Scale-Up Studies for Co/Ni Separations in Intensified Reactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
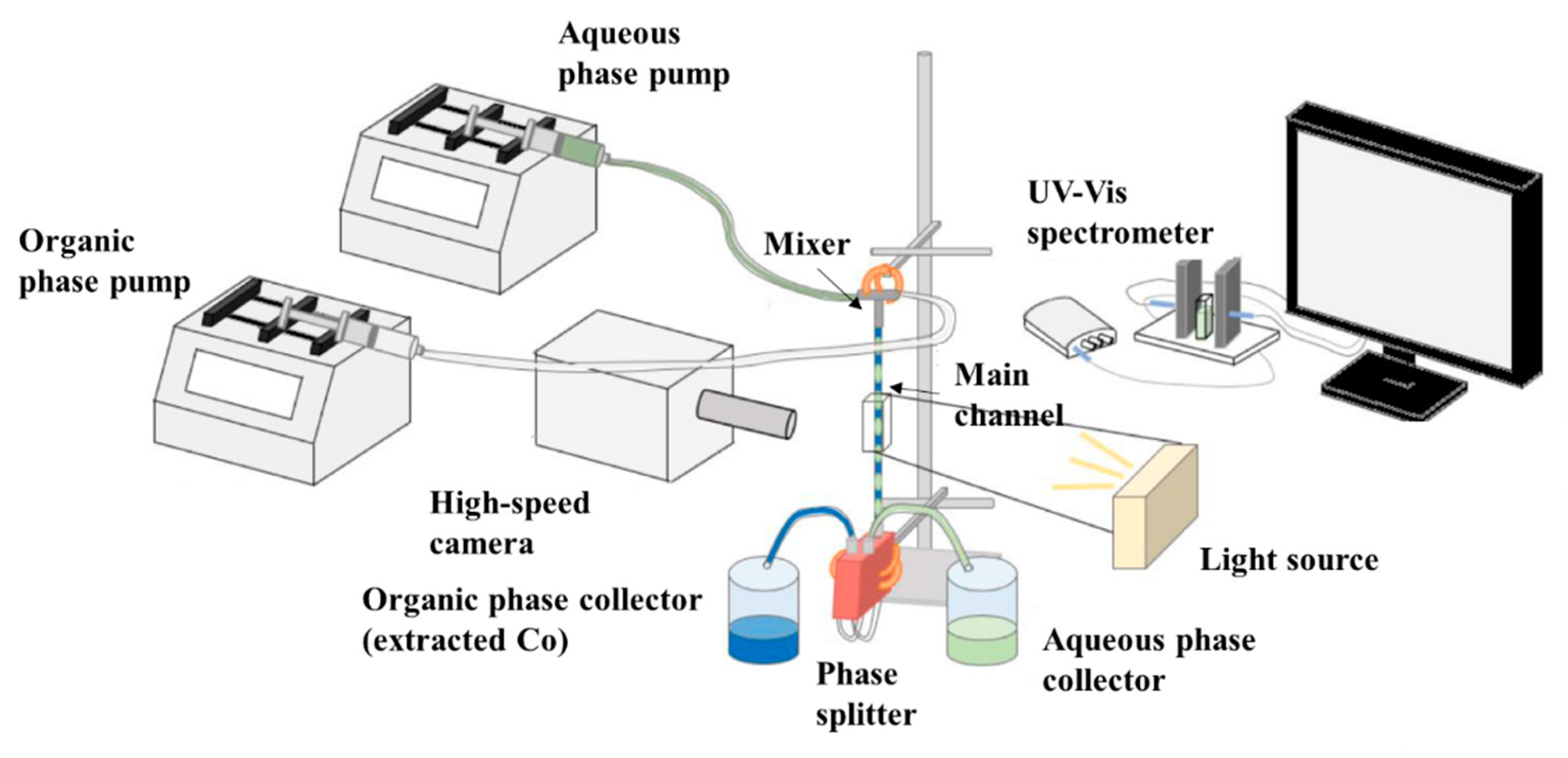
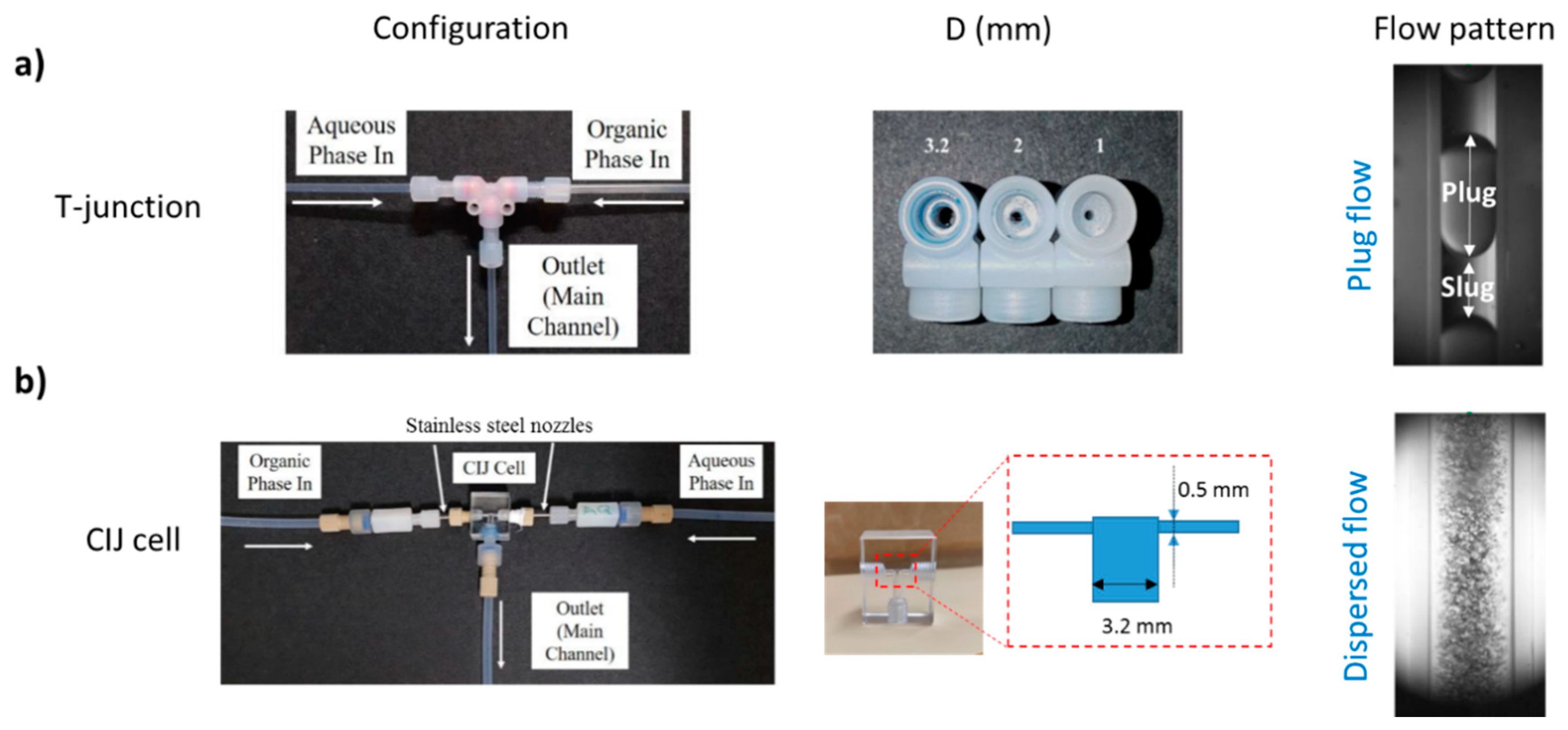
2.2. Experimental Setup and Procedure
3. Results and Discussion
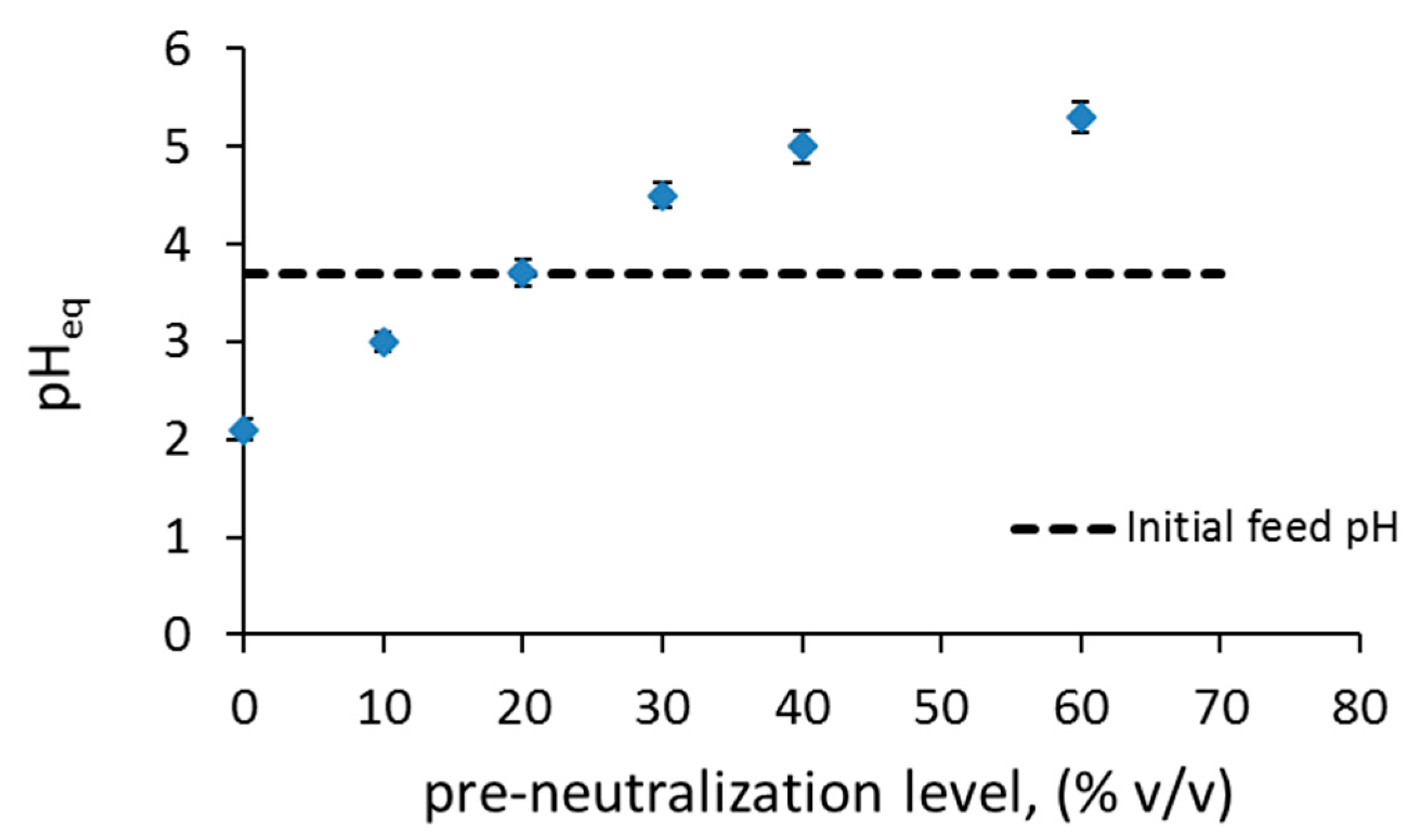
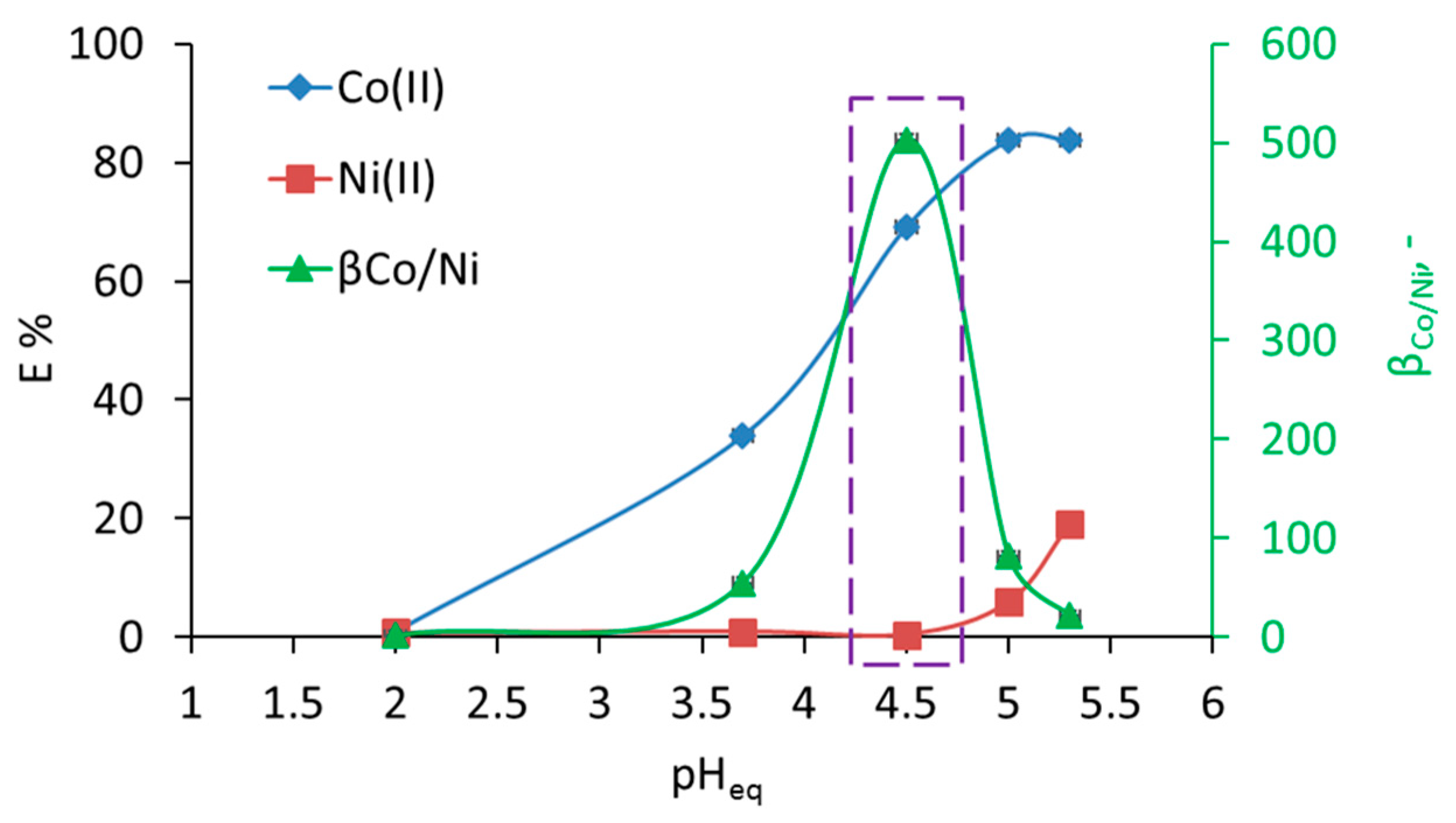
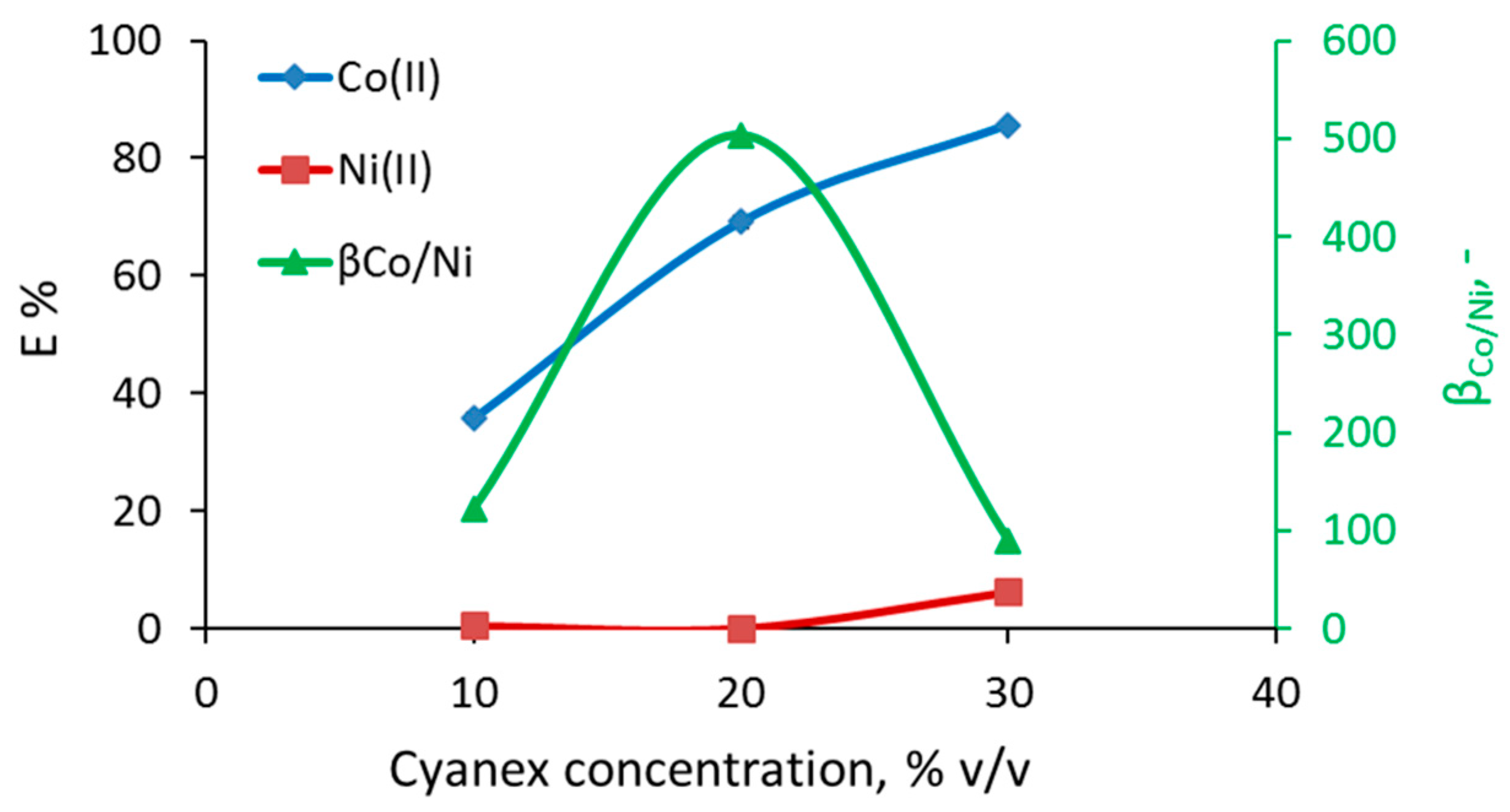
3.1. Separation of Co from Ni/Co Mixtures at Equilibrium
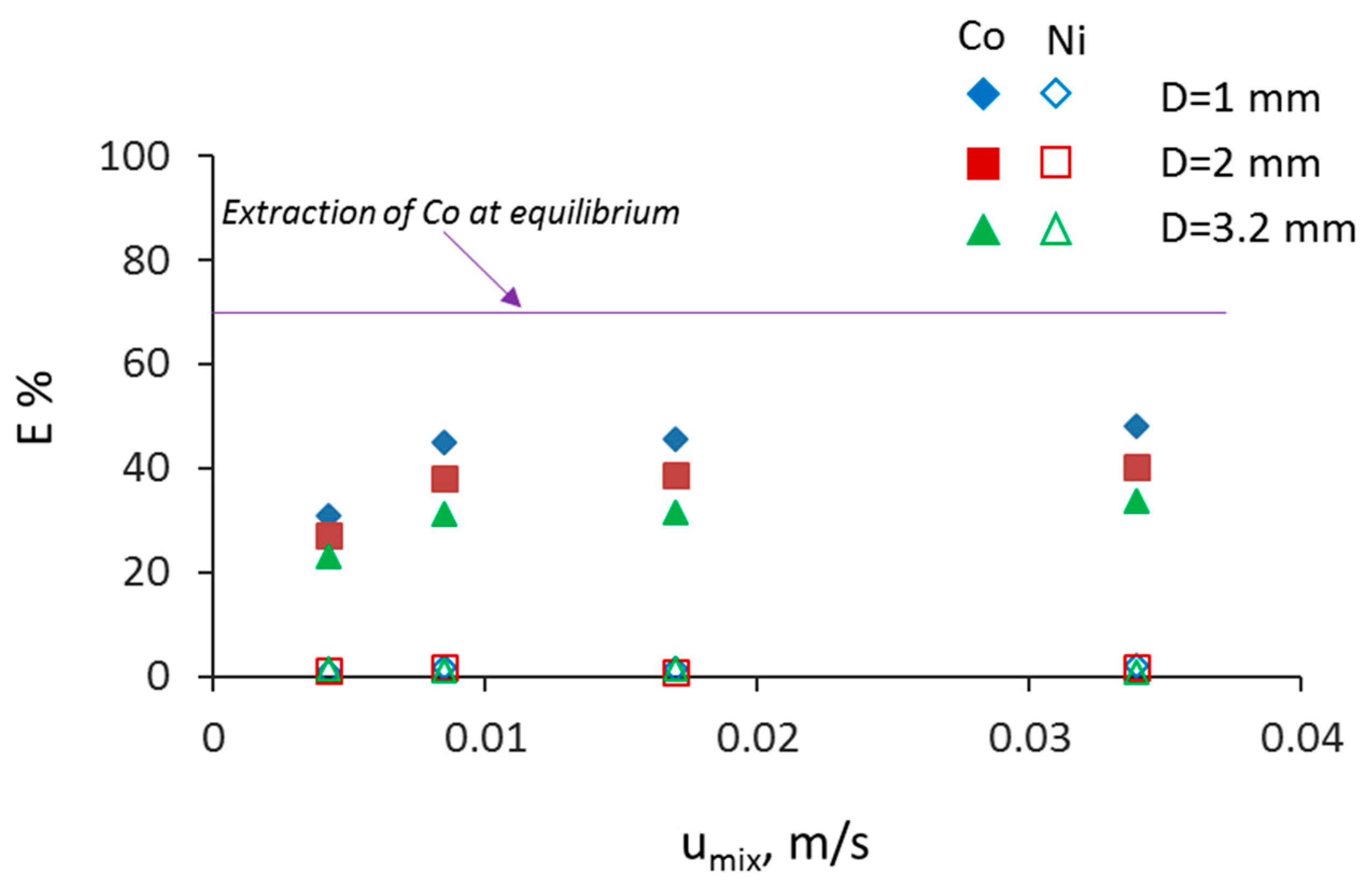
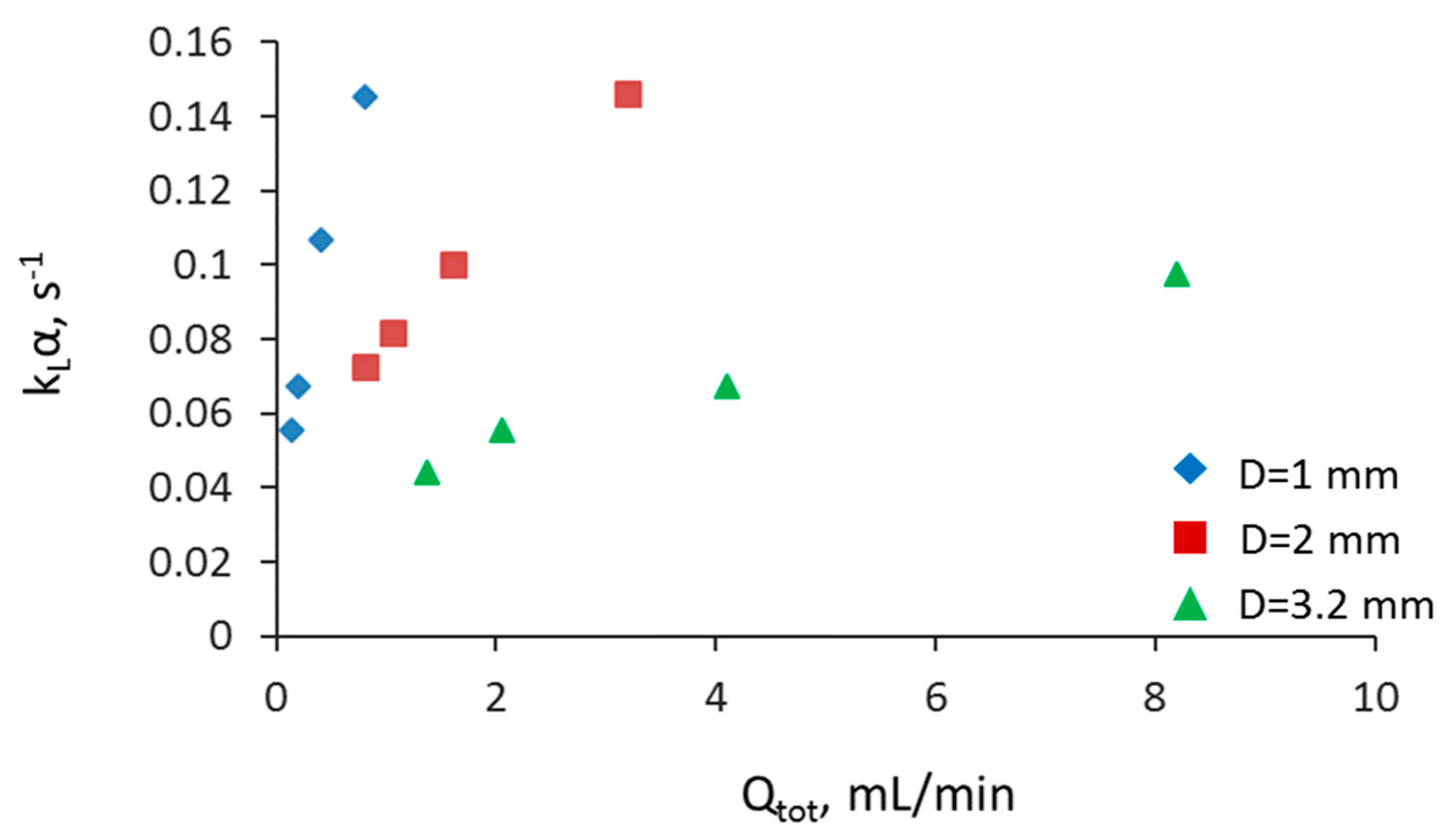
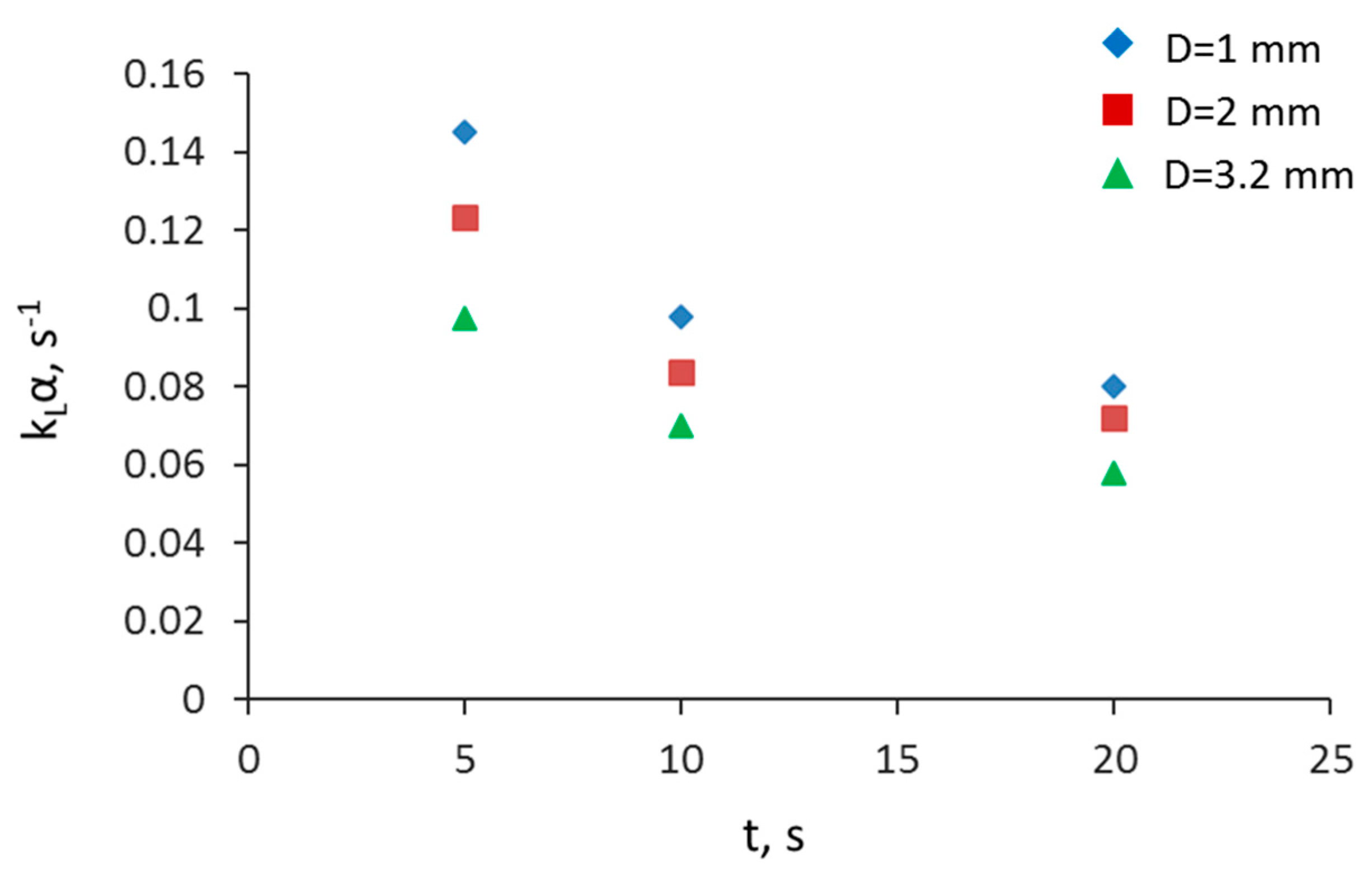
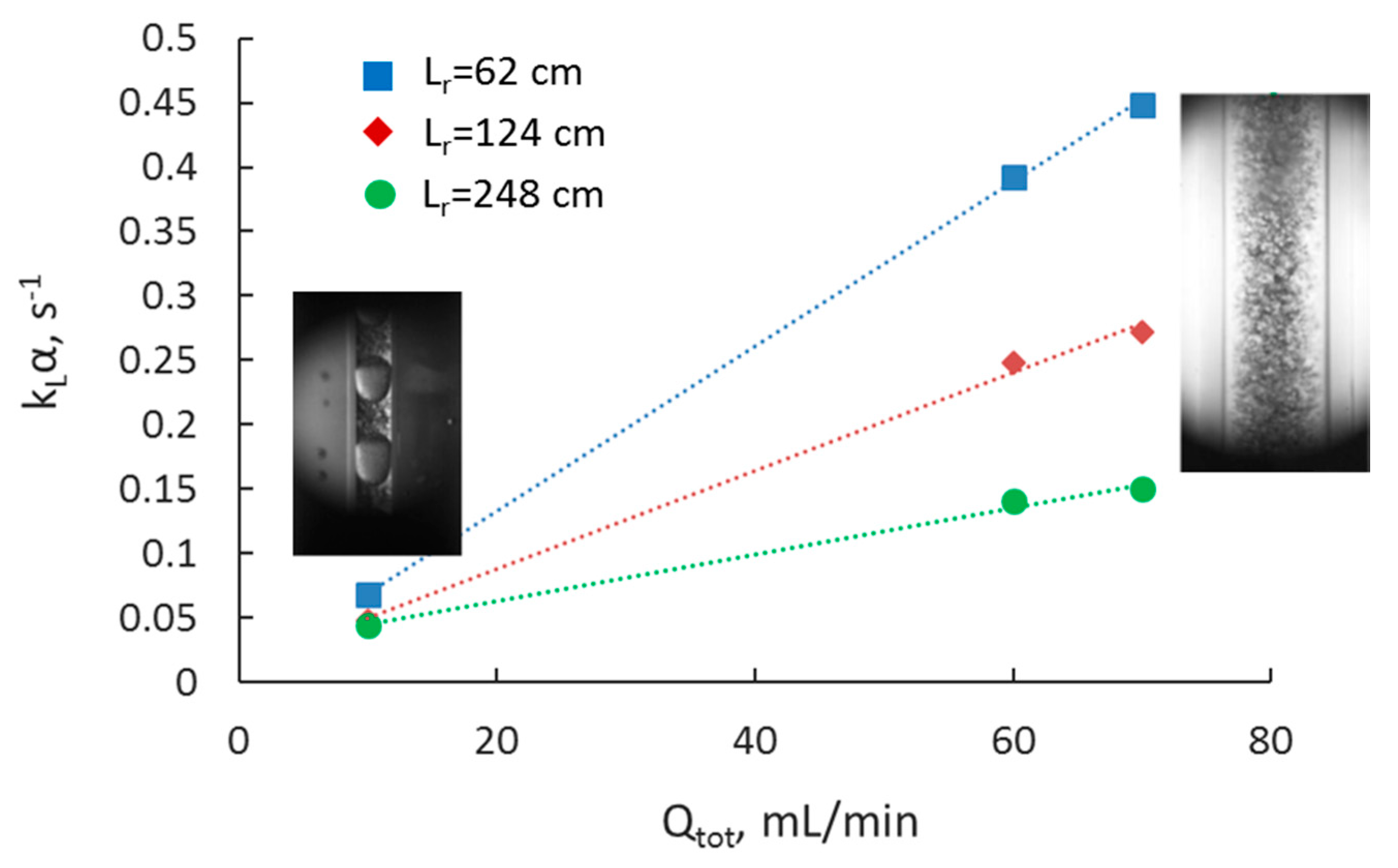
3.2. Separation during Flow—Effect of Scaling-Up
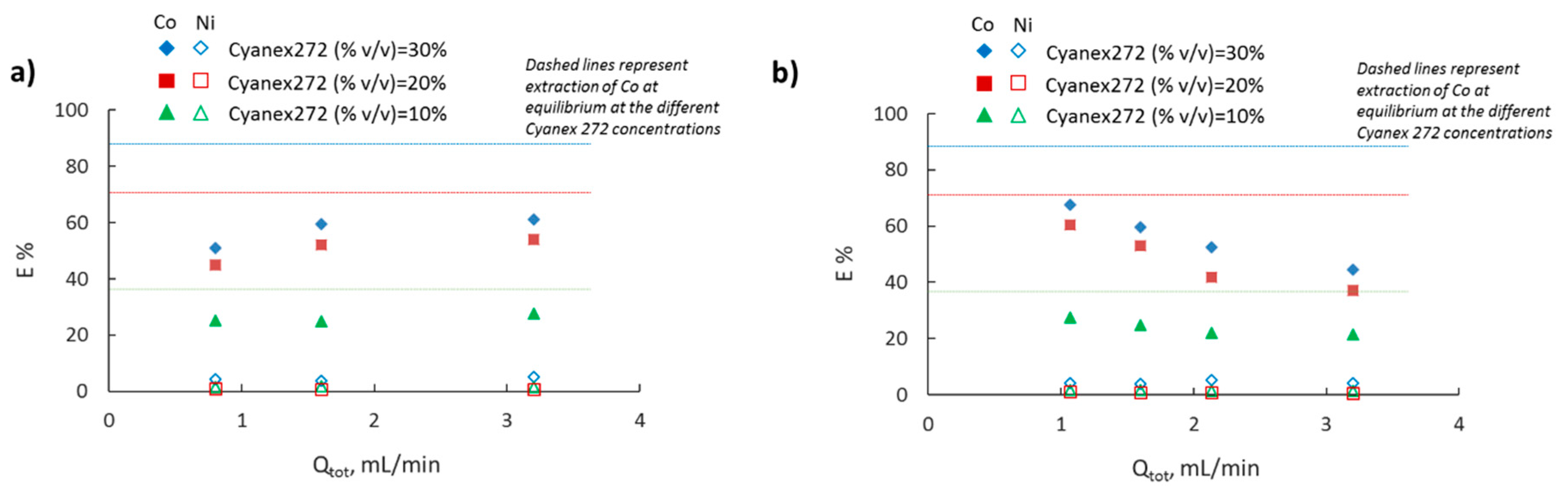
3.3. Effect of Cyanex 272 Concentration
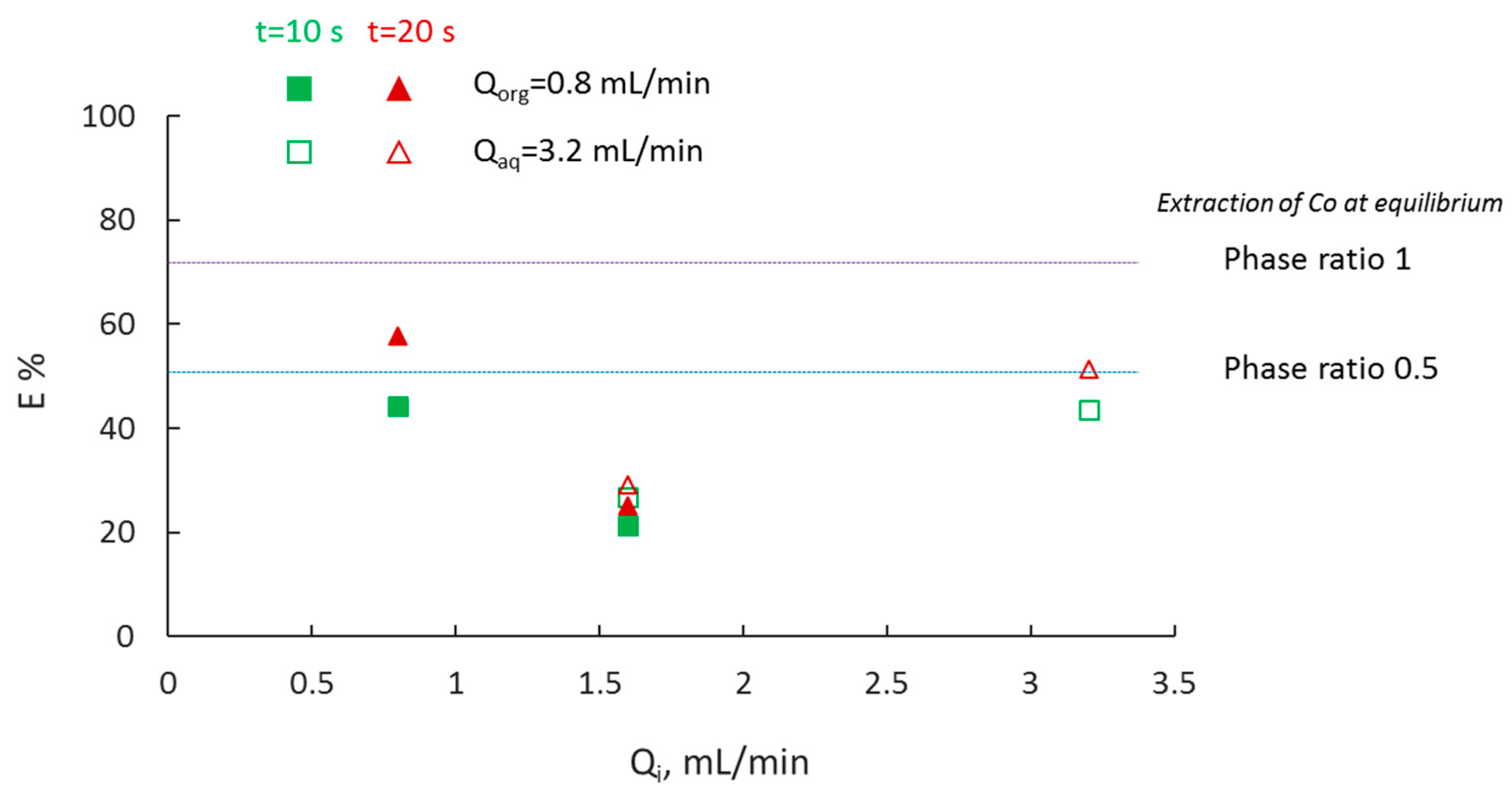
3.4. Effect of Phase Ratio
3.5. Overall Volumetric Mass Transfer Coefficient and Comparison of Intensified Extractors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, C.P. Engineering Materials: Properties and Applications of Metals and Alloys; PHI Learning Pvt. Ltd.: Delhi, India, 2003. [Google Scholar]
- Cheng, C.Y. Solvent extraction of nickel and cobalt with synergistic systems consisting of carboxylic acid and aliphatic hydroxyoxime. Hydrometallurgy 2006, 84, 109–117. [Google Scholar] [CrossRef]
- Crundwell, M.F.; Moats, V.; Ramachandran, T.; Robinson, W.G. Davenport, Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Mubarok, M.Z.; Hanif, L.I. Cobalt and Nickel Separation in Nitric Acid Solution by Solvent Extraction Using Cyanex 272 and Versatic 10. Procedia Chem. 2016, 19, 743–750. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Ichlas, Z.T.; Kaya, M. Solvent extraction process for the recovery of nickel and cobalt from Caldag laterite leach solution: The first bench scale study. Hydrometallurgy 2017, 169, 135–141. [Google Scholar] [CrossRef]
- Chang, J.; Jia, F.; Mumford, K.A.; Yang, X.; Xie, Z. Intensified solvent extraction and separation of cobalt from Ni-rich leaching solution in impinging stream-rotating packed bed contactor. Geosyst. Eng. 2019, 23, 251–253. [Google Scholar] [CrossRef]
- Reddy, B.R.; Rao, S.V.; Park, K.H. Solvent extraction separation and recovery of cobalt and nickel from sulphate medium using mixtures of TOPS 99 and TIBPS extractants. Miner. Eng. 2009, 22, 500–505. [Google Scholar] [CrossRef]
- Sarangi, K.; Reddy, B.; Das, R. Extraction studies of cobalt (II) and nickel (II) from chloride solutions using Na-Cyanex 272.: Separation of Co (II)/Ni (II) by the sodium salts of D2EHPA, PC88A and Cyanex 272 and their mixtures. Hydrometallurgy 1999, 52, 253–265. [Google Scholar] [CrossRef]
- Pinto, G.A.; Durão, F.O.; Fiúza, A.M.A.; Guimarães, M.M.B.L.; Madureira, C.M.N. Design optimisation study of solvent extraction: Chemical reaction, mass transfer and mixer–settler hydrodynamics. Hydrometallurgy 2004, 74, 131–147. [Google Scholar] [CrossRef]
- Angeli, P.; Ortega, E.G.; Tsaoulidis, D.; Earle, M. Intensified Liquid-Liquid Extraction Technologies in Small Channels: A Review. Johns. Matthey Technol. Rev. 2019, 63, 299–310. [Google Scholar] [CrossRef]
- Wang, K.; Luo, G. Microflow extraction: A review of recent development. Chem. Eng. Sci. 2017, 169, 18–33. [Google Scholar] [CrossRef]
- Yin, C.-Y.; Nikoloski, A.N.; Wang, M. Microfluidic solvent extraction of platinum and palladium from a chloride leach solution using Alamine 336. Miner. Eng. 2013, 45, 18–21. [Google Scholar] [CrossRef]
- Angeli, P.; Tsaoulidis, D.; Weheliye, W.H. Studies on mass transfer of europium(III) in micro-channels using a micro Laser Induced Fluorescence technique. Chem. Eng. J. 2019, 372, 1154–1163. [Google Scholar] [CrossRef]
- Li, Q.; Angeli, P. Intensified Eu (III) extraction using ionic liquids in small channels. Chem. Eng. Sci. 2016, 143, 276–286. [Google Scholar] [CrossRef]
- Garciadiego-Ortega, E.; Tsaoulidis, D.; Pineda, M.; Fraga, E.S.; Angeli, P. Hydrodynamics and mass transfer in segmented flow small channel contactors for uranium extraction. Chem. Eng. Process. Process. Intensif. 2020, 153, 107921. [Google Scholar] [CrossRef]
- Tsaoulidis, D.; Angeli, P. Effect of channel size on mass transfer during liquid–liquid plug flow in small scale extractors. Chem. Eng. J. 2015, 262, 785–793. [Google Scholar] [CrossRef]
- Tsaoulidis, D.; Dore, V.; Angeli, P.; Plechkova, N.V.; Seddon, K.R. Dioxouranium(VI) extraction in microchannels using ionic liquids. Chem. Eng. J. 2013, 227, 151–157. [Google Scholar] [CrossRef]
- Tsaoulidis, D.; Dore, V.; Angeli, P.; Plechkova, N.V.; Seddon, K.R. Extraction of dioxouranium(VI) in small channels using ionic liquids. Chem Eng. Res. Des. 2013, 91, 681–687. [Google Scholar] [CrossRef]
- Tsaoulidis, D.; Dore, V.; Angeli, P.; Plechkova, N.V.; Seddon, K.R. Flow patterns and pressure drop of ionic liquid–water two-phase flows in microchannels. Int. J. Multiph. Flow 2013, 54, 1–10. [Google Scholar] [CrossRef]
- Dore, V.; Tsaoulidis, D.; Angeli, P. Mixing patterns in water plugs during water/ionic liquid segmented flow in microchannels. Chem. Eng. Sci. 2012, 80, 334–341. [Google Scholar] [CrossRef]
- Siddiqui, S.W.; Norton, I.T. Oil-in-water emulsification using confined impinging jets. J. Colloid Interface Sci. 2012, 377, 213–221. [Google Scholar] [CrossRef]
- Tsaoulidis, D.; Angeli, P. Liquid-liquid dispersions in intensified impinging-jets cells. Chem. Eng. Sci. 2017, 171, 149–159. [Google Scholar] [CrossRef]
- Tsaoulidis, D.; Ortega, E.G.; Angeli, P. Intensified extraction of uranium (VI) in impinging-jets contactors. Chem. Eng. J. 2018, 342, 251–259. [Google Scholar] [CrossRef]
- Zhang, L.; Hessel, V.; Peng, J.; Wang, Q.; Zhang, L. Co and Ni extraction and separation in segmented micro-flow using a coiled flow inverter. Chem. Eng. J. 2017, 307, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Hessel, V.; Peng, J. Liquid-liquid extraction for the separation of Co(II) from Ni(II) with Cyanex 272 using a pilot scale Re-entrance flow microreactor. Chem. Eng. J. 2018, 332, 131–139. [Google Scholar] [CrossRef]
- Hereijgers, J.; Vandermeersch, T.; van Oeteren, N.; Verelst, H.; Song, H.; Cabooter, D.; Breugelmans, T.; de Malsche, W. Separation of Co(II)/Ni(II) with Cyanex 272 using a flat membrane microcontactor: Extraction kinetics study. J. Membr. Sci. 2016, 499, 370–378. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, L.; Yang, J.; Zhang, L.; Ju, S.; Luo, Y. Separation of Zn(II) from Zn-Ni-Co sulphate solution by di-(2-ethylhexyl)phosphoric acid (D2EHPA) using a slug flow microreactor. Chem. Eng. Process. Process. Intensif. 2019, 143, 107562. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Peng, J.-H.; Ju, S.-H.; Zhang, L.-B.; Dai, L.-Q.; Liu, N.-S. Microfluidic solvent extraction and separation of cobalt and nickel. RSC Adv. 2014, 4, 16081–16086. [Google Scholar] [CrossRef]
- Soldenhoff, K.; Shamieh, M.; Manis, A. Liquid–liquid extraction of cobalt with hollow fiber contactor. J. Membr. Sci. 2005, 252, 183–194. [Google Scholar] [CrossRef]
- Preston, J.S. Solvent extraction of cobalt and nickel by organophosphorus acids I. Comparison of phosphoric, phosphonic and phosphonic acid systems. Hydrometallurgy 1982, 9, 115–133. [Google Scholar] [CrossRef]
- Tsaoulidis, D.; Angeli, P. Effect of channel size on liquid-liquid plug flow in small channels. Aiche J. 2016, 62, 315–324. [Google Scholar] [CrossRef]



| Method and Device | Multi-Component System | kLα (s−1) | Reference |
|---|---|---|---|
| Micro-scale coiled flow inverter D = 1 mm | Co(II)/Ni(II) sulphate solution Cyanex 272 | 0.053–0.013 | [24] |
| 3 D conical microreactor, 12 mL internal volume | Co(II)/Ni(II) sulphate solution Cyanex 272 | 0.011–1.20 | [25] |
| Flat membrane microreactor 37–74 μL volume | Co(II)/Ni(II) sulphate solution Cyanex 272 | kL = 2.8–5.5 × 10−6 m/s | [26] |
| Capillary microreactors D = 1 mm | Zn-Ni-Co sulphate solution D2EHPA | 0.02–0.19 | [27] |
| Counter-current flow interdigital micromixer 40 μm width channels | Co(II)/Ni(II) sulphate solution PC88A | N/A | [28] |
| Impinging stream-rotating packed bed contactor 1.5 mm capillary nozzles | Co(II)/Ni(II) chloride solution P507 | 0.16 | [6] |
| Hollow fiber module with mixer-settler | Ionquest 801 Cyanex272 | kL = 4.3–7.3 × 10−7 m/s | [29] |
| Properties | Aqueous Solution (Co/Ni = 6/60 mg/mL) | Organic Solution (75% v/v Exxsol D80, 20% v/v Cyanex 272, 5% v/v TBP) |
|---|---|---|
| Viscosity (kg m−1 s−1) | 1.2 × 10−3 | 3.3 × 10−3 |
| Surface tension (N m−1) | 64.7 × 10−3 | 23.2 × 10−3 |
| Density (kg m−3) | 1137 | 831 |
| Interfacial tension (N m−1) | 11.1 × 10−3 | |
| Variables | Range |
|---|---|
| Main channel diameter, D (mm) | 1–3.2 |
| Cyanex 272 concentration, (% v/v) | 10–30 |
| Flowrate ratio, Qorg/Qaq | 0.5 and 1 |
| Residence time, t (s) | 2–60 |
| Reactor length (cm) | 8.5–250 |
| Mixture velocity, umix (m/s) | 0.0029–0.3 |
| Organic (aqueous) phase flowrate, Qorg (Qaq) (mL/min) | 10–70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsaoulidis, D.; Mamtora, M.; Gañet, M.M.; Garciadiego-Ortega, E.; Angeli, P. Scale-Up Studies for Co/Ni Separations in Intensified Reactors. Micromachines 2020, 11, 1106. https://doi.org/10.3390/mi11121106
Tsaoulidis D, Mamtora M, Gañet MM, Garciadiego-Ortega E, Angeli P. Scale-Up Studies for Co/Ni Separations in Intensified Reactors. Micromachines. 2020; 11(12):1106. https://doi.org/10.3390/mi11121106
Chicago/Turabian StyleTsaoulidis, Dimitrios, Milan Mamtora, Marta Mayals Gañet, Eduardo Garciadiego-Ortega, and Panagiota Angeli. 2020. "Scale-Up Studies for Co/Ni Separations in Intensified Reactors" Micromachines 11, no. 12: 1106. https://doi.org/10.3390/mi11121106
APA StyleTsaoulidis, D., Mamtora, M., Gañet, M. M., Garciadiego-Ortega, E., & Angeli, P. (2020). Scale-Up Studies for Co/Ni Separations in Intensified Reactors. Micromachines, 11(12), 1106. https://doi.org/10.3390/mi11121106






