3D Printed Reconfigurable Modular Microfluidic System for Generating Gel Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
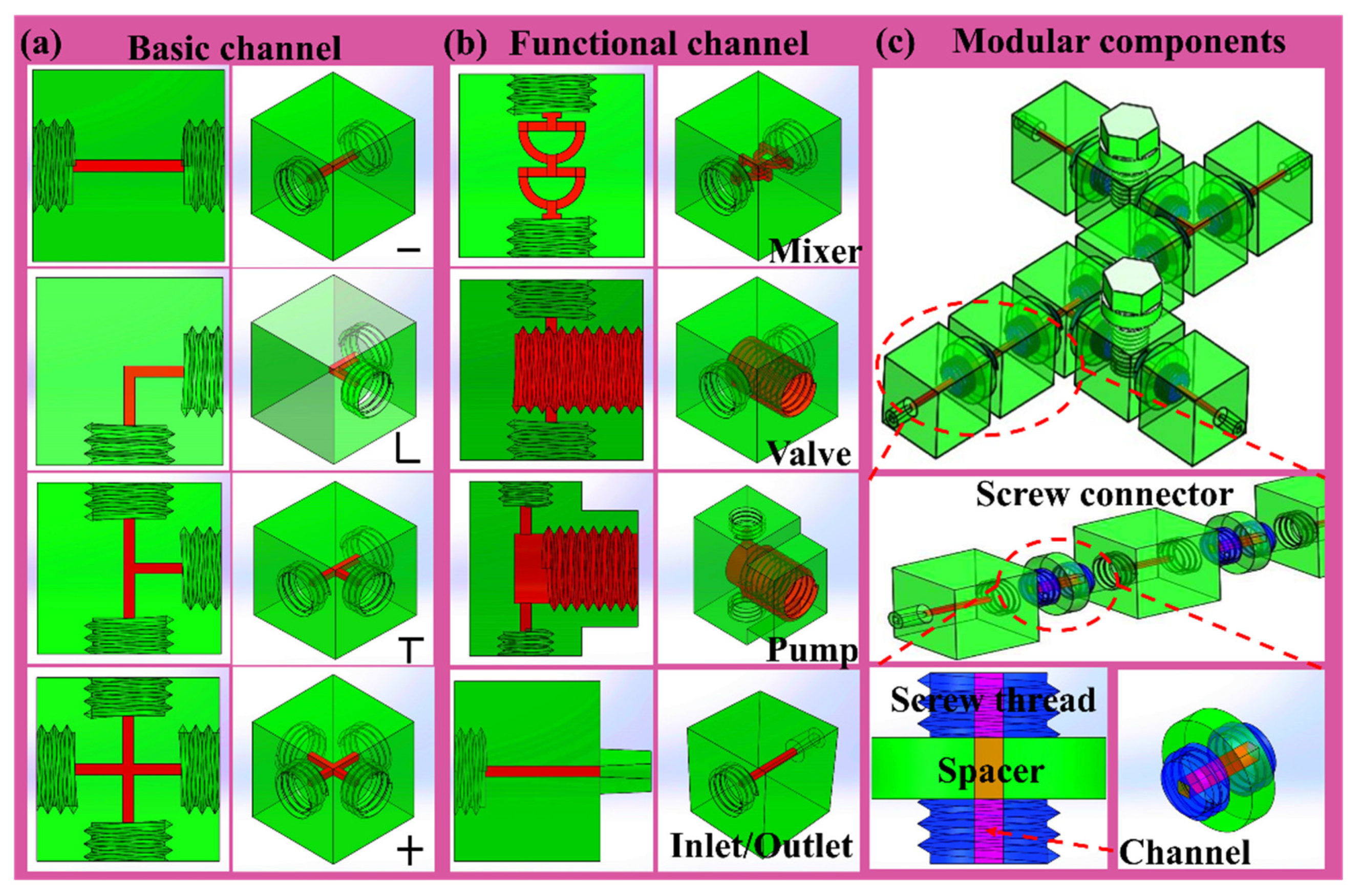
2.2. Fabrication of Basic Functional Components
3. Results and Discussion
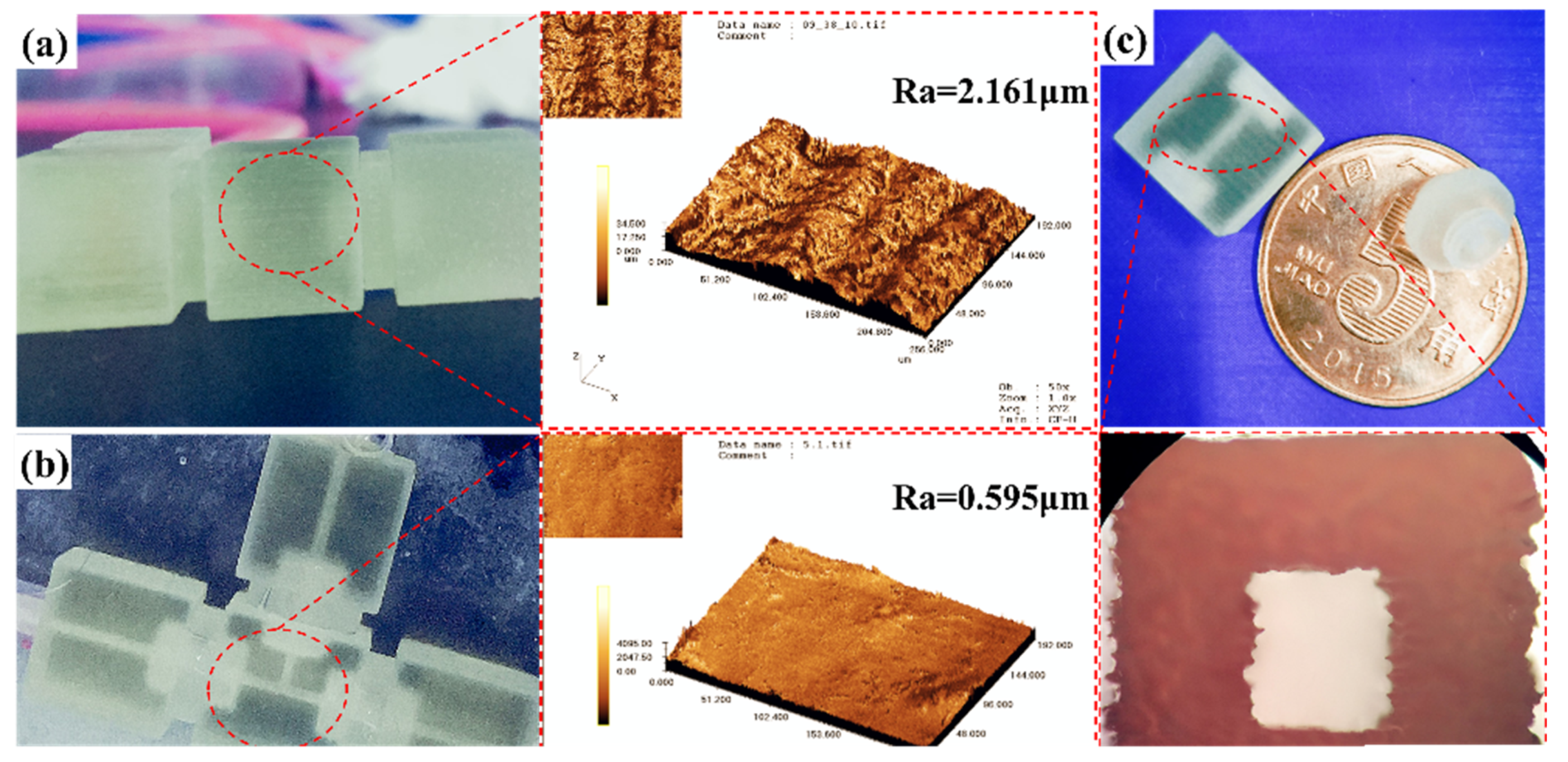
3.1. Surface Treatment and Leak Testing
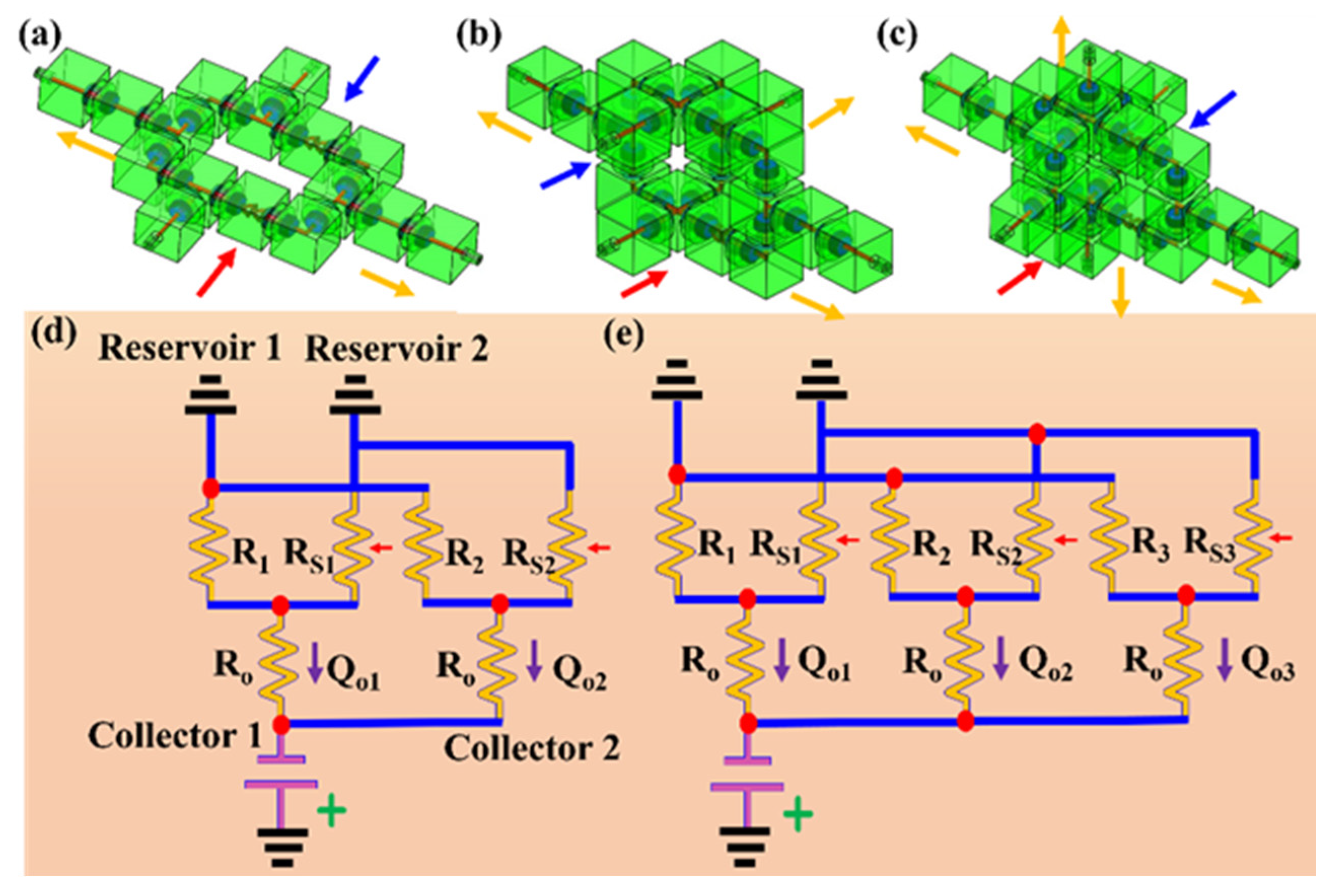
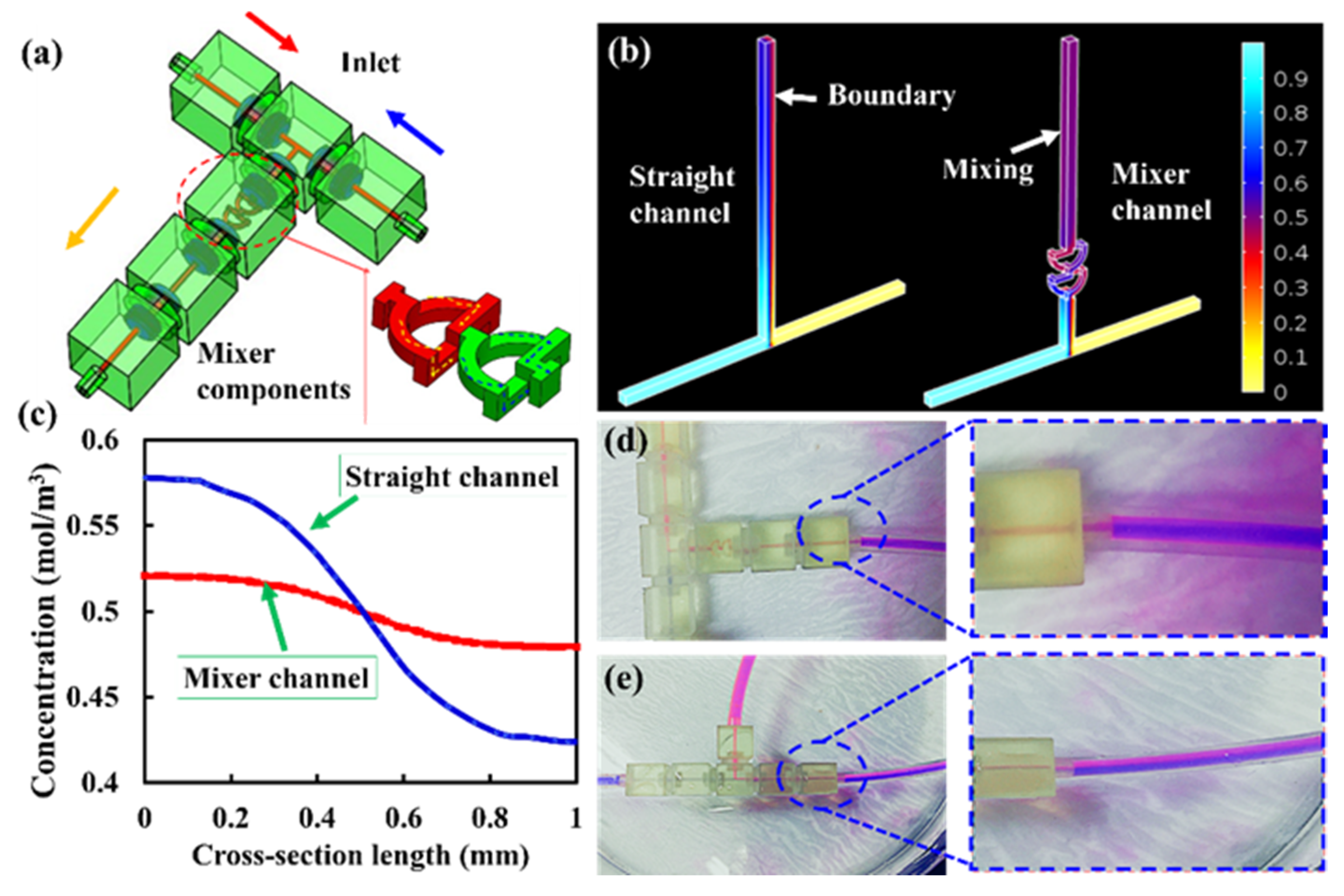
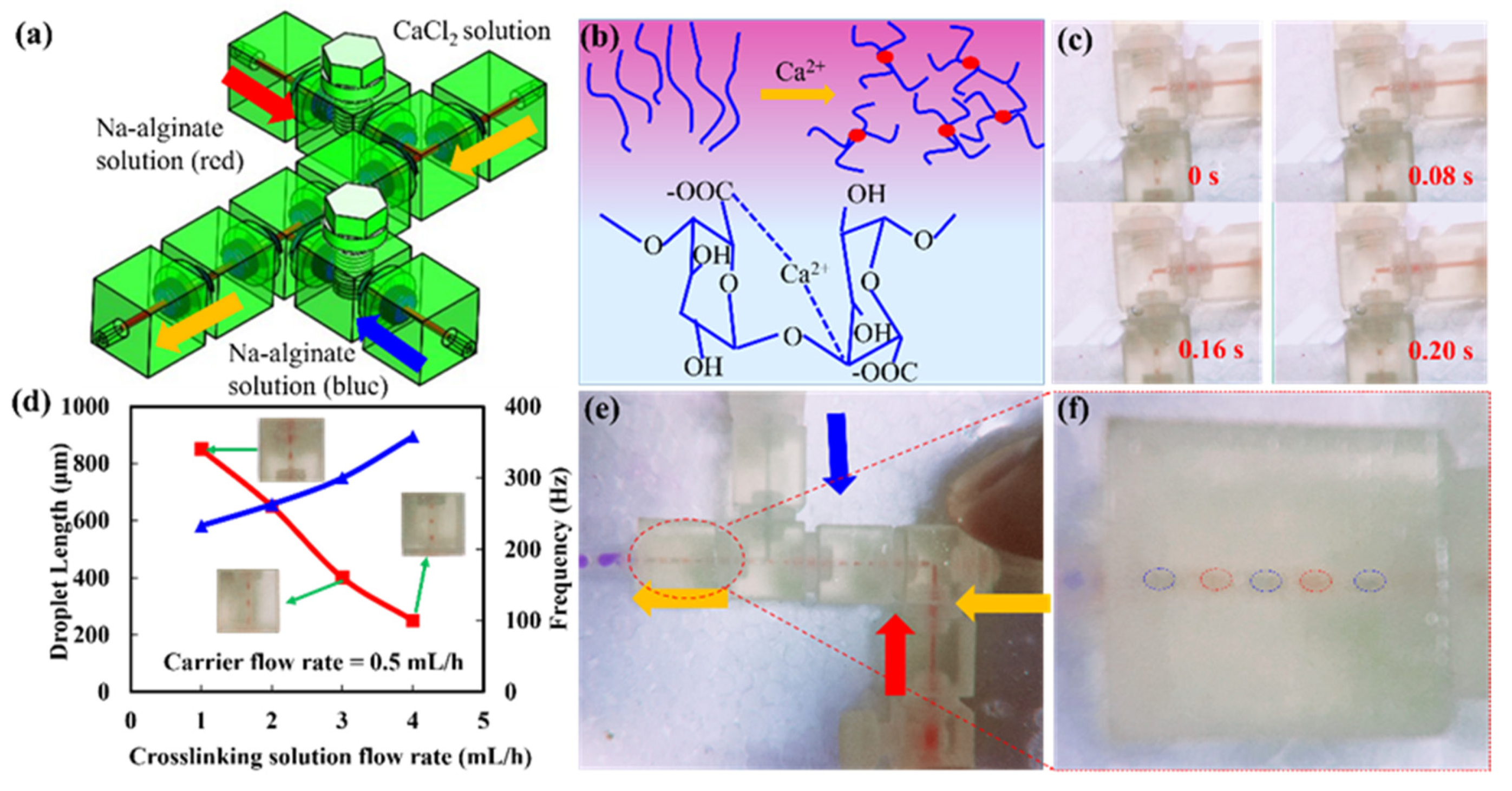
3.2. Reconfigurable Modular System Demonstration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Meng, Z.J.; Wang, W.; Liang, X.; Zheng, W.-C.; Deng, N.-N.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Plug-n-play microfluidic systems from flexible assembly of glass-based flow-control modules. Lab Chip 2015, 15, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Loskill, P.; Marcus, S.G.; Mathur, A.; Reese, W.M.; Healy, K.E. μOrgano: A Lego®-Like Plug & Play System for Modular Multi-Organ-Chips. PLoS ONE 2015, 10, e0139587. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Wang, Z.; Jiang, X. Modular microfluidics for gradient generation. Lab Chip 2008, 8, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, K.A.; Ryu, K.S.; Goluch, E.D.; Nam, J.M.; Liu, J.; Thaxton, C.S.; Chiesl, T.N.; Barron, A.E.; Lu, Y.; Mirkin, C.A. A modular microfluidic architecture for integrated biochemical analysis. Proc. Natl. Acad. Sci. USA 2005, 102, 9745–9750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, D.S.; Hsieh, Y.-F.; Kuo, L.-S.; Yang, C.-T.; Chen, P.-H. Modular component design for portable microfluidic devices. Microfluid. Nanofluid. 2011, 10, 465–474. [Google Scholar] [CrossRef]
- Hsieh, Y.F.; Yang, A.-S.; Chen, J.-W.; Liao, S.-K.; Su, T.-W.; Yeh, S.-H.; Chen, P.-J.; Chen, P.-H. A Lego®-like swappable fluidic module for bio-chem applications. Sens. Actuat. B Chem. 2014, 204, 489–496. [Google Scholar] [CrossRef]
- Bhargava, K.C.; Thompson, B.; Iqbal, D.; Malmstadt, N. Predicting the behavior of microfluidic circuits made from discrete elements. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Krisna, B.; Bryant, T.; Anoop, T.; Noah, M. Temperature Sensing in Modular Microfluidic Architectures. Micromachines 2016, 7, 11–23. [Google Scholar]
- Bhargava, K.C.; Thompson, B.; Malmstadt, N. Discrete elements for 3D microfluidics. Proc. Natl. Acad. Sci. USA 2014, 111, 15013–15018. [Google Scholar] [CrossRef] [Green Version]
- Rhee, M.; Burns, M.A. Microfluidic assembly blocks. Lab Chip 2008, 8, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Park, K.J.; Seok, S.; Shin, S.; Kim, D.H.; Park, J.Y.; Heo, Y.S.; Lee, S.J.; Lee, T.J. 3D printed modules for integrated microfluidic devices. RSC Adv. 2014, 4, 32876. [Google Scholar] [CrossRef]
- Farahani, R.D.; Dubé, M.; Therriault, D. Three-Dimensional Printing of Multifunctional Nanocomposites: Manufacturing Techniques and Applications. Adv. Mater. 2016, 28, 5794–5821. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Zhang, M.; Yeong, W.Y. Characterization and evaluation of 3D printed microfluidic chip for cell processing. Microfluid. Nanofluid. 2016, 20, 5. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-Printed Microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and Barriers. Lab Chip 2016, 16, 1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Method 2016, 8, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.K.; Zheng, H.Y.; Lim, R.Y.H.; Wang, Z.F.; Lam, Y.C. Improving surface smoothness of laser-fabricated microchannels for microfluidic application. J. Micromech. Microeng. 2011, 21, 095008. [Google Scholar] [CrossRef]
- Niall, M.P. Microsystems Manufacturing Technologies for Pharmaceutical Toxicity Testing. Ph.D. Thesis, University of Glasgow, Glasgow, UK, Novermber 2013. [Google Scholar]
- Yuen, P.K. A reconfigurable stick-n-play modular microfluidic system using magnetic interconnects. Lab Chip 2016, 16, 3700–3707. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, M.G.; Park, J.-K. Microfluidic parallel circuit for measurement of hydraulic resistance. Biomicrofluidics 2010, 4, 034110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Zheng, F.; Lu, J.; Shang, L.; Xie, Z.; Zhao, Y.; Chen, Y.; Gu, Z. Bioinspired Multicompartmental Microfibers from Microfluidics. Adv. Mater. 2014, 26, 5184–5190. [Google Scholar] [CrossRef] [PubMed]

| References | Connection Type | Manufacturing Characteristics | Fluid Pressure |
|---|---|---|---|
| Bhargava et al. [7,8,9] | Square interface embedded, comprising spacer and connector | 3D printed module, male−male connector aligned with female-type port, reversible strong | As high as 200 mL·h−1 |
| M. Rhee and M. A. Burn [10] | UV-curable glue bonding | PDMS coated glass substrate using the curing agent as the adhesive, reversible weak | 40 kPa |
| Lee et al. [11] | Insert connection, comprising rubber O–ring and metal pins | 3D printed module, concave and convex cone–shaped features, require auxiliary components. reversible strong | Up to 200 kPa |
| Yue [19] | Magnetic interconnects, comprising magnets and sealing gaskets | 3D printed module, simple, require auxiliary components | 6.8 kPa |
| Our work | Screw connector | 3D printed module, threaded connection, simple, reversible strong | Up to 500 kPa |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Mo, D.; Gong, M. 3D Printed Reconfigurable Modular Microfluidic System for Generating Gel Microspheres. Micromachines 2020, 11, 224. https://doi.org/10.3390/mi11020224
Chen X, Mo D, Gong M. 3D Printed Reconfigurable Modular Microfluidic System for Generating Gel Microspheres. Micromachines. 2020; 11(2):224. https://doi.org/10.3390/mi11020224
Chicago/Turabian StyleChen, Xiaojun, Deyun Mo, and Manfeng Gong. 2020. "3D Printed Reconfigurable Modular Microfluidic System for Generating Gel Microspheres" Micromachines 11, no. 2: 224. https://doi.org/10.3390/mi11020224





