Microelectrode Array based Functional Testing of Pancreatic Islet Cells
Abstract
1. Introduction
2. Materials and Methods
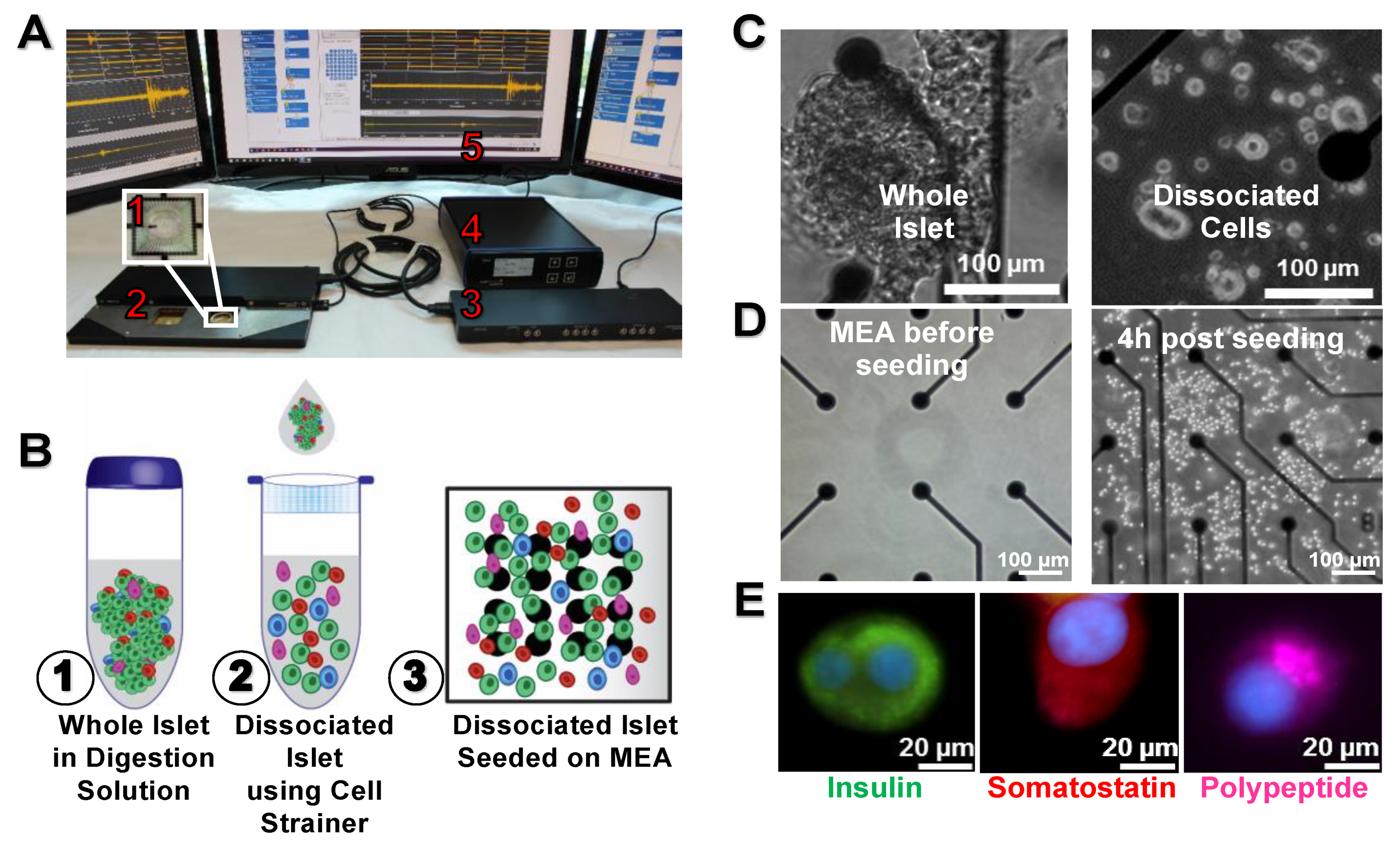
2.1. Islets Dissociation and Culture
2.2. Glucose-Stimulated Insulin Secretion (GSIS) Assay
2.3. Electrophysiological Recordings
2.4. Immunofluorescent Staining
2.5. Statistical Analyses
3. Results
3.1. Dissociation and Culture of Islets
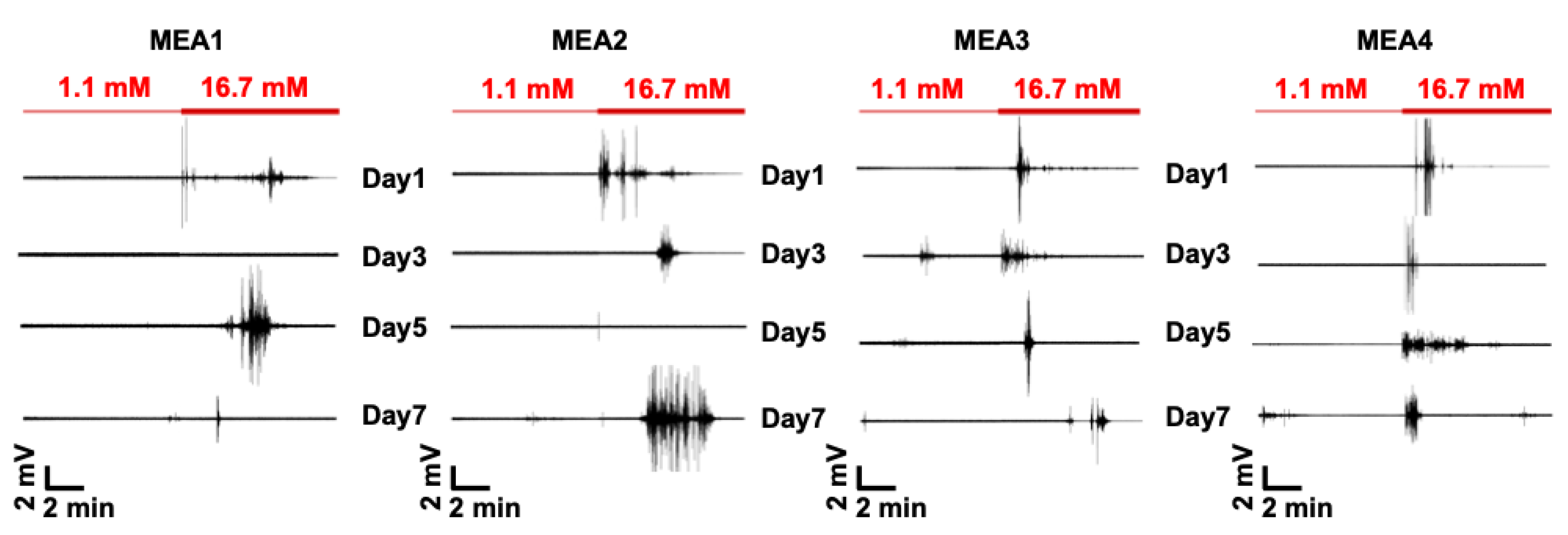
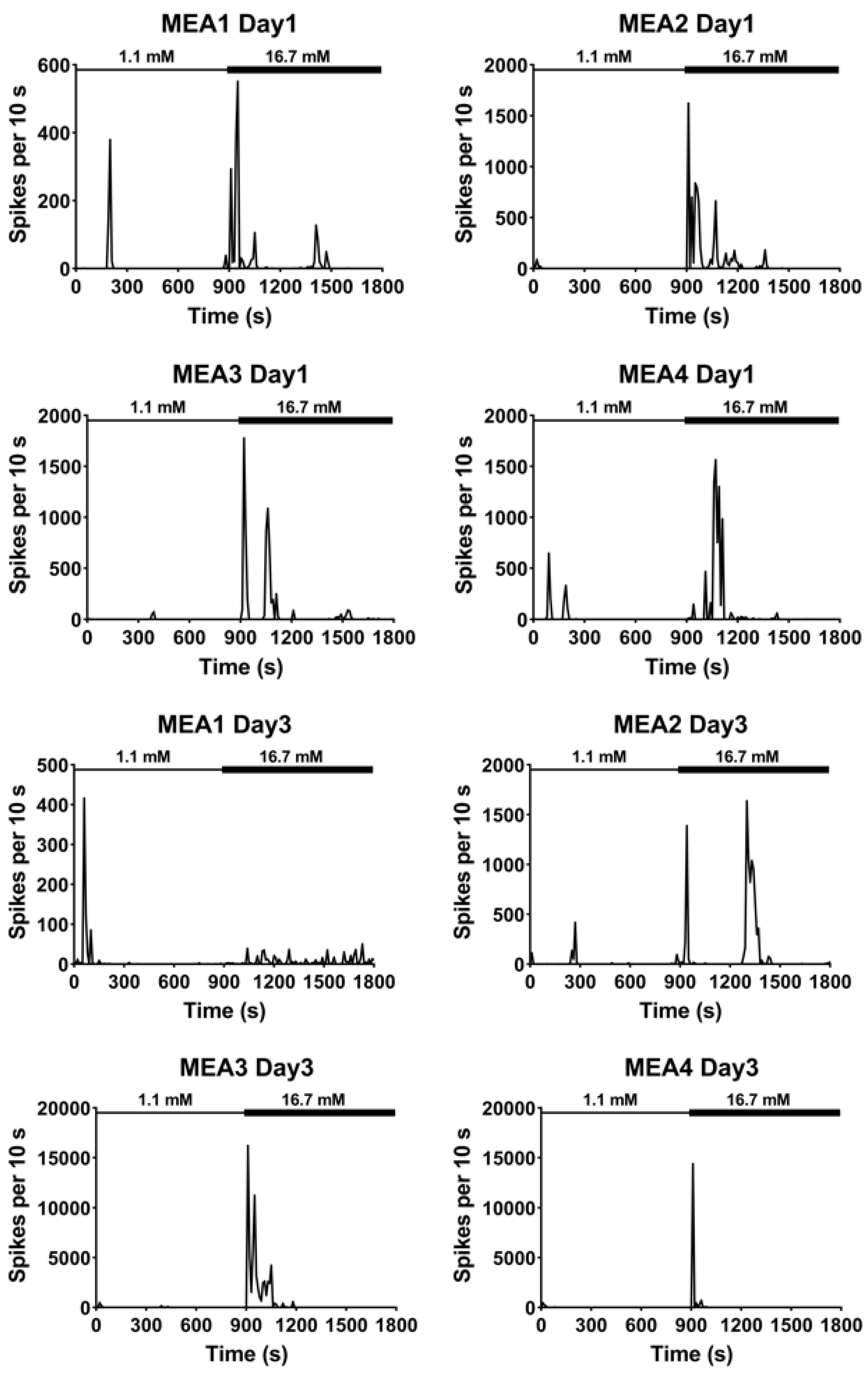
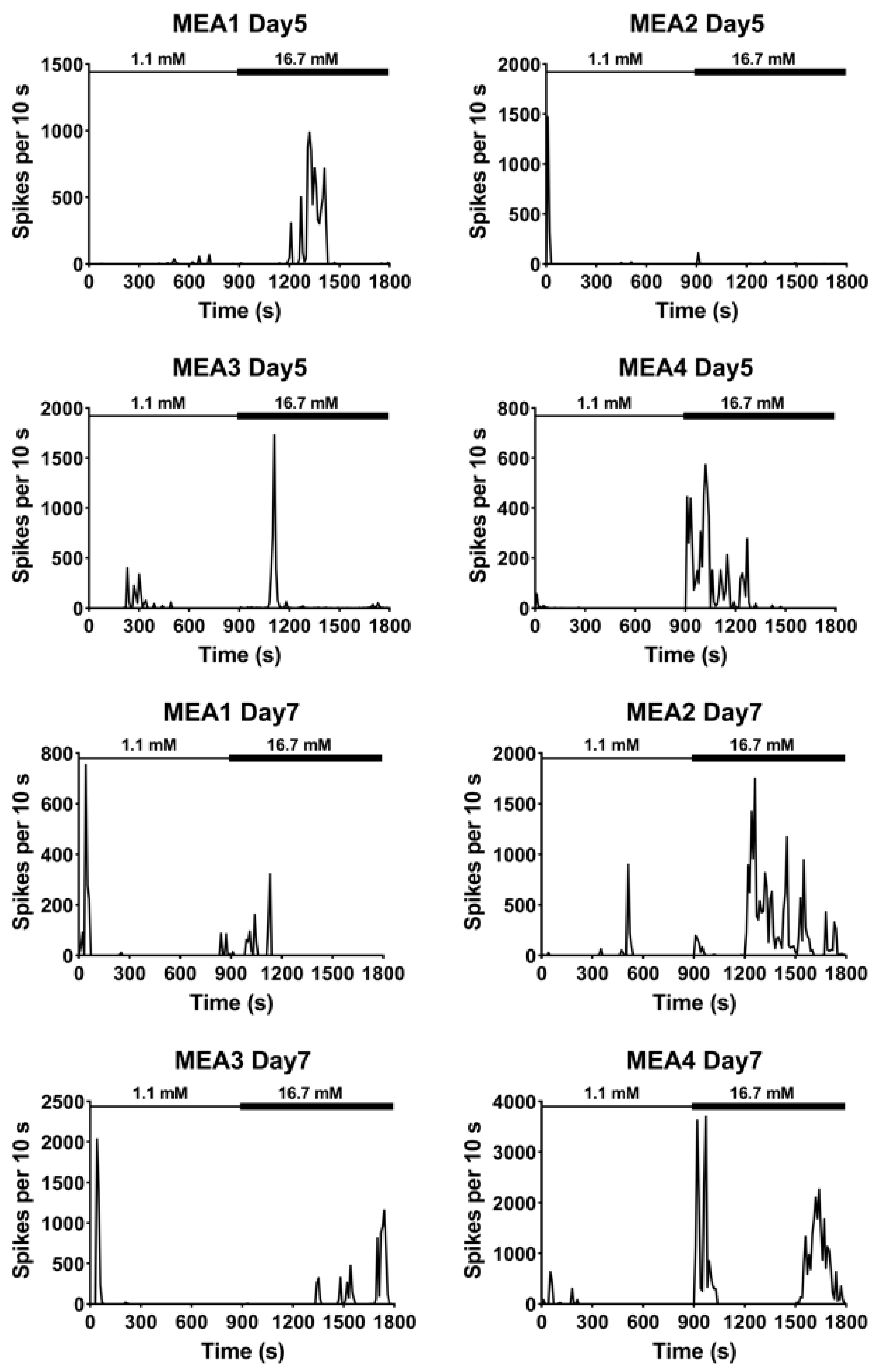
3.2. Extracellular Recordings of Dissociated Islets
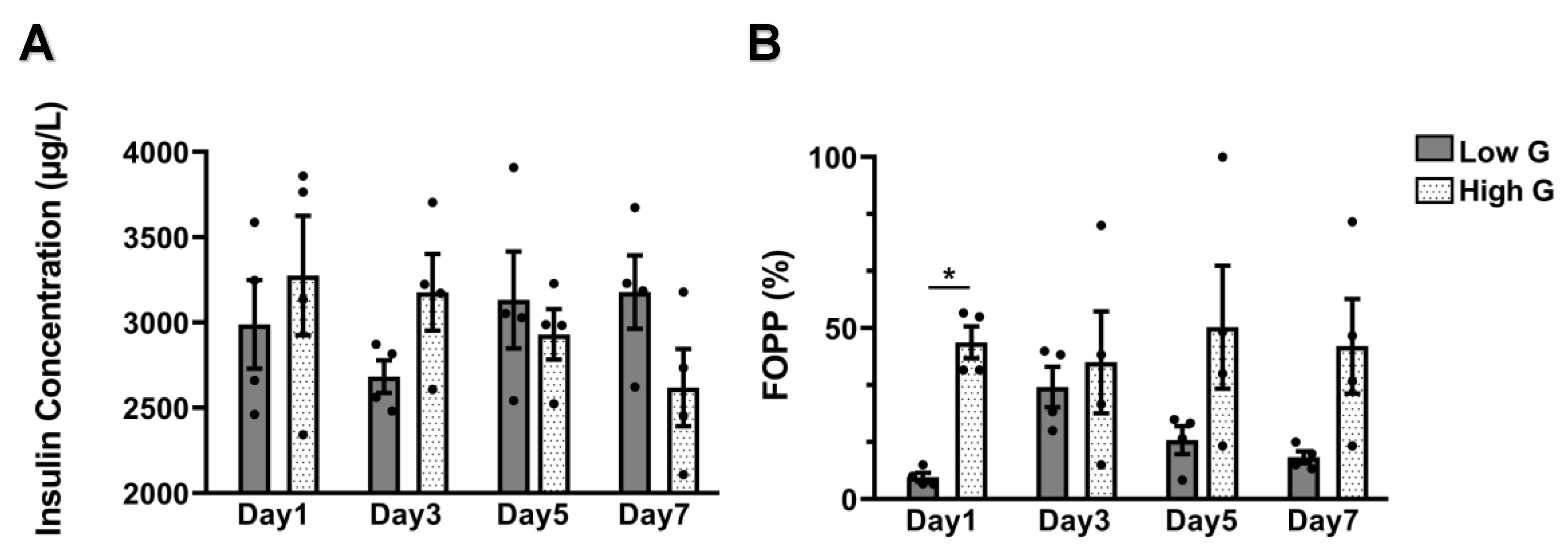
3.3. Insulin Secretion and FOPP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lehmann, R.; Zuellig, R.A.; Kugelmeier, P.; Baenninger, P.B.; Moritz, W.; Perren, A.; Clavien, P.A.; Weber, M.; Spinas, G.A. Superiority of small islets in human islet transplantation. Diabetes 2007, 56, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Elayat, A.A.; el-Naggar, M.M.; Tahir, M. An immunocytochemical and morphometric study of the rat pancreatic islets. J. Anat. 1995, 186 Pt 3, 629–637. [Google Scholar]
- Buse, J.; Polonsky, K.; Burant, C. Williams Textbook of Endocrinology, 11th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011; pp. 1371–1435. [Google Scholar]
- Burrack, A.L.; Martinov, T.; Fife, B.T. T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes. Front. Endocrinol. (Lausanne) 2017, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Drews, G.; Krippeit-Drews, P.; Düfer, M. Electrophysiology of islet cells. Adv. Exp. Med. Biol. 2010, 654, 115–163. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J. Regulation of insulin secretion: A matter of phase control and amplitude modulation. Clin. Exp. Diabetes Metab. 2009, 52, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Rorsman, P.; Gopel, S.O. Glucose-dependent regulation of rhythmic action potential firing in pancreatic beta-cells by K(ATP)-channel modulation. J. Physiol. 2002, 545, 501–507. [Google Scholar] [CrossRef]
- Shuvaev, A.N.; Salmin, V.V.; Kuvacheva, N.V.; Pozhilenkova, E.A.; Morgun, A.V.; Lopatina, O.L.; Salmina, A.B.; Illarioshkin, S.N. Current advances in cell electrophysiology: Applications for the analysis of intercellular communications within the neurovascular unit. Rev. Neurosci. 2016, 27, 365. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Kraushaar, U.; Düfer, M.; Schonecker, S.; Haspel, D.; Günther, E.; Drews, G.; Krippeit-Drews, P. Rapid functional evaluation of beta-cells by extracellular recording of membrane potential oscillations with microelectrode arrays. Eur. J. Physiol. 2011, 462, 835–840. [Google Scholar] [CrossRef]
- Brouwer, S.; Hoffmeister, T.; Gresch, A.; Schönhoff, L.; Düfer, M. Resveratrol influences pancreatic islets by opposing effects on electrical activity and insulin release. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Perrier, R.; Pirog, A.; Jaffredo, M.; Gaitan, J.; Catargi, B.; Renaud, S.; Raoux, M.; Lang, J. Bioelectronic organ-based sensor for microfluidic real-time analysis of the demand in insulin. Biosens. Bioelectron. 2018, 117, 253–259. [Google Scholar] [CrossRef]
- Schonecker, S.; Kraushaar, U.; Düfer, M.; Sahr, A.; Hrdtner, C.; Guenther, E.; Walther, R.; Lendeckel, U.; Barthlen, W.; Krippeit-Drews, P.; et al. Long-term culture and functionality of pancreatic islets monitored using microelectrode arrays. Integr. Biol. 2014, 6, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Raoux, M.; Bornat, Y.; Quotb, A.; Catargi, B.; Renaud, S.; Lang, J. Non-invasive long-term and real-time analysis of endocrine cells on micro-electrode arrays. J. Physiol. 2012, 590, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Micholt, E.; Jans, D.; Callewaert, G.; Bartic, C.; Lammertyn, J.; Nicolaï, B. Extracellular recordings from rat olfactory epithelium slices using micro electrode arrays. Sens. Actuators B Chem. 2013, 184, 40–47. [Google Scholar] [CrossRef]
- Phelps, E.; Cianciaruso, C.; Santo-Domingo, J.; Pasquier, M.; Galliverti, G.; Piemonti, L.; Berishvili, E.; Burri, O.; Wiederkehr, A.; Hubbell, J.; et al. Advances in pancreatic islet monolayer culture on glass surfaces enable super-resolution microscopy and insights into beta cell ciliogenesis and proliferation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Tovar, K.R.; Bridges, D.C.; Wu, B.; Randall, C.; Audouard, M.; Jang, J.; Hansma, P.K.; Kosik, K.S. Action potential propagation recorded from single axonal arbors using multielectrode arrays. J. Neurophysiol. 2018, 120, 306. [Google Scholar] [CrossRef]
- Fridlyand, L.E.; Tamarina, N.; Philipson, L.H. Bursting and calcium oscillations in pancreatic beta-cells: Specific pacemakers for specific mechanisms. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E517–E532. [Google Scholar] [CrossRef]
- Buchwald, P.; Tamayo-Garcia, A.; Manzoli, V.; Tomei, A.A.; Stabler, C.L. Glucose-stimulated insulin release: Parallel perifusion studies of free and hydrogel encapsulated human pancreatic islets. Biotechnol. Bioeng. 2018, 115, 232–245. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef]
- Aathira, R.; Jain, V. Advances in management of type 1 diabetes mellitus. World J. Diabetes 2014, 5, 689–696. [Google Scholar] [CrossRef]
- Bruni, A.; Gala-Lopez, B.; Pepper, A.R.; Abualhassan, N.S.; Shapiro, A.J. Islet cell transplantation for the treatment of type 1 diabetes: Recent advances and future challenges. Diabetes Metab. Syndr. Obes. 2014, 7, 211–223. [Google Scholar] [CrossRef]
- Correa-Giannella, M.L.; Raposo do Amaral, A.S. Pancreatic islet transplantation. Diabetol. Metab. Syndr. 2009, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Schonecker, S.; Kraushaar, U.; Günther, E.; Häring, H.-U.; Königsrainer, A.; Drews, G.; Krippeit-Drews, P.; Gerst, F.; Ullrich, S. Human islets exhibit electrical activity on microelectrode arrays (MEA). Exp. Clin. Endocrinol. Diabetes 2015, 123, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Castiello, F.R.; Heileman, K.; Tabrizian, M. Microfluidic perfusion systems for secretion fingerprint analysis of pancreatic islets: Applications, challenges and opportunities. Lab Chip 2016, 16, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Ashcroft, F.M. Pancreatic β-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef]
- Fridlyand, L.E.; Jacobson, D.A.; Philipson, L.H. Ion channels and regulation of insulin secretion in human β-cells: A computational systems analysis. Islets 2013, 5, 1–15. [Google Scholar] [CrossRef]
- Udo, K.; Sven, S.; Peter, K.-D.; Gisela, D.; Elke, G. Parallelization of MEA-based electrophysiological recordings of murine and human islets of Langerhans. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Lefèbvre, P.; Paolisso, G.; Scheen, A.; Henquin, J. Pulsatility of insulin and glucagon release: Physiological significance and pharmacological implications. Clin. Exp. Diabetes Metab. 1987, 30, 443–452. [Google Scholar] [CrossRef]
- Polonsky, K.S.; Given, B.D.; Van Cauter, E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J. Clin. Investig. 1988, 81, 442–448. [Google Scholar] [CrossRef]
- Henquin, J.-C.; Ishiyama, N.; Nenquin, M.; Ravier, M.A.; Jonas, J.-C. Signals and pools underlying biphasic insulin secretion. Diabetes 2002, 51 (Suppl. 1), S60–S67. [Google Scholar] [CrossRef]
- Henquin, J.-C.; Nenquin, M.; Stiernet, P.; Ahren, B. In Vivo and In Vitro Glucose-Induced Biphasic Insulin Secretion in the Mouse: Pattern and Role of Cytoplasmic Ca2+ and Amplification Signals in β-Cells. Diabetes 2006, 55, 441–451. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alassaf, A.; Ishahak, M.; Bowles, A.; Agarwal, A. Microelectrode Array based Functional Testing of Pancreatic Islet Cells. Micromachines 2020, 11, 507. https://doi.org/10.3390/mi11050507
Alassaf A, Ishahak M, Bowles A, Agarwal A. Microelectrode Array based Functional Testing of Pancreatic Islet Cells. Micromachines. 2020; 11(5):507. https://doi.org/10.3390/mi11050507
Chicago/Turabian StyleAlassaf, Ahmad, Matthew Ishahak, Annie Bowles, and Ashutosh Agarwal. 2020. "Microelectrode Array based Functional Testing of Pancreatic Islet Cells" Micromachines 11, no. 5: 507. https://doi.org/10.3390/mi11050507
APA StyleAlassaf, A., Ishahak, M., Bowles, A., & Agarwal, A. (2020). Microelectrode Array based Functional Testing of Pancreatic Islet Cells. Micromachines, 11(5), 507. https://doi.org/10.3390/mi11050507





