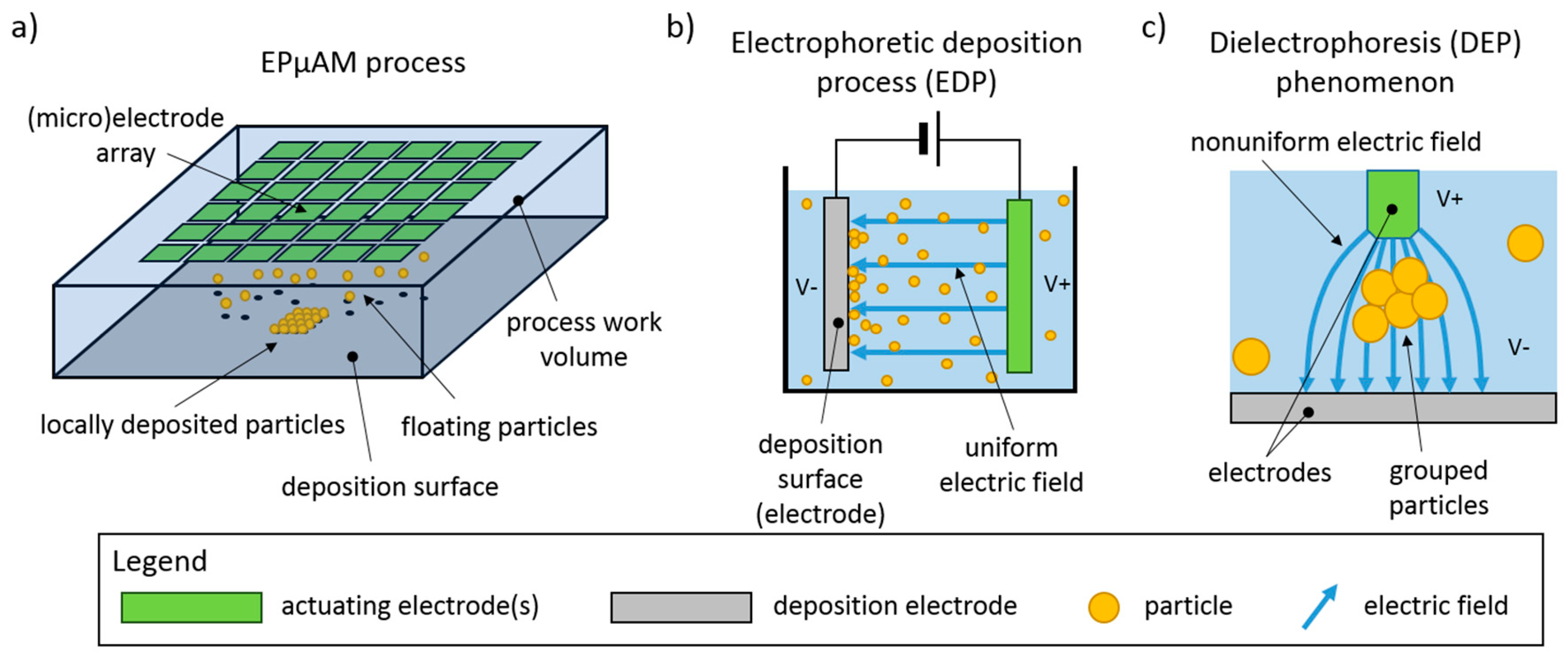
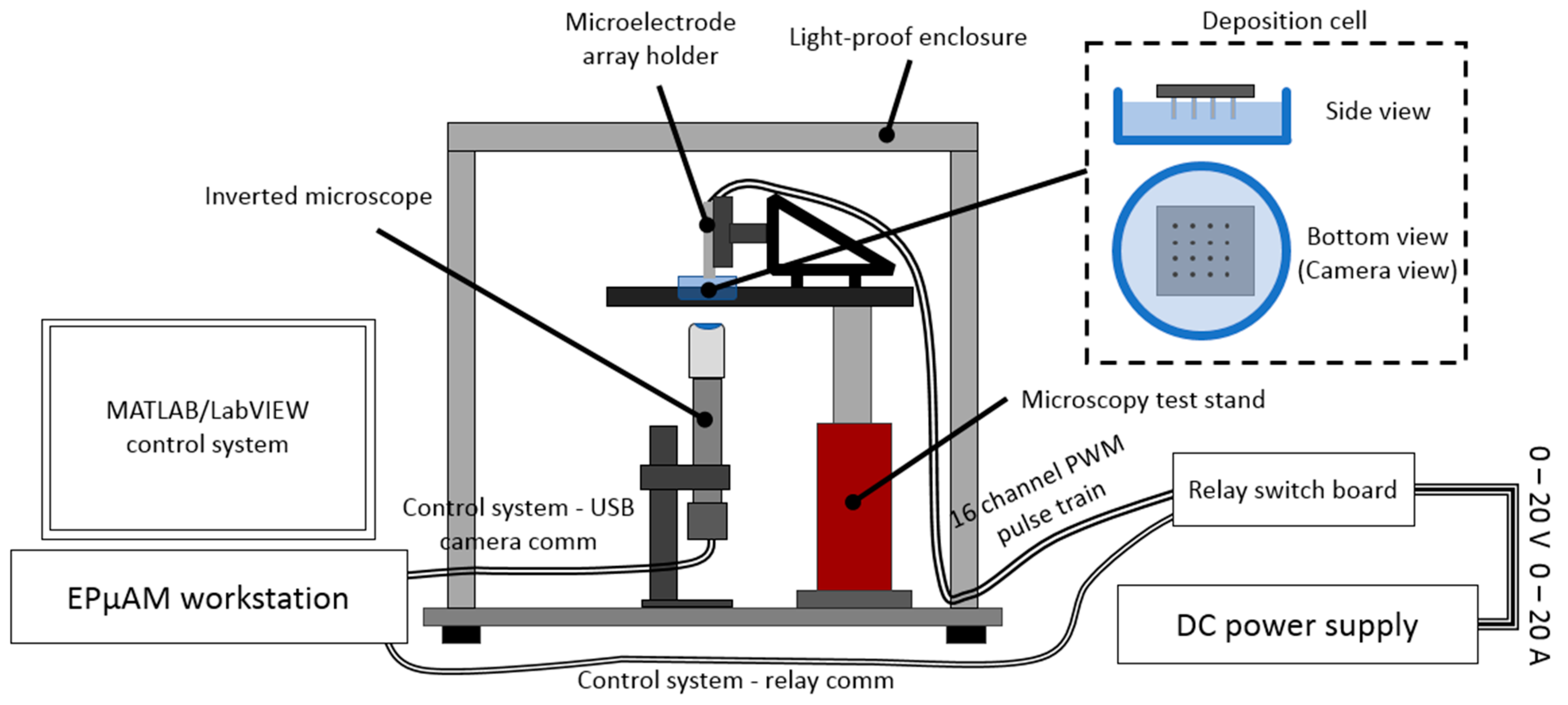
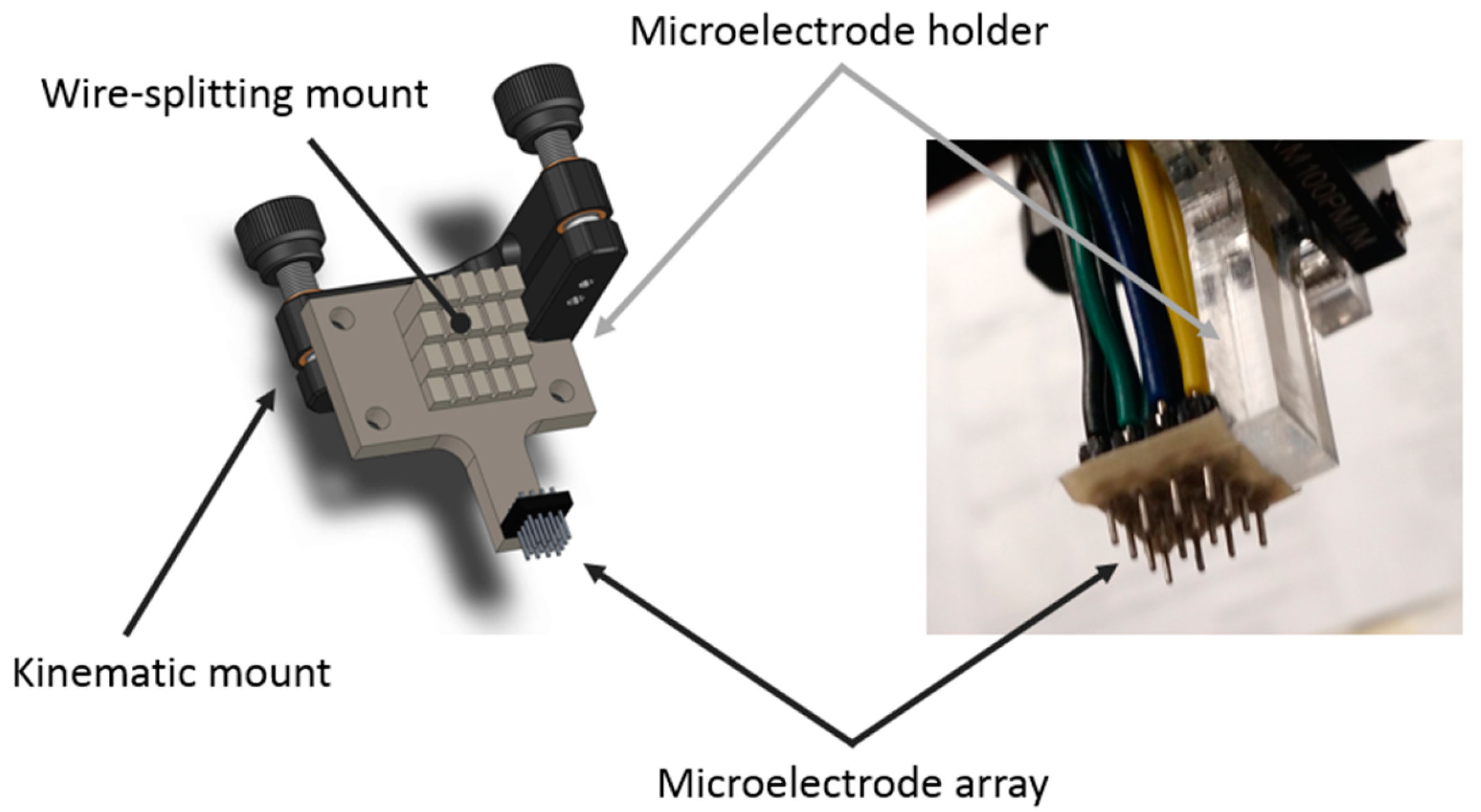
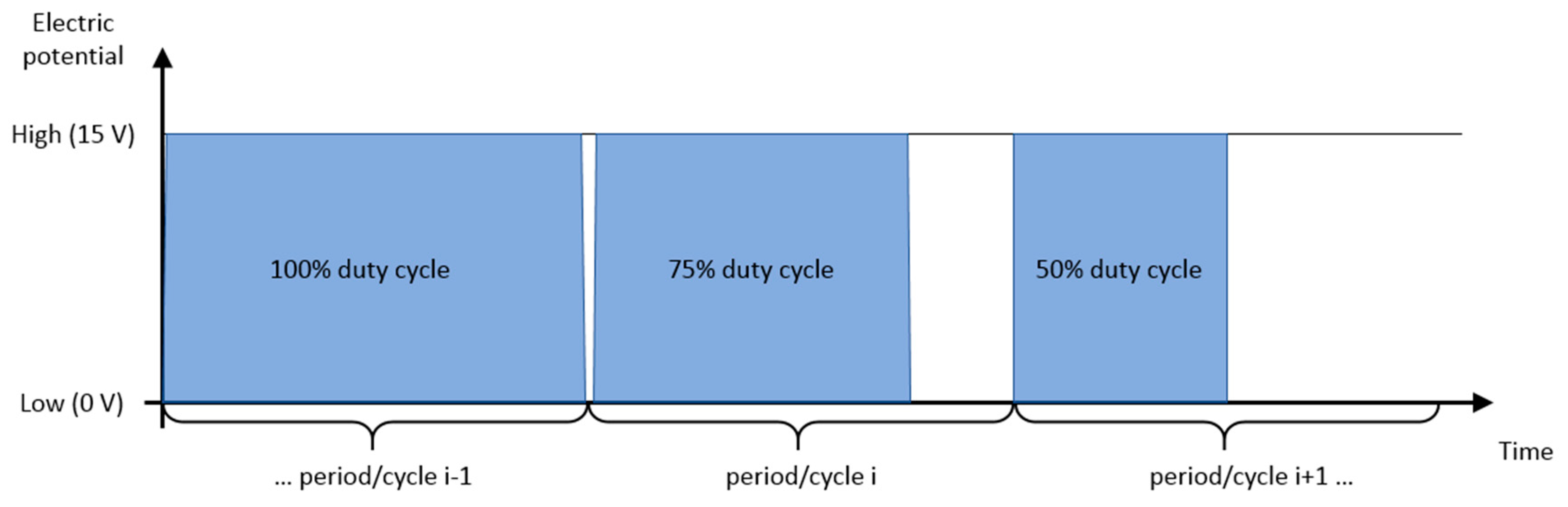
The Results section contains experimental results from three performed studies. In the first study, simple particle group manipulation is demonstrated and compared to the FEM results. The experimental runs were repeated with varying particle concentration and electric current density. Particle group locations and distributions from these characterization experiments were quantitively compared by computing the binary mask area of deposited particles and plotting them versus the experiment time. In the second study, an advanced manipulation technique was presented where particles were deposited between the electrodes. In the third study, a particle deposit was prepared for characterization and quantitative evaluation on a laser confocal microscopy instrument. These preliminary results demonstrated the EPμAM process capability to organize and order particles by pushing them into the desired location.
3.1. Simple Particle Group Manipulation and Process Characterization
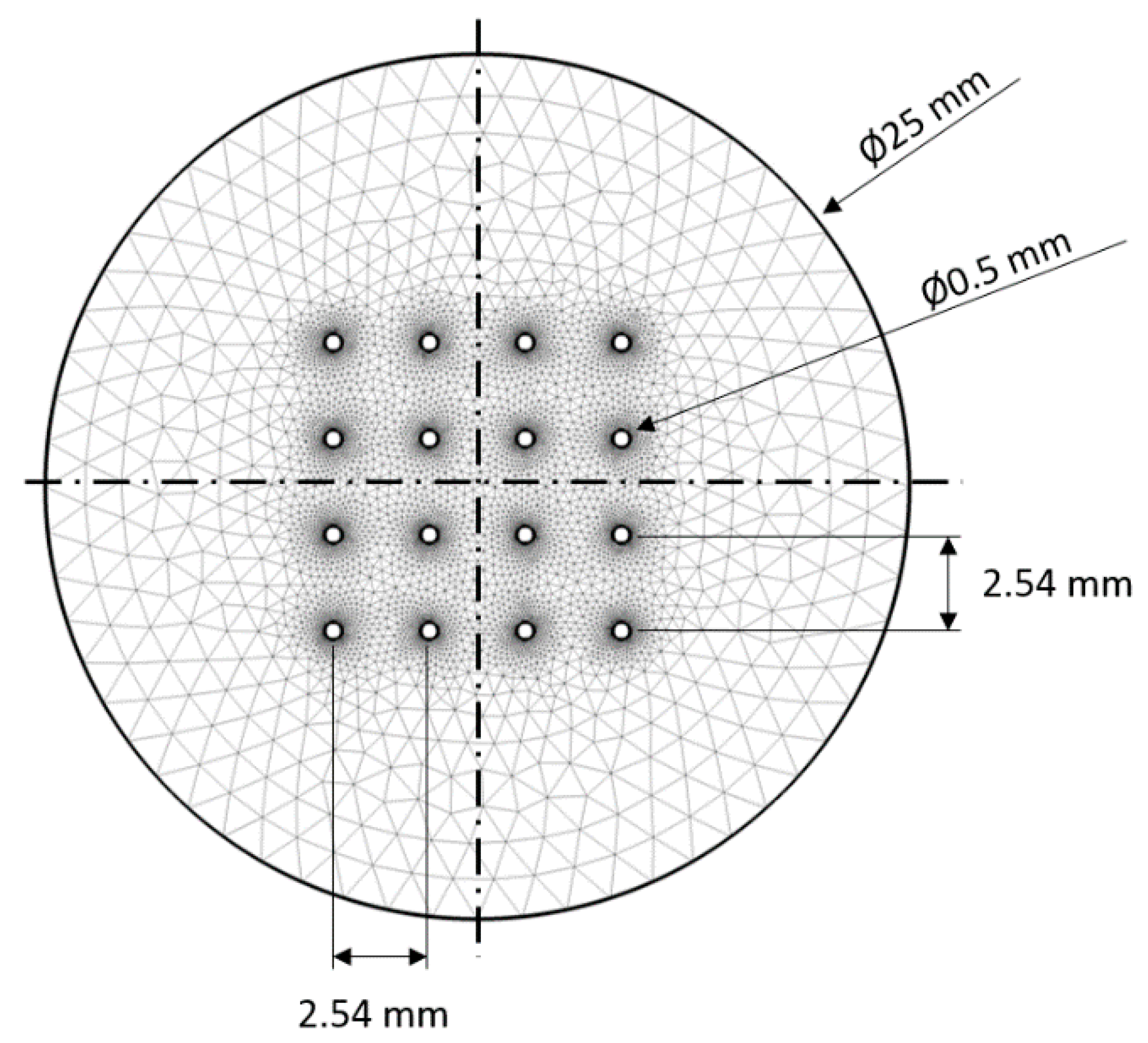
Preliminary deposition runs were designed to test the microelectrode array’s capability to move and deposit particle groups. At the beginning of each experimental run, a water solution (2% and 0.2% wt. solutions) of rutile titania (TiO2) particles were injected (100 to 200 μL injection volume) in the deposition cell with a syringe. The mini well was previously filled with 3 mL of water. The particles were left to diffuse and fall on the bottom of the cell before the electric fields were applied to them. This preparation stage usually lasted 10 to 20 s.
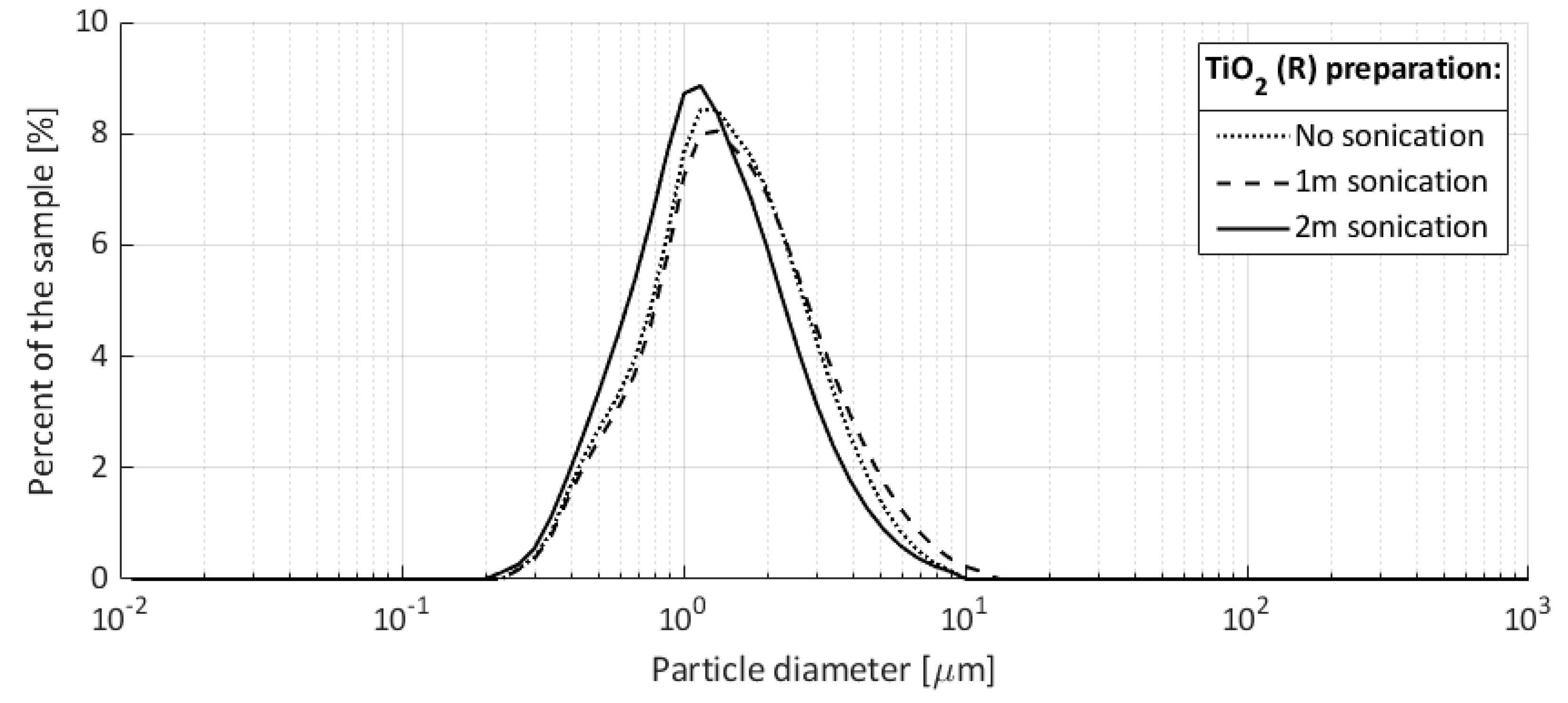
No detergents or chemical stabilizers were used for the preparation of the titania aqueous solution. Particle size measurements were performed with a Horiba LA-960 Laser Particle Size Analyzer, where no sonication, 1 min sonication, and 2 min sonication were applied to the sample prior to the measurement. These measurements were performed to check the colloidal stability. The obtained particle size distributions are shown in
Figure 9. The sample sonication preparation shows that the used particle-fluid system is stable under mechanical agitation and has an average particle size between 1 and 2 μm.
After the injected particles were settled on the bottom of the deposition cell, the manipulation tests were performed in the following sequence, as shown in
Figure 5: (i) Particles are focused in the middle of the array; (ii) then, particles are defocused from the middle of the array and grouped on the array’s edges; (iii) particles are translated to the third electrode array column (see upper right panel in
Figure 5); and (iv) particles are translated to the edge of the array, to the fourth array column. Each corresponding experimental step consisted of the application of 100 cycles of the appropriate control schemes, as shown in
Figure 5. During the particle movement, images were taken with the inverted microscopy setup every 10th cycle, which corresponded to every ~12 s of the process. In total, the whole experimental run, including the preparation stage, lasted for 500 to 510 s.
Experimental results for 2% wt. particle solution are shown in
Figure 10.
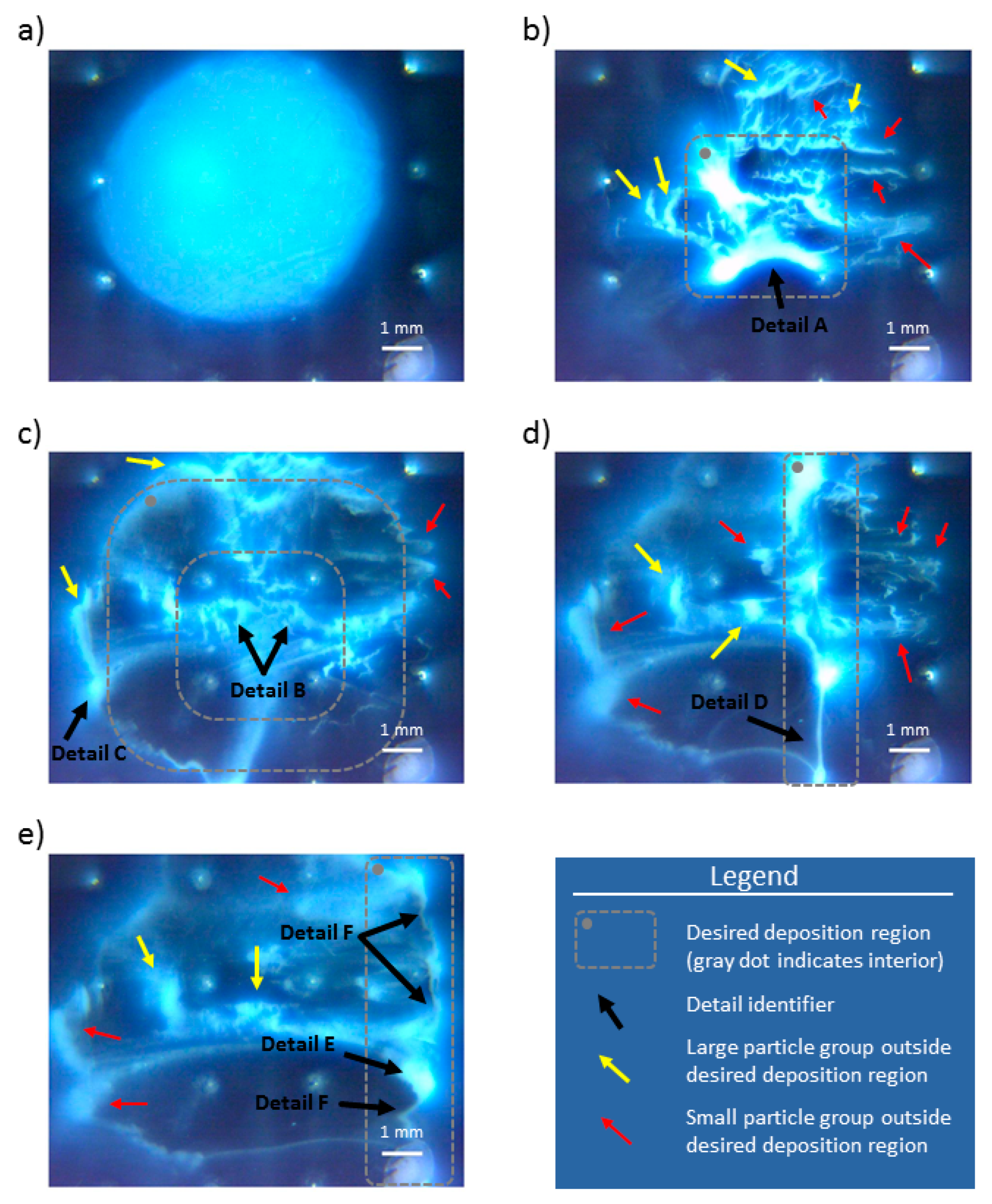
Figure 10a shows particle group after they have settled at the end of the preparation stage. Panels b–e in
Figure 10 show particle group distribution at the end of the 100th cycle for each experimental run (i–iv). In the bottom right corner of all images is the 1 mm white length bar.
Figure 11 shows the FEM model computed electric potential distribution for the control schemes A–D.
Figure 12 shows the initial and end test particle positions for the computed electric potential distribution cases given in
Figure 11.
Figure 10b depicts the final distribution of the focused particle group, delineated with the dashed gray rectangle in the middle of the electrode array. There are a few large particle groups left outside this deposition region, and they are marked with a yellow arrow (the readers are referred to the color version of
Figure 10). These large groups formed agglomerations on the bottom of the mini well, and they were sufficiently large to enable particle packing which prevented particle conveyance with the employed electric fields. These groups were broken up by subsequent application of electric fields in reverse, indicating that these groups formed semistable deposits outside of the desired deposition region. Smaller particle groups which ended outside the desired deposition region are indicated with the red arrows. These particles did not seem to be agglomerated and settled, but just smeared over the mini well’s surface and they were easily moved by applying additional cycles of the same electric field distribution. Detail A in
Figure 10a shows one notable particle formation, in the form of a deposited arch, that provided a good match with the obtained shape of the electric field distribution (Detail A,
Figure 11a) and final positions of the test particles (Detail A,
Figure 12b).
The obtained particle distribution after 100 application cycles of control scheme B is shown in
Figure 10c. The majority of the particles are located within the annulus of the desired deposition region (dashed gray annulus in
Figure 10c). It is worth noting that most particles within this region are not agglomerated (i.e., like large particle groups identified with yellow arrow), but lightly deposited, and with a form closer to the small particle groups, which are smeared (and identified with a red arrow in these figures). There are a few large groups of particles outside the desired deposition region and identified with a yellow arrow. Most likely, the application of electric potential caused them to overshoot and get latched to the electrodes (see, for example, Detail C in
Figure 10c). After latching, these particles started to behave like an extension of the electrode in contact and started to form undesired particle agglomerations. Some particle groups are left in the middle of the annulus (the hole in desired deposition region in
Figure 10c that encompasses the middle four electrodes), which was not a desired deposition region (see Detail B,
Figure 10c). These particles are left in the middle of the array due to the locally formed electric potential minima (created within the middle four electrodes) that prevented them from migrating away.
The end particle distribution obtained with control scheme C is shown in
Figure 10d. The majority of the agglomerated particles are located within the desired deposition region. Detail D in
Figure 10d shows formation of a long deposit of agglomerated particles, with high length-to-width aspect ratio that spans between the third and fourth rows of the third electrode array column (see upper right panel in
Figure 5 for definitions of these terms) that can be desirable in microelectronics (i.e., to form electrical or insulating connections) or surface science (e.g., for quick forming of surface textures or creating building blocks for more complicated formations). There are a few large groups of leftover particles, close to the middle of the electrode array which are most likely trapped due to the locally formed electric potential minimum (see Detail C in
Figure 11c). The locally formed electric field minima delineated with the contour plots equipotential surfaces that encompass Detail C in
Figure 11c, are most likely due to low electric field gradients (identified as large distances between the adjacent equipotential contour plots). They demonstrate the necessity of creating sufficiently strong electric field gradients in order to move the particle groups to the desired locations. If the gradient is not large enough, then, the overall force balance on the particles is insufficient to move the particles, and they remain stuck to that location until the electric field gradient is changed.
The final distribution of particles after migrating to the fourth column of the electrode array is shown in
Figure 10e. A large portion, but not the majority of the particles, is located within the desired deposition region, identified with the gray dashed rectangle with the rounded corners. There are a few large groups of particles (yellow arrows in
Figure 10e) and several small groups of particles (red arrows in
Figure 10e) that are outside of the desired deposition region. These “straggler” groups can be explained by the local electric field minima or insufficient electric field gradient which resulted from the applied electric field distribution (see
Figure 11d). These local minima and low gradient regions are located on the left side of the electrode array, between the first and third columns of the electrode array (the region with electric potential above 11 V and marked with red color). Detail E in
Figure 10e shows another example of particles latching to the electrode and acting as its extension. Detail F, in
Figure 10e, shows a preliminary formation of long particle deposits. These preliminary formations are similar to the smeared, small particle groups, which are identified with the red arrows in the panel. In
Figure 10e, F details have similar shapes with the computed particle positions, shown as Details D and F in
Figure 12d,e, respectively. They seem to be formed after the initial particle group gets stuck to the electrode and continues to chain-up with neighboring particle subgroups.
The common observation for all sets of experiments is that the particle groups do not deposit or stay in the regions with high electric field gradient and that it is possible to inadvertently trap particles in high electric potential regions where the electric field gradient is low. Additionally, stable particle groups tend to form in the low electric potential regions (<2.5 V), which corresponds to the dark blue regions of computed electric potential distribution, shown in
Figure 11.
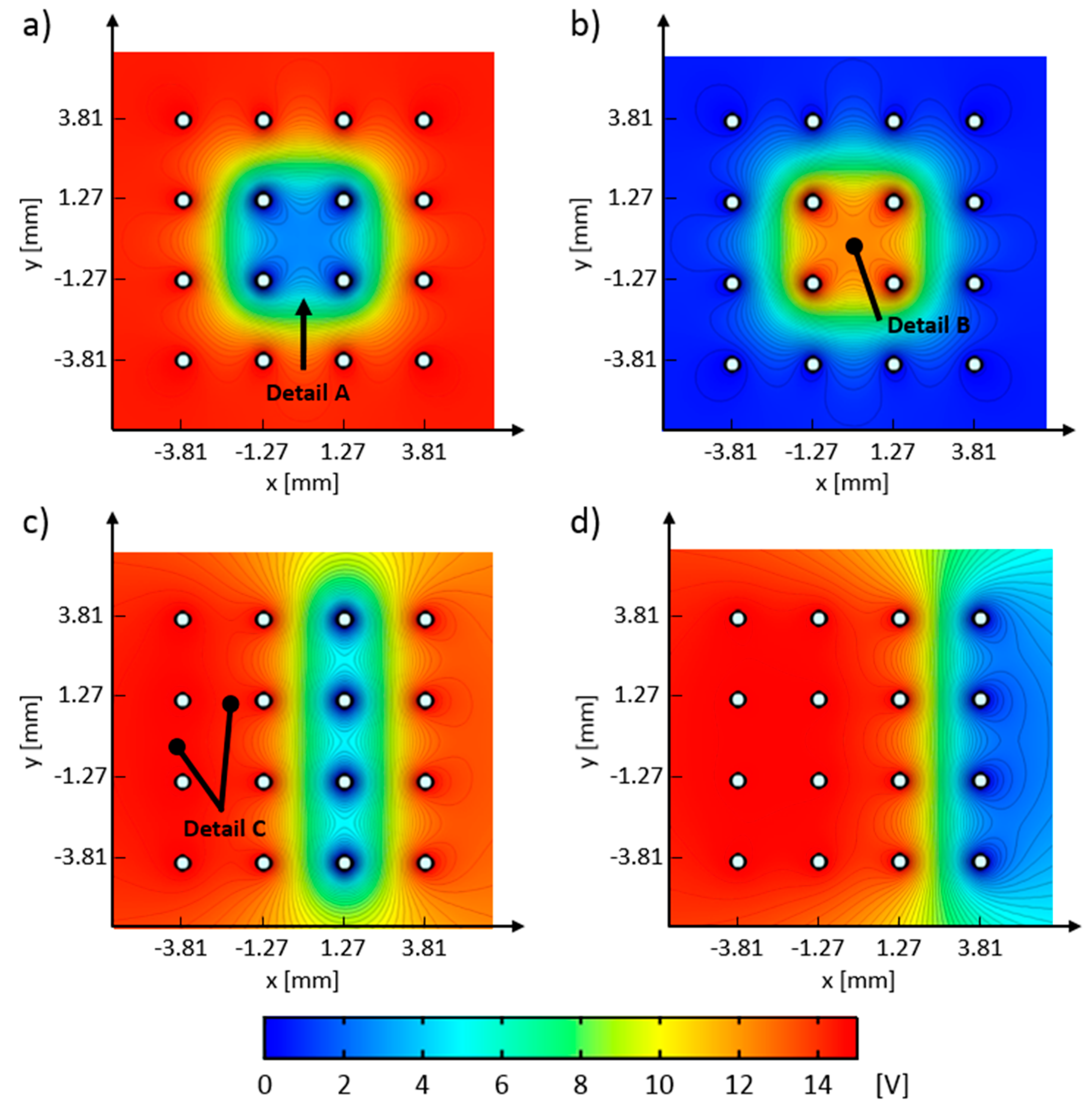
Electric potential distributions, computed with the COMSOL FEM model, are shown in
Figure 11. These figures are obtained by applying an electric potential to the electrodes’ circumferences, according to the control schemes (A–D) defined in
Figure 6a–d. Regions with high electric field gradients are identified with the closely spaced equipotential surfaces, whereas regions with low electric field gradients can be identified where equipotential surfaces are distanced further apart. All equipotential surfaces, denoted by the black contour plots, are spaced 0.34 V apart. The obtained computed electric potential distributions, which correspond to control schemes A–D, are shown in panels a–d in
Figure 11, respectively. Detail A, in
Figure 11a, indicates the region of electric potential that has a value of ~5 V, has a light blue color, and has the same arch shape as Detail A in
Figure 10b. By comparing these two details, it can be seen that very large particle groups form stable deposits and are in force equilibrium with electrical forces where the electric potential difference is approximately 5 V. Detail B, in
Figure 11b, shows the local electric field minima that have trapped the particle groups, as shown in Detail B in
Figure 10c.
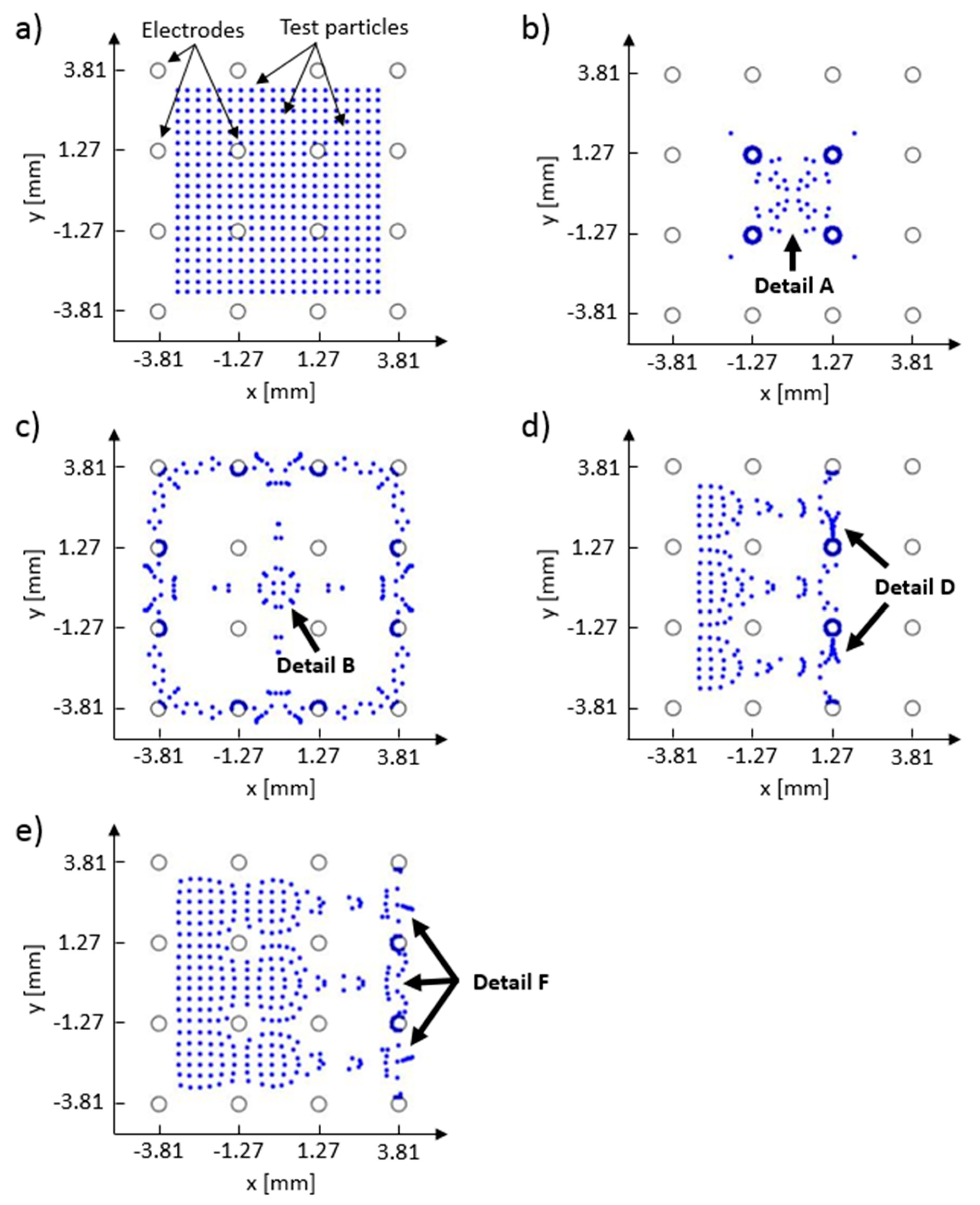
Computed test particle end positions are shown in
Figure 12. Test particle end positions that correspond to the control schemes A–D are shown in
Figure 12 panels b–e, respectively. Every simulation run had the same starting positions for all test particles and they are shown in
Figure 12a, where 400 test particles are equally spaced between −3.5 and 3.5 mm on both x and y axes. In this figure, test particles are shown as blue dots, and the positions of the electrodes are drawn with black circles (the reader is referred to the color version of
Figure 12). Detail A, in
Figure 12b, shows test particle positions which correspond to the arch-like particle deposits in Detail A in
Figure 10b, and to the electric potential distribution that has a 5-volt difference, as depicted in
Figure 11a’s Detail A. Because no interparticle and particle–electrode interaction potential was implemented in the current FEM model, the majority of the test particles have ended up on the surface of the electrodes with the lowest electric potential (i.e., blue dots which are in contact with the black circles). These full (
Figure 12b,d) or partial (
Figure 12c–e) electrode surface coverings can indicate probable locations where particles would latch on the electrode during the deposition process. Comparing
Figure 11a–d with
Figure 12b–e shows that the test particles have migrated away from the regions with high electric potential towards the regions with low electric potential. Some particle groups have remained close to their starting positions (e.g., particle group between the first and second array column in
Figure 12d, and particles between the first and third array column in
Figure 12e). Their positions, when compared to the equipotential contours in
Figure 11, indicate that the test particles have been stuck in the low electric field gradient regions.
To further characterize the EPμAM process, experiments presented in
Figure 10 were repeated twice. In these repetitions, two parameters were varied, particle concentration was decreased from 2% to 0.2% wt., and the total applied electric current was dropped from 1 A to 0.1 A. Case A experiments used 2% wt. solution and 1 A of total electric current, case B used 0.2% wt. solution and 1 A total electric current, and case C used 0.2% wt. solution and 0.1 A total electric current.
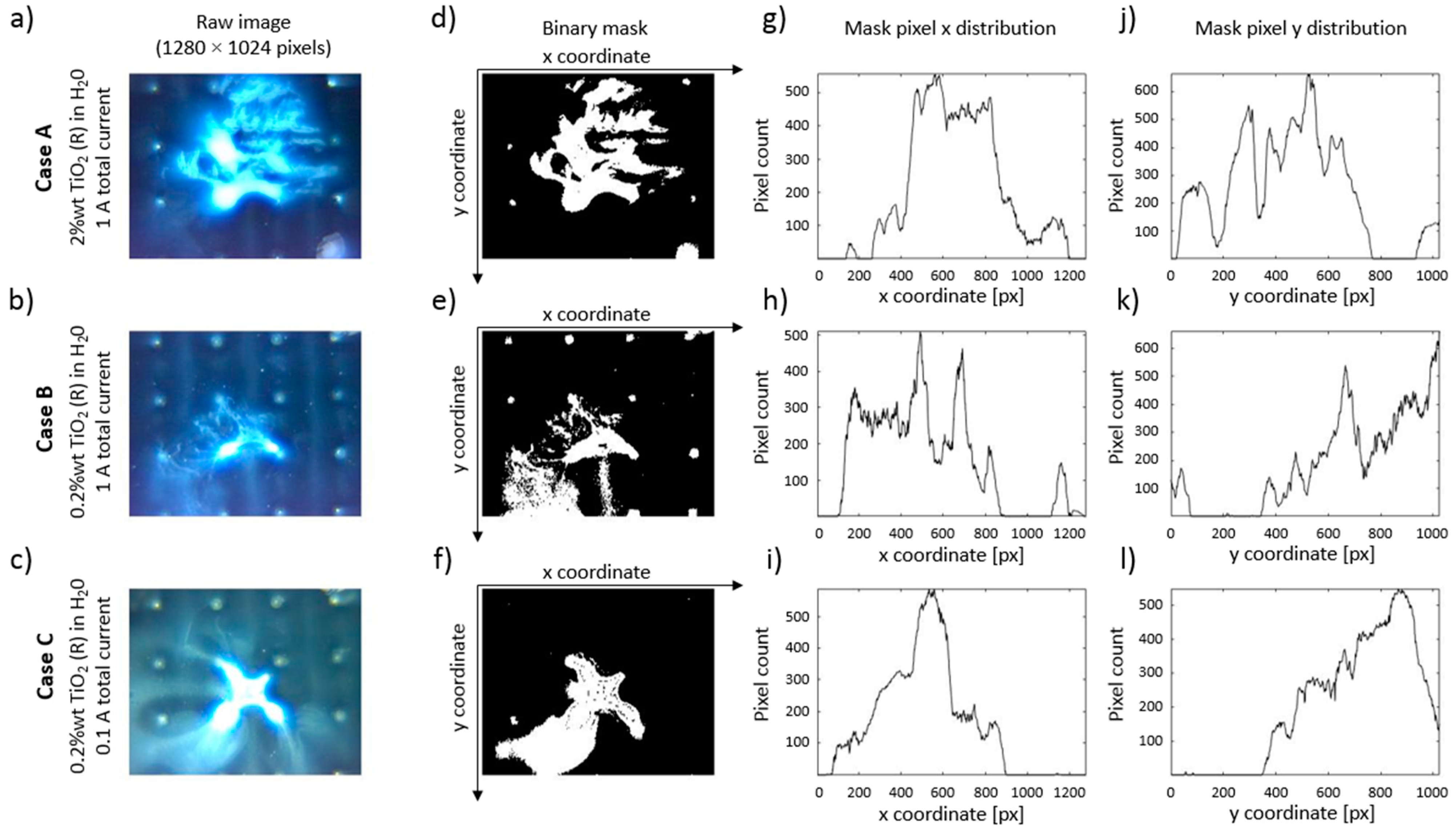
Captured images for each case were post processed in MATLAB, and the particle group locations were extracted as a binary mask. An example of post processing is shown in
Figure 13.
Figure 13a–c are raw images of particle distributions at the end particle of the focusing with control scheme B. It is noticeable from the raw image data that these particle group shapes are comparable (see the surrounding area of the middle two electrodes in the third row of the electrode array and take into account the shape of 5 V electric potential region in
Figure 11a, Detail A). A small exception to this observation is seen in
Figure 13b, where the lower particle concentration and a lower amount of injected particle solution volume managed to only partially cover the desired deposition region in the middle of the array.
The cases were quantitively compared in the following manner: The raw image data was transformed from RGB to HSV color space, and then a binary filter mask was built to extract the particle location data. Extracted binary masks of particle locations are shown 13d-f, which correspond to the images in
Figure 13a–c, respectively. Summed pixels along y- and x-axis of the images are shown in
Figure 13g–i and
Figure 13j–l, respectively. They depict the distribution of particles along the picture’s width and height and can be used for further analysis of particle redistribution during the experiments. Due to the sheer number of images used in all experiments, we employed a more aggregate approach, where the total binary mask area and rate of change in binary mask area were used.
The binary mask area and mask area change for performed experiments are presented as trendline plots in
Figure 14. The top plot in
Figure 14 shows the total number of pixels, which represent total deposition cell area covered with particles, throughout all four types of experiments, where control schemes A–D were used. To help the reader, we have marked the data from these experiments with gray and white regions in
Figure 14. Data shown in
Figure 13 corresponds to camera Frame 13, located at the beginning of the control scheme B region at the top of
Figure 14. The bottom plot in
Figure 14 shows binary mask area change, defined here as the total pixel count difference between the previous and the current camera frames.
The goal of these parameter runs was to determine the feasibility of the EPμAM process to manipulate and deposit particles when lower particle concentration and a lower electric current was used. The use of a lower particle concentration would result in material savings, and a lower electric current would allow the usage of faster and lower current-rated switching circuits. Faster switching circuits would, in turn, enable the use of a higher frequency range and expand the processing parameter space for the EPμAM process.
All three parameter runs behaved similarly when control scheme A was employed, as shown in
Figure 14 top (downward-facing slope for all three cases). The focusing runs managed to decrease the particle area approximately four times, demonstrating a reliable capability to group the particles. Higher focusing areas can be achieved by increasing the number of cycles from 100 to several hundred cycles; however, this was outside of the scope for these studies.
The parameter runs with control scheme B showed some variability. The binary mask area in these experiments seemed to increase fast for the first 30 to 50 s (see trendline values for camera Frames 11 to 15 in
Figure 14) and, then, started to decrease (from Frame 15 to Frame 20), at different rates for A and C cases. Cases B and C had a lower overall mask area as compared with the case A mask area. The total binary mask area for case B gradually increased throughout the control scheme B actuation. This “mask increase, then decrease” observation can be explained by the limited actuation range of each electrode in the following way: In the beginning, particles close to the actuating electrode are being moved away from it, which would increase the binary mask area. Then, after most of the particles are moved to the edges of the electrode’s effective actuation range, they are being deposited and grouped there which results in binary mask area reduction. From
Figure 14 data, it seems that lower particle concentration (as in cases B and C) and in combination with the lower electric current (as in case C) causes electrodes to have lower effective range.
Experimental runs, where particles are translated to the third column via the control scheme C, have the same trends, i.e., a gradual descent of trendlines for camera Frames 23 to 34, but with different offsets for total ray mask values. The results for the case B run can be explained by the overall decreased size of the binary mask (compare mask area from
Figure 13e with mask areas in
Figure 13d,f), which would translate into lower total mask area during the translation experiments. The variation in the case C run is mostly due to the binary mask filter limitation to capture image regions with lower particle concentrations (smeared particle groups, indicative of lower particle concentrations, are harder to contrast with their surroundings and background scene). Another possible explanation for these variations could be due to decreased capability of the electrode array to push away low concentration particles with a lower electric current.
Experiments, where control scheme D was used to translate particles to the fourth array column, show the same trends. All three cases run have constant mask areas, with cases A and C that have similar total value, and case B with significantly lower total mask area value than cases A and C. Case B run total binary mask area can be explained with a smaller starting group of particles (compare
Figure 13b to
Figure 13a,c).
3.3. Midline Particle Deposit Characterization
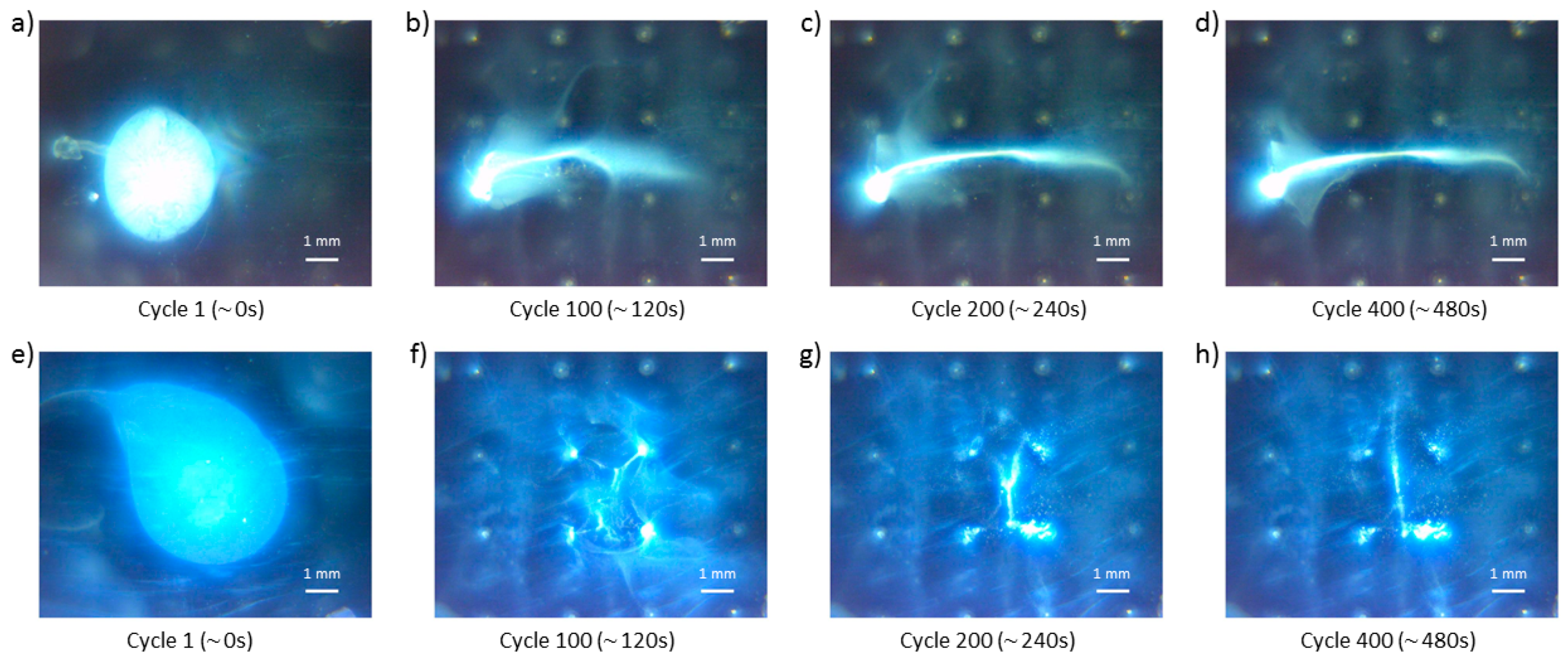
In order to characterize the obtained particle deposits, in terms of layer thickness and deposited particles’ shape, the horizontal midline deposit experiment was performed, and a high aspect ratio deposit was formed (
Figure 16). In this experiment, the initial particle blob was manipulated in the designated target region (compare panels a and e in
Figure 16) with subsequent application of control schemes E and F (refer to the panels e and f in
Figure 6). These control schemes were alternated four times each (eight alternations in total), and each control scheme was applied over 50 cycles during one alternation. The observed deposit formation is shown in
Figure 16a–e. During the deposition process, it was observed that two small particle groups, analogous to ones shown in
Figure 10 (i.e., yellow arrows), started to form in the center region of the deposit (Detail A in
Figure 16d). By the end of the deposition, these two groups merged with the whole particle deposit. Close to the end of the deposition procedure, a large particle group, analogous to the large particle groups shown in
Figure 10, started to form in the region near the middle leftmost electrodes (Detail B in
Figure 16e). However, this particle group did not latch to these electrodes and it remained in their vicinity. The overall particle deposit (
Figure 16e) was comparable in size and shape to the one shown in
Figure 15d. The reconstructed video of this midline deposit formation is provided for the reader’s reference, as
Video S1.
After the deposit was formed, the excess water (~2.5 mL) was gently removed with a syringe from the deposition cell. The remaining solution was left over to evaporate and dry. it took around 3 h for the whole cell to dry completely, under normal laboratory conditions. Then, the dried particle deposit was inspected on the Olympus OLS5000-EAF laser confocal microscope. The images in
Figure 17 were obtained with a LMPLFLN50xLEXT lens (1× zoom, 0.4 scan pitch, 50 brightness, 100 laser intensity, shading, and optical noise correction enabled set parameters). The resulting image’s size is 2423 × 12,121 pixels and was obtained with the stitching function (32 × 6 individual region scans) of the microscope’s control application.
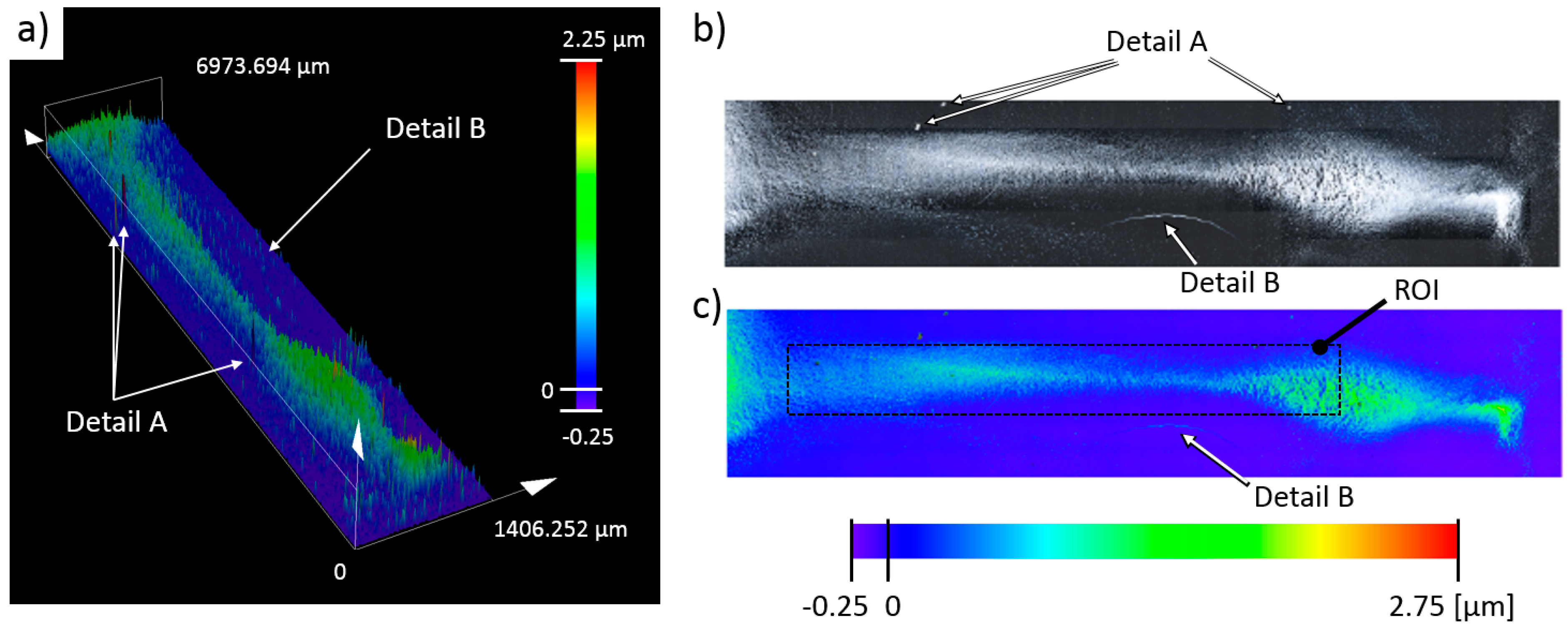
The 3D scan in
Figure 17a corresponds to the particle deposit shown in
Figure 16e. The sample scan had an approximate area of 10.5 mm
2 (7 × 1.5 mm), and the obtained particle deposit had a 1:5 (width-to-length) aspect ratio, in contrast to ~1:20 aspect ratio of the deposit, as shown in
Figure 16e. Another notable observation was the observed bending of the deposit shape, most likely due to suboptimal drying conditions, which caused partial movement of the deposit over the glass surface. Preliminary inspection of the scanned sample identified a few features (Details A and B in
Figure 17). Detail(s) A on this figure correspond to the deposits of nondispersed particle groups, deposited as a lump, which were observed with the microscope scan as a surface feature with large height. The second observation, best seen in the 2D sample intensity image in
Figure 17b is a particle deposit formed due to particle collection on the edge of the evaporating water droplet. The particles deposited in Detail B came from nondeposited particles that were freely floating in the vicinity of the deposited sample. These freely deposited particles were hard to detect on the microscope 3D height scan (compare Detail B in
Figure 17a with Detail B in
Figure 17b). The color bars in
Figure 17 have a minimum as a negative value (−0.25). This negative value originated from the tilt correction algorithm used in post processing of the scanned sample and not from the perceived negative depth. The glass substrate in the deposition cell was inspected and cleared for scratches and defects before the deposition experiment was performed.
The next step in the analysis of the deposited sample was the computation of areal roughness parameters within the OLS5000-EAF laser confocal microscope’s application, which are presented in
Table 2. The reader is referred to the technical manual for the details of the used parameters [
17]. The initial parameter computation was performed over the whole sample area, after which the computation was repeated over the region of interest (ROI) (see
Figure 17c) under the same conditions. The obtained results for the whole sample and the ROI areas are given in the second and the third column of
Table 2.
After areal parameters computation, several profile measurements were performed. In total, two vertical profile height measurements (named V1 and V2) and six horizontal profile height measurements (named H1, H2, H3, H4, H5, H6) were taken. The location of the selected profile lines is shown in
Figure 18. The dashed lines’ arrows indicate the scanning direction of the performed profile height measurements, shown in
Figure 19,
Figure 20,
Figure 21,
Figure 22,
Figure 23,
Figure 24,
Figure 25 and
Figure 26. In all these figures (
Figure 19,
Figure 20,
Figure 21,
Figure 22,
Figure 23,
Figure 24,
Figure 25 and
Figure 26), the gray lines represent the data points obtained from the profile measurement, and the black lines were obtained by applying a Gaussian filter with 100 and 50 bins for vertical and horizontal data profiles, respectively. The black line plots were used for better estimation of the observed particle deposit distributions.
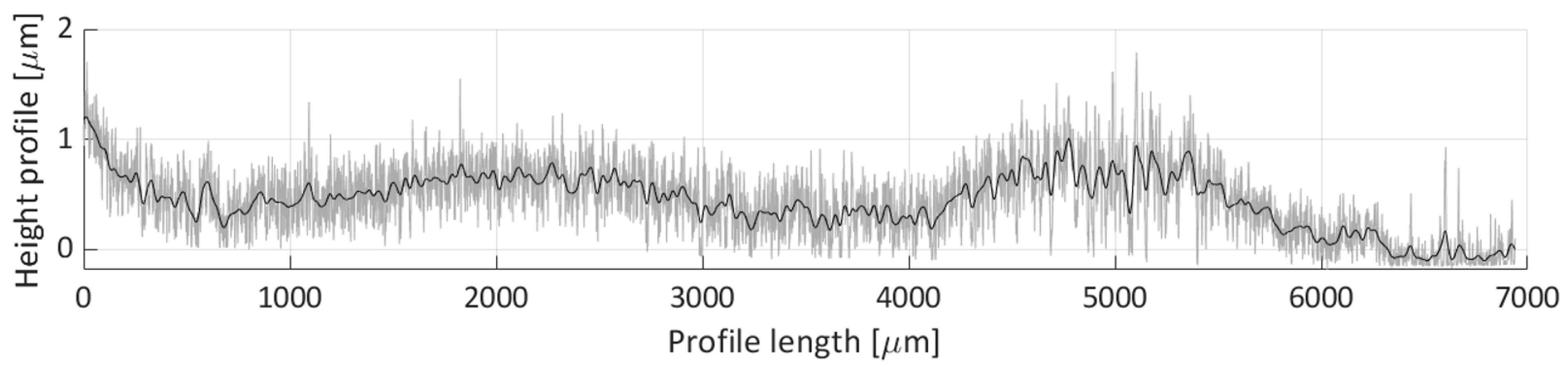
Figure 19 shows the obtained profile height for the V1 scan line. This profile covers the majority of the particle deposit’s length, where most of the detected height particle height is between 0.3 and 1 µm. The observed profile is rather nonuniform, with three specific distributions (0 to 500, 500 to 3500, and 3500 to 6000 µm over the profile’s length), which correspond to the three distinct particle groups shown in
Figure 18 (e.g., the group near the left edge of the image, the group between H2 and H3 lines, and the group between the H5 and H6 scan lines).
Figure 20 presents the height profile of the second vertical scan line (V2). This profile does not capture a lot of deposited particles. Three particle distributions are discernible from the plot (0 to 1500, 4500 to 6000, and 6000 to 6750 µm over the profile’s length). In the first two distributions, the majority of the profile height is below 1 µm height mark, and in the case of the third distribution, which is partially overlapped with the second, the profile height rises up to 2 µm.
Horizontal height profile 1 (H1) is shown in
Figure 21. The majority of the profile’s height is below 0.5 µm, with only one discernible particle distribution, spanning 200 to 1000 µm along the profile length. This profile shows the distribution of sparsely deposited particles, without clear deposit formation.
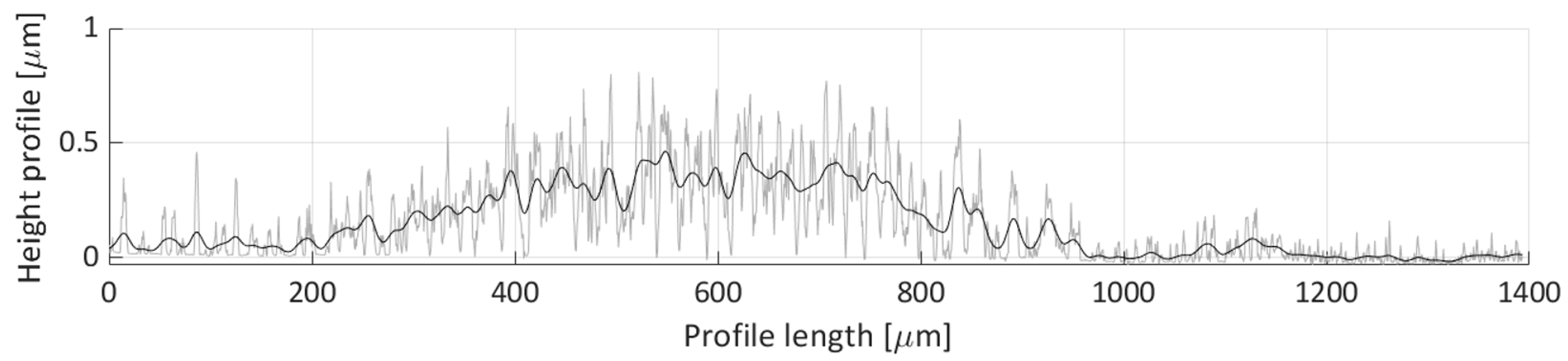
Figure 22 shows the H2 profile scan. In this figure, a single particle distribution can be detected, starting around 250 µm and ending at 800 µm on the profile length axis. The particles deposited along this line form a deposit with a ~300 µm width and an average height of around 0.5 µm.
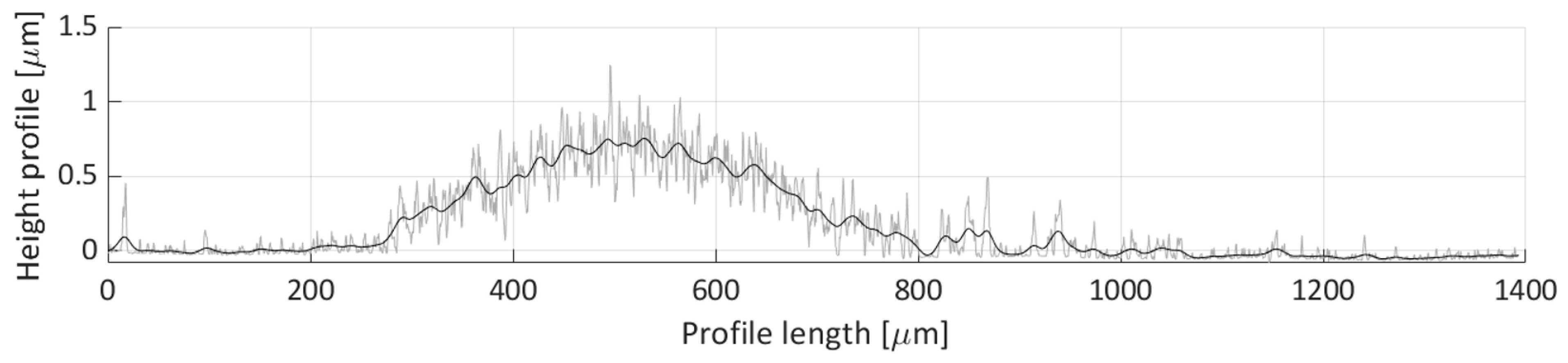
Figure 23 depicts the height profile scan for the H3 line. In this figure, only one particle distribution is present that spans from 200 µm to 800 µm along the profile length axis. Noticeable waviness in the distribution is indicative of loose particle grouping in this region, which corresponds to the particle deposit created from the particle groups denoted as Detail(s) A in
Figure 16. The average height of the profile line, within this distribution, is around 0.4 µm.
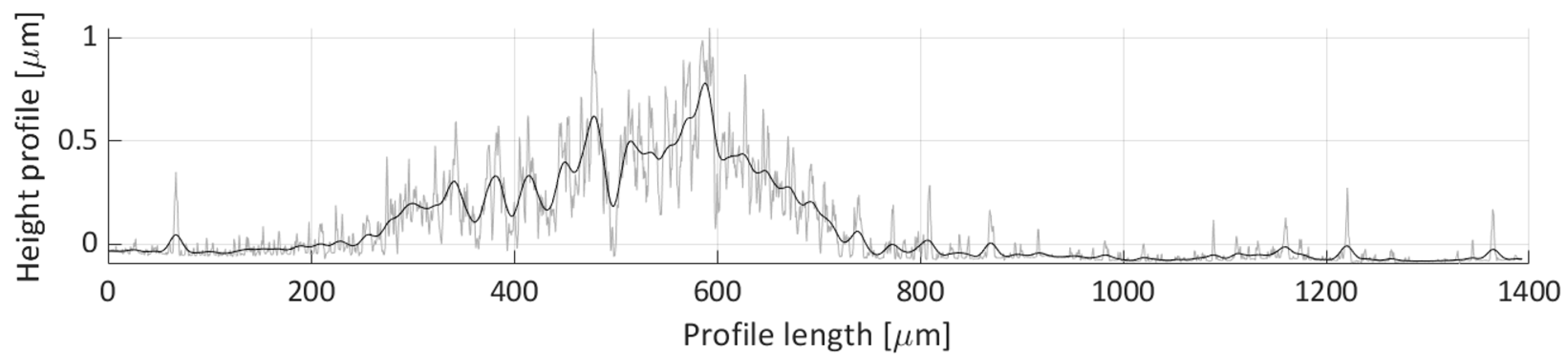
The horizontal profile scan H4 height profile is depicted in
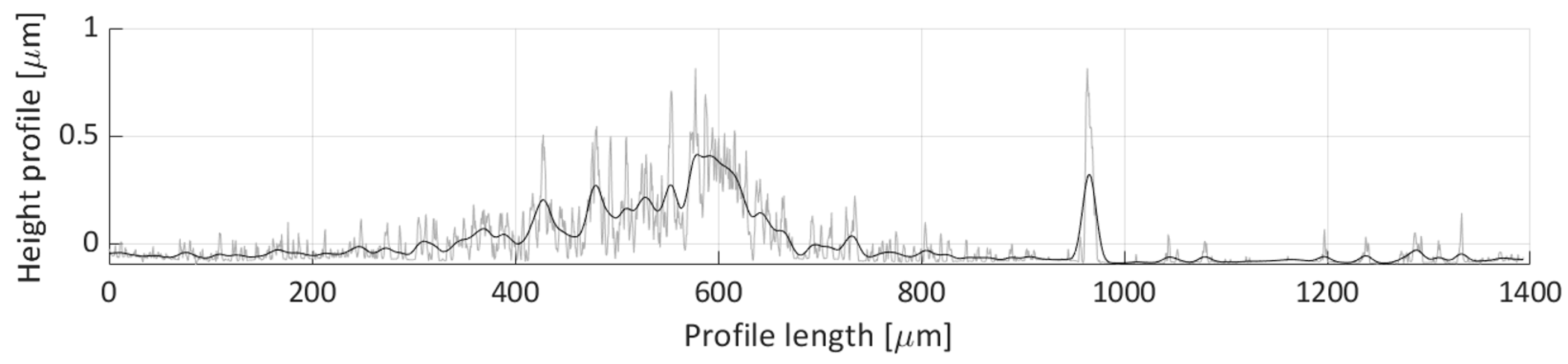
Figure 24. There seems not to be many particles deposited along this scan line, only two features can be seen on this figure. The first feature is the particle distribution spanning 400 to 600 µm profile length range, which corresponds to the small particle group deposit with an average height of around 0.250 µm. The second feature is a peak near the 975 µm length coordinate. This corresponds to the deposit height of the particle groups shown in Detail B in
Figure 17, which stems from the particle grouping on the edge of the water droplet during the droplet’s evaporation.
Figure 25 shows the height profile for the horizontal scan H5 line. A single, nonuniform distribution is noticeable between 200 and 1000 µm profile length range. The average height of the distribution profile, 0.5 µm, in this scan is larger than the others which is a possible indicator of a multilayered deposit. An apparent dip, near 700 µm, corresponds to the crack in the particle deposit, most likely due to the nonuniform drying of the particle deposit in this location.
The horizontal profile for the H6 scan line is shown in
Figure 26. In this profile, a single distribution of particles is seen, spanning from 400 to 1100 µm on the profile length axis. The average profile height in this distribution is around 0.5 µm.