A Microfluidic Chip Architecture Enabling a Hypoxic Microenvironment and Nitric Oxide Delivery in Cell Culture
Abstract
1. Introduction
2. Materials and Methods
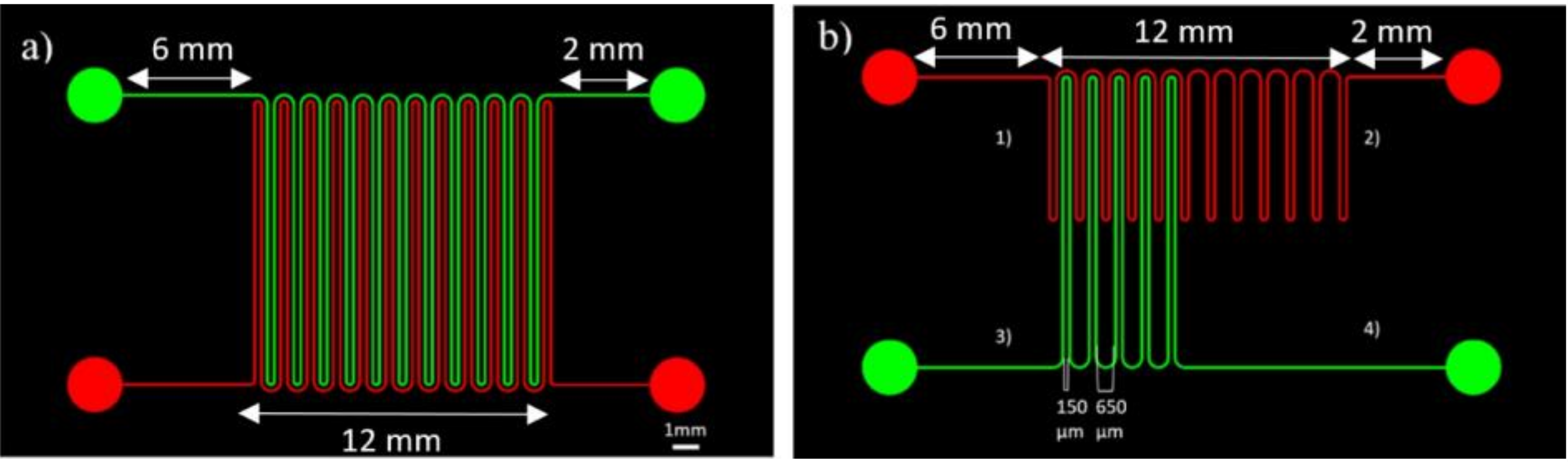
2.1. Chip Fabrication
2.2. Oxygen Measurements Using Oxygen Sensing Foil
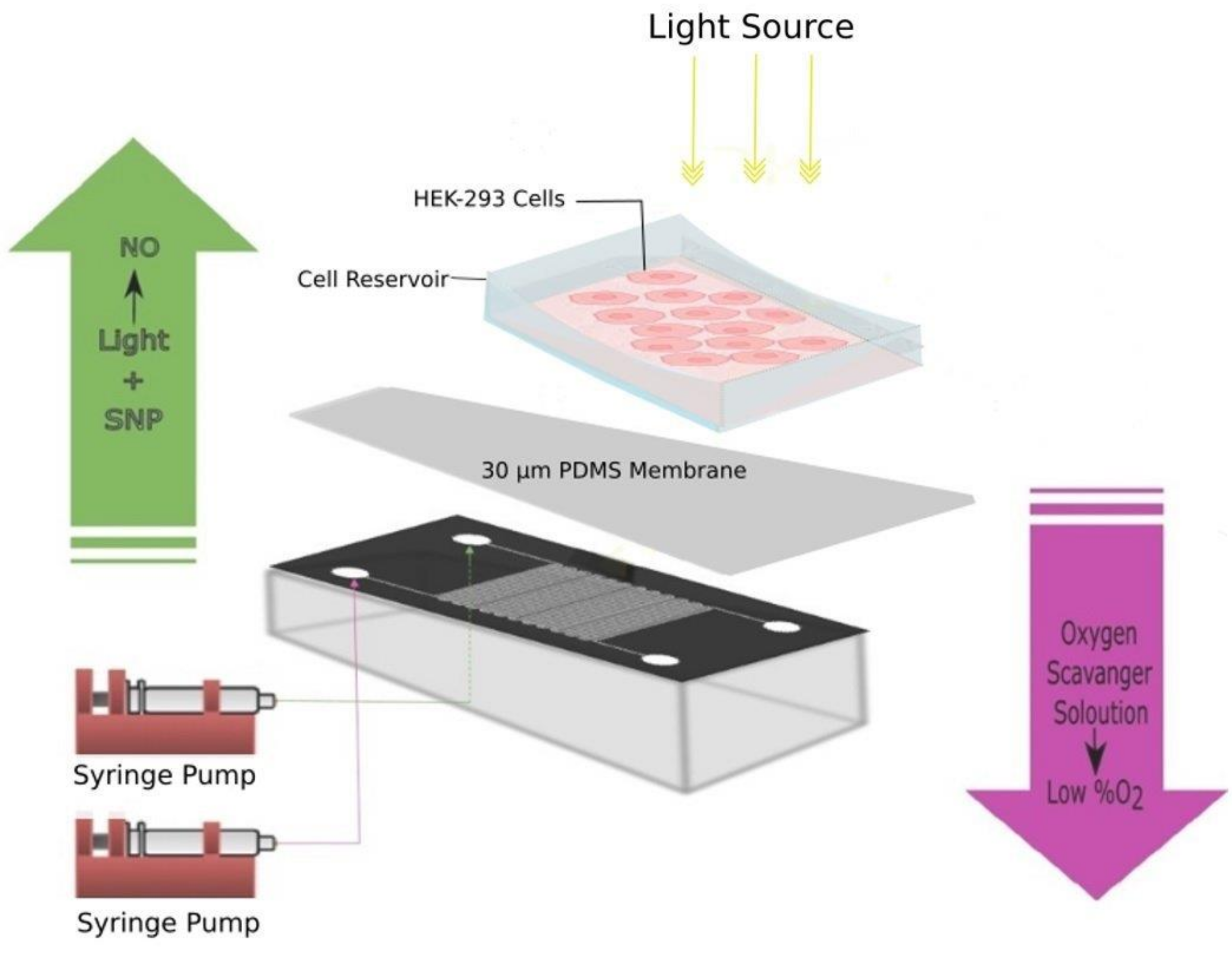
2.3. Preparation of Microfluidic Chips Setup
2.4. Cell Culture Experiments on Microchips
2.5. Hypoxia Response
2.6. Measurements of Nitrite on Microchip
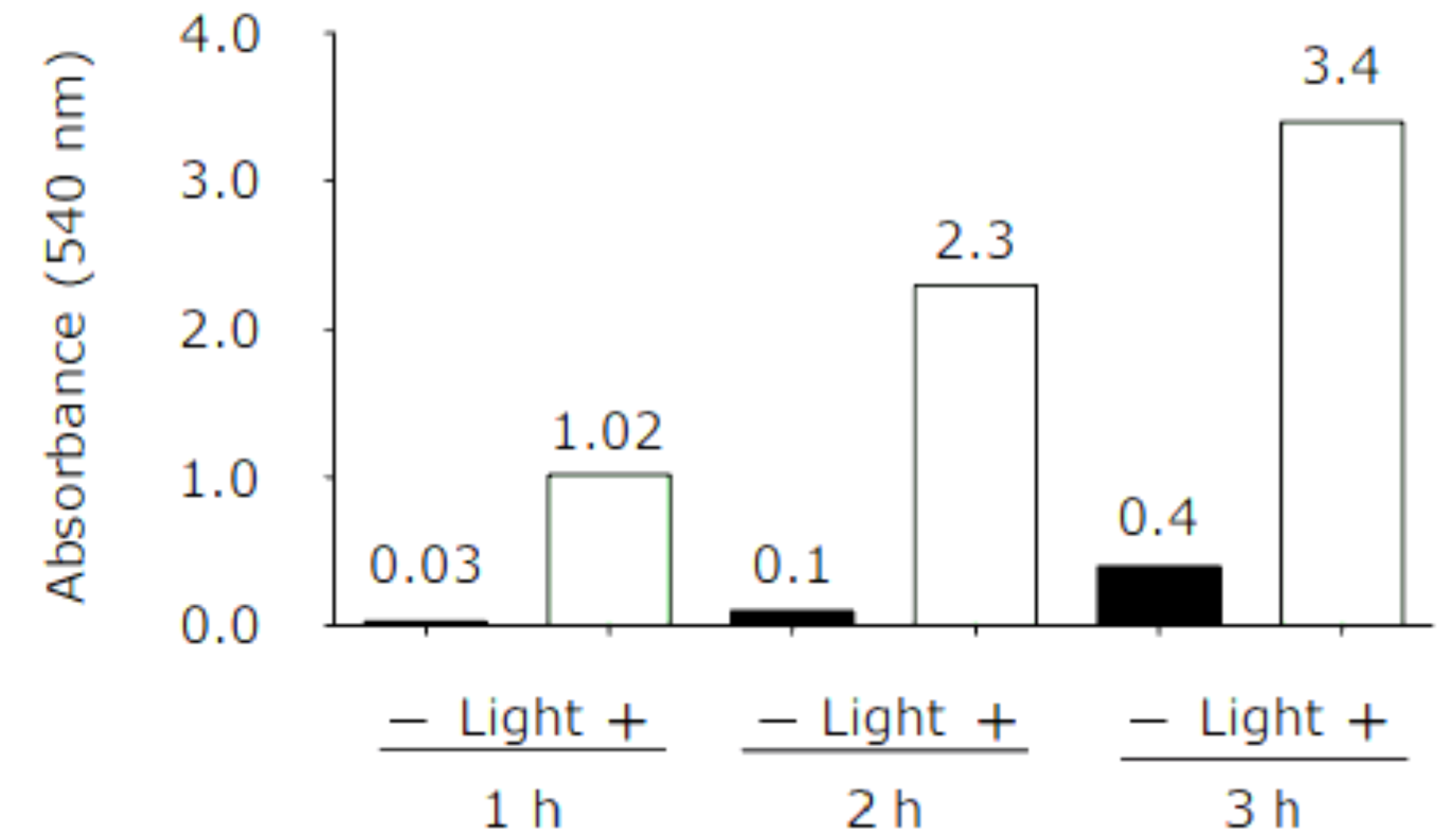
2.7. Nitric Oxide Response in Cells
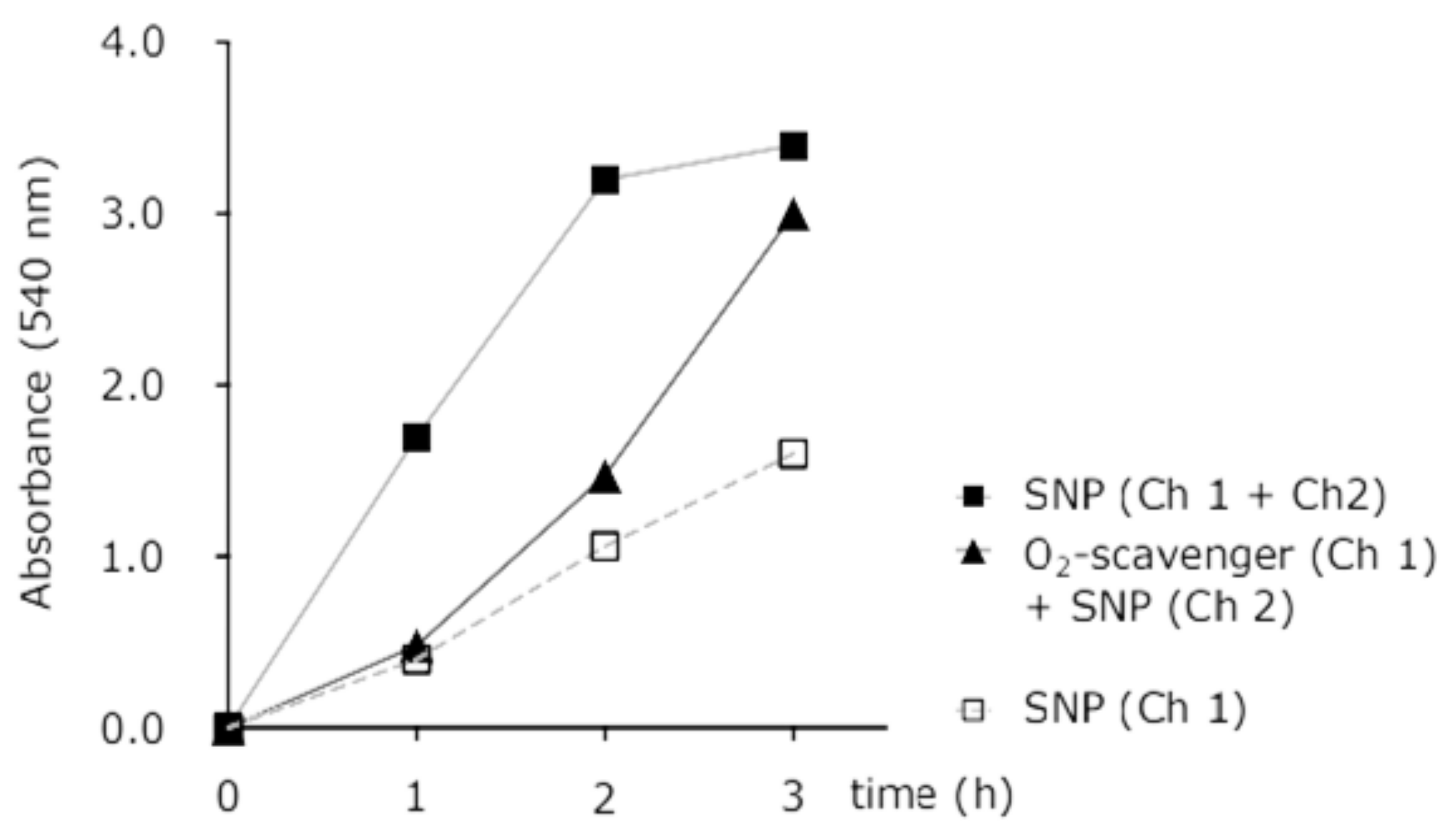
2.8. Simultaneous Recording of NO and Hypoxia Signals
2.9. Image Analysis
3. Results and Discussion
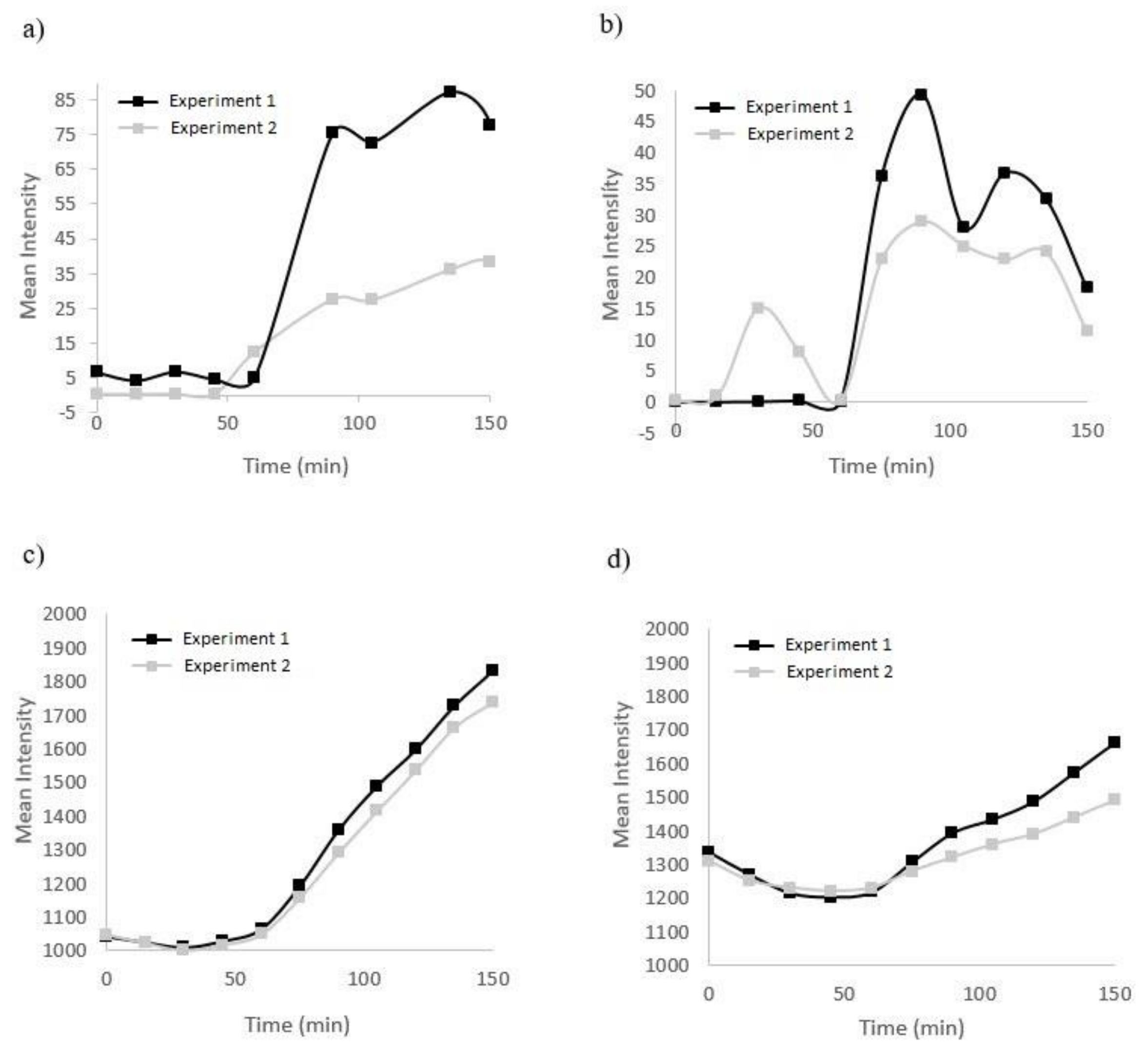
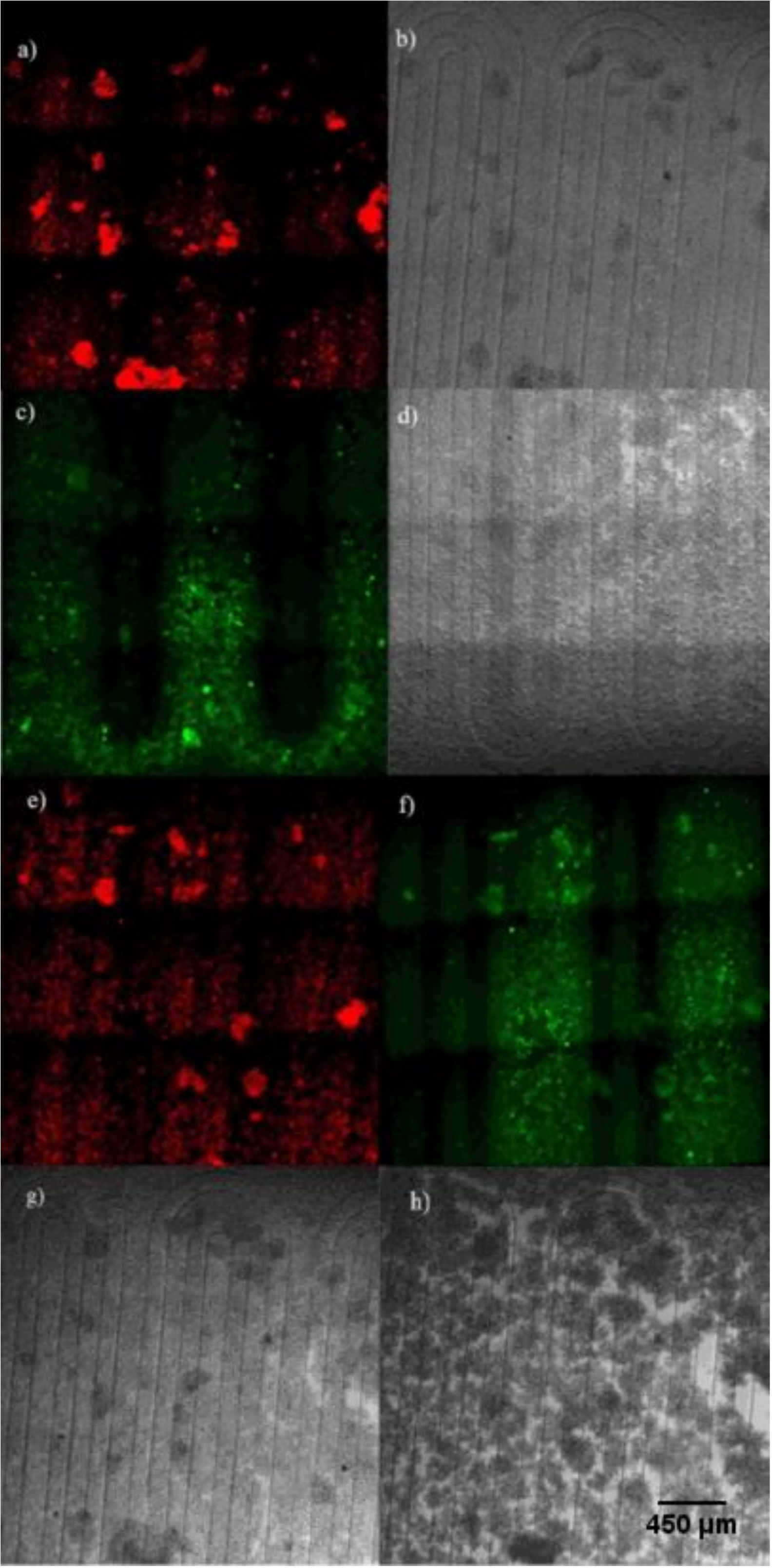
3.1. Simultaneous Hypoxia and Nitric Oxide Response in Cells
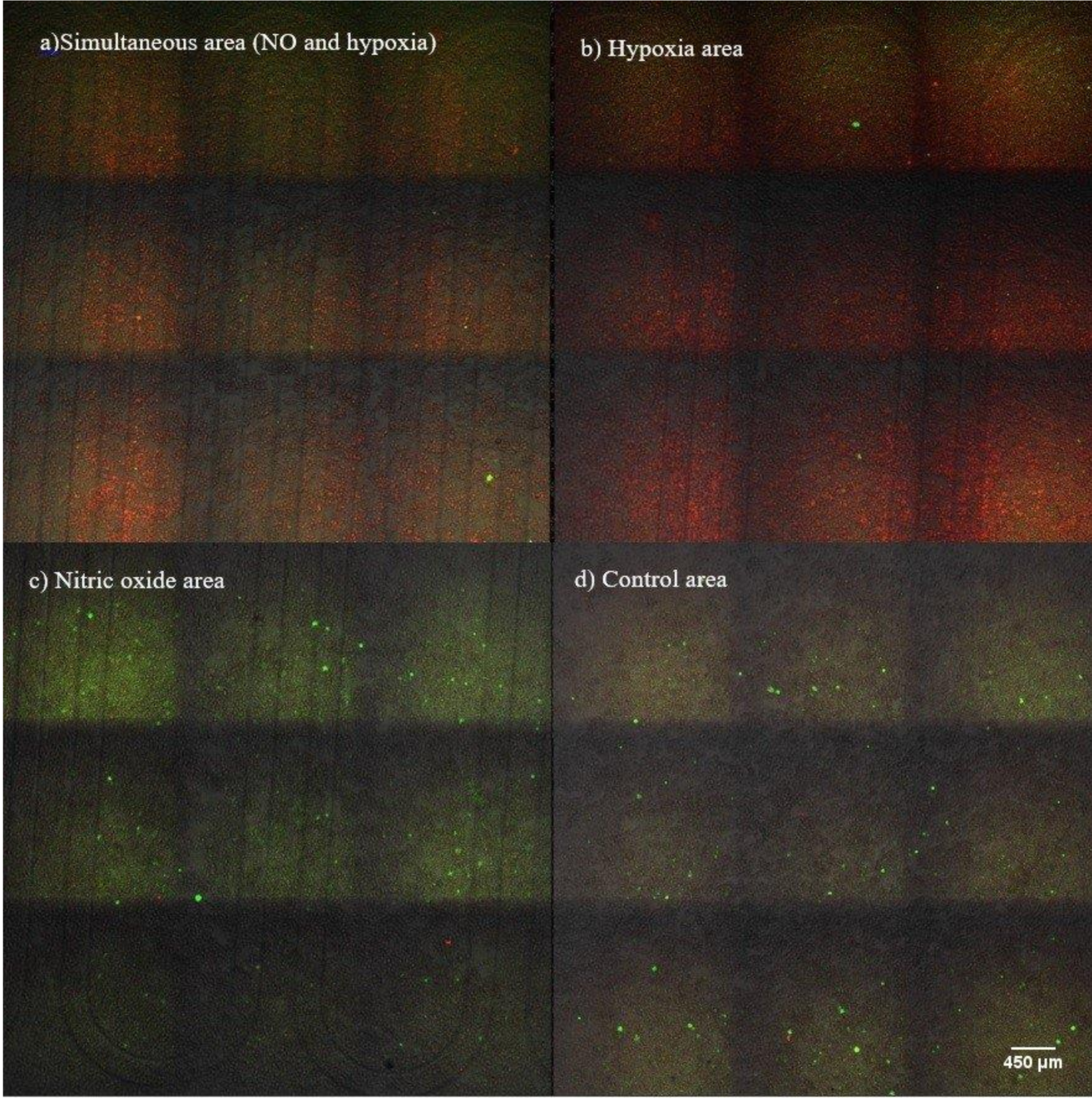
3.2. Microenvironment Patterning
3.3. Microenvironment Patterning over Millimetre Scale
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, R. Overview of Gasotransmitters and the Related Signaling Network. Gasotransmitters 2018, 1–28. [Google Scholar] [CrossRef]
- Shefa, U.; Yeo, S.G.; Kim, M.S.; Song, I.O.; Jung, J.; Jeong, N.Y.; Huh, Y. Role of Gasotransmitters in Oxidative Stresses, Neuroinflammation, and Neuronal Repair. Biomed. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Snijder, P.M.; van den Berg, E.; Whiteman, M.; Bakker, S.J.L.; Leuvenink, H.G.D.; van Goor, H. Emerging role of gasotransmitters in renal transplantation. Am. J. Transplant. 2013, 12, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 1035. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Chandra, M.; Tuteja, R.; Misra, M.K. Nitric Oxide as a Unique Bioactive Signaling Messenger in Physiology and Pathophysiology. J. Biomed. Biotechnol. 2004, 4, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Garini, G.; Savazzi, G.; Borghetti, A. The L-arginine-nitric oxide system: Physiology, physiopathology and clinical relevance. Recenti Prog. Med. 1997, 2, 90–99. [Google Scholar]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Aspects Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- Napoli, C.; Ignarro, L.J. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch. Pharm. Res. 2009, 8, 1103–1108. [Google Scholar] [CrossRef]
- Allen, C.B.; Schneider, B.K.; White, C.W. Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L1021–L1027. [Google Scholar] [CrossRef]
- Byrne, M.B.; Leslie, M.T.; Gaskins, H.R.; Kenis, P.J.A. Methods to study the tumor microenvironment under controlled oxygen conditions. Trends Biotechnol. 2014, 32, 556–563. [Google Scholar] [CrossRef]
- Wu, H.M.; Lee, T.A.; Ko, P.L.; Chiang, H.J.; Peng, C.C.; Tung, Y.C. Review of microfluidic cell culture devices for the control of gaseous microenvironments in vitro. J. Micromech. Microeng. 2018, 28. [Google Scholar] [CrossRef]
- Mehta, G.; Lee, J.; Cha, W.; Tung, Y.C.; Linderman, J.J.; Takayama, S. Hard top soft bottom microfluidic devices for cell culture and chemical analysis. Anal. Chem. 2009, 81, 3714–3722. [Google Scholar] [CrossRef] [PubMed]
- Pinelis, M.; Shamban, L.; Jovic, A.; Maharbiz, M.M. A high-yield method for generating mass-transfer gradients in elastomer microfluidics using impermeable capillaries. Biomed. Microdevices 2008, 6, 807–811. [Google Scholar] [CrossRef]
- Adler, M.; Polinkovsky, M.; Gutierrez, E.; Groisman, A. Generation of oxygen gradients with arbitrary shapes in a microfluidic device. Lab Chip 2010, 3, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Peng, C.C.; Cheng, Y.J.; Wu, J.G.; Tung, Y.C. Generation of nitric oxide gradients in microfluidic devices for cell culture using spatially controlled chemical reactions. Biomicrofluidics 2013, 6, 64104. [Google Scholar] [CrossRef]
- Mehta, G.; Mehta, K.; Sud, D.; Song, J.W.; Bersano-Begey, T.; Futai, N.; Heo, Y.S.; Mycek, M.A.; Linderman, J.J.; Takayama, S. Quantitative measurement and control of oxygen levels in microfluidic poly(dimethylsiloxane) bioreactors during cell culture. Biomed. Microdevices 2007, 2, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Polinkovsky, M.; Gutierrez, E.; Levchenko, A.; Groisman, A. Fine temporal control of the medium gas content and acidity and on-chip generation of series of oxygen concentrations for cell cultures. Lab Chip 2009, 8, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Cheng, Y.J.; Tu, M.; Chen, Y.H.; Peng, C.C.; Liao, W.H.; Tung, Y.C. A polydimethylsiloxane-polycarbonate hybrid microfluidic device capable of generating perpendicular chemical and oxygen gradients for cell culture studies. Lab Chip 2014, 19, 3762–3772. [Google Scholar] [CrossRef]
- Brennan, M.D.; Rexius-Hall, M.L.; Elgass, L.J.; Eddington, D.T. Oxygen control with microfluidics. Lab Chip 2014, 22, 4305–4318. [Google Scholar] [CrossRef]
- Peng, C.C.; Liao, W.H.; Chen, Y.H.; Wu, C.Y.; Tung, Y.C. A microfluidic cell culture array with various oxygen tensions. Lab Chip 2013, 16, 3239–3245. [Google Scholar] [CrossRef]
- Chen, Y.A.; King, A.D.; Shih, H.C.; Peng, C.C.; Wu, C.Y.; Liao, W.H.; Tung, Y.C. Generation of oxygen gradients in microfluidic devices for cell culture using spatially confined chemical reactions. Lab Chip 2011, 21, 3626–3633. [Google Scholar] [CrossRef] [PubMed]
- Oppegard, S.C.; Nam, K.H.; Carr, J.R.; Skaalure, S.C.; Eddington, D.T. Modulating temporal and spatial oxygenation over adherent cellular cultures. PLoS ONE 2009, 9, e90972. [Google Scholar] [CrossRef]
- Funamoto, K.; Zervantonakis, I.K.; Liu, Y.; Ochs, C.J.; Kim, C.; Kamm, R.D. A novel microfluidic platform for high-resolution imaging of a three-dimensional cell culture under a controlled hypoxic environment. Lab Chip 2012, 22, 4855–4863. [Google Scholar] [CrossRef]
- Skolimowski, M.; Nielsen, M.W.; Emnéus, J.; Molin, S.R.; Taboryski, R.; Sternberg, C.; Dufva, M.; Geschke, O. Microfluidic dissolved oxygen gradient generator biochip as a useful tool in bacterial biofilm studies. Lab Chip 2010, 10, 2162–2169. [Google Scholar] [CrossRef]
- Lo, J.F.; Brennan, M.; Merchant, Z.; Chen, L.; Guo, S.; Eddington, D.T.; DiPietro, L.A. Microfluidic wound bandage: Localized oxygen modulation of collagen maturation. Wound Repair Regen. 2013, 2, 226–234. [Google Scholar] [CrossRef]
- Forry, S.P.; Locascio, L.E. On-chip CO2 control for microfluidic cell culture. Lab Chip 2011, 23, 4041–4046. [Google Scholar] [CrossRef] [PubMed]
- Barmaki, S.; Jokinen, V.; Obermaier, D.; Blokhina, D.; Korhonen, M.; Ras, R.H.A.; Vuola, J.; Franssila, S.; Kankuri, E. A microfluidic oxygen sink to create a targeted cellular hypoxic microenvironment under ambient atmospheric condition. Acta Biomater. 2018, 73, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Oomen, P.E.; Skolimowski, M.D.; Verpoorte, E. Implementing oxygen control in chip-based cell and tissue culture systems. Lab Chip 2016, 16, 3394–3414. [Google Scholar] [CrossRef]
- Yamamoto, T.; Bing, R.J. Nitric oxide donors. Proc. Soc. Exp. Biol. Med. 2000, 3, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Griess, J.P. Bemerkungen zu der abhandlung der H.H. Weselsky und Benedikt-Ueber einige azoverbindungen. Chem. Ber. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 1–26. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzch, T.; Preibisch, S.; Rueden, C.; Saalfed, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 7, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Zacharia, I.G.; Deen, W.M. Diffusivity and solubility of nitric oxide in water and saline. Ann. Biomed. Eng. 2005, 33, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Markov, D.A.; Lillie, E.M.; Garbett, S.P.; McCawley, L.J. Variation in diffusion of gases through PDMS due to plasma surface treatment and storage conditions. Biomed. Microdevices 2014, 16, 91–96. [Google Scholar] [CrossRef]
- Ren, H.; Bull, J.L.; Meyerhoff, M.E. Transport of Nitric Oxide (NO) in Various Biomedical grade Polyurethanes: Measurements and Modeling Impact on NO Release Properties of Medical Devices. ACS Biomater. Sci. Eng. 2016, 2, 1483–1492. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barmaki, S.; Obermaier, D.; Kankuri, E.; Vuola, J.; Franssila, S.; Jokinen, V. A Microfluidic Chip Architecture Enabling a Hypoxic Microenvironment and Nitric Oxide Delivery in Cell Culture. Micromachines 2020, 11, 979. https://doi.org/10.3390/mi11110979
Barmaki S, Obermaier D, Kankuri E, Vuola J, Franssila S, Jokinen V. A Microfluidic Chip Architecture Enabling a Hypoxic Microenvironment and Nitric Oxide Delivery in Cell Culture. Micromachines. 2020; 11(11):979. https://doi.org/10.3390/mi11110979
Chicago/Turabian StyleBarmaki, Samineh, Daniela Obermaier, Esko Kankuri, Jyrki Vuola, Sami Franssila, and Ville Jokinen. 2020. "A Microfluidic Chip Architecture Enabling a Hypoxic Microenvironment and Nitric Oxide Delivery in Cell Culture" Micromachines 11, no. 11: 979. https://doi.org/10.3390/mi11110979
APA StyleBarmaki, S., Obermaier, D., Kankuri, E., Vuola, J., Franssila, S., & Jokinen, V. (2020). A Microfluidic Chip Architecture Enabling a Hypoxic Microenvironment and Nitric Oxide Delivery in Cell Culture. Micromachines, 11(11), 979. https://doi.org/10.3390/mi11110979





