Swelling Studies of Porous and Nonporous Semi-IPN Hydrogels for Sensor and Actuator Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
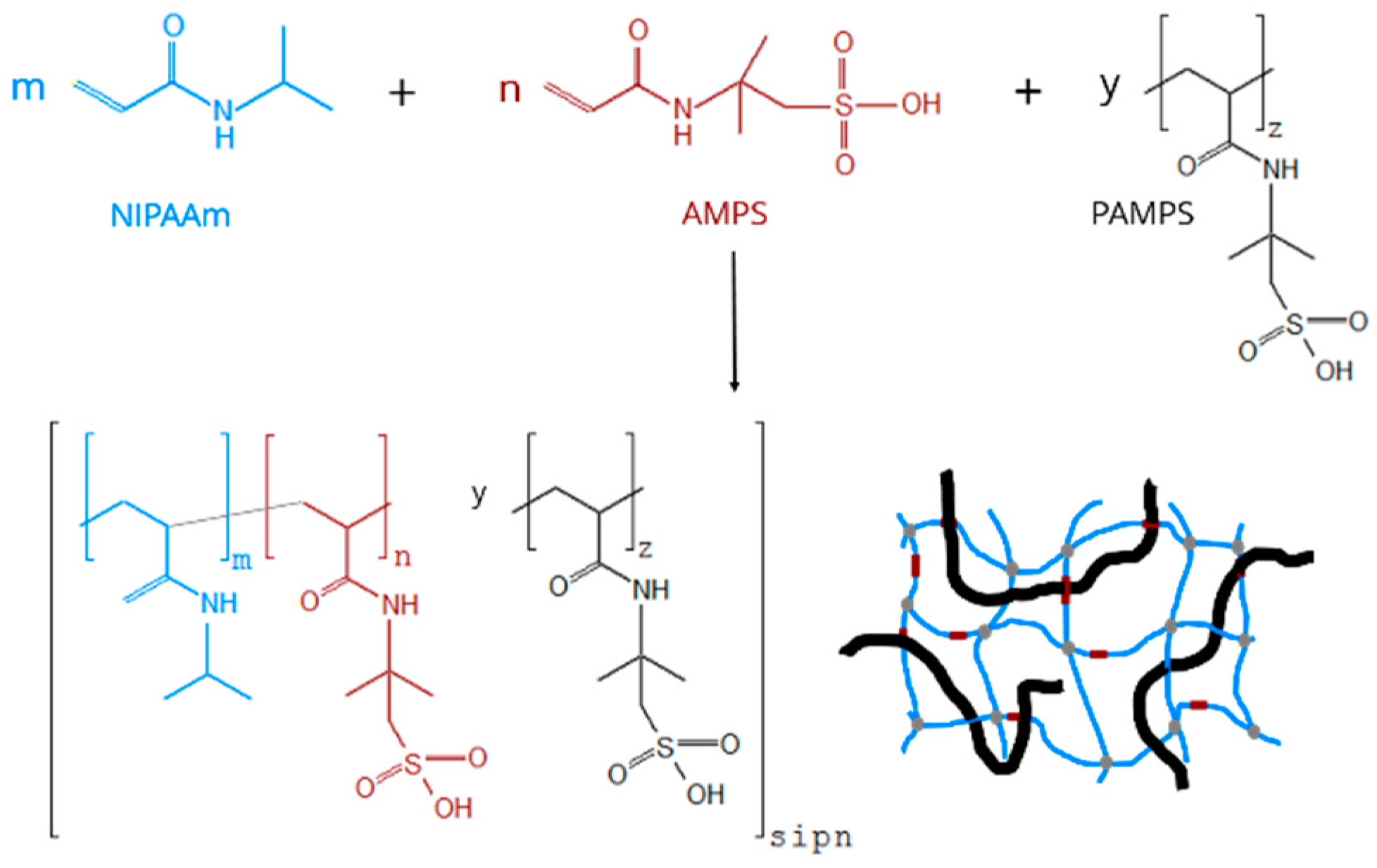
2.2. Syntheses
2.3. Scanning Electron Microscopy (SEM) Observation
2.4. Swelling Kinetics
2.5. Static Degrees of Swelling
3. Results and Discussion
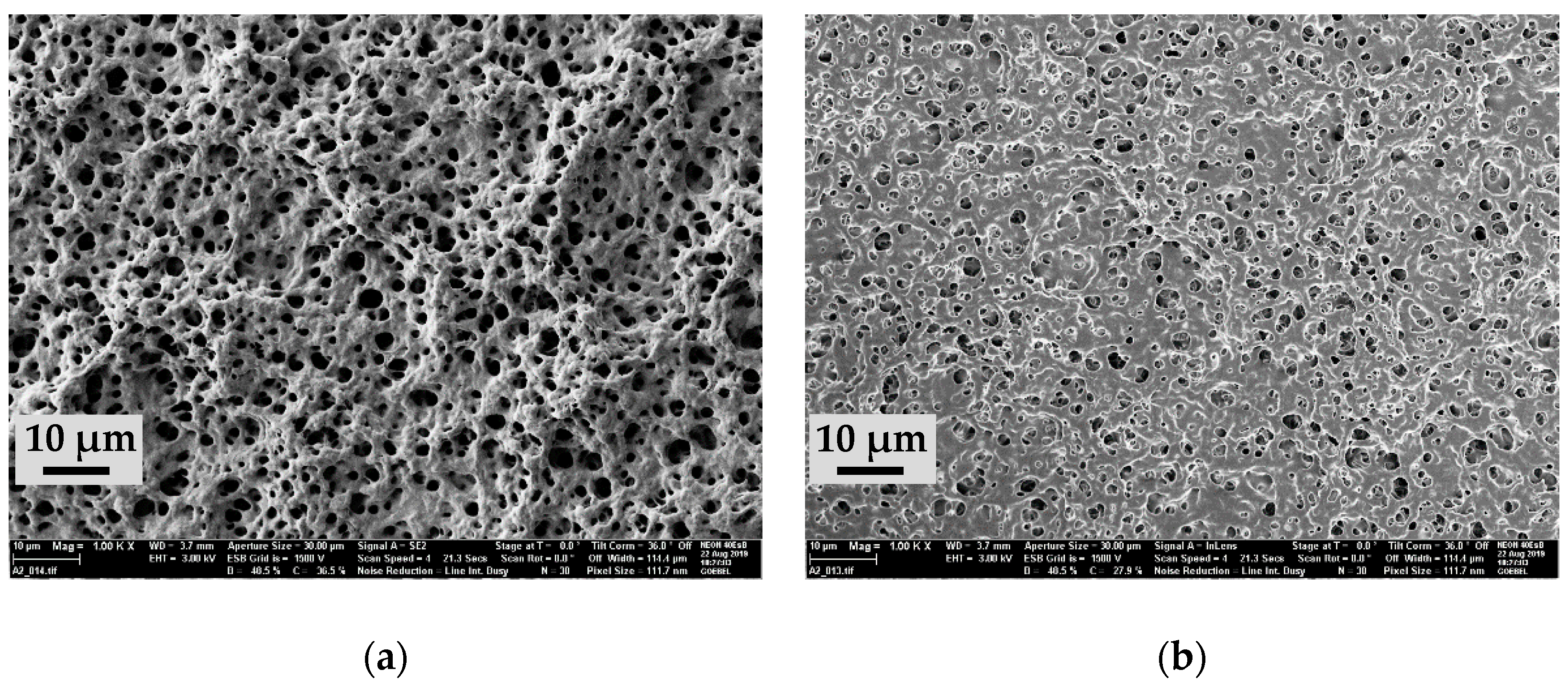
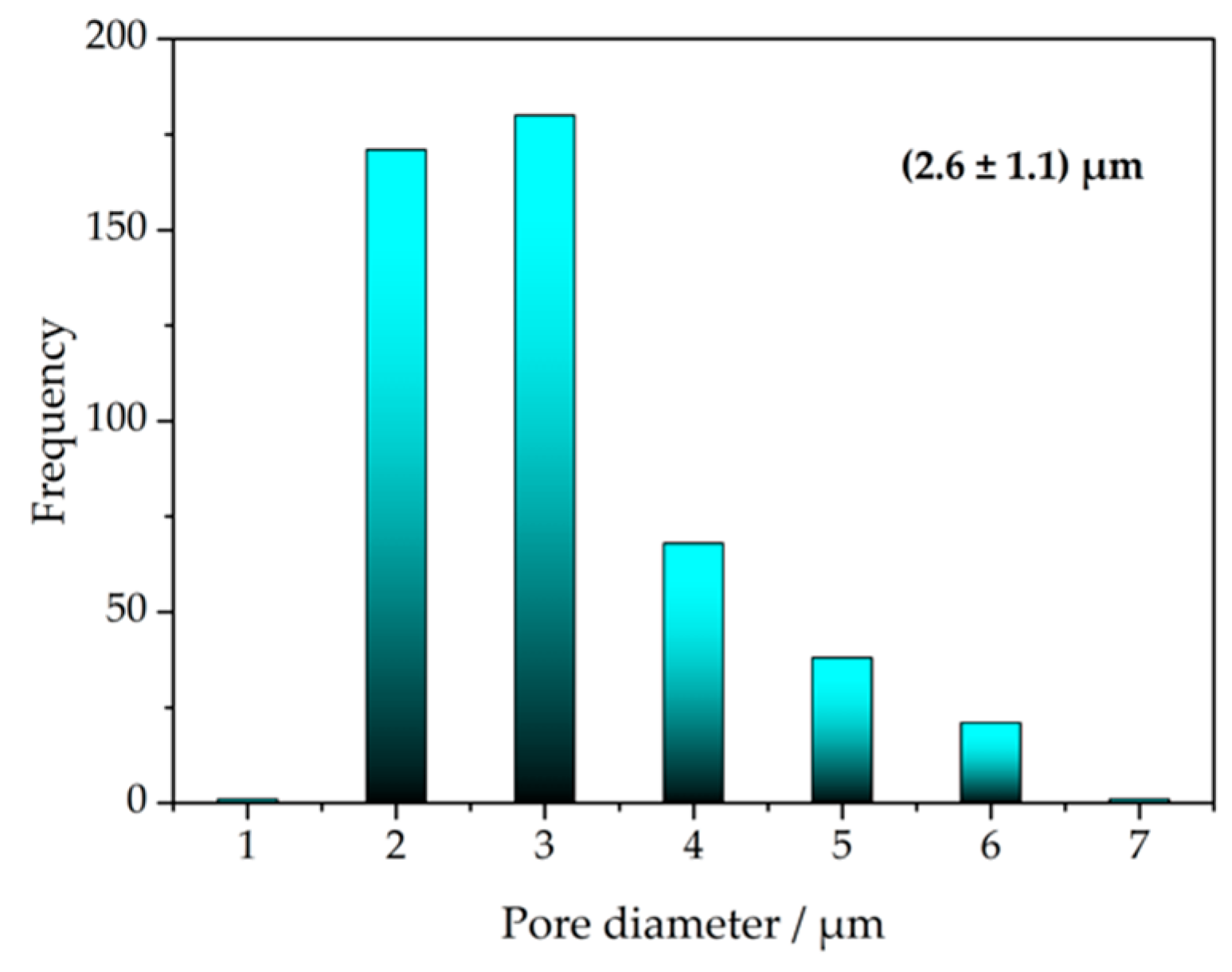
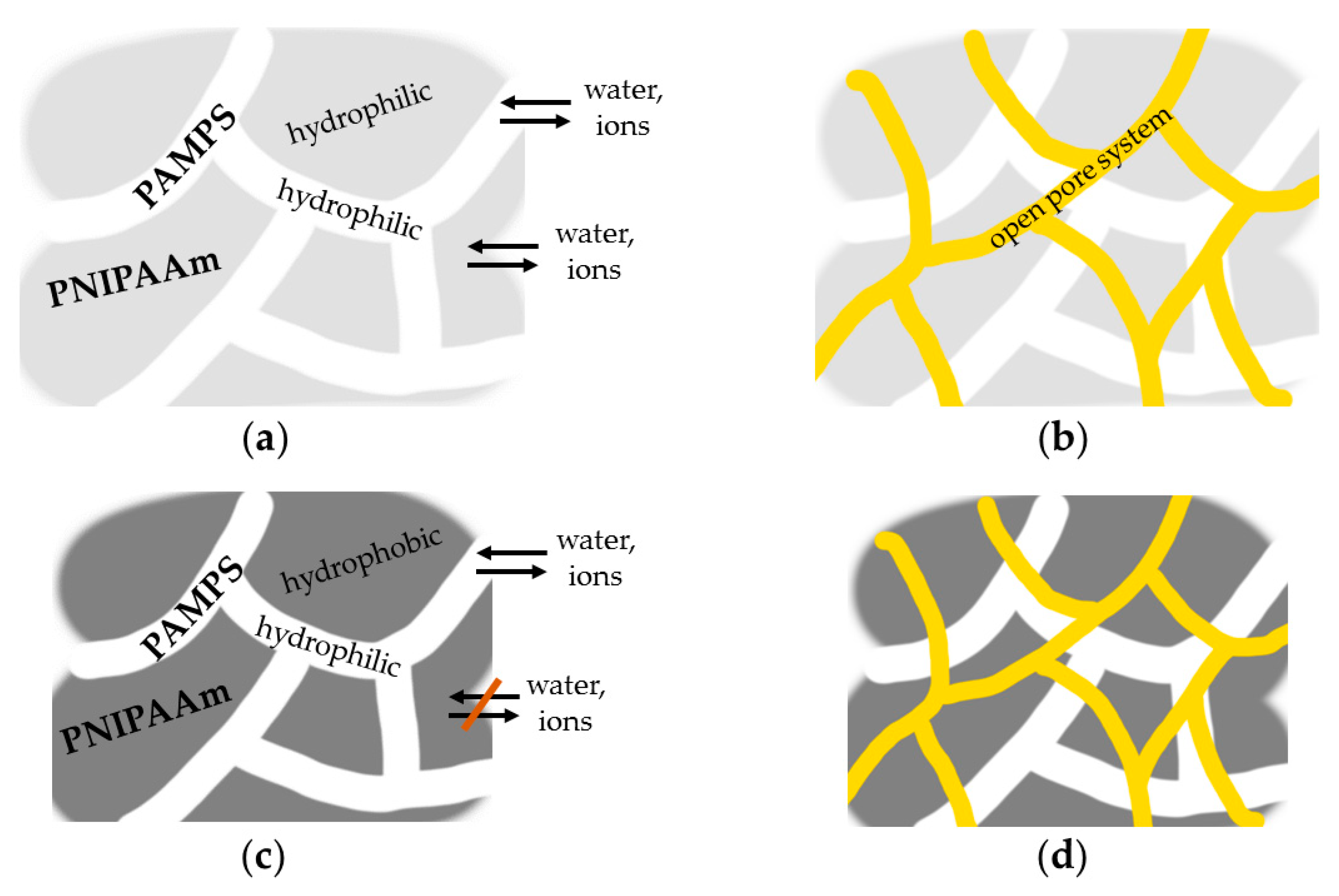
3.1. Scanning Electron Microscopy (SEM)
3.2. Swelling Kinetics
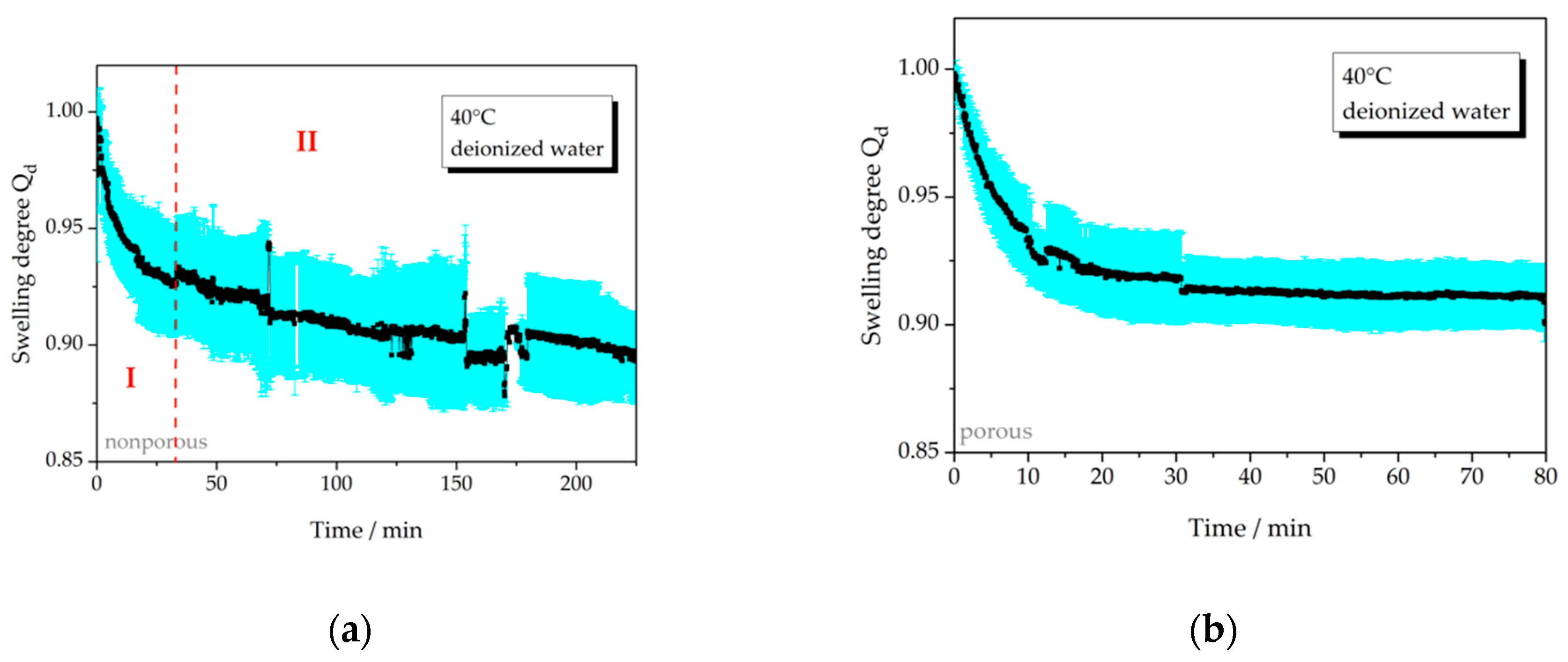
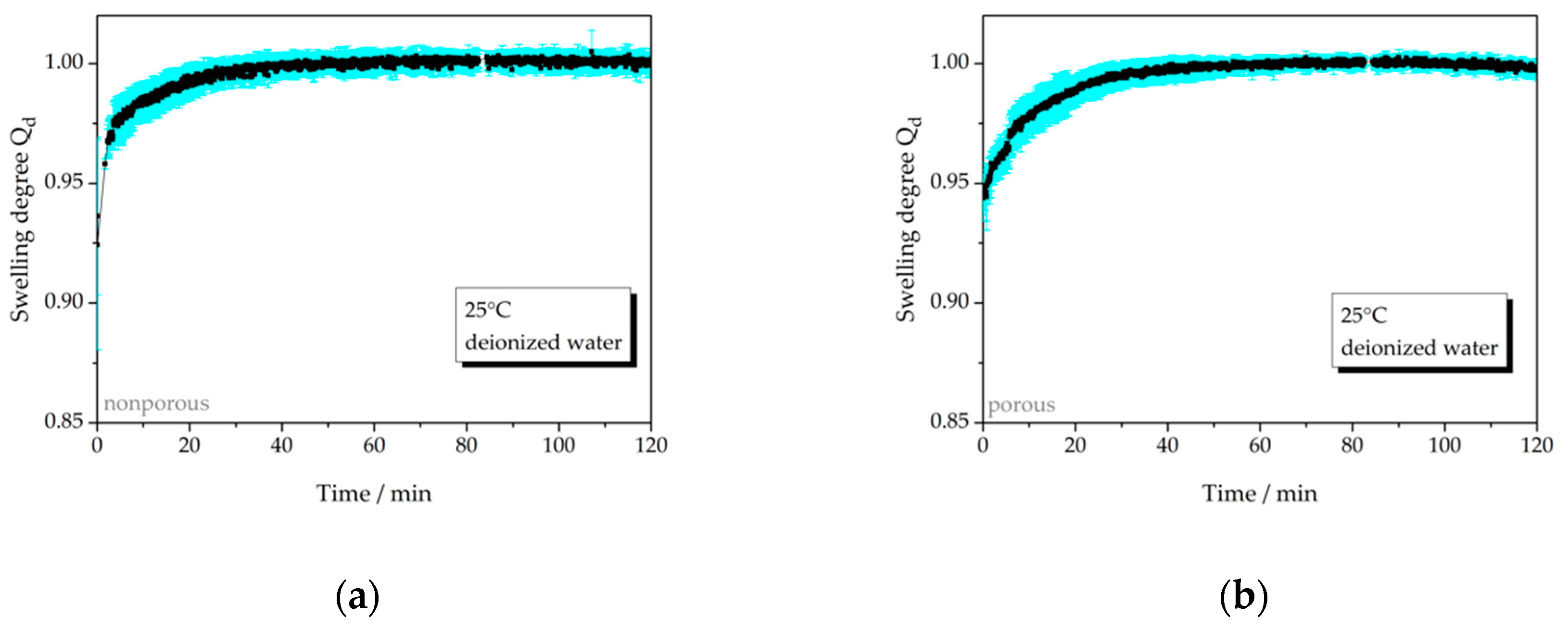
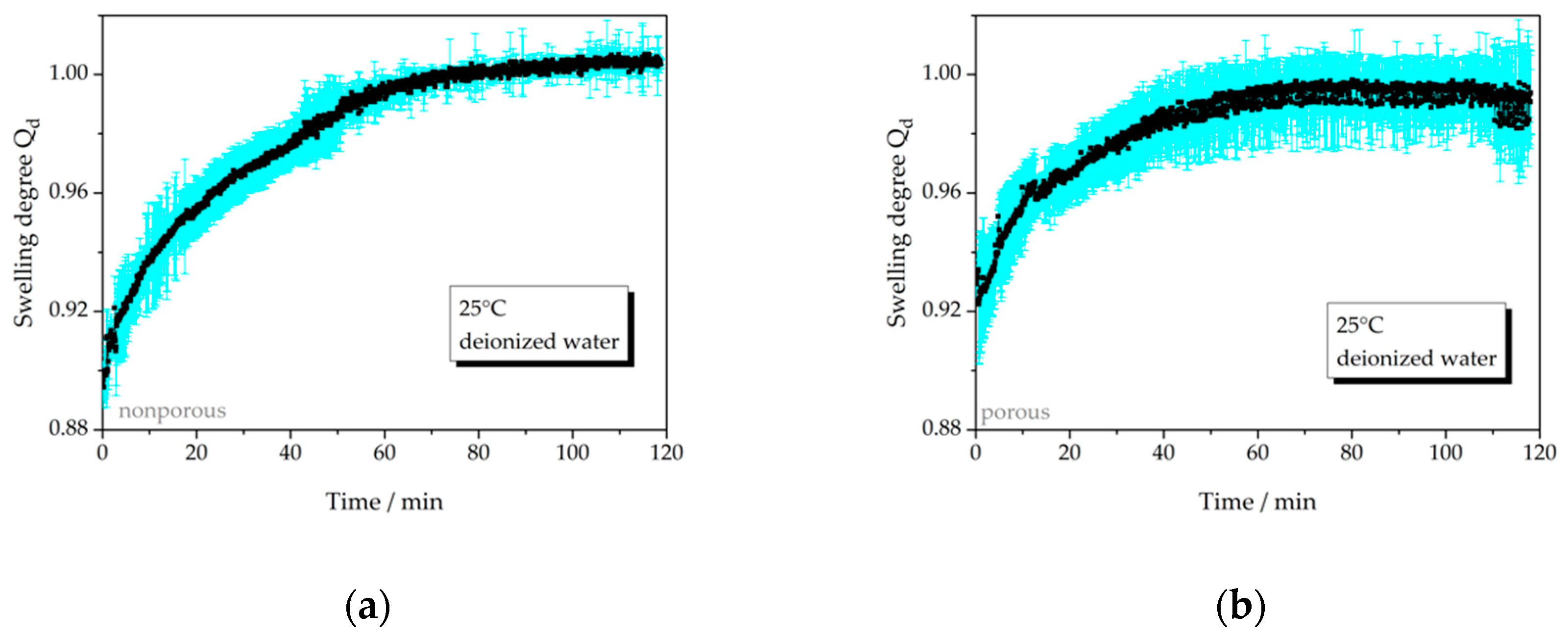
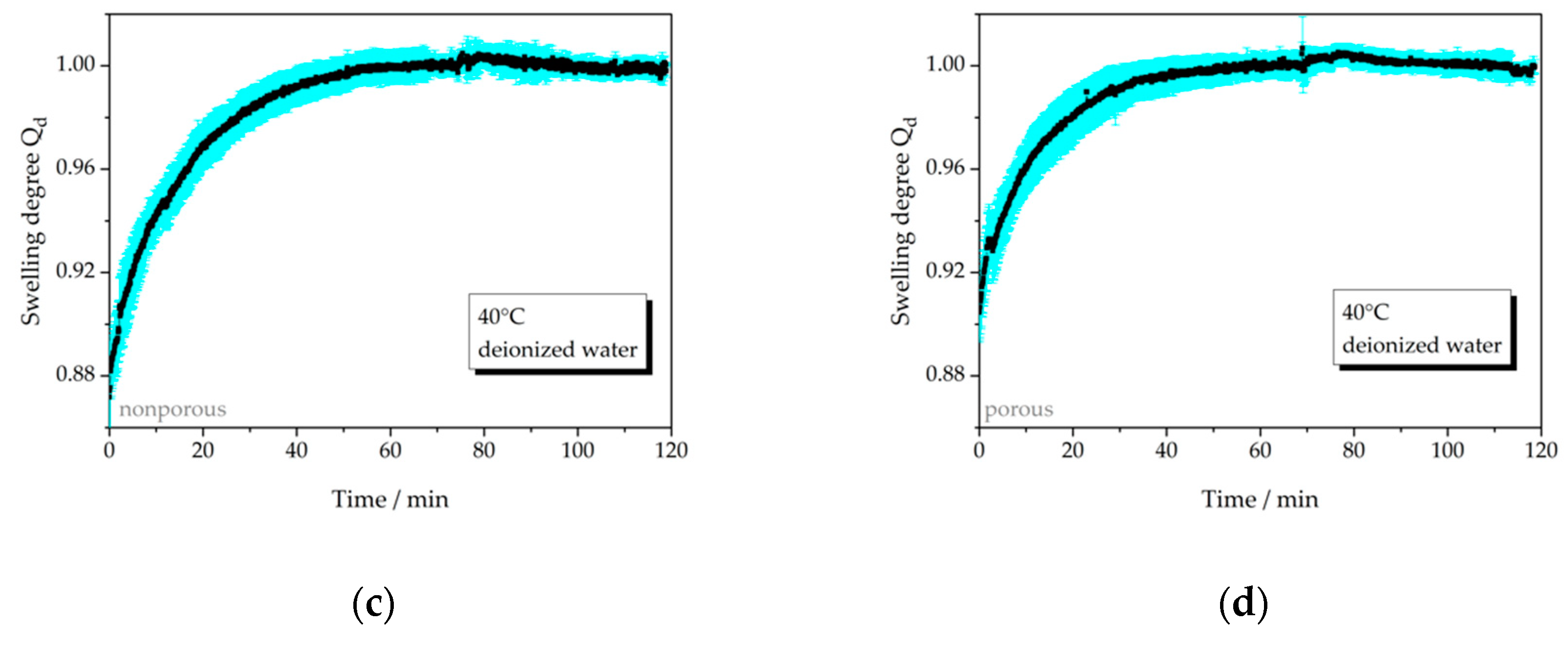
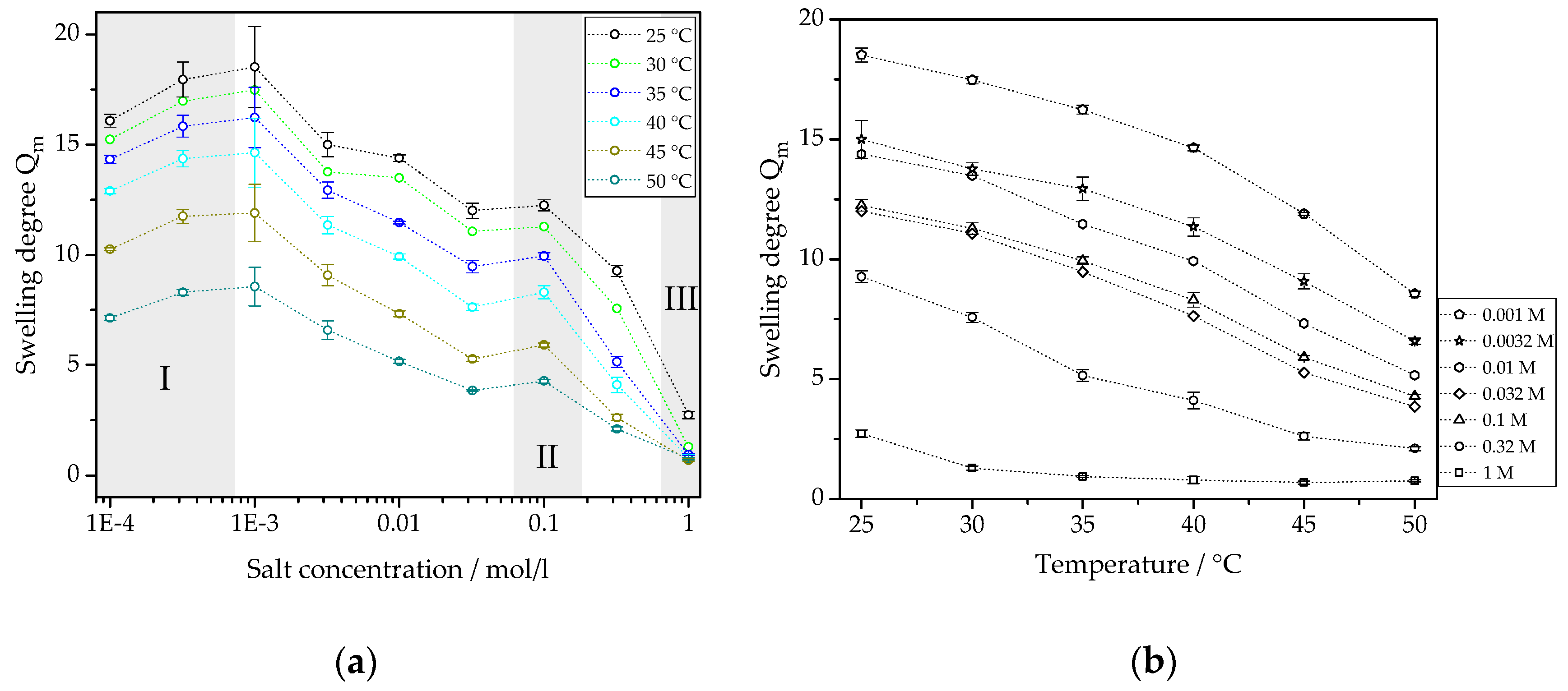
3.2.1. Temperature-Dependent Swelling Behavior
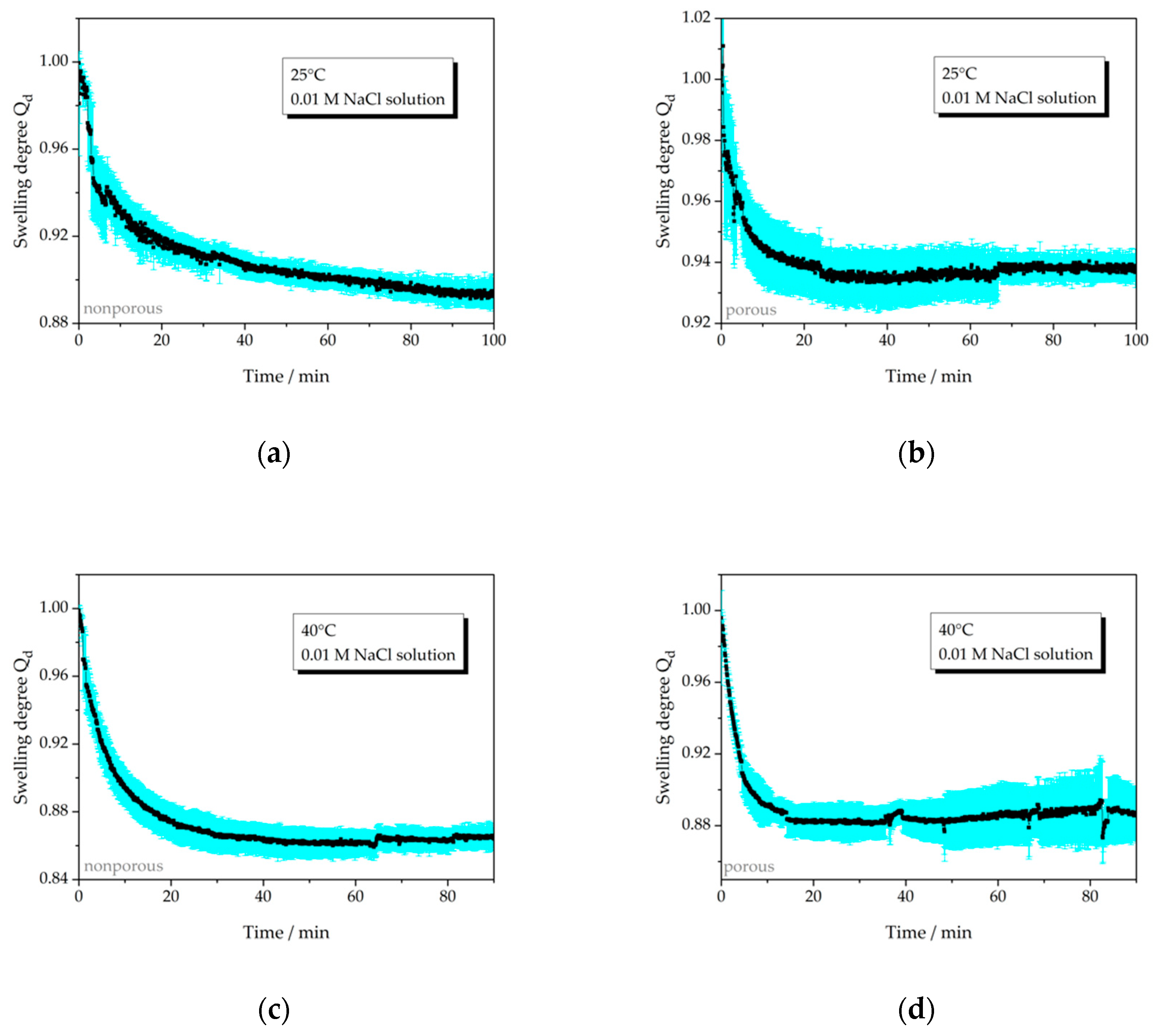
3.2.2. Swelling Behavior Depending on Salt Concentration
3.2.3. Discussion of Deswelling and Swelling Mechanisms in Salt Solution
- (1)
- Mass transport of water based on osmotic pressure,
- (2)
- Mass transport of sodium and chloride ions based on the Gibbs–Donnan effect,
- (3)
- Water transport based on ionic exchange at the ionic groups of the hydrogel (rules: smaller H+ ions are exchanged for larger Na+ ions, and larger ions have a smaller hydration shell),
- (4)
- Diffusion of sodium and chloride ions according to Fick‘s law based on a concentration gradient and
- (5)
- Convection.
Deswelling
Swelling
3.3. Static Degrees of Swelling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Binder, S.; Gerlach, G. Intramolecular force-compensated hydrogel-based sensors with reduced response times. tm–Tech. Mess. 2019, 86, 227–236. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Lu, Z.; Li, X.; Sarker, A.K.; Hu, L.; Choi, P.; Li, X.; Hakobyan, N.; Serpe, M.J. Responsive polymers for analytical applications: A review. Anal. Chim. Acta 2013, 789, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Dušek, K.; Patterson, D. Transition in swollen polymer networks induced by intramolecular condensation. J. Polym. Sci. Part A-2 1968, 6, 1209–1216. [Google Scholar] [CrossRef]
- Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Orakdogen, N.; Okay, O. Reentrant conformation transition in poly(N,N-dimethylacrylamide) hydrogels in water-organic solvent mixtures. Polymer 2006, 47, 561–568. [Google Scholar] [CrossRef]
- Sumaru, K.; Ohi, K.; Takagi, T.; Kanamor, T.; Shinbo, T. Photoresponsive properties of poly(N-isopropylacrylamide) hydrogel partly modified with spirobenzopyran. Langmuir 2006, 22, 4353–4356. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on hydrogel-based pH sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Stayton, P.S. Bioconjugates of smart polymers and proteins: Synthesis and applications. Macromol. Symp. 2004, 207, 139–151. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution: Unexpected properties from known building blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef]
- Koda, Y.; Terashima, T.; Sawamoto, M. LCST-type phase separation of poly[poly(ethylene glycol) methyl ether methacrylate]s in hydrofluorocarbon. ACS Macro Lett. 2015, 4, 1366–1369. [Google Scholar] [CrossRef]
- Nayak, S.; Lyon, L.A. Soft nanotechnology with soft nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 7686–7708. [Google Scholar] [CrossRef] [PubMed]
- Dhara, D.; Nisha, C.K.; Chatterji, P.R. Super absorbent hydrogels: Interpenetrating networks of poly(acrylamide-co-acrylic acid) and poly(vinyl alcohol): Swelling behavior and structural parameters. J. Macromol. Sci.-Pure Appl. Chem. 1999, 36, 197–210. [Google Scholar] [CrossRef]
- Hooper, H.H.; Baker, J.P.; Blanch, H.W.; Prausnitz, J.M. Swelling equilibria for positively ionized polyacrylamide hydrogels. Macromolecules 1990, 23, 1096–1104. [Google Scholar] [CrossRef]
- Jeon, C.H.; Makhaeva, E.E.; Khokhlov, A.R. Swelling behavior of polyelectrolyte gels in the prsences of salts. Macromol. Chem. Phys. 1998, 199, 2665–2670. [Google Scholar] [CrossRef]
- Horkay, F.; Tasaki, I.; Basser, P.J. Osmotic swelling of polyacrylate hydrogels in physiological salt solutions. Biomacromolecules 2000, 1, 84–90. [Google Scholar] [CrossRef]
- Gerlach, G.; Arndt, K.-F. Hydrogel Sensors and Actuators; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2009; Chapters 5 and 6; Volume 6. [Google Scholar]
- Richter, A.; Paschew, G. Optoelectrothermic control of highly integrated polymer-based MEMS applied in an artificial skin. Adv. Mater. 2009, 21, 979–983. [Google Scholar] [CrossRef]
- Richter, A.; Klatt, S.; Paschew, G.; Klenke, C. Micropumps operated by swelling and shrinking of temperature-sensitive hydrogels. Lab Chip 2009, 9, 613–618. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Kratochvíl, P.; Stepto, R.F.T.; Suter, U.W. Glossary of basic terms in polymer science. Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Mishra, S.; Rani, P.; Sen, G.; Dey, K.P. Chapter 6 Preparation, properties and application of hydrogels: A Review. In Hydrogels–Recent Advances, 1st ed.; Thakur, V.K., Thakur, M.K., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; Volume 1, pp. 145–173. [Google Scholar]
- Myung, D.; Waters, D.; Wiseman, M.; Duhamel, P.-E.; Noolandi, J.; Ta, C.N.; Frank, C.W. Progress in the development of interpenetrating polymer network hydrogels. Polym. Adv. Technol. 2008, 19, 647–657. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Kahovec, J.; Kratochvíl, P.; Jenkins, A.D.; Mita, I.; Papisov, I.M.; Sperling, L.H.; Stepto, R.F.T. Source-based nomenclature for non-linear macromolecules and macromolecular assemblies. Pure Appl. Chem. 1997, 69, 2511–2521. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Schulz, V.; Zschoche, S.; Zhang, H.P.; Voit, B.; Gerlach, G. Macroporous smart hydrogels for fast-responsive piezoresistive chemical microsensors. Procedia Eng. 2011, 25, 1141–1144. [Google Scholar] [CrossRef]
- Franke, D.; Binder, S.; Gerlach, G. Performance of fast-responsive, porous crosslinked poly(N-isopropylacrylamide) in a piezoresistive microsensor. IEEE Sens. Lett. 2017, 1, 1500904. [Google Scholar] [CrossRef]
- Hentze, H.-P.; Antonietii, M. Template synthesis of porous organic polymers. Curr. Opin. Solid State Mater. Sci. 2001, 5, 343–353. [Google Scholar] [CrossRef]
- Available online: https://imagej.net/ (accessed on 30 March 2020).
- Schreiber, S. PIA–Python Image Analyzer, 2019; in press.
- Wu, C.; Zhou, S. Volume phase transition of swollen gels: Discontinuous or continuous? Macromolecules 1997, 30, 574–576. [Google Scholar] [CrossRef]


| Swelling Conditions | Figure | ∆Q | t/min |
|---|---|---|---|
| 40 °C, np | 4a | >0.10 | − |
| 40 °C, p | 4b | 0.08 | 20 |
| 25 °C, np | 5a | not relevant | 50 |
| 25 °C, p | 5b | not relevant | 45 |
| Swelling Conditions | Figure | ∆Q | t/min |
|---|---|---|---|
| 25 °C, np, salt | 6a | >0.10 | − |
| 25 °C, p, salt | 6b | 0.06 | 25 |
| 40 °C, np, salt | 6c | 0.14 | 43 |
| 40 °C, p, salt | 6d | 0.12 | 15 |
| 25 °C, np, water | 7a | not relevant | − |
| 25 °C, p, water | 7b | not relevant | 60 |
| 40 °C, np, water | 7c | not relevant | 60 |
| 40 °C, p, water | 7d | not relevant | 50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franke, D.; Gerlach, G. Swelling Studies of Porous and Nonporous Semi-IPN Hydrogels for Sensor and Actuator Applications. Micromachines 2020, 11, 425. https://doi.org/10.3390/mi11040425
Franke D, Gerlach G. Swelling Studies of Porous and Nonporous Semi-IPN Hydrogels for Sensor and Actuator Applications. Micromachines. 2020; 11(4):425. https://doi.org/10.3390/mi11040425
Chicago/Turabian StyleFranke, Daniela, and Gerald Gerlach. 2020. "Swelling Studies of Porous and Nonporous Semi-IPN Hydrogels for Sensor and Actuator Applications" Micromachines 11, no. 4: 425. https://doi.org/10.3390/mi11040425
APA StyleFranke, D., & Gerlach, G. (2020). Swelling Studies of Porous and Nonporous Semi-IPN Hydrogels for Sensor and Actuator Applications. Micromachines, 11(4), 425. https://doi.org/10.3390/mi11040425





