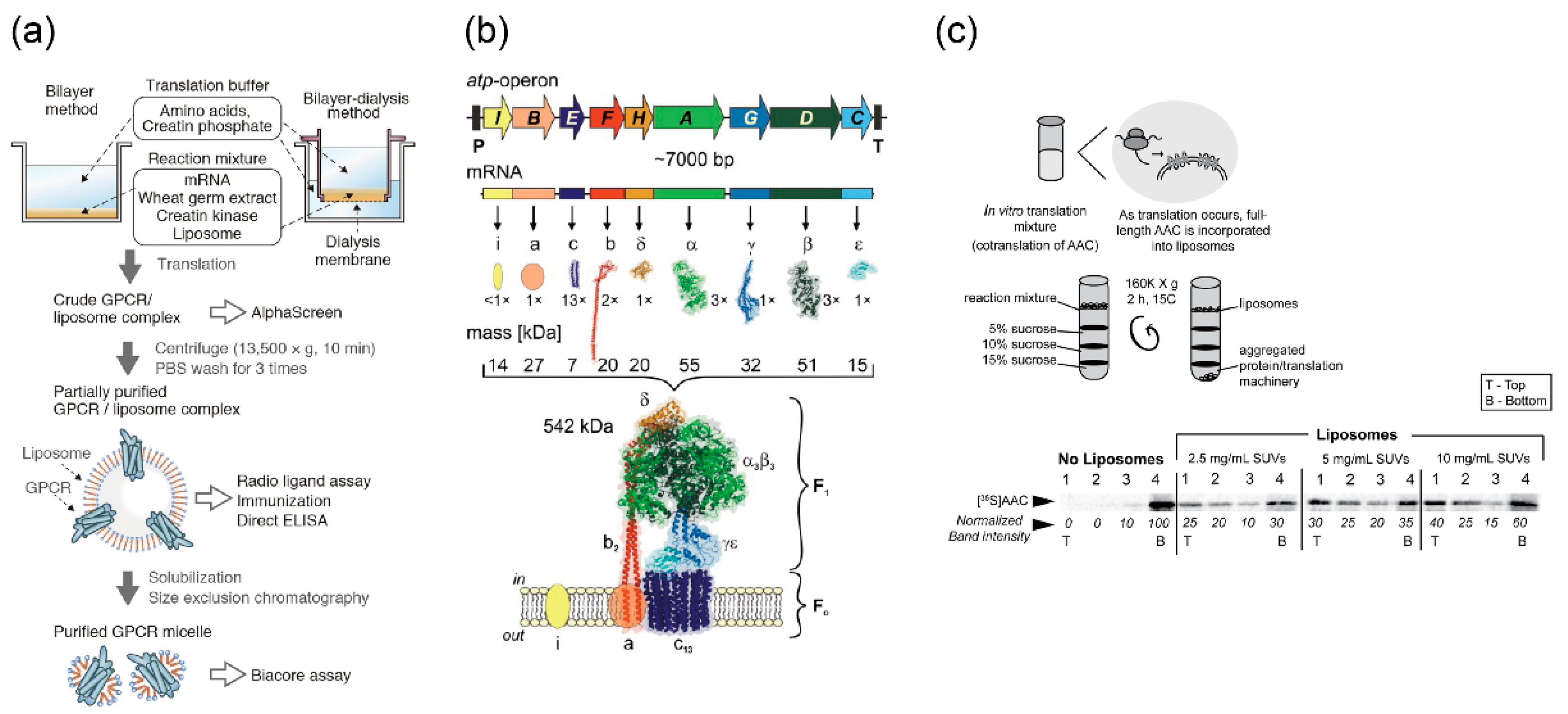
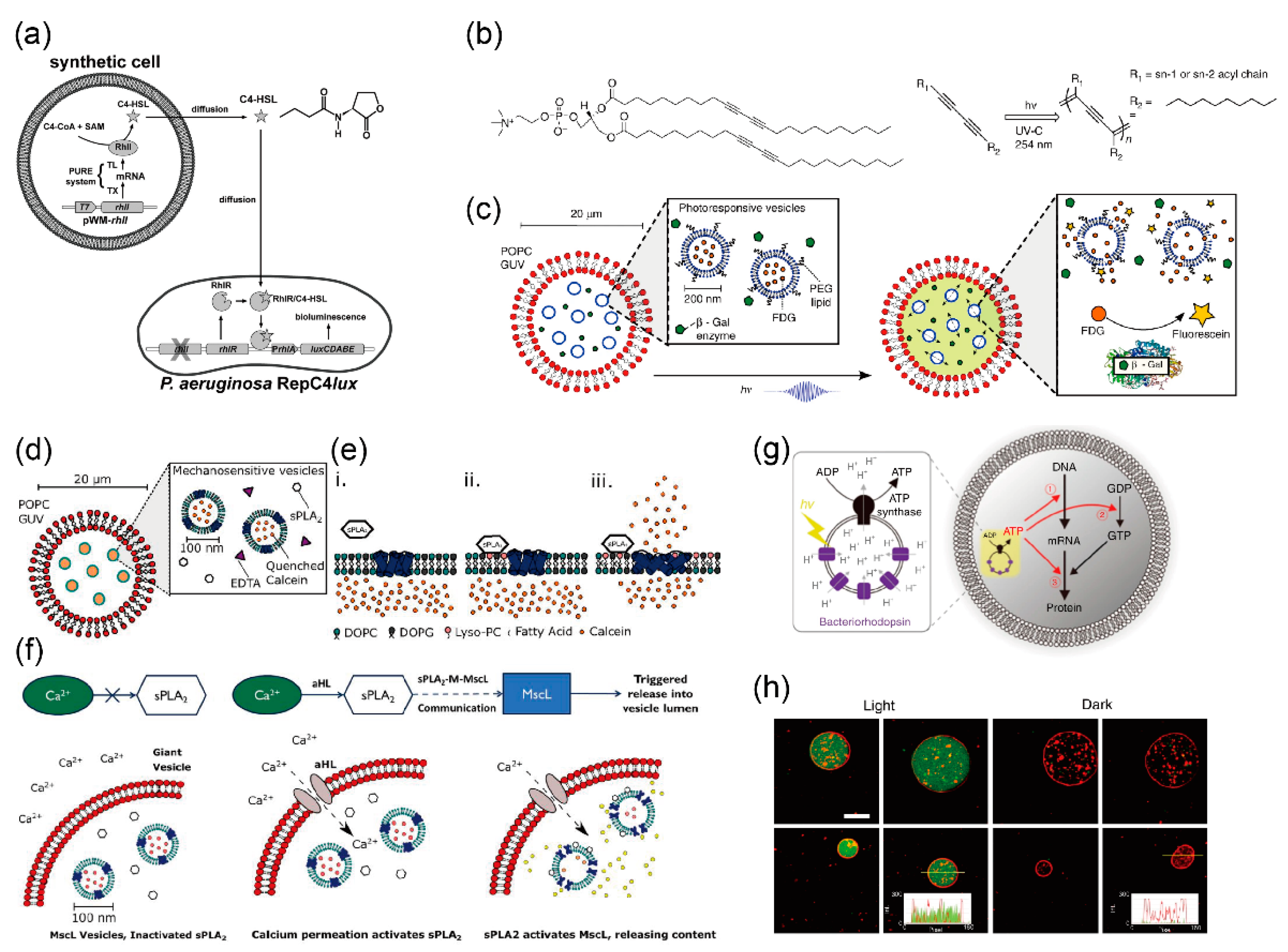
| Properties | Droplet Transfer Method | Pulsed-Jet Flow Method | Double/Triple-Emulsion Method | Gentle Hydration Method |
|---|---|---|---|---|
| Unilamellarity | High | High | High | Low |
| Monodisperse | High | High | Middle | Low |
| Highly encapsulated | Yes | Yes | Yes | No |
| Asymmetric membrane | Yes | Yes | Yes | No |
| Production amount | Middle | Large | Low | Large |
| Organic solvent | Large | Small | Less | No |
| Long-term stability | Middle | Yes | Yes | Yes |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiya, K. Development of Artificial Cell Models Using Microfluidic Technology and Synthetic Biology. Micromachines 2020, 11, 559. https://doi.org/10.3390/mi11060559
Kamiya K. Development of Artificial Cell Models Using Microfluidic Technology and Synthetic Biology. Micromachines. 2020; 11(6):559. https://doi.org/10.3390/mi11060559
Chicago/Turabian StyleKamiya, Koki. 2020. "Development of Artificial Cell Models Using Microfluidic Technology and Synthetic Biology" Micromachines 11, no. 6: 559. https://doi.org/10.3390/mi11060559
APA StyleKamiya, K. (2020). Development of Artificial Cell Models Using Microfluidic Technology and Synthetic Biology. Micromachines, 11(6), 559. https://doi.org/10.3390/mi11060559





