Reliability Analysis of an Epileptic Seizure Detector Powered by an Energy Harvester
Abstract
:1. Introduction
2. Materials and Methods
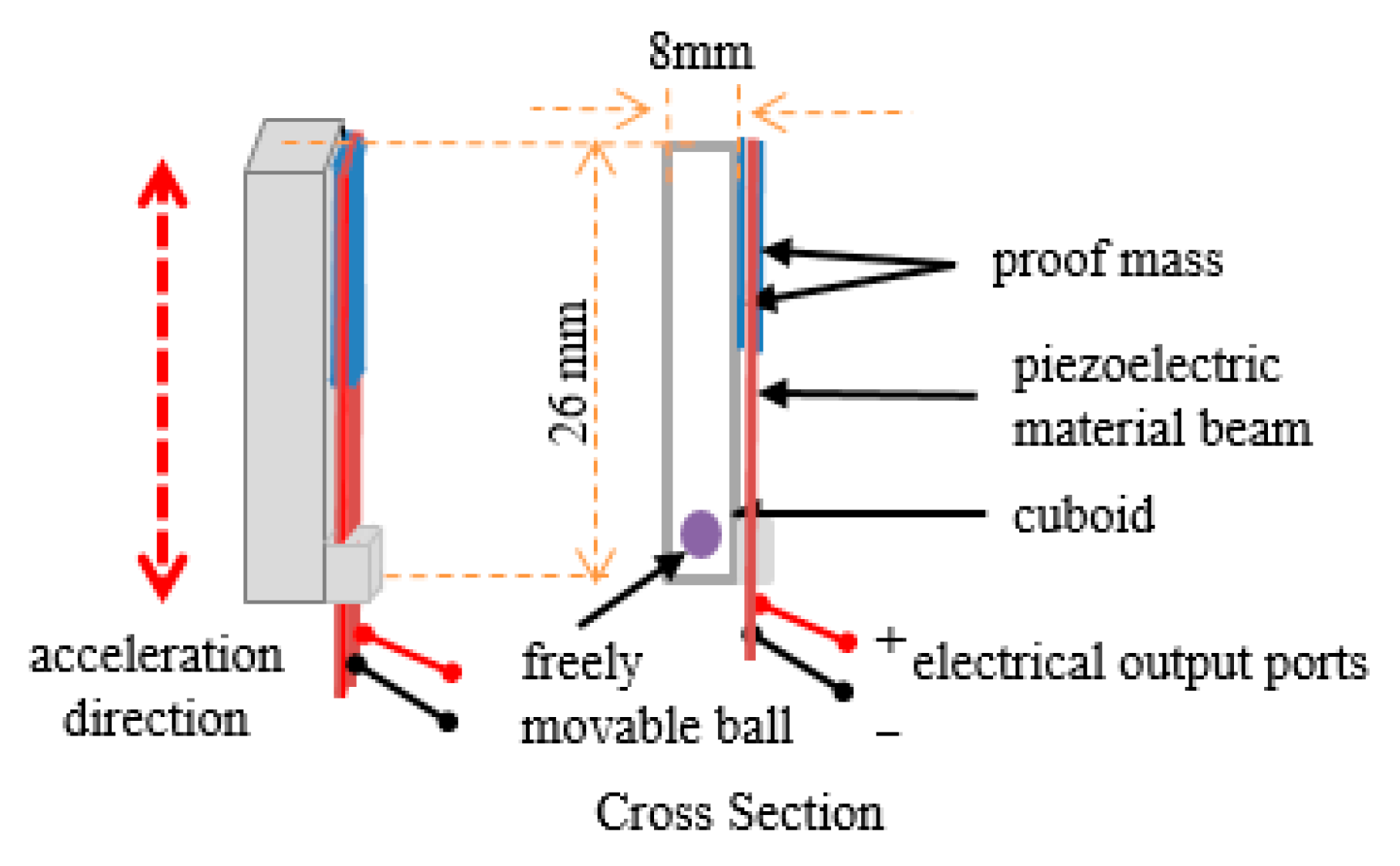
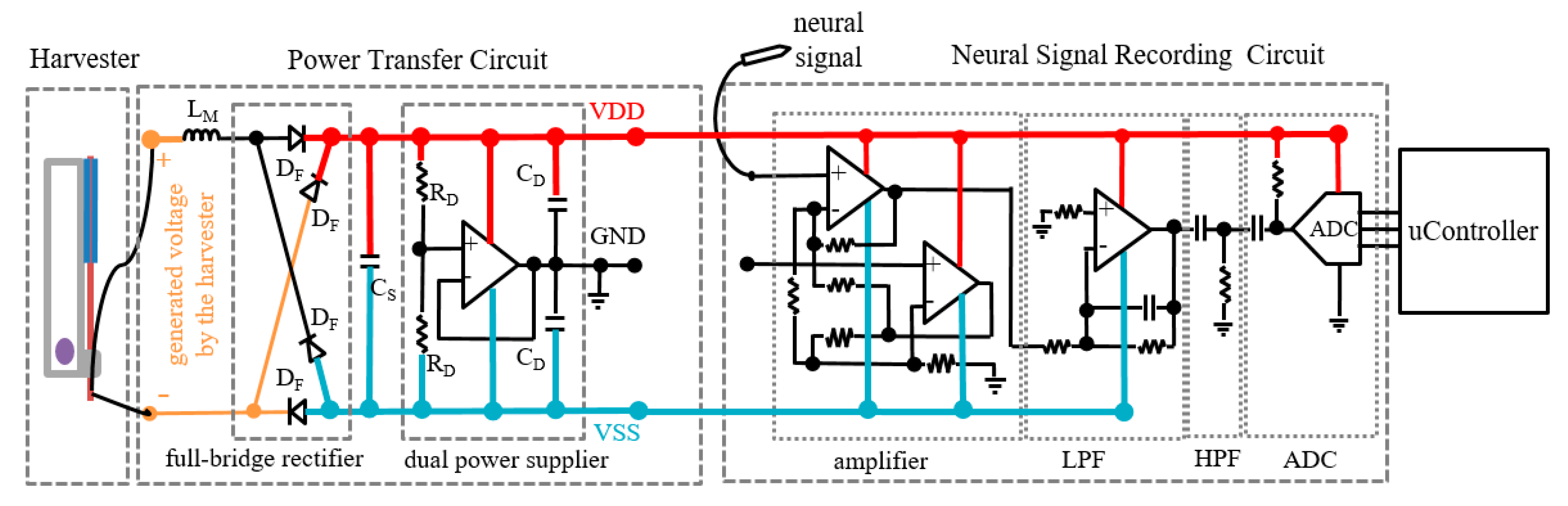
2.1. Harvester and Circuits
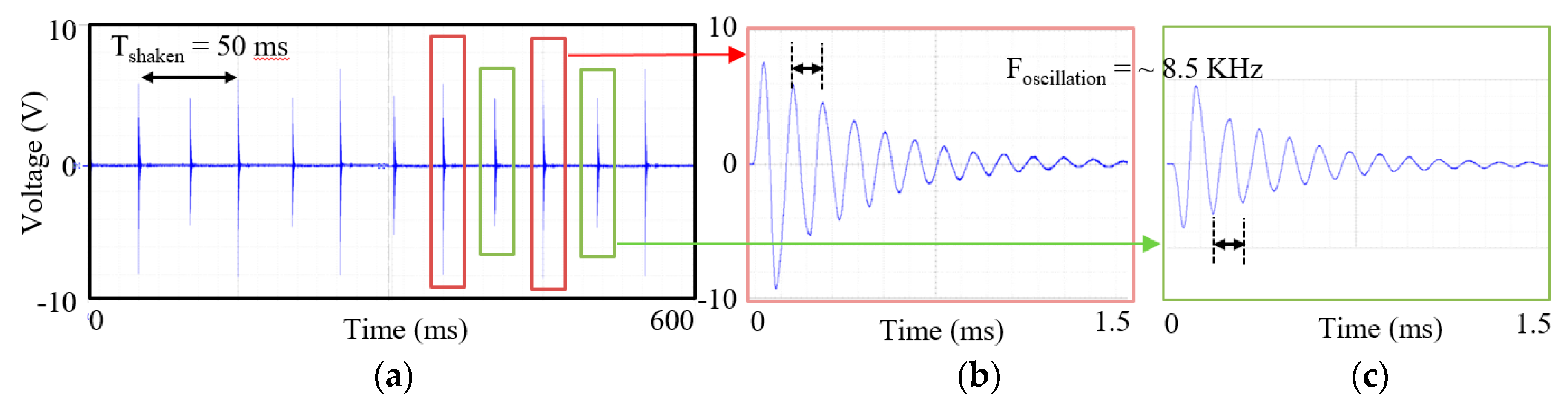
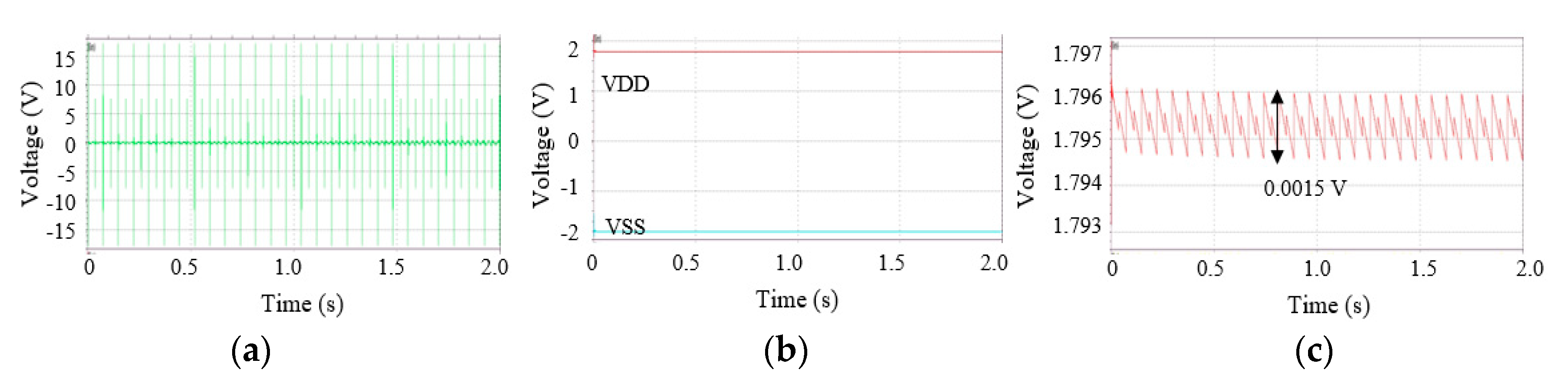
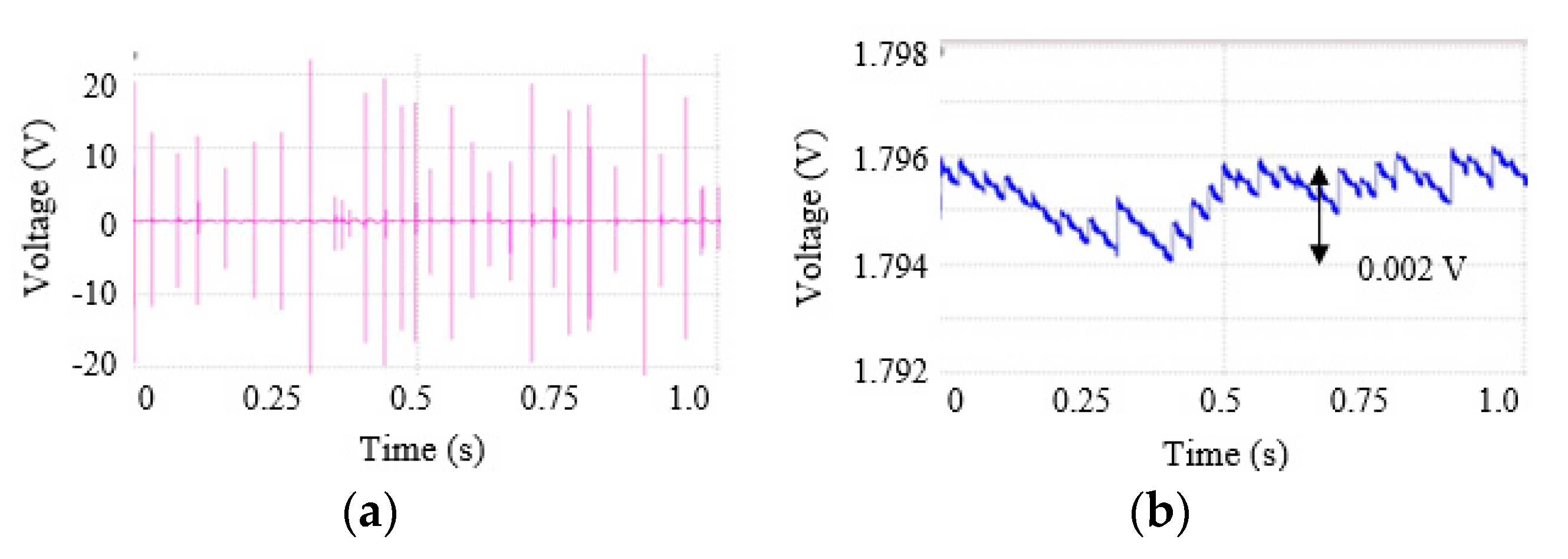
2.2. Test of the Harvester and the Circuits
2.3. Seizure Detection Algorithm
2.3.1. Filtering
2.3.2. Calculating Foreground Sequences
2.3.3. Calculating Background Sequences
2.3.4. Decision
2.4. Simulation
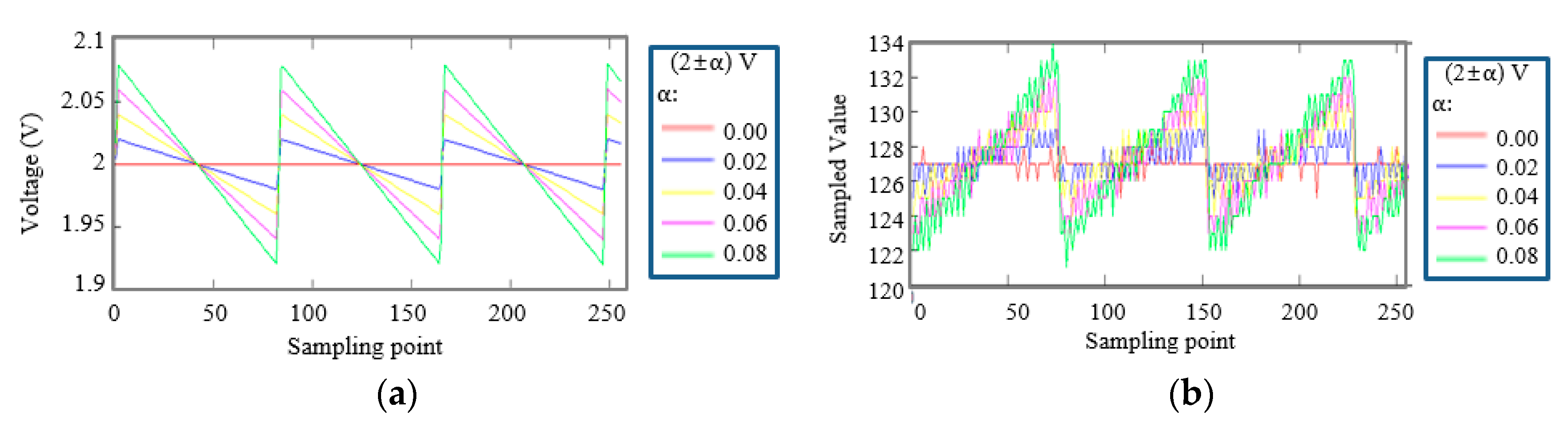
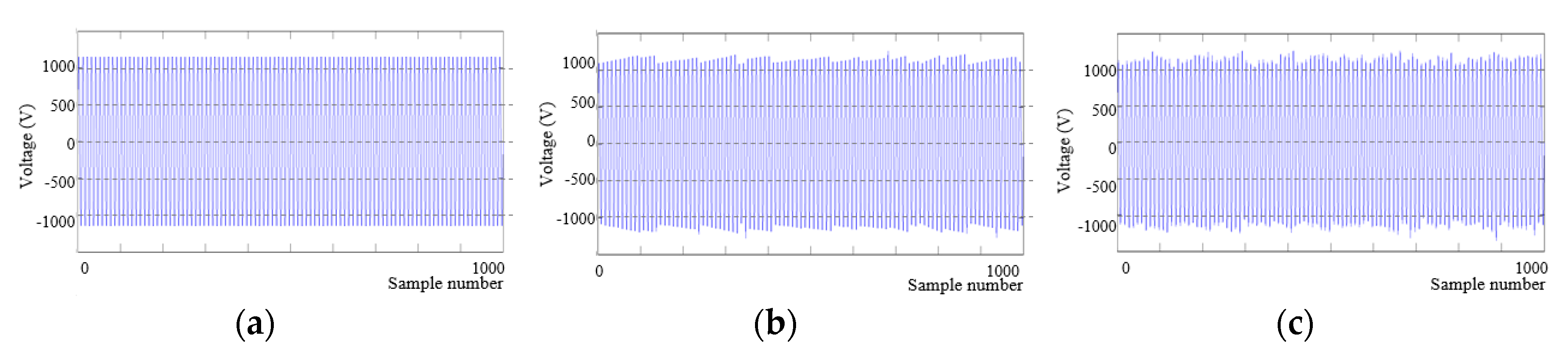
2.4.1. Supply Voltage Conditions
2.4.2. Simulation Method
2.4.3. Performance Measures
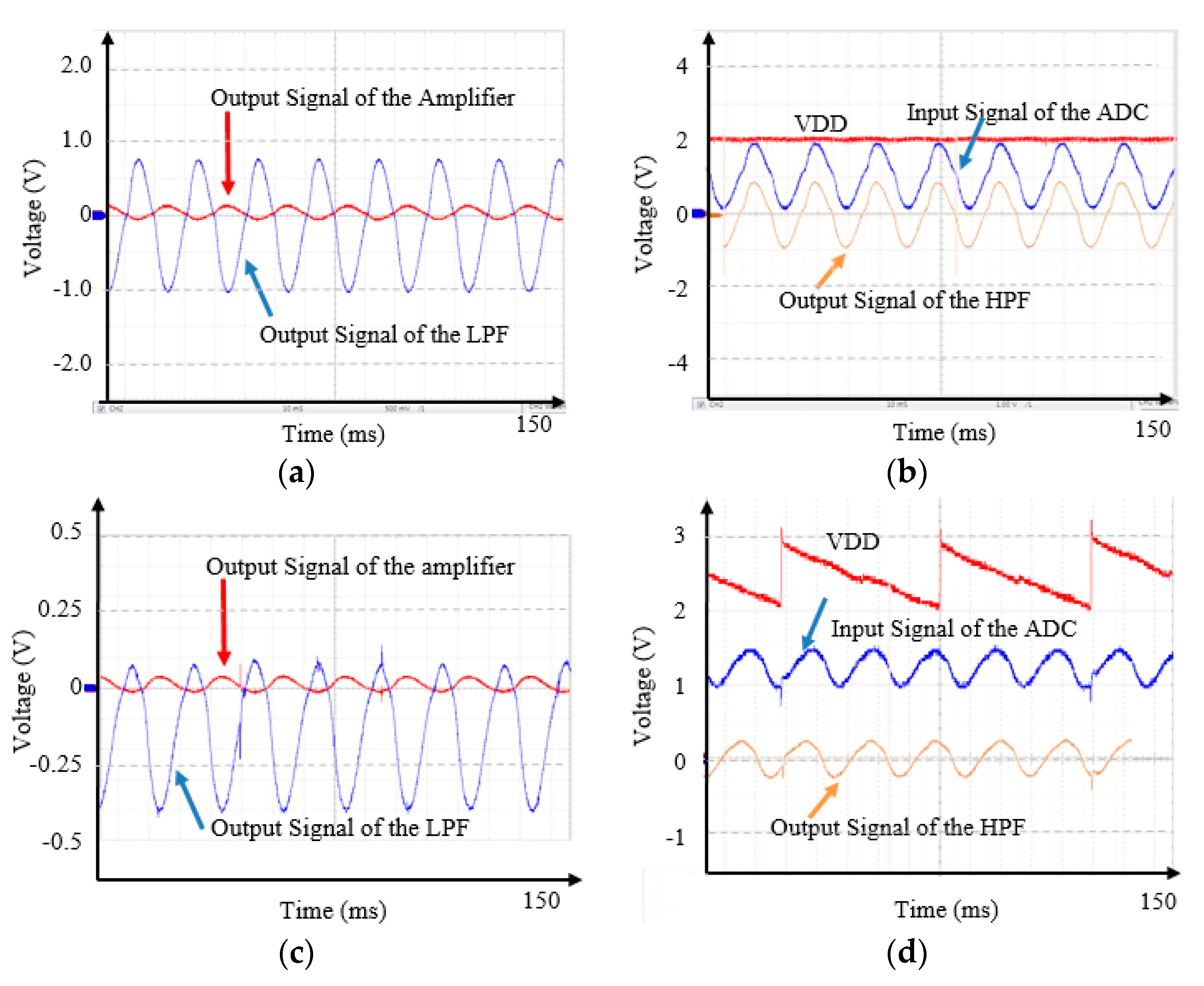
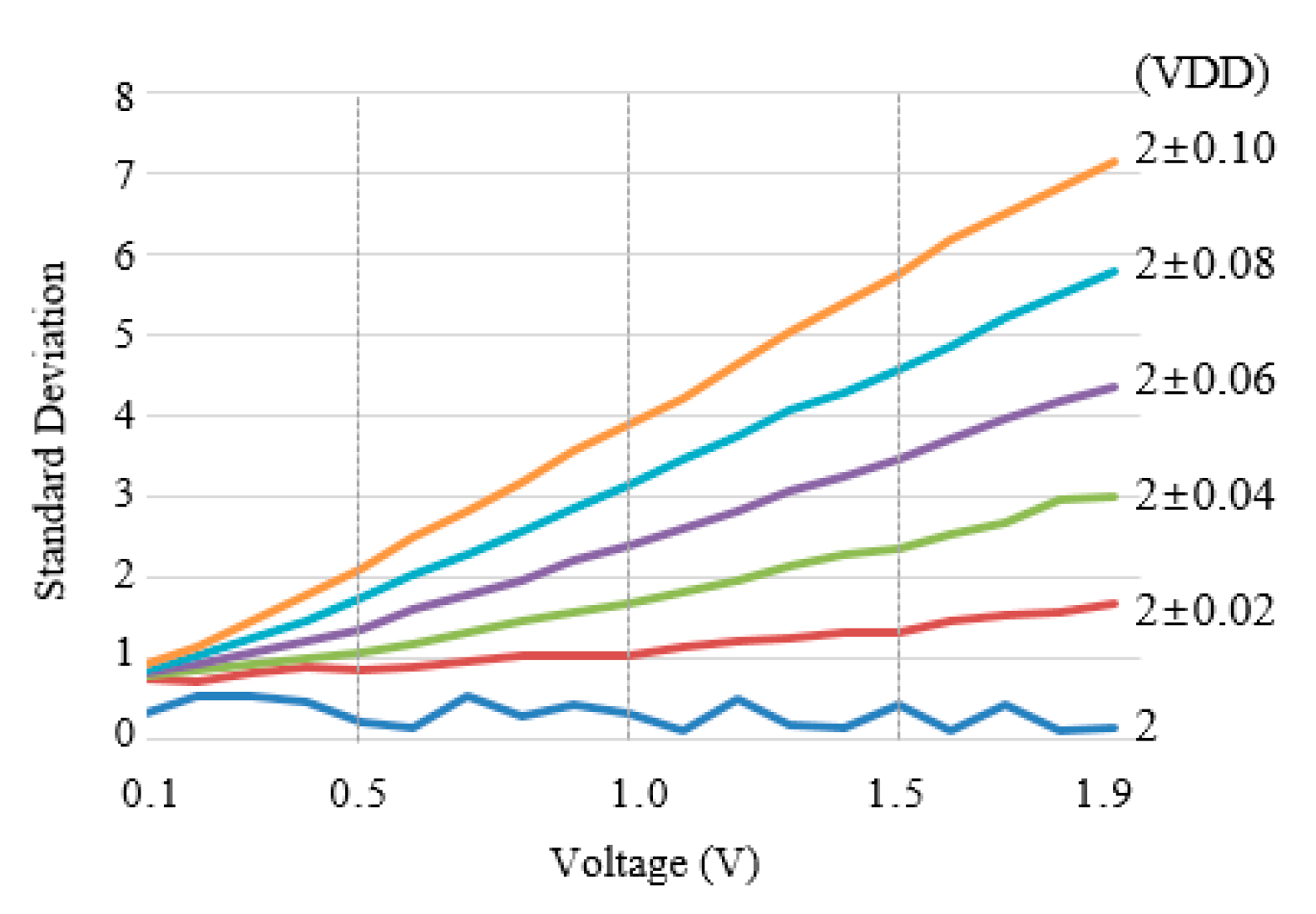
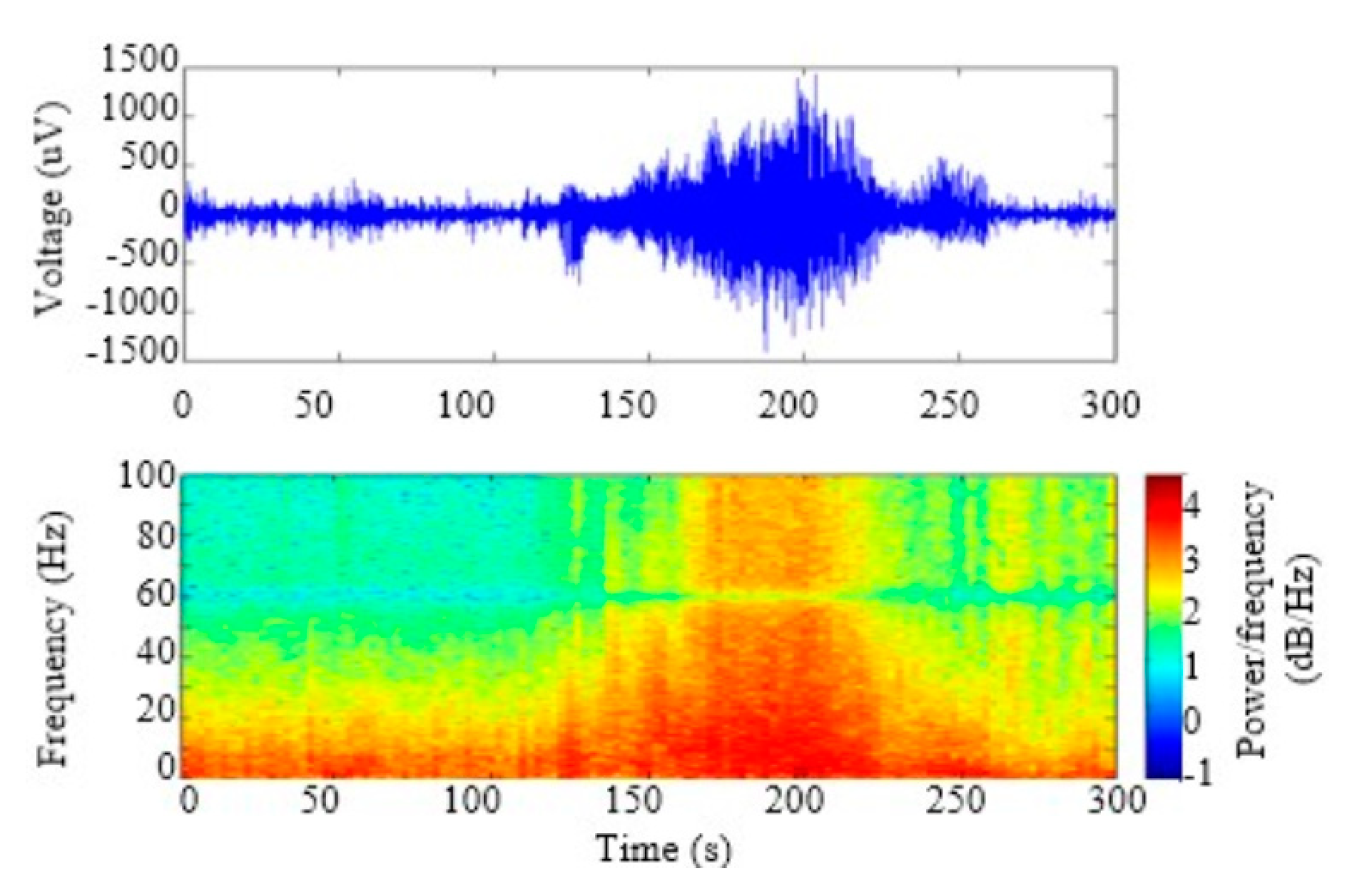
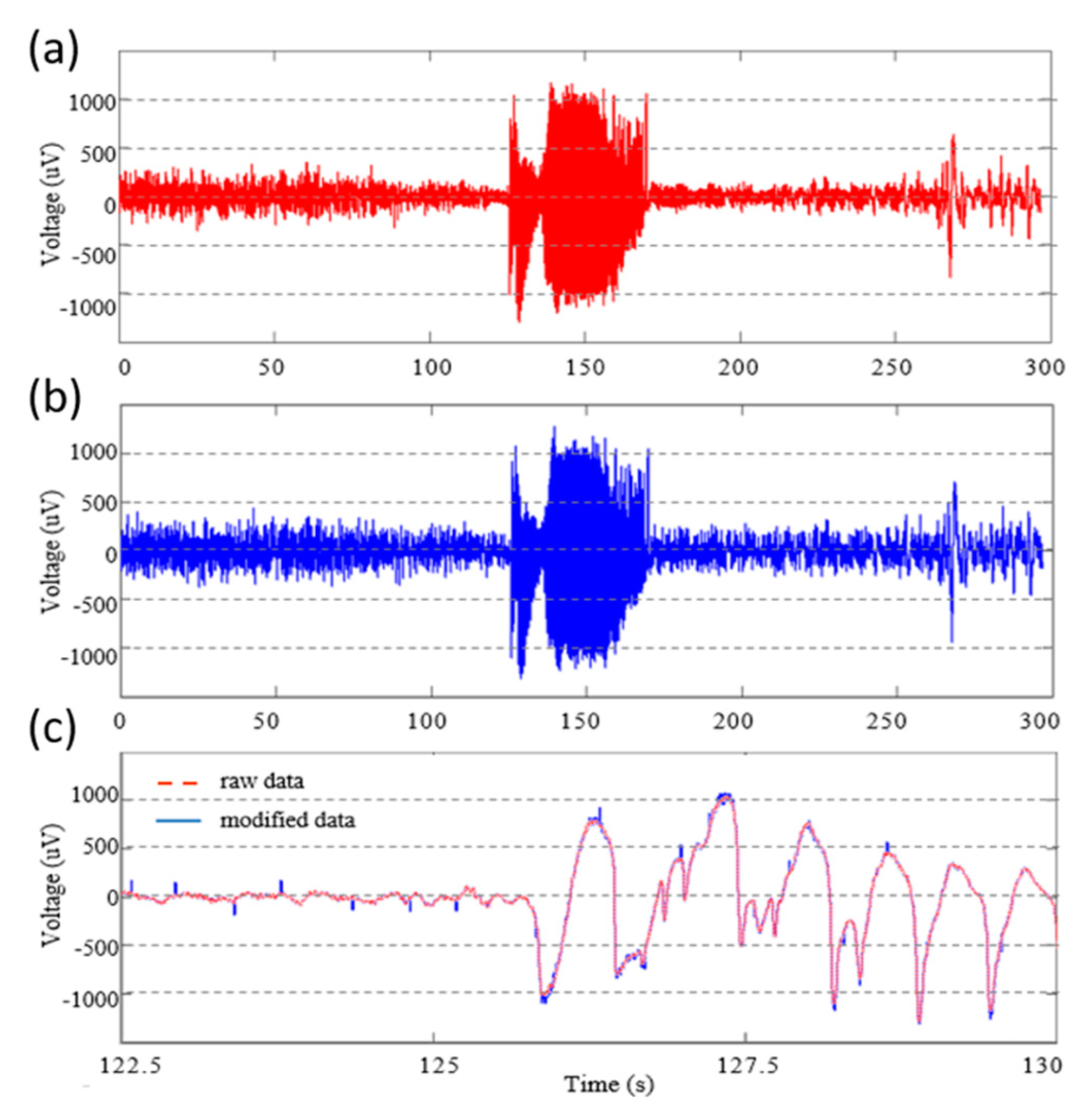
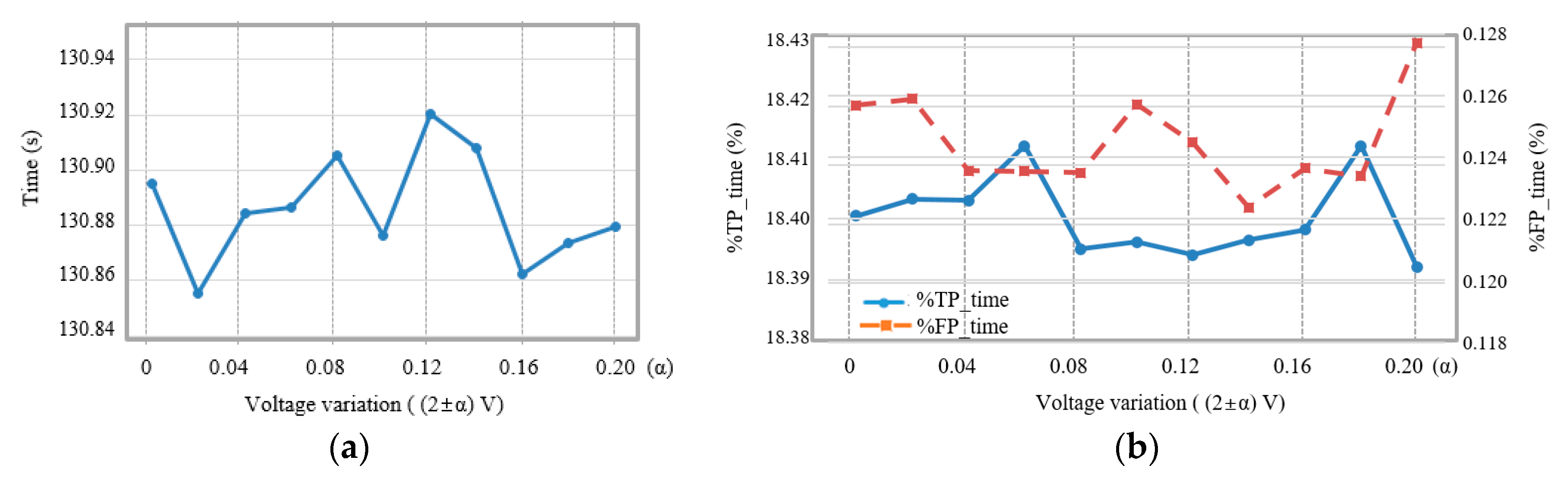
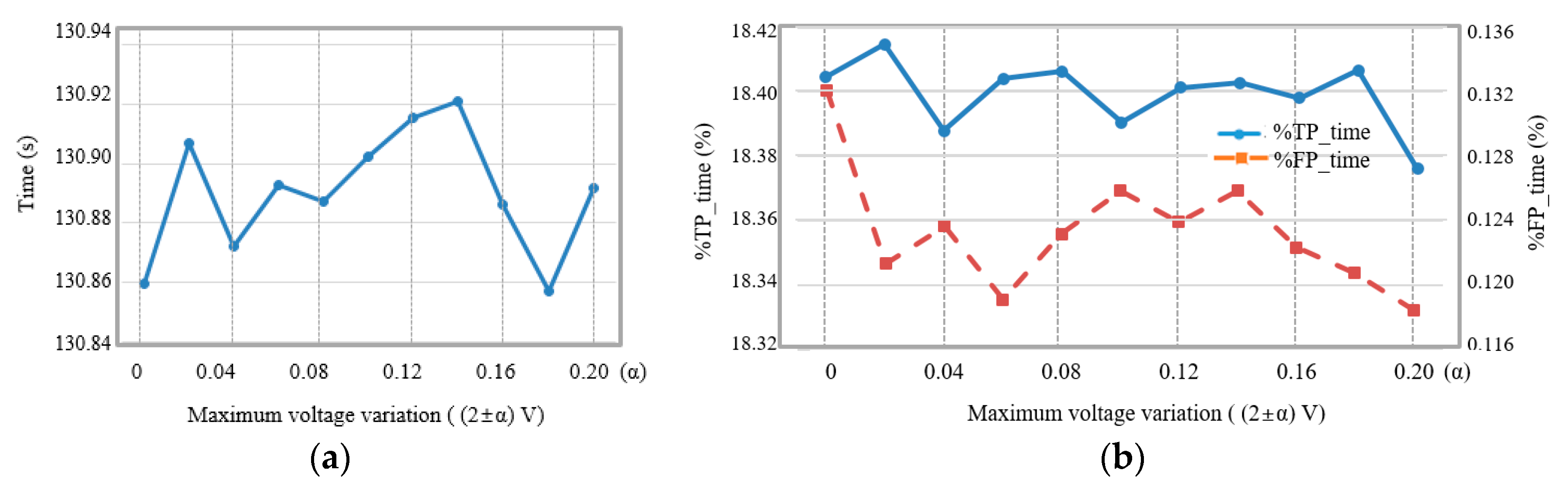
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Young, C.P.; Liang, S.F.; Chang, D.W.; Liao, Y.C.; Shaw, F.Z.; Hsieh, C.H. A portable wireless online closed-loop seizure controller in freely moving rats. IEEE Trans. Instrum. Meas. 2011, 60, 513–521. [Google Scholar] [CrossRef]
- Carey, J. Brain Facts: A Primer on the Brain and Nervous System. Available online: http://www.brainfacts.org/book (accessed on 10 December 2019).
- Bhavaraju, N.; Frei, M.; Osorio, I. Analog Seizure Detection and Performance Evaluation. IEEE Trans. Biomed. Eng. 2006, 53, 238–245. [Google Scholar] [CrossRef] [PubMed]
- What is Epilepsy? Available online: http://www.epilepsy.com/learn/epilepsy-101/what-epilepsy (accessed on 10 December 2019).
- Epilepsy Health Center. Available online: http://www.webmd.com/epilepsy/ (accessed on 10 December 2019).
- Engel, J.; Pedley, T.A.; Aicardi, J. Epilepsy: A Comprehensive Textbook, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Cadei, A.; Dionisi, A.; Sardini, E.; Serpelloni, M. Kinetic and thermal energy harvesters for implantable medical devices and biomedical autonomous sensors. Meas. Sci. Technol. 2014, 25, 012003. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, X.-J.; Liu, J. Suitability of a thermoelectric power generator for implantable medical electronic devices. J. Phys. D: Appl. Phys. 2007, 40, 5790–5800. [Google Scholar] [CrossRef]
- Almouahed, S.; Gouriou, M.; Hamitouche, C.; Stindel, E.; Roux, C. The Use of Piezoceramics As Electrical Energy Harvesters Within Instrumented Knee Implant During Walking. IEEE/ASME Trans. Mechatronics 2011, 16, 799–807. [Google Scholar] [CrossRef]
- Luciano, V.; Sardini, E.; Serpelloni, M.; Baronio, G. Analysis of an electromechanical generator implanted in a human total knee prosthesis. In Proceedings of the 2012 IEEE Sensors Applications Symposium Proceedings, Brescia, Italy, 7–9 February 2012. [Google Scholar]
- Nasiri, A.; Zabalawi, S.A.; Jeutter, D.C. A Linear Permanent Magnet Generator for Powering Implanted Electronic Devices. IEEE Trans. Power Electron. 2011, 26, 192–199. [Google Scholar] [CrossRef]
- Tashiro, R.; Kabei, N.; Katayama, K.; Tsuboi, E.; Tsuchiya, K. Development of an electrostatic generator for a cardiac pacemaker that harnesses the ventricular wall motion. J. Artif. Organs 2002, 5, 239–245. [Google Scholar] [CrossRef]
- Erturk, A. Electromechanical Modeling of Piezoelectric Energy Harvesters. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2009. [Google Scholar]
- Shen, D. Piezoelectric Energy Harvesting Devices for Low Frequency Vibration Applications. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2009. [Google Scholar]
- Ashraf, K.; Khir, M.H.M.; O Dennis, J.; Baharudin, Z. A wideband, frequency up-converting bounded vibration energy harvester for a low-frequency environment. Smart Mater. Struct. 2013, 22, 25018. [Google Scholar] [CrossRef]
- Ju, S.; Chae, S.H.; Choi, Y.; Jun, S.; Park, S.M.; Lee, S.J.; Lee, H.W.; Ji, C.H. Impact Based Piezoelectric Vibration Energy Harvester Using Spherical Metal Ball and MFC. In Proceedings of the 7th Asia-Pacific Conference on Transducers and Micro/Nano Technologies, Daegu, Korea, 29 June–2 July 2014. [Google Scholar]
- Kim, S.H.; Ju, S.; Ji, C.H.; Lee, S.J. Equivalent circuit model of an impact-based piezoelectric energy harvester. J. Phys. Conf. Ser. 2014, 557, 012094. [Google Scholar] [CrossRef] [Green Version]
- Osorio, I.; Frei, M.G.; Wilkinson, S.B. Real-time automated detection and quantitative analysis of seizures and short-term prediction of clinical onset. Epilepsia 1998, 39, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S.; Cole, R. Low complexity algorithms for heart rate and epileptic seizure detection. In Proceedings of the 2009 2nd International Symposium on Applied Sciences in Biomedical and Communication Technologies, Bratislava, Slovakia, 24–27 November 2009; pp. 1–5. [Google Scholar]
- Sridhara, S.R.; DiRenzo, M.; Lingam, S.; Lee, S.-J.; Blazquez, R.; Maxey, J.; Ghanem, S.; Lee, Y.-H.; Abdallah, R.; Singh, P.; et al. Microwatt Embedded Processor Platform for Medical System-on-Chip Applications. IEEE J. Solid-State Circuits 2011, 46, 721–730. [Google Scholar] [CrossRef]
- Liu, X.; Hao, H.; Yang, L.; Li, L.; Zhang, J.; Yang, A.; Ma, Y. Epileptic seizure detection with the local field potential of anterior thalamic of rats aiming at real time application. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; Volume 2011, pp. 6781–6784. [Google Scholar]
- Raghunathan, S.; Gupta, S.K.; Markandeya, H.S.; Roy, K.; Irazoqui, P.P. A hardware-algorithm co-design approach to optimize seizure detection algorithms for implantable applications. J. Neurosci. Methods 2010, 193, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ju, S.; Ji, C.-H.; Lee, S. Realistic Circuit Model of an Impact-Based Piezoelectric Energy Harvester. J. Semicond. Technol. Sci. 2015, 15, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Choi, Y.S.; Choi, K.; Lee, J.; Lee, B.-U.; Lee, H.W.; Lee, S. Performance Estimation of an Implantable Epileptic Seizure Detector with a Low-power On-chip Oscillator. J. Biomed. Eng. Res. 2015, 36, 169–176. [Google Scholar] [CrossRef] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Ju, S.; Ji, C.-H. Reliability Analysis of an Epileptic Seizure Detector Powered by an Energy Harvester. Micromachines 2020, 11, 45. https://doi.org/10.3390/mi11010045
Kim S, Ju S, Ji C-H. Reliability Analysis of an Epileptic Seizure Detector Powered by an Energy Harvester. Micromachines. 2020; 11(1):45. https://doi.org/10.3390/mi11010045
Chicago/Turabian StyleKim, Sunhee, Suna Ju, and Chang-Hyeon Ji. 2020. "Reliability Analysis of an Epileptic Seizure Detector Powered by an Energy Harvester" Micromachines 11, no. 1: 45. https://doi.org/10.3390/mi11010045
APA StyleKim, S., Ju, S., & Ji, C.-H. (2020). Reliability Analysis of an Epileptic Seizure Detector Powered by an Energy Harvester. Micromachines, 11(1), 45. https://doi.org/10.3390/mi11010045



