AC Electroosmosis Effect on Microfluidic Heterogeneous Immunoassay Efficiency
Abstract
1. Introduction
2. Description and Formulation of the Problem
2.1. Immunoassay Geometry Model
2.2. Electroosmotic Flow Modeling
2.3. Electric Modeling
2.4. Analyte Concentration Modeling
2.5. Immunoassay Surface Reaction Modeling
2.6. Numerical Method
3. Results and Discussions
3.1. Electroosmotic Flow
3.2. Immunoassay Binding Reaction Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rath, D.; Panda, S. Contribution of rotational diffusivity towards the transport of antigens in heterogeneous immunosensors. Analyst 2015, 140, 6579–6587. [Google Scholar] [CrossRef] [PubMed]
- Chaiken, I.; Rose, S.; Karlsson, R. Analysis of macromolecular interactions using immobilized ligands. Anal. Biochem. 1992, 201, 197–210. [Google Scholar] [CrossRef]
- Vijayendran, R.A.; Ligler, F.S.; Leckband, D.E. A computational reaction−diffusion model for the analysis of transport-limited kinetics. Anal. Chem. 1999, 71, 5405–5412. [Google Scholar] [CrossRef]
- Sigurdson, M.; Wang, D.; Meinhart, C.D. Electrothermal stirring for heterogeneous immunoassays. Lab Chip 2005, 5, 1366–1373. [Google Scholar] [CrossRef]
- Hu, G.; Gao, Y.; Li, D. Modeling micropatterned antigen–antibody binding kinetics in a microfluidic chip. Biosens. Bioelectron. 2007, 22, 1403–1409. [Google Scholar] [CrossRef]
- Huang, K.-R.; Chang, J.S.; Chao, S.D.; Wu, K.C.; Yang, C.K.; Lai, C.Y.; Chen, S.H. Simulation on binding efficiency of immunoassay for a biosensor with applying electrothermal effect. J. Appl. Phys. 2008, 104, 064702. [Google Scholar] [CrossRef]
- Lebedev, K.; Mafe, S.; Stroeve, P. Convection, diffusion and reaction in a surface-based biosensor: Modeling of cooperativity and binding site competition on the surface and in the hydrogel. J. Colloid Interface Sci. 2006, 296, 527–537. [Google Scholar] [CrossRef]
- Mason, T.; Pineda, A.R.; Wofsy, C.; Goldstein, B. Effective rate models for the analysis of transport-dependent biosensor data. Math. Biosci. 1999, 159, 123–144. [Google Scholar] [CrossRef]
- Sikavitsas, V.; Nitsche, J.; Mountziaris, T. Transport and kinetic processes underlying biomolecular interactions in the BIACORE optical biosensor. Biotechnol. Prog. 2002, 18, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Lynn, N.S., Jr.; Martínez-López, J.I.; Bocková, M.; Adam, P.; Coello, V.; Siller, H.R.; Homola, J. Biosensing enhancement using passive mixing structures for microarray-based sensors. Biosens. Bioelectron. 2014, 54, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Green, N.G.; Ramos, A.; González, A.; Morgan, H.; Castellanos, A. Fluid flow induced by nonuniform ac electric fields in electrolytes on microelectrodes. I. Experimental measurements. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 61, 4011–4018. [Google Scholar]
- Ramos, A.; Morgan, H.; Green, N.G.; Castellanos, A. Ac electrokinetics: A review of forces in microelectrode structures. J. Phys. D Appl. Phys. 1998, 31, 2338. [Google Scholar] [CrossRef]
- Meinhart, C.; Wang, D.; Turner, K. Measurement of AC electrokinetic flows. Biomed. Microdevices 2003, 5, 139–145. [Google Scholar] [CrossRef]
- Hu, X.; Bessette, P.H.; Qian, J.; Meinhart, C.D.; Daugherty, P.S.; Soh, H.T. Marker-specific sorting of rare cells using dielectrophoresis. Proc. Natl. Acad. Sci. USA 2005, 102, 15757–15761. [Google Scholar] [CrossRef] [PubMed]
- Coll De Peña, A.; Redzuan, M.; Humaira, N.; Abajorga, M.K.; Hill, N.; Thomas, J.A.; Lapizco-Encinas, B.H. Analysis of bacteriophages with insulator-based dielectrophoresis. Micromachines 2019, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.; Lapizco-Encinas, B.H. Continuous flow separation of particles with insulator-based dielectrophoresis chromatography. Anal. Bioanal. Chem. 2020, 1–12. [Google Scholar] [CrossRef]
- Zhao, K.; Li, D. Continuous separation of nanoparticles by type via localized DC-dielectrophoresis using asymmetric nano-orifice in pressure-driven flow. Sens. Actuators B Chem. 2017, 250, 274–284. [Google Scholar] [CrossRef]
- Zhao, K.; Li, D. Tunable droplet manipulation and characterization by AC-DEP. ACS Appl. Mater. Interfaces 2018, 10, 36572–36581. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, Y.; Tao, Y.; Hou, L.; Jiang, H. High-Throughput Separation, Trapping, and Manipulation of Single Cells and Particles by Combined Dielectrophoresis at a Bipolar Electrode Array. Anal. Chem. 2018, 90, 11461–11469. [Google Scholar] [CrossRef]
- Sun, H.; Ren, Y.; Hou, L.; Tao, Y.; Liu, W.; Jiang, T.; Jiang, H. Continuous particle trapping, switching, and sorting utilizing a combination of dielectrophoresis and alternating current electrothermal flow. Anal. Chem. 2019, 91, 5729–5738. [Google Scholar] [CrossRef]
- Feldman, H.C.; Sigurdson, M.; Meinhart, C.D. AC electrothermal enhancement of heterogeneous assays in microfluidics. Lab Chip 2007, 7, 1553–1559. [Google Scholar] [CrossRef]
- Salari, A.; Thompson, M. Recent advances in AC electrokinetic sample enrichment techniques for biosensor development. Sens. Actuators B Chem. 2018, 255, 3601–3615. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y. Simultaneous microfluidic pumping and mixing using an array of asymmetric 3D ring electrode pairs in a cylindrical microchannel by the AC electroosmosis effect. Eur. J. Mech. B/Fluids 2019, 75, 361–371. [Google Scholar] [CrossRef]
- Selmi, M.; Gazzah, M.H.; Belmabrouk, H. Numerical study of the electrothermal effect on the kinetic reaction of immunoassays for a microfluidic biosensor. Langmuir 2016, 32, 13305–13312. [Google Scholar] [CrossRef]
- Selmi, M.; Khemiri, R.; Echouchene, F.; Belmabrouk, H. Electrothermal effect on the immunoassay in a microchannel of a biosensor with asymmetrical interdigitated electrodes. Appl. Therm. Eng. 2016, 105, 77–84. [Google Scholar] [CrossRef]
- Selmi, M.; Echouchene, F.; Gazzah, M.H.; Belmabrouk, H. Flow confinement enhancement of heterogeneous immunoassays in microfluidics. IEEE Sens. J. 2015, 15, 7321–7328. [Google Scholar] [CrossRef]
- Selmi, M.; Gazzah, M.H.; Belmabrouk, H. Optimization of microfluidic biosensor efficiency by means of fluid flow engineering. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sin, M.L.; Gau, V.; Liao, J.C.; Wong, P.K. Electrothermal fluid manipulation of high-conductivity samples for laboratory automation applications. J. Assoc. Lab. Autom. 2010, 15, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-R.; Chang, J.-S. Three dimensional simulation on binding efficiency of immunoassay for a biosensor with applying electrothermal effect. Heat Mass Transf. 2013, 49, 1647–1658. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Chang, J.S.; Chao, S.D.; Wu, K.C.; Huang, L. Improving the binding efficiency of quartz crystal microbalance biosensors by applying the electrothermal effect. Biomicrofluidics 2014, 8, 054116. [Google Scholar] [CrossRef]
- Hibbert, D.B.; Gooding, J.J.; Erokhin, P. Kinetics of irreversible adsorption with diffusion: Application to biomolecule immobilization. Langmuir 2002, 18, 1770–1776. [Google Scholar] [CrossRef]
- Friedrich, D.; Please, C.; Melvin, T. Optimisation of analyte transport in integrated microfluidic affinity sensors for the quantification of low levels of analyte. Sens. Actuators B Chem. 2008, 131, 323–332. [Google Scholar] [CrossRef]
- Kim, D.R.; Zheng, X. Numerical characterization and optimization of the microfluidics for nanowire biosensors. Nano Lett. 2008, 8, 3233–3237. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Mirzakhanloo, M.; Dabirzadeh, M.R. Numerical simulation for efficient mixing of newtonian and non-newtonian fluids in an electro-osmotic micro-mixer. Chem. Eng. Process. Process Intensif. 2016, 107, 11–20. [Google Scholar] [CrossRef]
- Li, D. Electrokinetics in Microfluidics; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Honegger, T.; Peyrade, D. Comprehensive analysis of alternating current electrokinetics induced motion of colloidal particles in a three-dimensional microfluidic chip. J. Appl. Phys. 2013, 113, 194702. [Google Scholar] [CrossRef]
- Islam, N.; Lian, M.; Wu, J. Enhancing microcantilever capability with integrated AC electroosmotic trapping. Microfluid. Nanofluid. 2007, 3, 369–375. [Google Scholar] [CrossRef]
- Chiou, P.-Y.; Ohta, A.T.; Jamshidi, A.; Hsu, H.Y.; Wu, M.C. Light-actuated AC electroosmosis for nanoparticle manipulation. J. Microelectromech. Syst. 2008, 17, 525–531. [Google Scholar] [CrossRef]
- Chen, Z. Finite Element Methods and Their Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
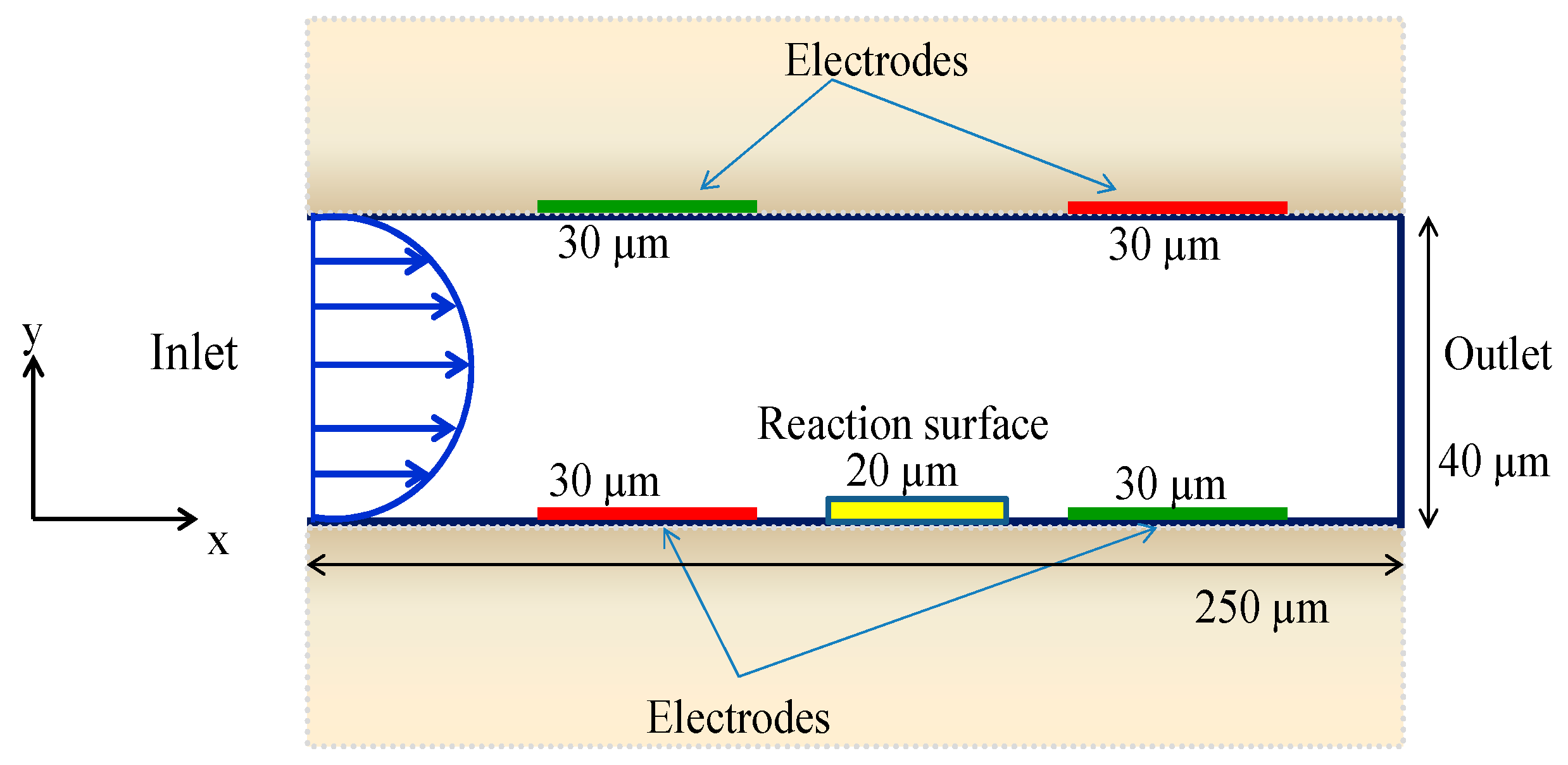
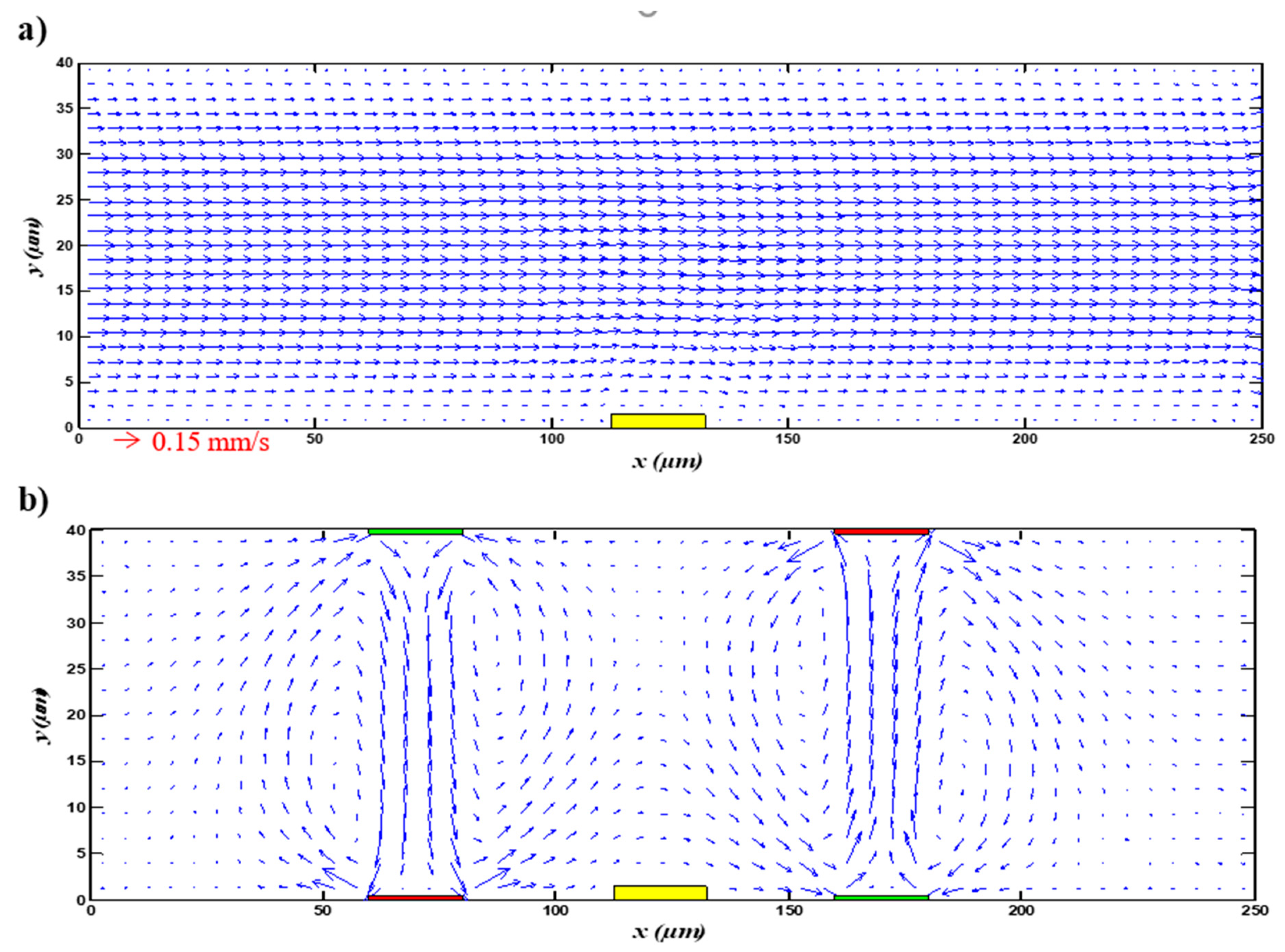
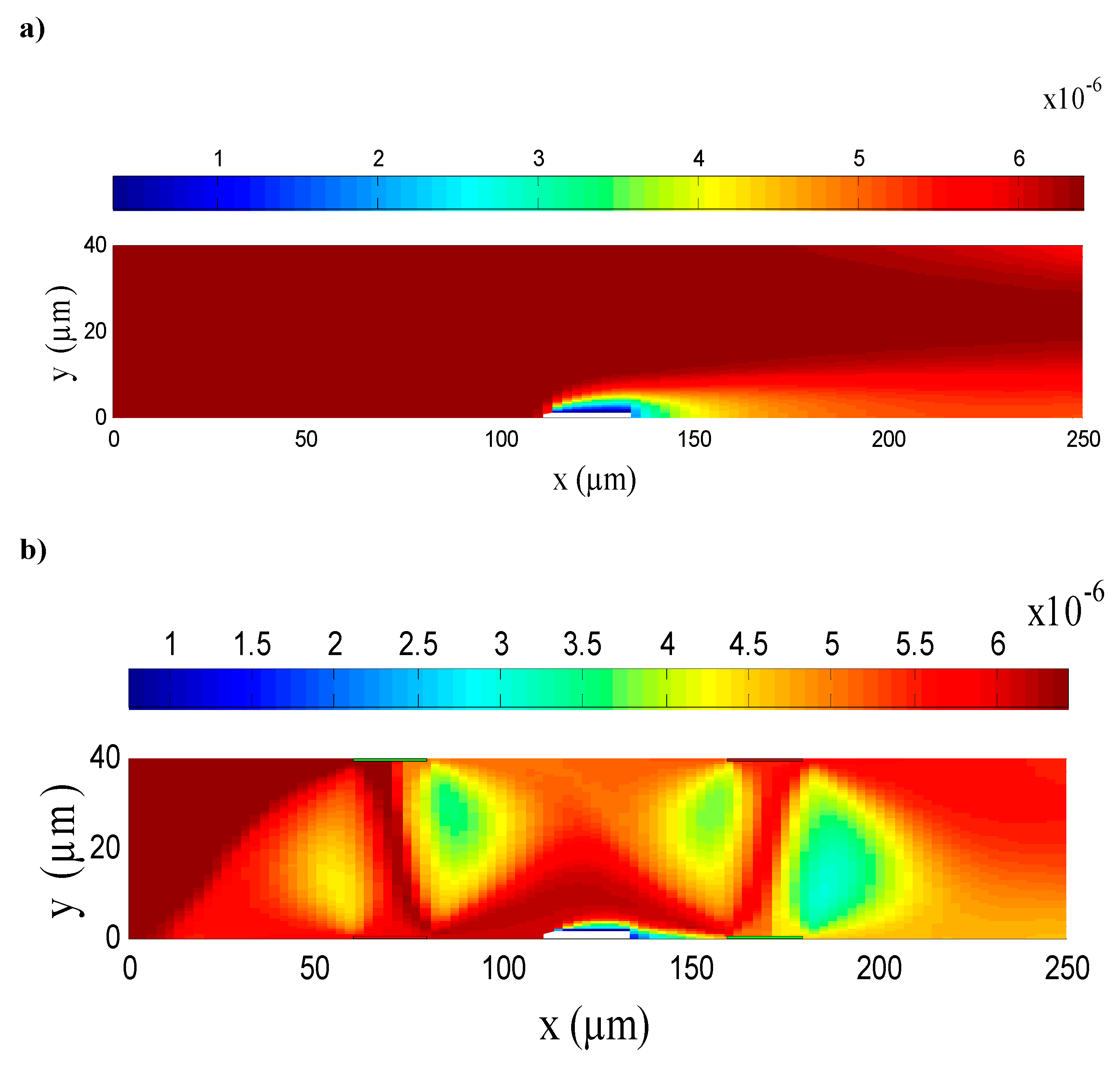

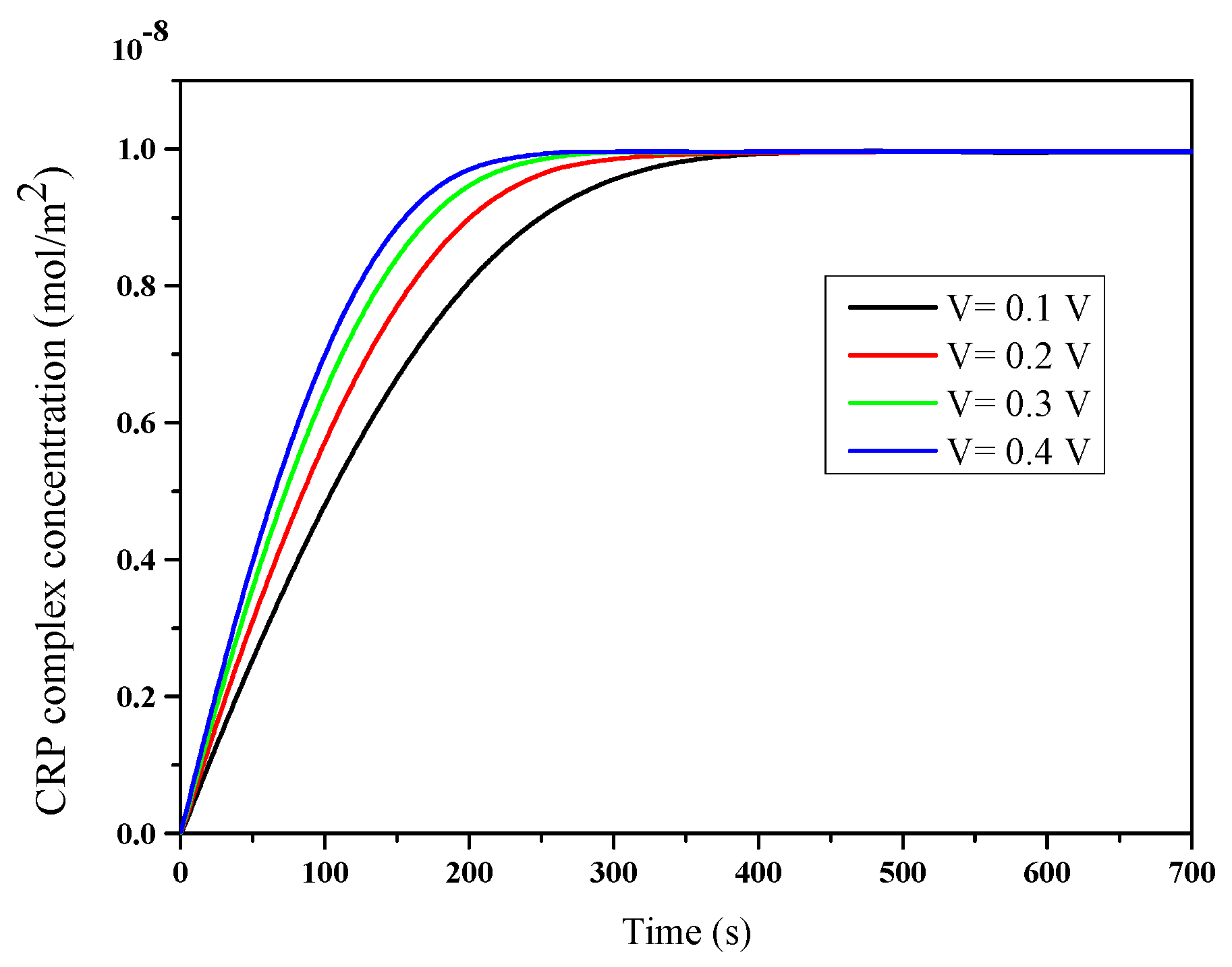
| Case | Detection Time (s) |
|---|---|
| Without ACEO effect | 417 |
| With ACEO effect | 180 |
| Applied voltage (V) | Detection Time (s) | Reduction Percentage (%) |
|---|---|---|
| 0.1 | 291 | 30.21 |
| 0.2 | 232 | 44.36 |
| 0.3 | 200 | 52.03 |
| 0.4 | 180 | 56.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selmi, M.; Belmabrouk, H. AC Electroosmosis Effect on Microfluidic Heterogeneous Immunoassay Efficiency. Micromachines 2020, 11, 342. https://doi.org/10.3390/mi11040342
Selmi M, Belmabrouk H. AC Electroosmosis Effect on Microfluidic Heterogeneous Immunoassay Efficiency. Micromachines. 2020; 11(4):342. https://doi.org/10.3390/mi11040342
Chicago/Turabian StyleSelmi, Marwa, and Hafedh Belmabrouk. 2020. "AC Electroosmosis Effect on Microfluidic Heterogeneous Immunoassay Efficiency" Micromachines 11, no. 4: 342. https://doi.org/10.3390/mi11040342
APA StyleSelmi, M., & Belmabrouk, H. (2020). AC Electroosmosis Effect on Microfluidic Heterogeneous Immunoassay Efficiency. Micromachines, 11(4), 342. https://doi.org/10.3390/mi11040342





