A Quantitative Study of the Secondary Acoustic Radiation Force on Biological Cells during Acoustophoresis
Abstract
1. Introduction
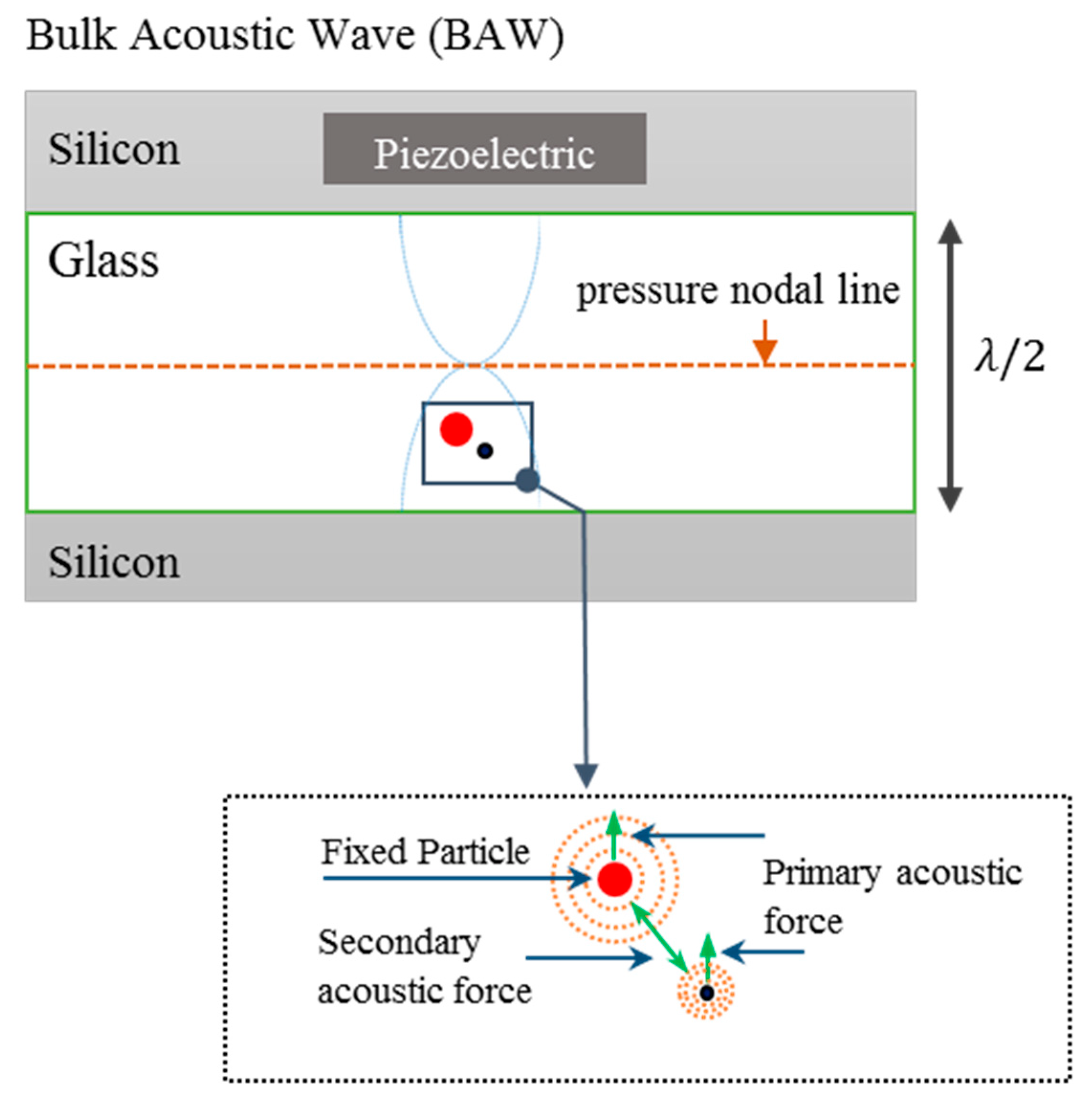
2. Interparticle Forces in Acoustophoresis
2.1. Acoustic Forces
2.2. Non-Acoustic Forces
2.2.1. Lubrication Force
2.2.2. Hydrodynamic Force
3. Materials and Methods
3.1. Experimental Apparatus
3.2. Fluid and Particle and Cell Properties
3.3. Experimental Procedure
4. Results and Discussion
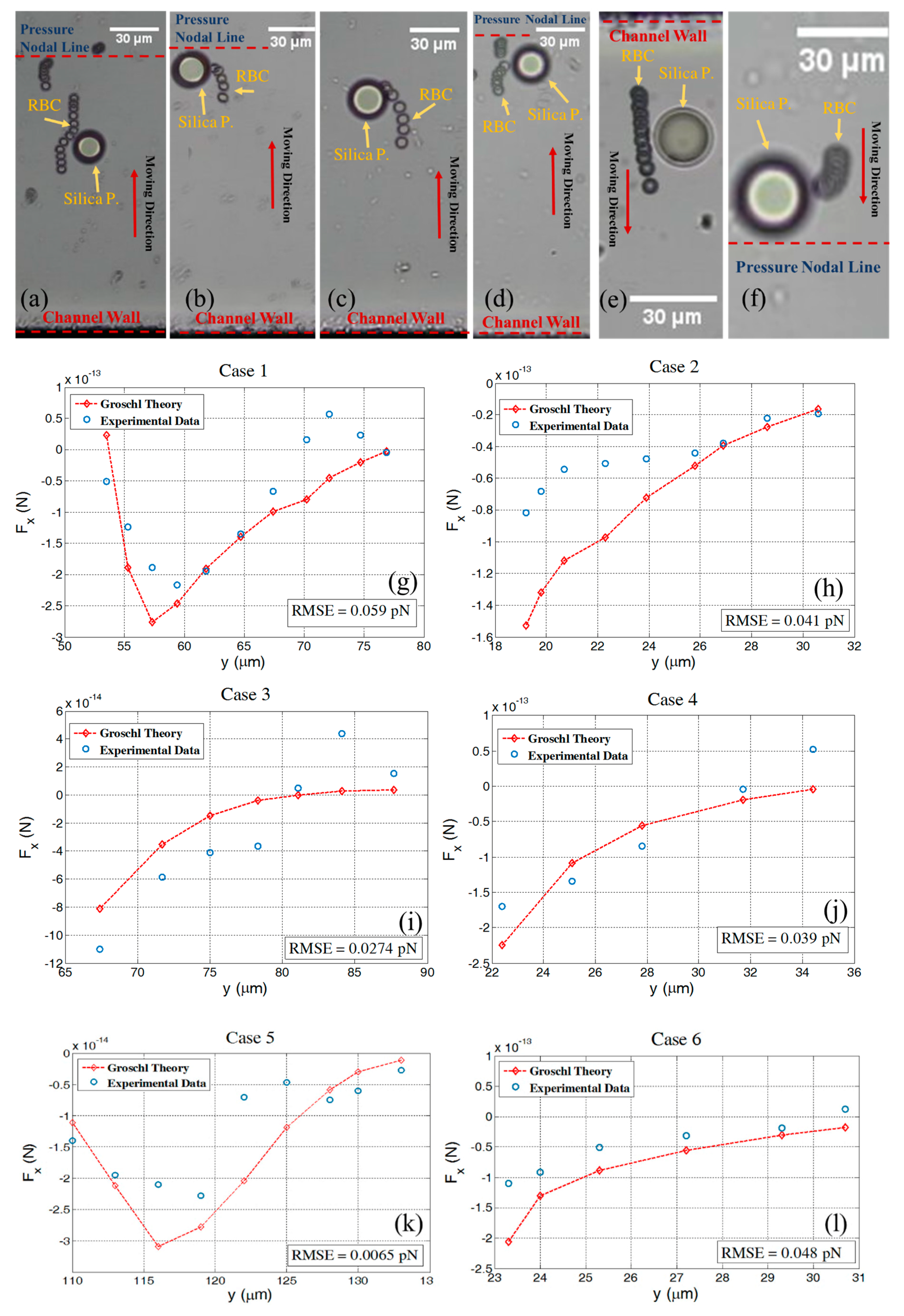
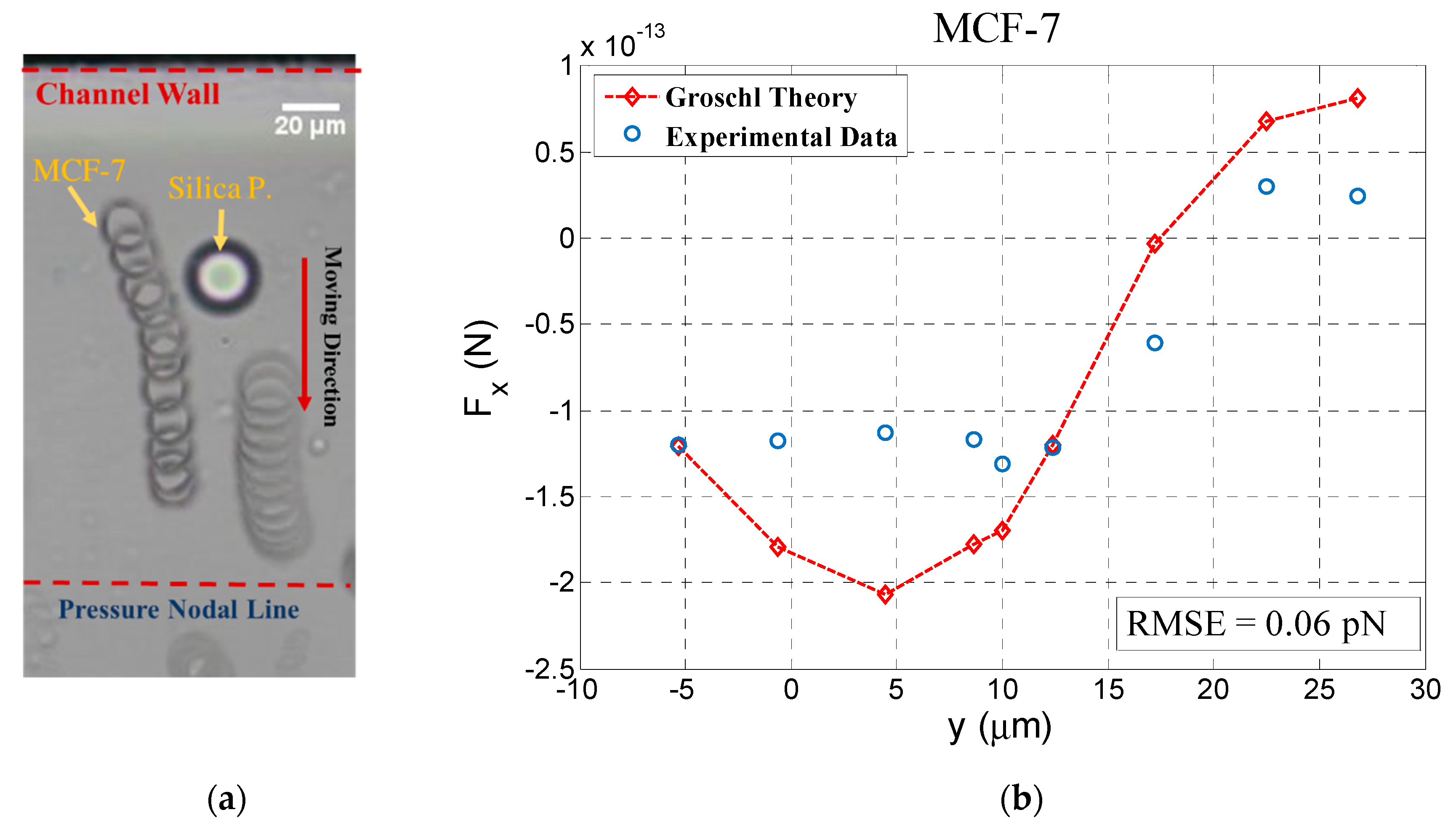
4.1. Interparticle Force Estimation
4.2. Energy Density in the Channel
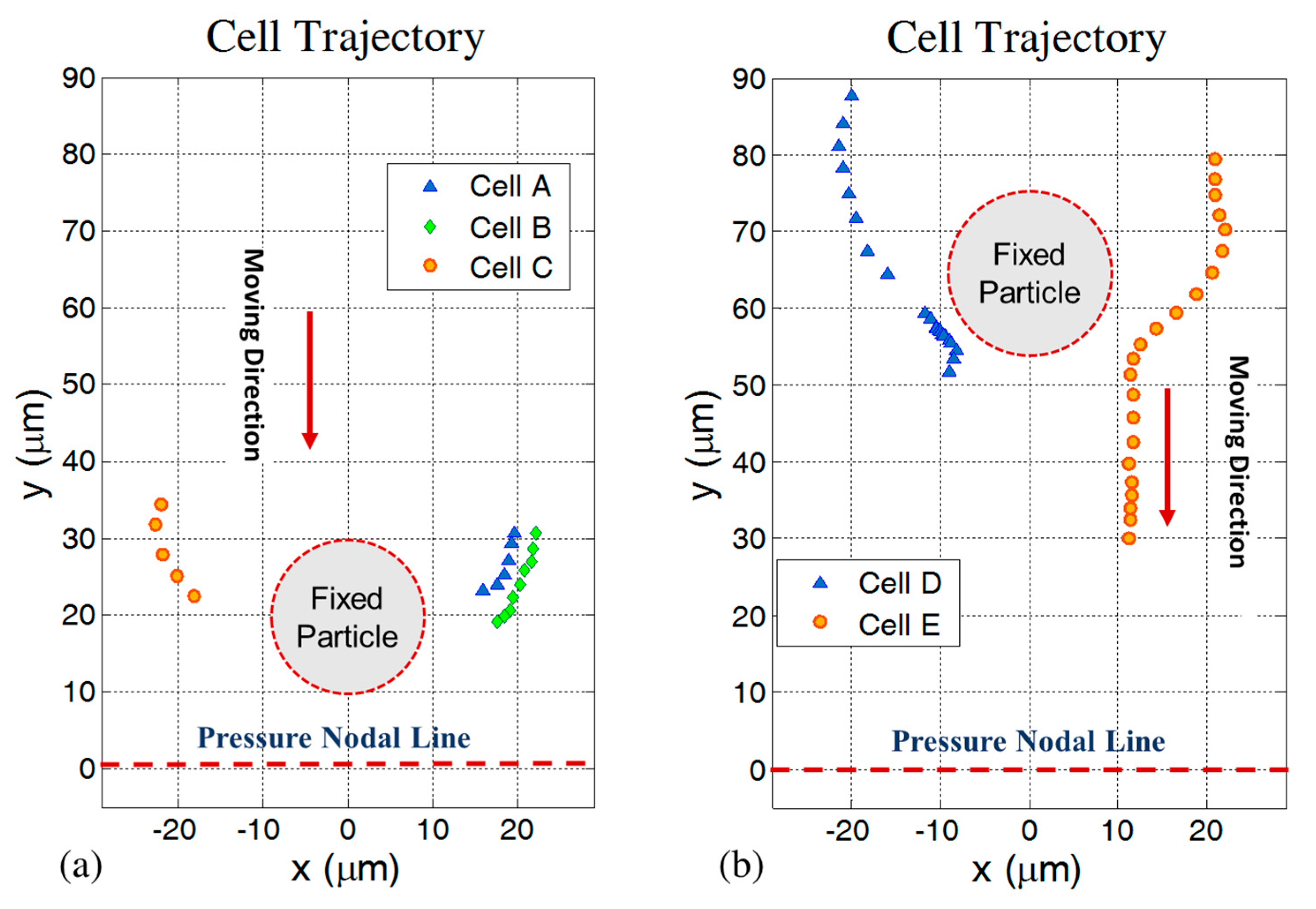
4.3. Effect of the Secondary Acoustic Force on Cell Movement
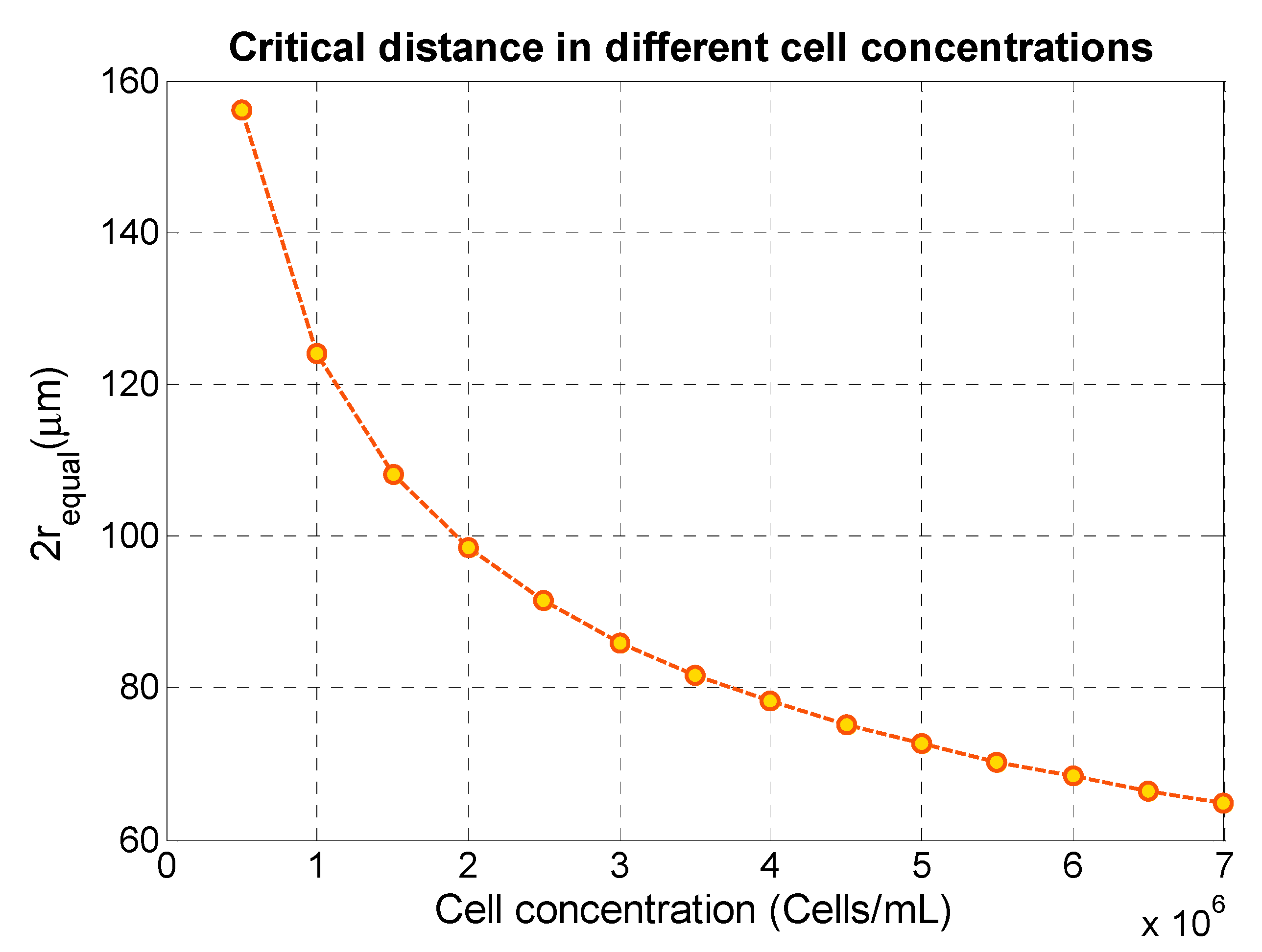
4.4. Effect of Cell Concentration on the Secondary Acoustic Force
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- King, L.V. On the acoustic radiation pressure on spheres. Proceedings of the Royal Society of London. Series A Math. Phys. Sci. 1934, 147, 212–240. [Google Scholar]
- Gor’kov, L.P. On the forces acting on a small particle in an acoustical field in an ideal fluid. Sov. Phys. Dokl. 1962, 6, 773–775. [Google Scholar]
- Yosioka, K.; Kawasima, Y. Acoustic radiation pressure on a compressible sphere. Acustica 1955, 5, 167–173. [Google Scholar]
- Hasegawa, T. Acoustic-Radiation Force on a Solid Elastic Sphere. J. Acoust. Soc. Am. 1969, 46, 1139–1143. [Google Scholar] [CrossRef]
- Doinikov, A.A. Acoustic radiation force on a spherical particle in a viscous heat-conducting fluid. I. General formula. J. Acoust. Soc. Am. 1997, 101, 713–721. [Google Scholar] [CrossRef]
- Doinikov, A.A. Acoustic radiation force on a spherical particle in a viscous heat-conducting fluid. III. Force on a liquid drop. J. Acoust. Soc. Am. 1997, 101, 731–740. [Google Scholar] [CrossRef]
- Bruus, H.; Dual, J.; Hawkes, J.; Hill, M.; Laurell, T.; Nilsson, J.; Radel, S.; Sadhal, S.; Wiklund, M. Forthcoming Lab on a Chip tutorial series on acoustofluidics: Acoustofluidics-exploiting ultrasonic standing wave forces and acoustic streaming in microfluidic systems for cell and particle manipulation. Lab Chip 2011, 11, 3579–3580. [Google Scholar] [CrossRef]
- Lenshof, A.; Magnusson, C.; Laurell, T. Acoustofluidics 8: Applications of acoustophoresis in continuous flow microsystems. Lab Chip 2012, 12, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Cai, F.; Jin, Q.; Niu, L.; Jiang, C.; Wang, Z.; Wu, J.; Zheng, H. Acoustic aligning and trapping of microbubbles in an enclosed PDMS microfluidic device. Sens. Actuators B Chem. 2011, 160, 1599–1605. [Google Scholar] [CrossRef]
- Roux-Marchand, T.; Beyssen, D.; Sarry, F.; Elmazria, O. Rayleigh surface acoustic wave as an efficient heating system for biological reactions: Investigation of microdroplet temperature uniformity. IEEE Trans Ultrason. Ferroelectr. Freq. Control. 2015, 62, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.B.; Bruus, H. Theoretical study of time-dependent, ultrasound-induced acoustic streaming in microchannels. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2015, 92, 063018. [Google Scholar] [CrossRef] [PubMed]
- Lighthill, S.J. Acoustic streaming. J. Sound Vib. 1978, 61, 391–418. [Google Scholar] [CrossRef]
- Wiklund, M.; Green, R.; Ohlin, M. Acoustofluidics 14: Applications of acoustic streaming in microfluidic devices. Lab Chip 2012, 12, 2438–2451. [Google Scholar] [CrossRef] [PubMed]
- Settnes, M.; Bruus, H. Forces acting on a small particle in an acoustical field in a viscous fluid. Phys. Rev. E 2012, 85, 016327. [Google Scholar] [CrossRef] [PubMed]
- Gröschl, M. Ultrasonic Separation of Suspended Particles-Part I: Fundamentals. Acustica 1998, 84, 432–447. [Google Scholar]
- Bjerknes, V.F.K. Fields of Force; Columbia University Press: New York, NY, USA, 1906. [Google Scholar]
- Crum, L.A. Bjerknes forces on bubbles in a stationary sound field. J. Acoust. Soc. Am. 1975, 57, 1363–1370. [Google Scholar] [CrossRef]
- Doinikov, A.A. Bjerknes forces between two bubbles in a viscous fluid. J. Acoust. Soc. Am. 1999, 106, 3305–3312. [Google Scholar] [CrossRef]
- Zheng, X.; Apfel, R.E. Acoustic interaction forces between two fluid spheres in an acoustic field. J. Acoust. Soc. Am. 1995, 97, 2218–2226. [Google Scholar] [CrossRef]
- Garcia-Sabaté, A.; Castro, A.; Hoyos, M.; González-Cinca, R. Experimental study on inter-particle acoustic forces. J. Acoust. Soc. Am. 2014, 135, 1056–1063. [Google Scholar] [CrossRef]
- Silva, G.T.; Bruus, H. Acoustic interaction forces between small particles in an ideal fluid. Phys. Rev. E 2014, 90, 063007. [Google Scholar] [CrossRef]
- Sepehrirahnama, S.; Lim, K.M.; Chau, F.S. Numerical study of interparticle radiation force acting on rigid spheres in a standing wave. J. Acoust. Soc. Am. 2015, 137, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Baasch, T.; Leibacher, I.; Dual, J. Multibody dynamics in acoustophoresis. J. Acoust. Soc. Am. 2017, 141, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Allen, J.S.; Ardekani, A.M. Unsteady particle motion in an acoustic standing wave field. Eur. J. Comput. Mech. 2017, 26, 115–130. [Google Scholar] [CrossRef]
- Habibi, R.; Devendran, C.; Neild, A. Trapping and patterning of large particles and cells in a 1D ultrasonic standing wave. Lab Chip 2017, 17, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.R.; Sepehrirahnama, S.; Lim, K. Experimental measurement of interparticle acoustic radiation force in the Rayleigh limit. Phys. Rev. E 2018, 97, 053105. [Google Scholar] [CrossRef]
- Saeidi, D.; Saghafian, M.; Javanmard, S.; Hammarstrom, B.; Wiklund, M. Acoustic dipole and monopole effects in solid particle interaction dynamics during acoustophoresis. J. Acoust. Soc. Am. 2019, 145, 3311–3319. [Google Scholar] [CrossRef]
- Apfel, R.E. Acoustically induced square law forces and some speculations about gravitation. Am. J. Phys. 1988, 56, 726–729. [Google Scholar] [CrossRef]
- Weiser, M.A.; Apfel, R.E.; Neppiras, E.A. Interparticle forces on red cells in a standing wave field. Acta Acust. United Acust. 1984, 56, 114–119. [Google Scholar]
- Crum, L.A. Acoustic force on a liquid droplet in an acoustic stationary wave. J. Acoust. Soc. Am. 1971, 50, 157–163. [Google Scholar] [CrossRef]
- Vázquez-Quesada, A.; Ellero, M. Analytical solution for the lubrication force between two spheres in a bi-viscous fluid. Phys. Fluids 2016, 28, 073101. [Google Scholar] [CrossRef]
- Lambert, B.; Weynans, L.; Bergmann, M. Local lubrication model for spherical particles within incompressible Navier-Stokes flows. Phy. Rev. E 2018, 97, 033313. [Google Scholar] [CrossRef] [PubMed]
- Manneberg, O.; Svennebring, J.; Hertz, H.M.; Wiklund, M. Wedge transducer design for two-dimensional ultrasonic manipulation in a microfluidic chip. J. Micromech. Microeng. 2008, 18, 095025. [Google Scholar] [CrossRef]
- Mishra, P.; Hill, M.; Glynne-Jones, P. Deformation of red blood cells using acoustic radiation forces. Biomicrofluidics 2014, 8, 034109. [Google Scholar] [CrossRef] [PubMed]
- Hartono, D.; Liu, Y.; Tan, P.L.; Then, X.Y.S.; Yung, L.-Y.L.; Lim, K.-M. On-chip measurements of cell compressibility via acoustic radiation. Lab Chip 2011, 11, 4072–4080. [Google Scholar] [CrossRef]
- Brown, D. Tracker Free Video Analysis and Modeling Tool for Physics Education. Edition 4.11.0, Open Source Physics compadre. Available online: https://arxiv.org/abs/1308.2614 (accessed on 30 January 2020).
- Barnkob, R.; Augustsson, P.; Laurell, T.; Bruus, H. Measuring the local pressure amplitude in microchannel acoustophoresis. Lab Chip 2010, 10, 563–570. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeidi, D.; Saghafian, M.; Haghjooy Javanmard, S.; Wiklund, M. A Quantitative Study of the Secondary Acoustic Radiation Force on Biological Cells during Acoustophoresis. Micromachines 2020, 11, 152. https://doi.org/10.3390/mi11020152
Saeidi D, Saghafian M, Haghjooy Javanmard S, Wiklund M. A Quantitative Study of the Secondary Acoustic Radiation Force on Biological Cells during Acoustophoresis. Micromachines. 2020; 11(2):152. https://doi.org/10.3390/mi11020152
Chicago/Turabian StyleSaeidi, Davood, Mohsen Saghafian, Shaghayegh Haghjooy Javanmard, and Martin Wiklund. 2020. "A Quantitative Study of the Secondary Acoustic Radiation Force on Biological Cells during Acoustophoresis" Micromachines 11, no. 2: 152. https://doi.org/10.3390/mi11020152
APA StyleSaeidi, D., Saghafian, M., Haghjooy Javanmard, S., & Wiklund, M. (2020). A Quantitative Study of the Secondary Acoustic Radiation Force on Biological Cells during Acoustophoresis. Micromachines, 11(2), 152. https://doi.org/10.3390/mi11020152




