Generation of Ultra-Thin-Shell Microcapsules Using Osmolarity-Controlled Swelling Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
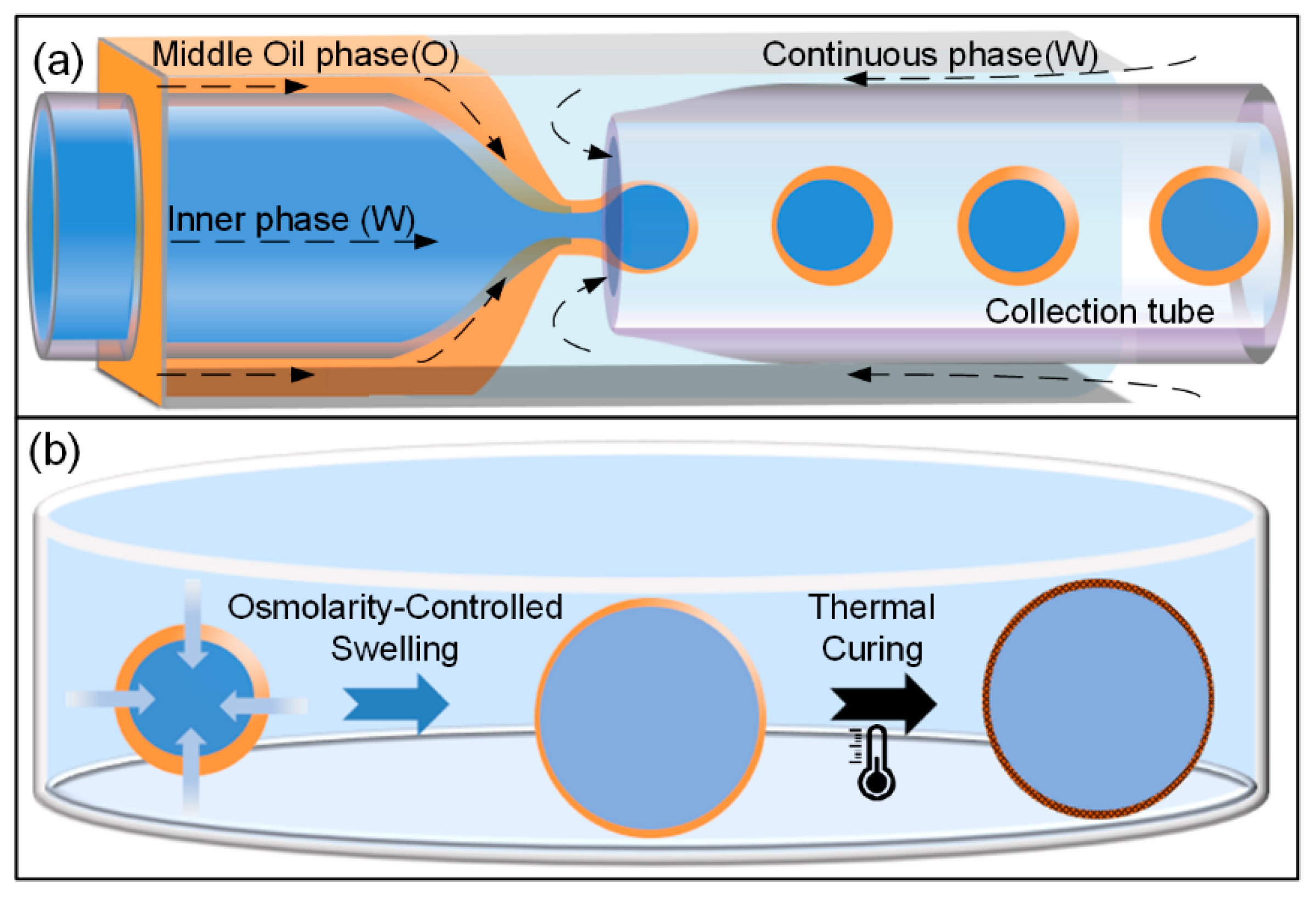
2.2. Fabrication of the Glass Capillary Microfluidic Device
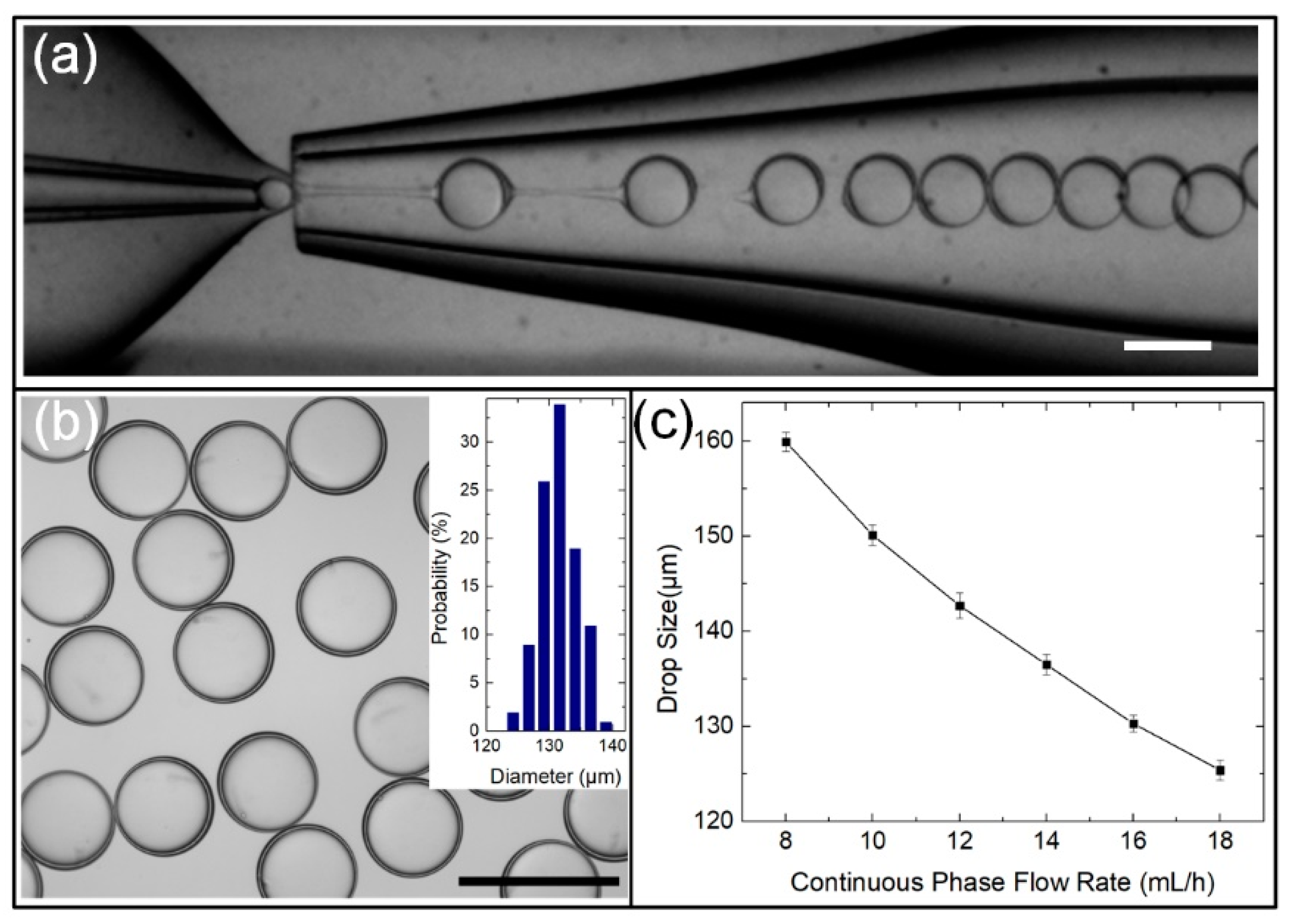
2.3. Generation of Monodisperse W/O/W Double-Emulsion Drops
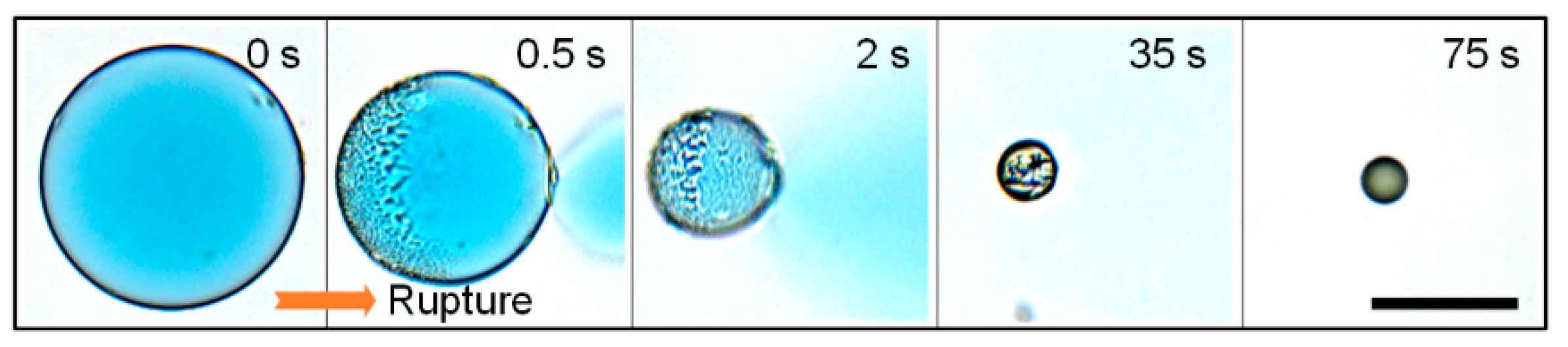
2.4. Electrotriggered Rupturing of W/O/W Double-Emulsion Drops
3. Results and Discussion
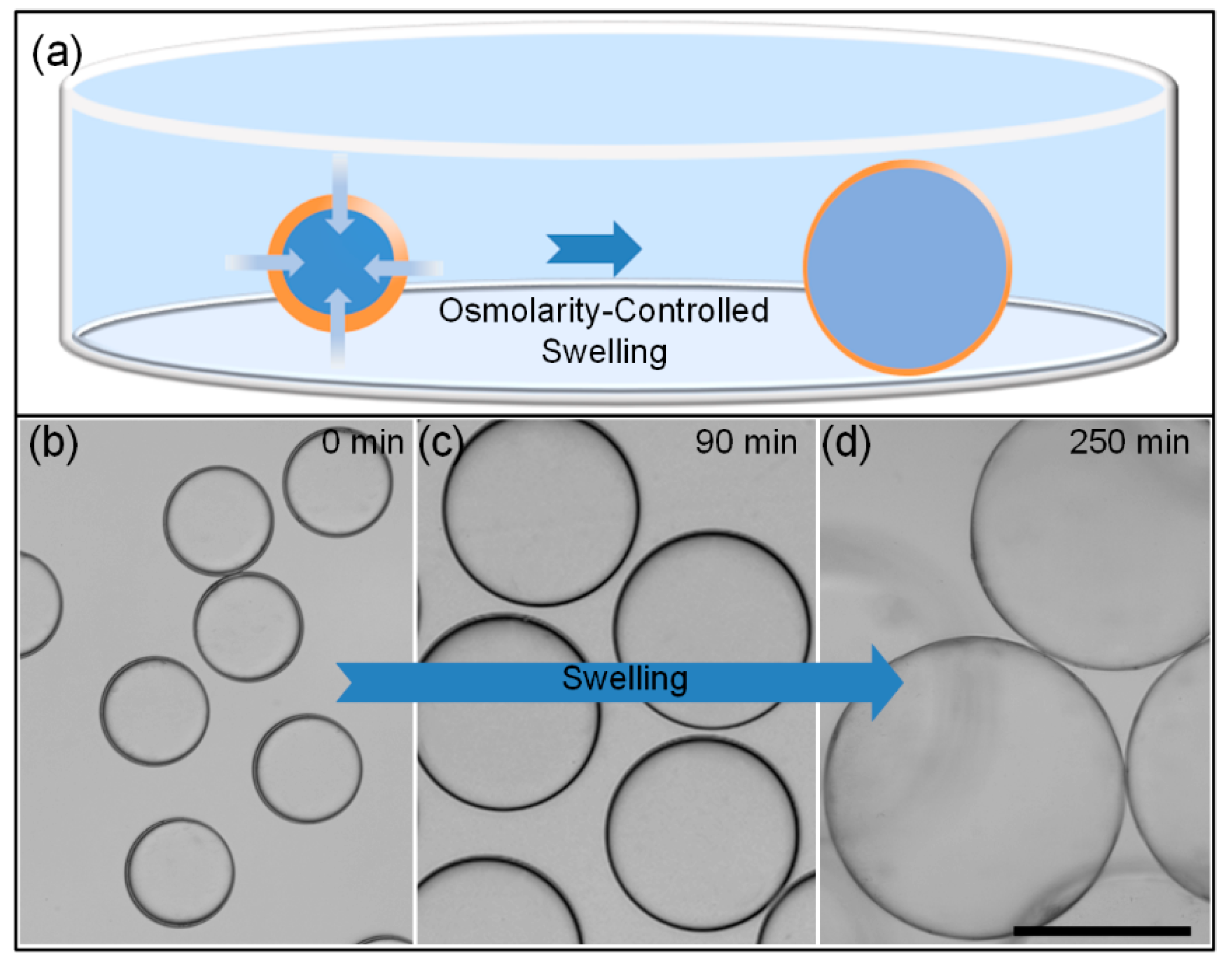
3.1. Osmolarity-Controlled Swelling Behavior
3.2. Measuring of the Shell Thickness
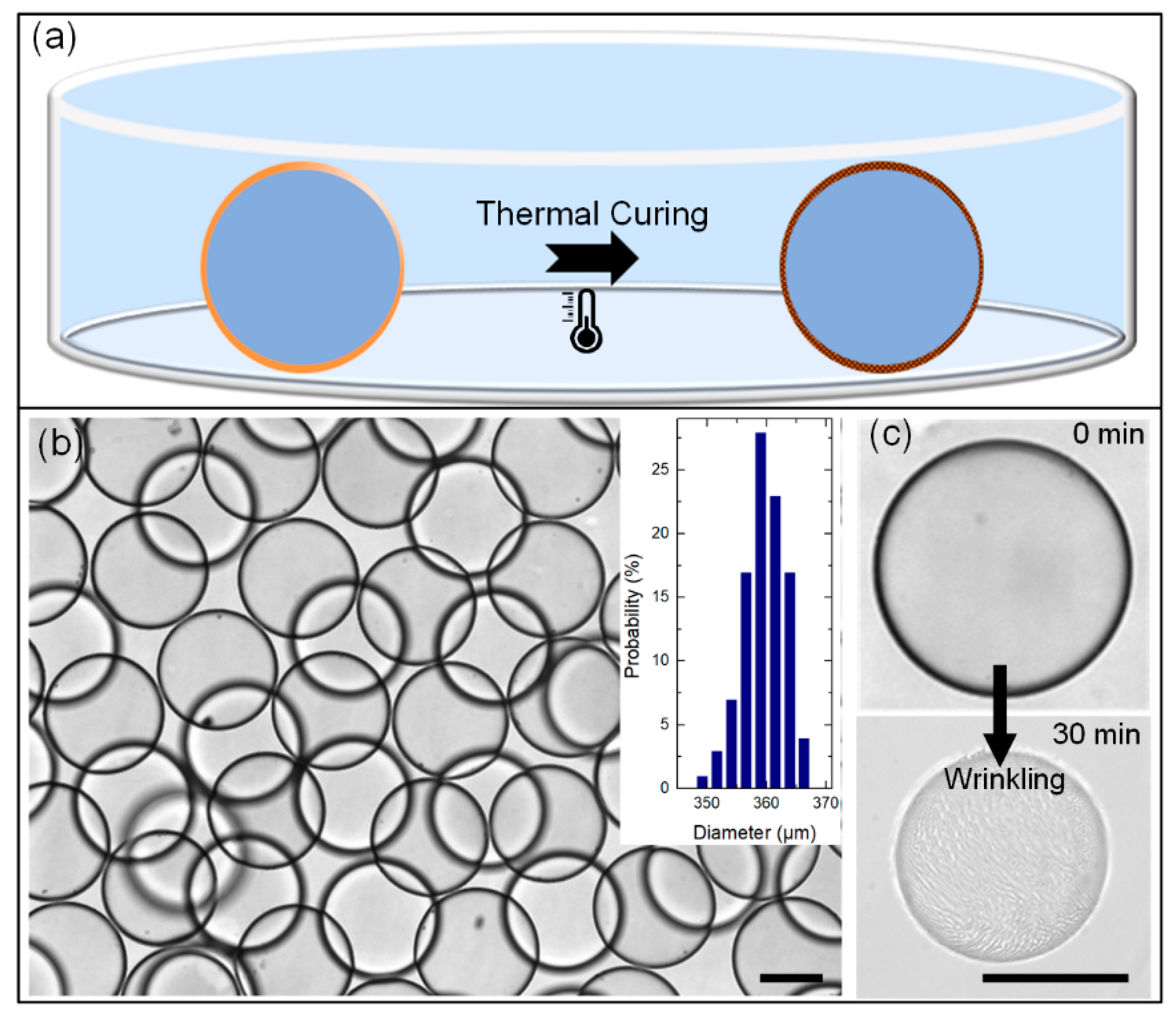
3.3. Curing Process – Transform the Double-Emulsion Drops to Solid Core-Shell Microcapsules
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Datta, S.S.; Abbaspourrad, A.; Amstad, E.; Fan, J.; Kim, S.H.; Romanowsky, M.; Shum, H.C.; Sun, B.; Utada, A.S.; Windbergs, M.; et al. 25th anniversary article: Double emulsion templated solid microcapsules: Mechanics and controlled release. Adv. Mater. 2014, 26, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Barea, J.; Lee, J.; Kang, D.K. Recent Advances in Droplet-based Microfluidic Technologies for Biochemistry and Molecular Biology. Micromachines (Basel) 2019, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Chen, J.; He, T.; Hu, Y.; Dong, X.; Zhang, H.; Huang, W.; Ko, F.; Zhou, W. Electrospray biodegradable microcapsules loaded with curcumin for drug delivery systems with high bioactivity. RSC Adv. 2017, 7, 1724–1734. [Google Scholar] [CrossRef]
- Lee, W.L.; Tan, J.W.; Tan, C.N.; Loo, S.C. Modulating drug release from gastric-floating microcapsules through spray-coating layers. PLoS ONE 2014, 9, e114284. [Google Scholar] [CrossRef] [PubMed]
- You, X.R.; Ju, X.J.; He, F.; Wang, Y.; Liu, Z.; Wang, W.; Xie, R.; Chu, L.Y. Polymersomes with Rapid K(+)-Triggered Drug-Release Behaviors. ACS Appl. Mater. Interfaces 2017, 9, 19258–19268. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dong, H.; Tang, G.; Ma, T.; Cao, X. Controllable microfluidic fabrication of Janus and microcapsule particles for drug delivery applications. RSC Adv. 2015, 5, 23181–23188. [Google Scholar] [CrossRef]
- Sun, S.; Liang, N.; Gong, X.; An, W.; Kawashima, Y.; Cui, F.; Yan, P. Multifunctional Composite Microcapsules for Oral Delivery of Insulin. Int. J. Mol. Sci. 2016, 18, 54. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, J.G.; Choi, T.M.; Manoharan, V.N.; Weitz, D.A. Osmotic-pressure-controlled concentration of colloidal particles in thin-shelled capsules. Nat. Commun. 2014, 5, 3068. [Google Scholar] [CrossRef]
- Choi, T.M.; Je, K.; Park, J.G.; Lee, G.H.; Kim, S.H. Photonic Capsule Sensors with Built-In Colloidal Crystallites. Adv. Mater. 2018, 30, e1803387. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Z.; Gu, H.; Jin, L.; Zhao, X.; Wang, B.; Gu, Z. Multifunctional photonic crystal barcodes from microfluidics. NPG Asia Mater. 2012, 4, e25. [Google Scholar] [CrossRef]
- Niepa, T.H.; Hou, L.; Jiang, H.; Goulian, M.; Koo, H.; Stebe, K.J.; Lee, D. Microbial Nanoculture as an Artificial Microniche. Sci. Rep. 2016, 6, 30578. [Google Scholar] [CrossRef]
- Chang, C.B.; Wilking, J.N.; Kim, S.H.; Shum, H.C.; Weitz, D.A. Monodisperse Emulsion Drop Microenvironments for Bacterial Biofilm Growth. Small 2015, 11, 3954–3961. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, W.; Abbaspourrad, A.; Ahn, J.; Bader, A.; Bose, S.; Vegas, A.; Lin, J.; Tao, J.; Hang, T.; et al. Microfluidic Fabrication of Colloidal Nanomaterials-Encapsulated Microcapsules for Biomolecular Sensing. Nano Lett. 2017, 17, 2015–2020. [Google Scholar] [CrossRef]
- Chen, P.W.; Erb, R.M.; Studart, A.R. Designer polymer-based microcapsules made using microfluidics. Langmuir 2012, 28, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef]
- Goertz, J.P.; DeMella, K.C.; Thompson, B.R.; White, I.M.; Raghavan, S.R. Responsive capsules that enable hermetic encapsulation of contents and their thermally triggered burst-release. Mater. Horiz. 2019, 6, 1238–1243. [Google Scholar] [CrossRef]
- Grolman, J.M.; Inci, B.; Moore, J.S. pH-Dependent Switchable Permeability from Core–Shell Microcapsules. ACS Macro Lett. 2015, 4, 441–445. [Google Scholar] [CrossRef]
- Zhou, S.; Fan, J.; Datta, S.S.; Guo, M.; Guo, X.; Weitz, D.A. Thermally Switched Release from Nanoparticle Colloidosomes. Adv. Funct. Mater. 2013, 23, 5925–5929. [Google Scholar] [CrossRef]
- Windbergs, M.; Zhao, Y.; Heyman, J.; Weitz, D.A. Biodegradable core-shell carriers for simultaneous encapsulation of synergistic actives. J. Am. Chem. Soc. 2013, 135, 7933–7937. [Google Scholar] [CrossRef] [PubMed]
- DiLauro, A.M.; Abbaspourrad, A.; Weitz, D.A.; Phillips, S.T. Stimuli-Responsive Core–Shell Microcapsules with Tunable Rates of Release by Using a Depolymerizable Poly(phthalaldehyde) Membrane. Macromolecules 2013, 46, 3309–3313. [Google Scholar] [CrossRef]
- Abbaspourrad, A.; Datta, S.S.; Weitz, D.A. Controlling release from pH-responsive microcapsules. Langmuir 2013, 29, 12697–12702. [Google Scholar] [CrossRef]
- Abbaspourrad, A.; Carroll, N.J.; Kim, S.H.; Weitz, D.A. Polymer microcapsules with programmable active release. J. Am. Chem. Soc. 2013, 135, 7744–7750. [Google Scholar] [CrossRef]
- Amstad, E.; Kim, S.H.; Weitz, D.A. Photo- and thermoresponsive polymersomes for triggered release. Angew. Chem. Int. Ed. Engl. 2012, 51, 12499–12503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Luo, J.; Zhang, D.X.; Jing, T.F.; Li, B.X.; Liu, F. Porous microcapsules with tunable pore sizes provide easily controllable release and bioactivity. J. Colloid Interface Sci. 2018, 517, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.-L.; Wang, W.; Li, Z.-L.; Ju, X.-J.; Xie, R.; Deng, N.-N.; Wei, J.; Liu, Z.; Chu, L.-Y. Trojan-Horse-Like Stimuli-Responsive Microcapsules. Adv. Sci. 2018, 5, 1700960. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, T.Y.; Amstad, E.; Kim, S.H. Microfluidic Production of Capsules-in-Capsules for Programed Release of Multiple Ingredients. Adv. Mater. Technol.-US 2018, 3, 1800006. [Google Scholar] [CrossRef]
- Sun, B.J.; Shum, H.C.; Holtze, C.; Weitz, D.A. Microfluidic melt emulsification for encapsulation and release of actives. ACS Appl. Mater. Interfaces 2010, 2, 3411–3416. [Google Scholar] [CrossRef]
- Wang, W.; Luo, T.; Ju, X.J.; Xie, R.; Liu, L.; Chu, L.Y. Microfluidic Preparation of Multicompartment Microcapsules for Isolated Co-encapsulation and Controlled Release of Diverse Components. Int. J. Nonlinear Sci. Numer. Simul. 2012, 13, 325–332. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.W.; Kim, D.H.; Han, S.H.; Weitz, D.A. Polymersomes containing a hydrogel network for high stability and controlled release. Small 2013, 9, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Wu, W.; Ju, X.-J.; Wang, W.; Xie, R.; Mou, C.-L.; Zheng, W.-C.; Liu, Z.; Chu, L.-Y. Smart microcapsules for direction-specific burst release of hydrophobic drugs. RSC Adv. 2014, 4, 46568–46575. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Y.; Chen, L.; Zhong, J.; Johri, A.M.; Zhou, J. Multimodality imaging-guided local injection of eccentric magnetic microcapsules with electromagnetically controlled drug release. Cancer Rep. 2018, 2, e1154. [Google Scholar] [CrossRef]
- Huang, J.; Luo, C.; Li, W.; Li, Y.; Zhang, Y.S.; Zhou, J.; Jiang, Q. Eccentric magnetic microcapsules for orientation-specific and dual stimuli-responsive drug release. J. Mater. Chem. B 2015, 3, 4530–4538. [Google Scholar] [CrossRef]
- Katifori, E.; Alben, S.; Cerda, E.; Nelson, D.R.; Dumais, J. Foldable structures and the natural design of pollen grains. Proc. Natl. Acad. Sci. USA 2010, 107, 7635–7639. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.W.; Cho, J.C.; Weitz, D.A. Double-emulsion drops with ultra-thin shells for capsule templates. Lab Chip 2011, 11, 3162–3166. [Google Scholar] [CrossRef]
- Arriaga, L.R.; Datta, S.S.; Kim, S.H.; Amstad, E.; Kodger, T.E.; Monroy, F.; Weitz, D.A. Ultrathin shell double emulsion templated giant unilamellar lipid vesicles with controlled microdomain formation. Small 2014, 10, 950–956. [Google Scholar] [CrossRef]
- Zhao, C.X.; Chen, D.; Hui, Y.; Weitz, D.A.; Middelberg, A.P.J. Controlled Generation of Ultrathin-Shell Double Emulsions and Studies on Their Stability. Chemphyschem 2017, 18, 1393–1399. [Google Scholar] [CrossRef]
- Zhang, W.X.; Abbaspourrad, A.; Chen, D.; Campbell, E.; Zhao, H.; Li, Y.W.; Li, Q.N.; Weitz, D.A. Osmotic Pressure Triggered Rapid Release of Encapsulated Enzymes with Enhanced Activity. Adv. Funct. Mater. 2017, 27, 1700975. [Google Scholar] [CrossRef]
- Lee, T.Y.; Ku, M.; Kim, B.; Lee, S.; Yang, J.; Kim, S.H. Microfluidic Production of Biodegradable Microcapsules for Sustained Release of Hydrophilic Actives. Small 2017, 13, 1700646. [Google Scholar] [CrossRef]
- Kim, B.; Jeon, T.Y.; Oh, Y.K.; Kim, S.H. Microfluidic Production of Semipermeable Microcapsules by Polymerization-Induced Phase Separation. Langmuir 2015, 31, 6027–6034. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, A.S.; Josephides, D.N.; Sajjadi, S. Large ultrathin shelled drops produced via non-confined microfluidics. Chemphyschem 2015, 16, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Hou, L.; Ren, Y.; Deng, X.; Lang, Q.; Jia, Y.; Hu, Q.; Tao, Y.; Liu, J.; Jiang, H. A dual-core double emulsion platform for osmolarity-controlled microreactor triggered by coalescence of encapsulated droplets. Biomicrofluidics 2016, 10, 034111. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Ren, Y.; Jia, Y.; Chen, X.; Deng, X.; Tang, Z.; Hu, Q.; Tao, Y.; Jiang, H. Osmolarity-controlled swelling behaviors of dual-cored double-emulsion drops. Microfluid. Nanofluid. 2017, 21, 60. [Google Scholar] [CrossRef]
- Lee, D.; Weitz, D.A. Double Emulsion-Templated Nanoparticle Colloidosomes with Selective Permeability. Adv. Mater. 2008, 20, 3498–3503. [Google Scholar] [CrossRef]
- Hennequin, Y.; Pannacci, N.; de Torres, C.P.; Tetradis-Meris, G.; Chapuliot, S.; Bouchaud, E.; Tabeling, P. Synthesizing microcapsules with controlled geometrical and mechanical properties with microfluidic double emulsion technology. Langmuir 2009, 25, 7857–7861. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Ren, Y.; Hou, L.; Liu, W.; Jiang, T.; Deng, X.; Tao, Y.; Jiang, H. Electrically controlled rapid release of actives encapsulated in double-emulsion droplets. Lab Chip 2018, 18, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Ren, Y.; Hou, L.; Liu, W.; Jia, Y.; Jiang, H. Electric Field-Induced Cutting of Hydrogel Microfibers with Precise Length Control for Micromotors and Building Blocks. ACS Appl. Mater. Interfaces 2018, 10, 40228–40237. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Ren, Y.; Hou, L.; Liu, W.; Jiang, T.; Jiang, H. Compound-Droplet-Pairs-Filled Hydrogel Microfiber for Electric-Field-Induced Selective Release. Small 2019, 15, e1903098. [Google Scholar] [CrossRef]
- Tu, F.; Lee, D. Controlling the stability and size of double-emulsion-templated poly(lactic-co-glycolic) acid microcapsules. Langmuir 2012, 28, 9944–9952. [Google Scholar] [CrossRef]
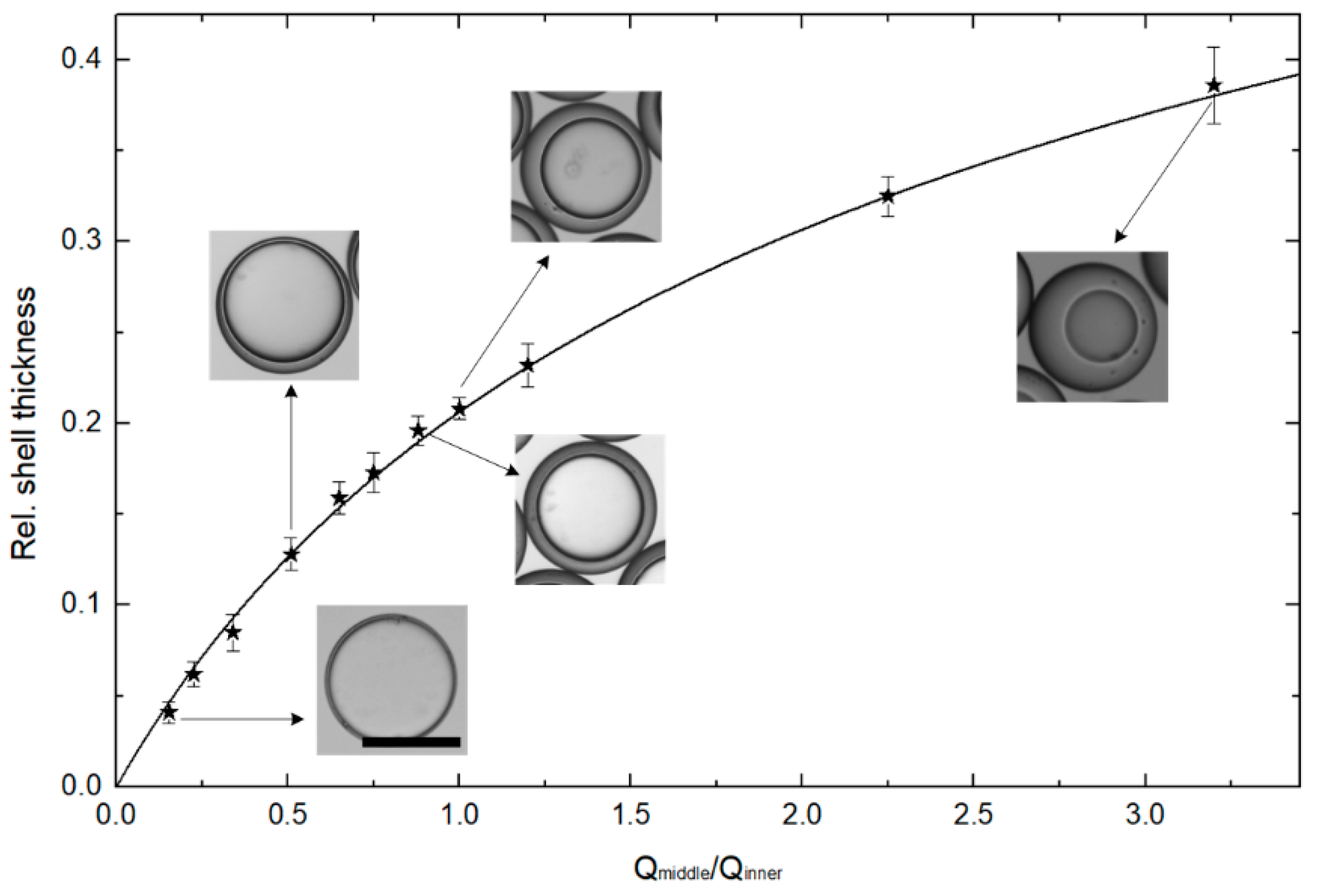
| Qmiddle/Qinner | Rel. Shell Thickness | D (μm) | h (μm) |
|---|---|---|---|
| 0.154 | 0.041 | 132.5 | 2.7 |
| 0.511 | 0.128 | 139.6 | 8.9 |
| 0.881 | 0.196 | 136.6 | 13.4 |
| 1.00 | 0.208 | 133.5 | 13.9 |
| 3.20 | 0.386 | 132.0 | 25.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Hou, L.; Hou, J.; Yu, J.; Hu, Q. Generation of Ultra-Thin-Shell Microcapsules Using Osmolarity-Controlled Swelling Method. Micromachines 2020, 11, 444. https://doi.org/10.3390/mi11040444
Guo J, Hou L, Hou J, Yu J, Hu Q. Generation of Ultra-Thin-Shell Microcapsules Using Osmolarity-Controlled Swelling Method. Micromachines. 2020; 11(4):444. https://doi.org/10.3390/mi11040444
Chicago/Turabian StyleGuo, Jianhua, Lihua Hou, Junpeng Hou, Jiali Yu, and Qingming Hu. 2020. "Generation of Ultra-Thin-Shell Microcapsules Using Osmolarity-Controlled Swelling Method" Micromachines 11, no. 4: 444. https://doi.org/10.3390/mi11040444
APA StyleGuo, J., Hou, L., Hou, J., Yu, J., & Hu, Q. (2020). Generation of Ultra-Thin-Shell Microcapsules Using Osmolarity-Controlled Swelling Method. Micromachines, 11(4), 444. https://doi.org/10.3390/mi11040444




