Nanopiezoelectric Devices for Energy Generation Based on ZnO Nanorods/Flexible-Conjugated Copolymer Hybrids Using All Wet-Coating Processes
Abstract
1. Introduction
2. Experiment Section
2.1. Preparation of Conductive Copolymer of EDOT (3,4-Ethylenedioxythiophene) and Aniline
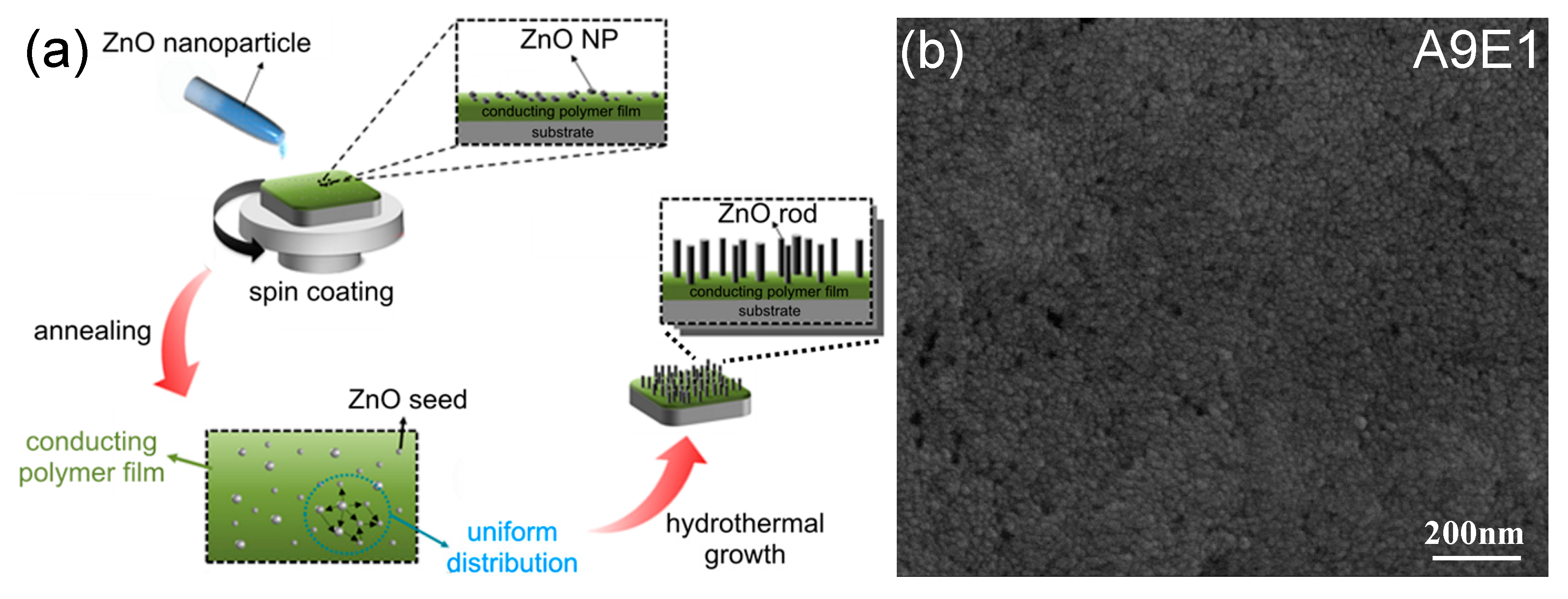
2.2. Deposition of ZnO Seed Layer on Polyaniline-Based Copolymer Coating
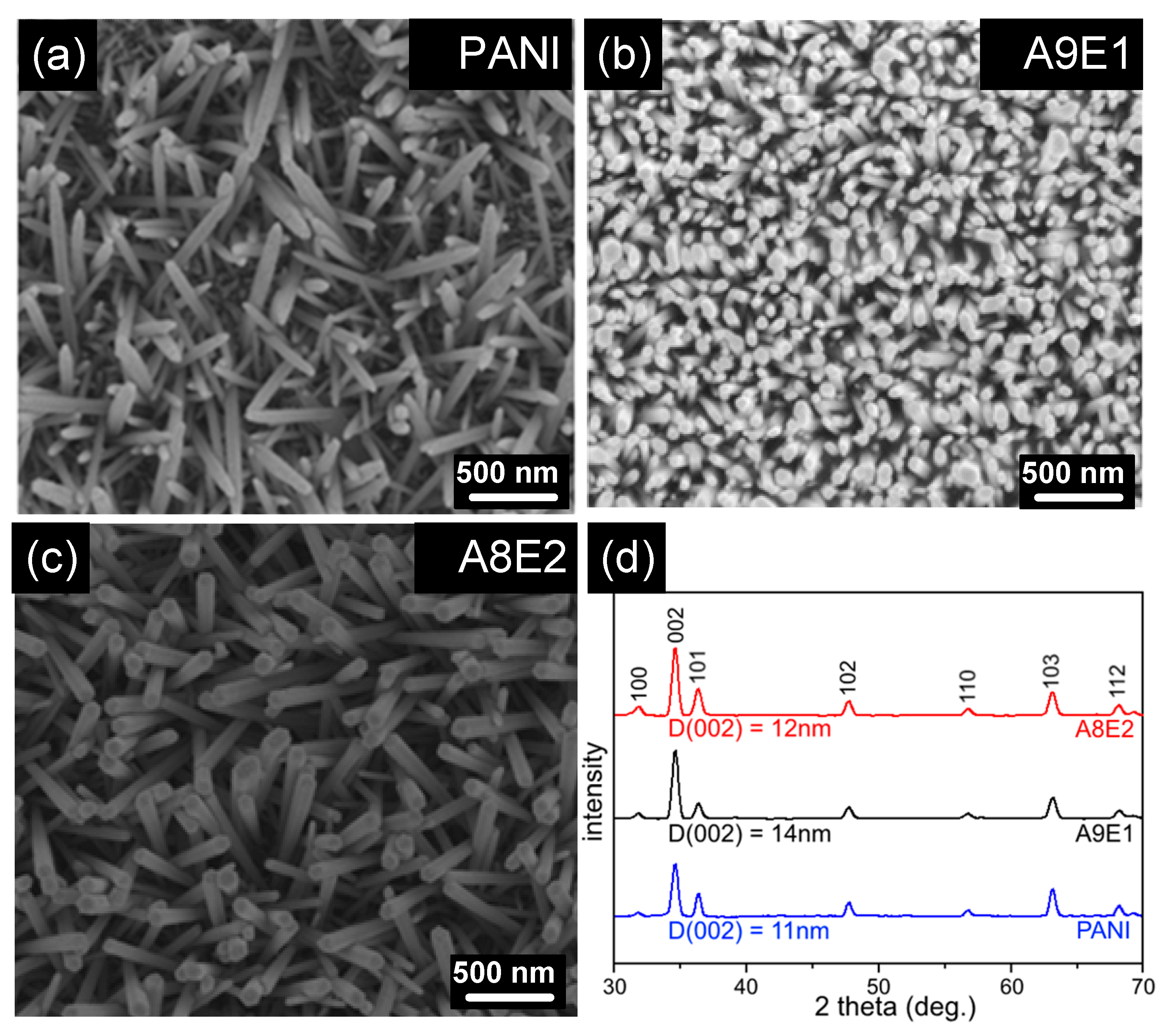
2.3. Hydrothermal Growth of ZnO Nanorods
2.4. Field Emission Scanning Electron Microscopy (FE-SEM)
2.5. Thickness Measurement
2.6. X-ray Diffraction (XRD) Analysis
2.7. Conductive Atomic Force Microscopy (c-AFM) for Piezoelectric Current Measurement
2.8. Fabrication of Large Area NG Devices for Piezoelectric Current Measurement
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rajendar, V.; Dayakar, T.; Shobhan, K.; Srikanth, I.; Rao, K.V. Systematic approach on the fabrication of Co doped ZnO semiconducting nanoparticles by mixture of fuel approach for antibacterial applications. Superlattices Microstruct. 2014, 75, 551. [Google Scholar] [CrossRef]
- Dingle, R. Luminescent transitions associated with divalent copper impurities and the green emission from semiconducting zinc oxide. Phys. Rev. Lett. 1969, 23, 579. [Google Scholar] [CrossRef]
- Kumar, B.; Kim, S.-W. Energy harvesting based on semiconducting piezoelectric ZnO nanostructures. Nano Energy 2012, 1, 342. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, J.; Song, J.; Liu, J.; Xu, N.; Wang, Z.L. Piezoelectric field effect transistor and nanoforce sensor based on a single ZnO nanowire. Nano Lett. 2006, 6, 2768. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lee, Y.-H.; Dai, C.-A.; Hsiao, C.-C.; Chen, S.-H.; Nurmalasari, N.P.D.; Chen, J.-C.; Cheng, Y.-Y.; Shih, W.-P.; Chang, P.-Z. A large area bimaterial sheet of piezoelectric nanogenerators for energy harvesting: Effect of RF sputtering on ZnO nanorod. Microelectron. Eng. 2011, 88, 2236. [Google Scholar] [CrossRef]
- Salehi, R.; Arami, M.; Mahmoodi, N.M.; Bahrami, H.; Khorramfar, S. Novel biocompatible composite (chitosan–zinc oxide nanoparticle): preparation, characterization and dye adsorption properties. Colloids Surf. B Biointerfaces 2010, 80, 86. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Hwang, S.; Su, Y.; Kim, S.; Cheng, H.; Gur, O.; Haney, R.; Omenetto, F.G.; Huang, Y.; Rogers, J.A. Transient, biocompatible electronics and energy harvesters based on ZnO. Small 2013, 9, 3398. [Google Scholar] [CrossRef]
- Moussodia, R.-O.; Balan, L.; Merlin, C.; Mustin, C.; Schneider, R. Biocompatible and stable ZnO quantum dots generated by functionalization with siloxane-core PAMAM dendrons. J. Mater. Chem. 2010, 20, 1147. [Google Scholar] [CrossRef]
- Vispute, R.D.; Talyansky, V.; Choopun, S.; Sharma, R.P.; Venkatesan, T. Heteroepitaxy of ZnO on GaN and its implications for fabrication of hybrid optoelectronic devices. Appl. Phys. Lett. 1998, 73, 348. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: material properties and device applications. Prog. Quantum Electron. 2010, 34, 191. [Google Scholar] [CrossRef]
- Choopun, S.; Vispute, R.D.; Noch, W.; Balsamo, A.; Sharma, R.P.; Venkatesan, T. Oxygen pressure-tuned epitaxy and optoelectronic properties of laser-deposited ZnO films on sapphire. Appl. Phys. Lett. 1999, 75, 3947. [Google Scholar] [CrossRef]
- Lim, J.H.; Shim, J.H.; Choi, J.H.; Joo, J.; Park, K.; Jeon, H.; Moon, M.R.; Jung, D.; Kim, H.; Lee, H.-J. Solution-processed InGaZnO-based thin film transistors for printed electronics applications. Appl. Phys. Lett. 2009, 95, 012108. [Google Scholar] [CrossRef]
- Subramanian, V.; Bakhishev, T.; Redinger, D.; Volkman, S.K. Solution-processed zinc oxide transistors for low-cost electronics applications. J. Disp. Technol. 2009, 5, 525. [Google Scholar] [CrossRef]
- Wager, J.F. Transparent electronics. Science 2003, 300, 1245–1246. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.; Kirchner, C.; Kling, R.; Reuss, F. ZnMgO epilayers and ZnO–ZnMgO quantum wells for optoelectronic applications in the blue and UV spectral region. Appl. Phys. Lett. 2004, 84, 5359. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P. Catalytic growth of zinc oxide nanowires by vapor transport. Adv. Mater. 2001, 13, 113. [Google Scholar] [CrossRef]
- Pauporté, T.; Lincot, D. Electrodeposition of semiconductors for optoelectronic devices: results on zinc oxide. Electrochim. Acta 2000, 45, 3345. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184. [Google Scholar] [CrossRef]
- Xiong, H.-M.; Xu, Y.; Ren, Q.-G.; Xia, Y.-Y. Stable aqueous ZnO@ polymer core− shell nanoparticles with tunable photoluminescence and their application in cell imaging. J. Am. Chem. Soc. 2008, 130, 7522. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, W.; Formo, E.; Sun, Y.; Xia, Y. Ceramic nanofibers fabricated by electrospinning and their applications in catalysis, environmental science, and energy technology. Polym. Adv. Technol. 2011, 22, 326. [Google Scholar] [CrossRef]
- Lai, X.; Halpert, J.E.; Wang, D. Recent advances in micro-/nano-structured hollow spheres for energy applications: From simple to complex systems. Energy Environ. Sci. 2012, 5, 5604. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Chou, S.C.; Chang, J.A. Development of flexible piezoelectric nanogenerator: Toward all wet chemical method. Microelectron. Eng. 2011, 88, 3015. [Google Scholar] [CrossRef]
- Umar, A.; Akhtar, M.S.; Al-Hajry, A.; Al-Assiri, M.S.; Almehbad, N.Y. Hydrothermally grown ZnO nanoflowers for environmental remediation and clean energy applications. Mater. Res. Bull. 2012, 47, 2407. [Google Scholar] [CrossRef]
- Fei, P.; Yeh, P.-H.; Zhou, J.; Xu, S.; Gao, Y.; Song, J.; Gu, Y.; Huang, Y.; Wang, Z.L. Piezoelectric potential gated field-effect transistor based on a free-standing ZnO wire. Nano Lett. 2009, 9, 3435. [Google Scholar] [CrossRef]
- Kwon, S.; Hong, W.; Jo, G.; Maeng, J.; Kim, T.; Song, S.; Lee, T. Piezoelectric effect on the electronic transport characteristics of ZnO nanowire field-effect transistors on bent flexible substrates. Adv. Mater. 2008, 20, 4557. [Google Scholar] [CrossRef]
- Gao, Z.; Zhou, J.; Gu, Y.; Fei, P.; Hao, Y.; Bao, G.; Wang, Z.L. Effects of piezoelectric potential on the transport characteristics of metal-ZnO nanowire-metal field effect transistor. J. Appl. Phys. 2009, 105, 113707. [Google Scholar] [CrossRef]
- He, J.; Hsin, C.; Liu, J.; Chen, L.; Wang, Z. Piezoelectric gated diode of a single ZnO nanowire. Adv. Mater. 2007, 19, 781. [Google Scholar] [CrossRef]
- Zhou, J.; Fei, P.; Gu, Y.; Mai, W.; Gao, Y.; Yang, R.; Bao, G.; Wang, Z.L. Piezoelectric-potential-controlled polarity-reversible Schottky diodes and switches of ZnO wires. Nano Lett. 2008, 8, 3973. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qi, J.; Liao, Q.; Li, H.; Wang, Y.; Tang, L.; Zhang, Y. High-performance piezoelectric gate diode of a single polar-surface dominated ZnO nanobelt. Nanotechnology 2009, 20, 125201. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Choi, D.; Jin, M.; Kim, I.; Kim, S.; Choi, J.; Lee, S.Y.; Kim, J.M.; Kim, S. Mechanically powered transparent flexible charge-generating nanodevices with piezoelectric ZnO nanorods. Adv. Mater. 2009, 21, 2185. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Li, G. In situ ZnO nanowire growth to promote the PVDF piezo phase and the ZnO–PVDF hybrid self-rectified nanogenerator as a touch sensor. Phys. Chem. Chem. Phys. 2014, 16, 5475. [Google Scholar] [CrossRef]
- Song, J.; Zhou, J.; Wang, Z.L. Piezoelectric and semiconducting coupled power generating process of a single ZnO belt/wire. A technology for harvesting electricity from the environment. Nano Lett. 2006, 6, 1656. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chen, K.-C. Improving piezoelectric nanogenerator comprises ZnO nanowires by bending the flexible PET substrate at low vibration frequency. J. Phys. Chem. C 2012, 116, 9351. [Google Scholar] [CrossRef]
- Lyu, S.C.; Zhang, Y.; Ruh, H.; Lee, H.-J.; Shim, H.-W.; Suh, E.-K.; Lee, C.J. Low temperature growth and photoluminescence of well-aligned zinc oxide nanowires. Chem. Phys. Lett. 2002, 363, 134. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.H.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251. [Google Scholar]
- Guo, L.; Ji, Y.L.; Xu, H.; Simon, P.; Wu, Z. Regularly shaped, single-crystalline ZnO nanorods with wurtzite structure. J. Am. Chem. Soc. 2002, 124, 14864. [Google Scholar] [CrossRef]
- Umar, A.; Hahn, Y.B. ZnO nanosheet networks and hexagonal nanodiscs grown on silicon substrate: growth mechanism and structural and optical properties. Nanotechnology 2006, 17, 2174. [Google Scholar] [CrossRef]
- Gao, P.; Wang, Z.L. High-yield synthesis of single-crystal nanosprings of ZnO. Small 2005, 1, 945. [Google Scholar] [CrossRef] [PubMed]
- Bakharev, P.V.; Dobrokhotov, V.V.; McIlroy, D.N. A method for integrating ZnO coated nanosprings into a low cost redox-based chemical sensor and catalytic tool for determining gas phase reaction kinetics. Chemosensors 2014, 2, 56. [Google Scholar] [CrossRef]
- Dobrokhotov, V.; Oakes, L.; Sowell, D.; Larin, A.; Hall, J.; Barzilov, A.; Kengne, A.; Bakharev, P.; Corti, G.; Cantrell, T.; et al. Thermal and optical activation mechanisms of nanospring-based chemiresistors. Sensors 2012, 12, 5608. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Qu, F.; Zhang, X.; Cai, W.; Shen, G. Fabrication of ZnO ring-like nanostructures at a moderate temperature via a thermal evaporation process. J. Alloys Compd. 2009, 486, L13. [Google Scholar] [CrossRef]
- Liu, W.C.; Cai, W. Synthesis and characterization of ZnO nanorings with ZnO nanowires array aligned at the inner surface without catalyst. J. Crystal Growth 2008, 310, 843. [Google Scholar] [CrossRef]
- Tu, Z.C.; Li, Q.X.; Hu, X. Theoretical determination of the necessary conditions for the formation of ZnO nanorings and nanohelices. Phys. Rev. B 2006, 73, 115402. [Google Scholar] [CrossRef]
- Hughes, W.L.; Wang, Z.L. Formation of piezoelectric single-crystal nanorings and nanobows. J. Am. Chem. Soc. 2004, 126, 6703. [Google Scholar] [CrossRef]
- Hughes, W.L.; Wang, Z.L. Controlled synthesis and manipulation of ZnO nanorings and nanobows. Appl. Phys. Lett. 2005, 86, 043106. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, X.T.; Zhou, M.Y.; Zhang, Z.G.; Wang, X.Z. Growth of novel ZnO nanohelices modified by SiO2-sheathed ZnO discs. Nanotechnology 2007, 18, 065601. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, X.; Zhou, M.; Zhang, E.; Zhang, Z. Super-uniform ZnO nanohelices synthesized via thermal evaporation. Solid State Commun. 2006, 140, 455. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, D.H.; Jung, S.-W.; Yi, G.-C. Metalorganic vapor-phase epitaxial growth of vertically well-aligned ZnO nanorods. Appl. Phys. Lett. 2002, 80, 4232. [Google Scholar] [CrossRef]
- Feng, X.; Feng, L.; Jin, M.; Zhai, J.; Jiang, L.; Zhu, D. Reversible super-hydrophobicity to super-hydrophilicity transition of aligned ZnO nanorod films. J. Am. Chem. Soc. 2004, 126, 62. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.H. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 2004, 84, 3654. [Google Scholar] [CrossRef]
- Kong, Y.C.; Yu, D.P.; Zhang, B.; Fang, W.; Feng, S.Q. Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl. Phys. Lett. 2001, 78, 407. [Google Scholar] [CrossRef]
- Beek, W.; Wienk, M.; Janssen, R. Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer. Adv. Mater. 2004, 16, 1009. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866. [Google Scholar] [CrossRef]
- Corso, A.D.; Posternak, M.; Resta, R.; Baldereschi, A. Ab initio study of piezoelectricity and spontaneous polarization in ZnO. Phys. Rev. B 1994, 50, 10715. [Google Scholar] [CrossRef]
- Emanetoglu, N.W.; Gorla, C.; Liu, Y.; Liang, S.; Lu, Y. Epitaxial ZnO piezoelectric thin films for saw filters. Mater. Sci. Semicond. Process. 1999, 2, 247. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Mohan, R.; Thiyagarajana, K.; Kim, S.-J. Fabrication of a ZnO nanogenerator for eco-friendly biomechanical energy harvesting. RSC Adv. 2013, 3, 16646. [Google Scholar] [CrossRef]
- Liu, C.; Yu, A.; Peng, M.; Song, M.; Liu, W.; Zhang, Y.; Zhai, J. Improvement in the piezoelectric performance of a ZnO nanogenerator by a combination of chemical doping and interfacial modification. J. Phys. Chem. C 2016, 120, 6971. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, Y.; Wang, P.; Xing, L.; Xue, X. Highly stable piezo-immunoglobulin-biosensing of a SiO2/ZnO nanogenerator as a self-powered/active biosensor arising from the field effect influenced piezoelectric screening effect. Nanoscale 2015, 7, 1904. [Google Scholar] [CrossRef] [PubMed]
- Vayssieres, L.; Keis, K.; Lindquist, S.-E.; Hagfeldt, A. Purpose-built anisotropic metal oxide material: 3D highly oriented microrod array of ZnO. J. Phys. Chem. B 2001, 105, 3350. [Google Scholar] [CrossRef]
- Lokhande, B.J.; Uplane, M.D. Structural, optical and electrical studies on spray deposited highly oriented ZnO films. Appl. Surf. Sci. 2000, 167, 243. [Google Scholar] [CrossRef]
- de Leeuw, D.M.; Kraakman, P.A.; Bongaerts, P.F.G.; Mutsaers, C.M.J.; Klaassen, D.B.M. Electroplating of conductive polymers for the metallization of insulators. Synth. Met. 1994, 66, 263. [Google Scholar] [CrossRef]
- Ha, Y.-H.; Nikolov, N.; Pollack, S.; Mastrangelo, J.; Martin, B.; Shashidhar, R. Towards a transparent, highly conductive Poly (3, 4-ethylenedioxythiophene). Adv. Funct. Mater. 2004, 14, 615. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X.; Wang, Z.L. Microfibre–nanowire hybrid structure for energy scavenging. Nature 2008, 451, 809. [Google Scholar] [CrossRef]
- Yang, R.; Qin, Y.; Dai, L.; Wang, Z.L. Power generation with laterally packaged piezoelectric fine wires. Nat. Nano 2009, 4, 34. [Google Scholar] [CrossRef]
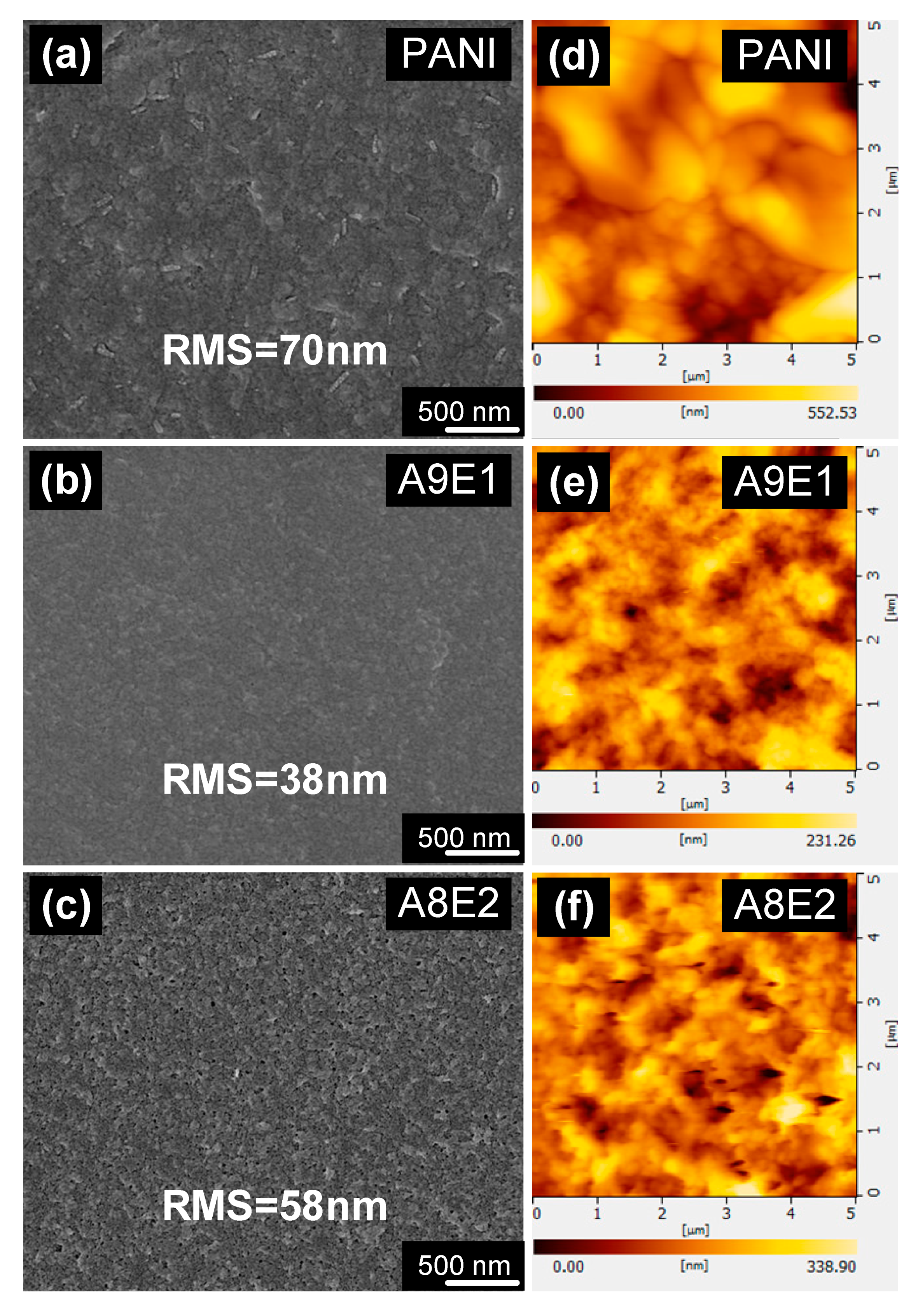
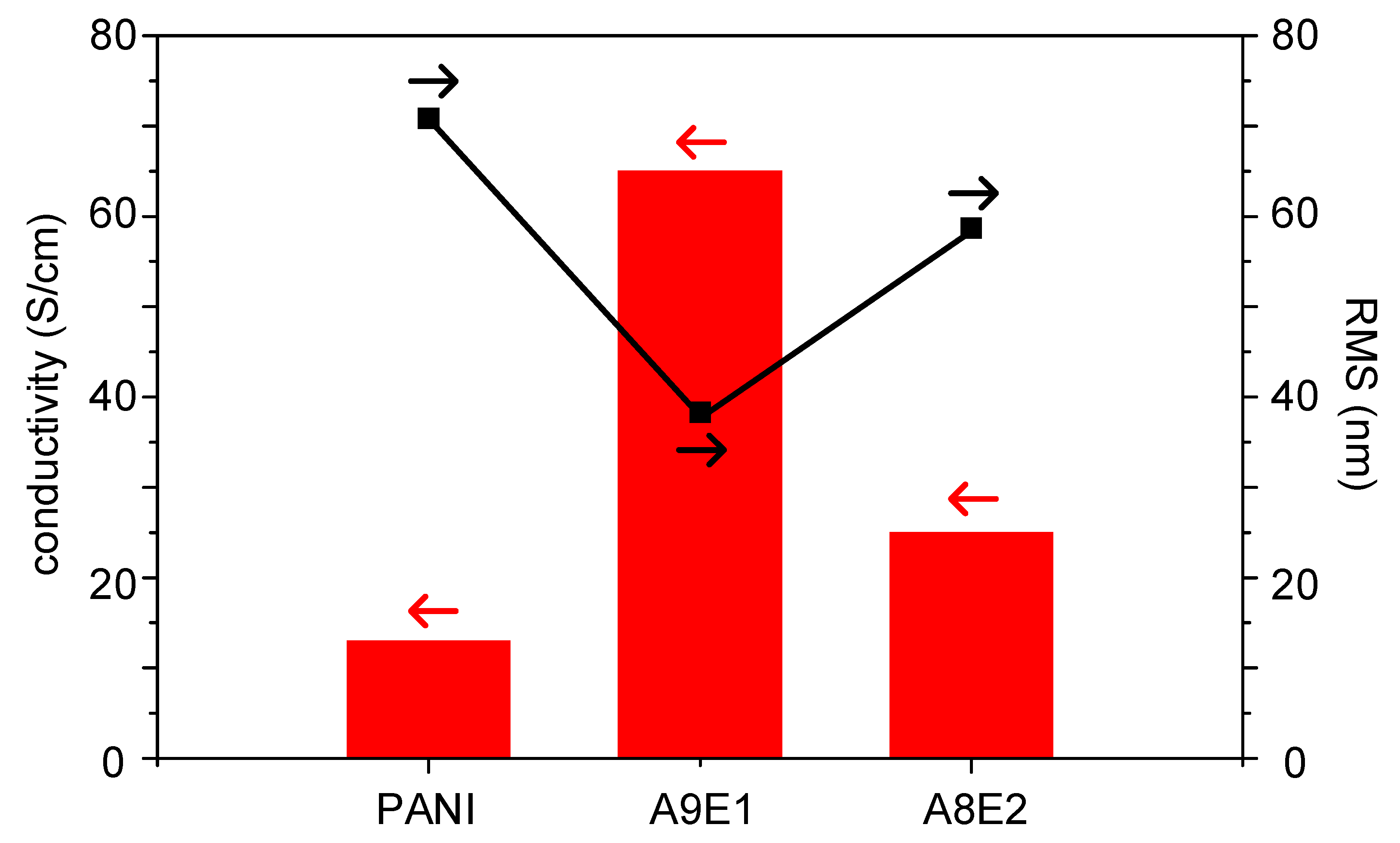
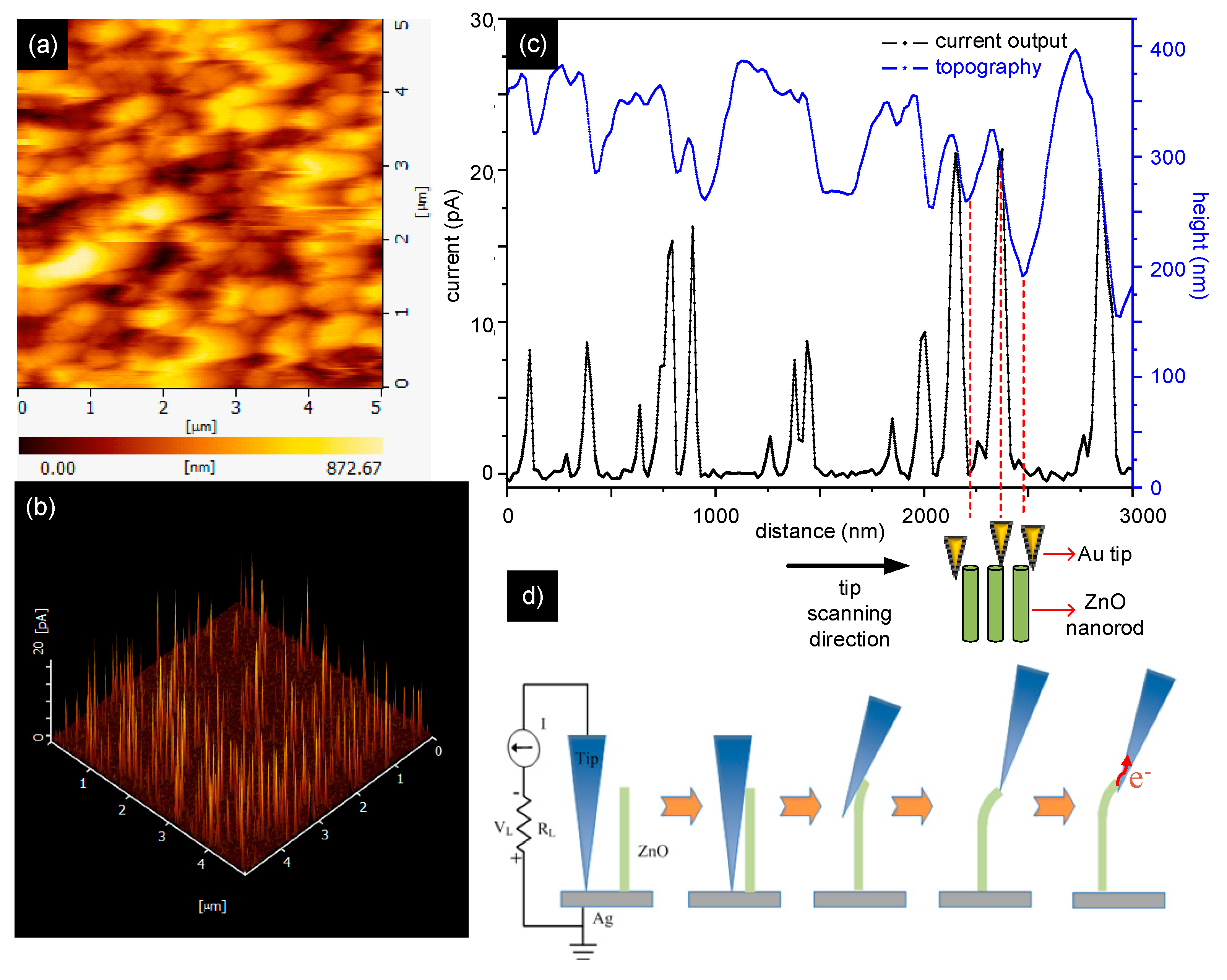
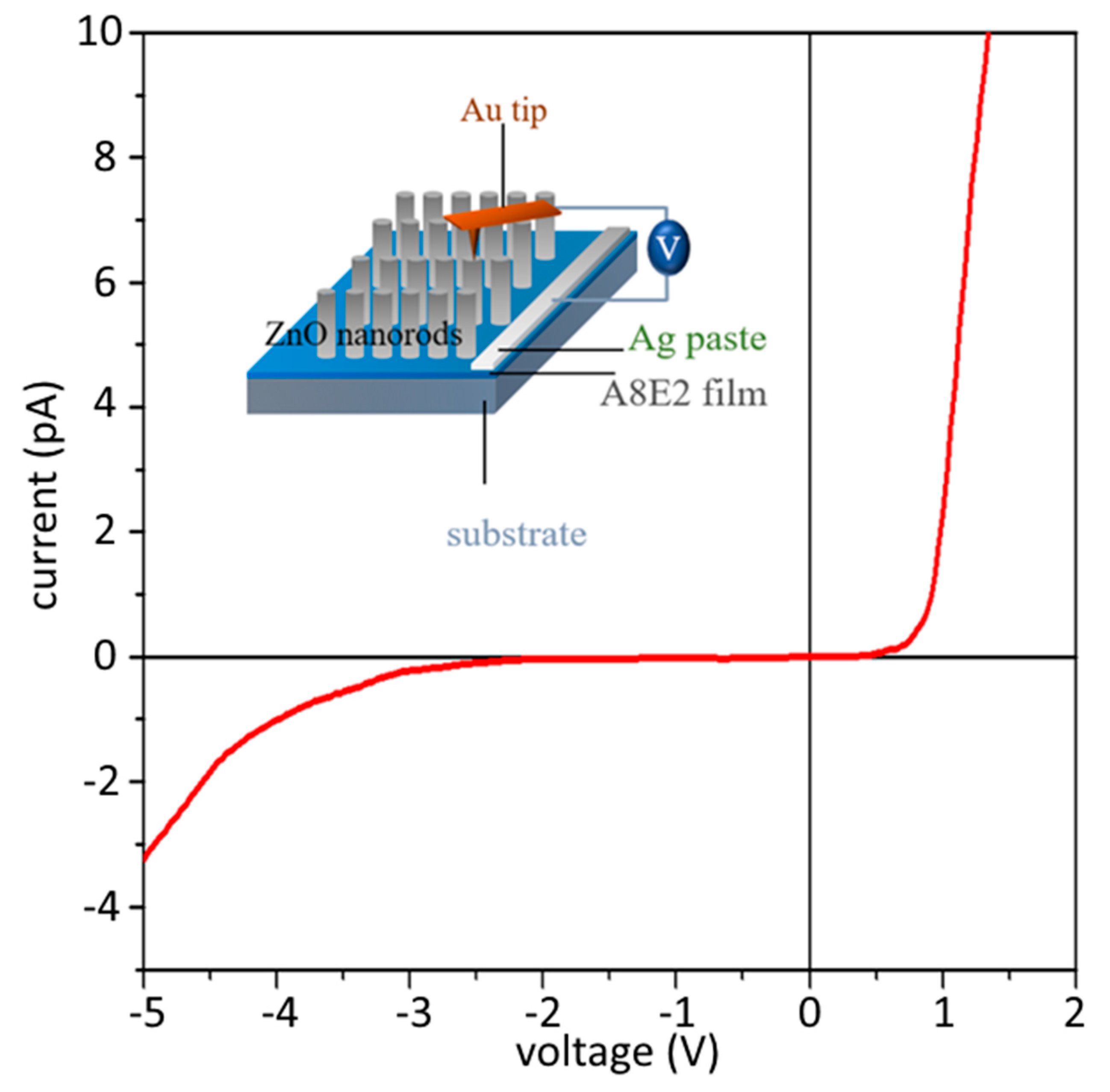
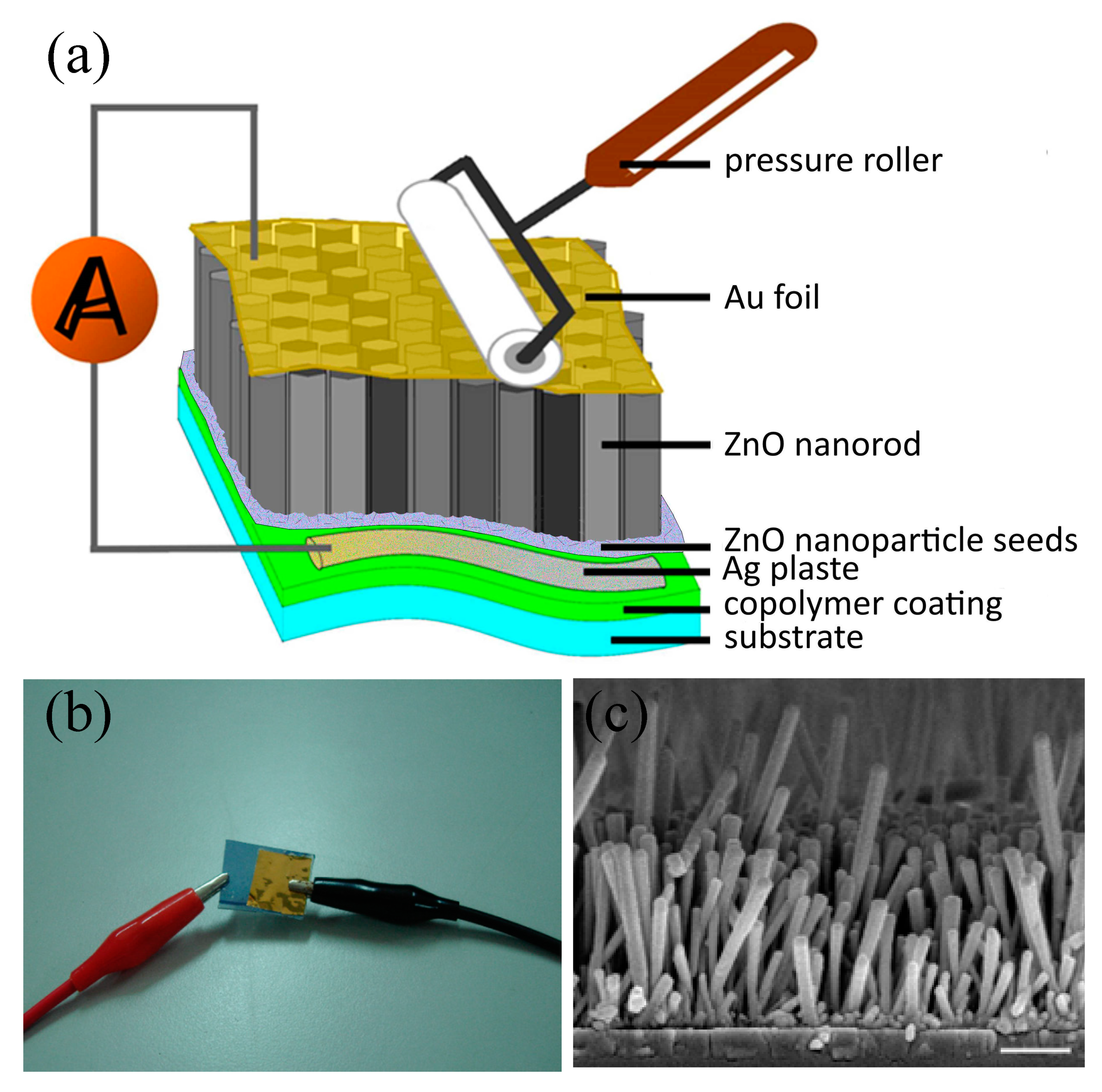
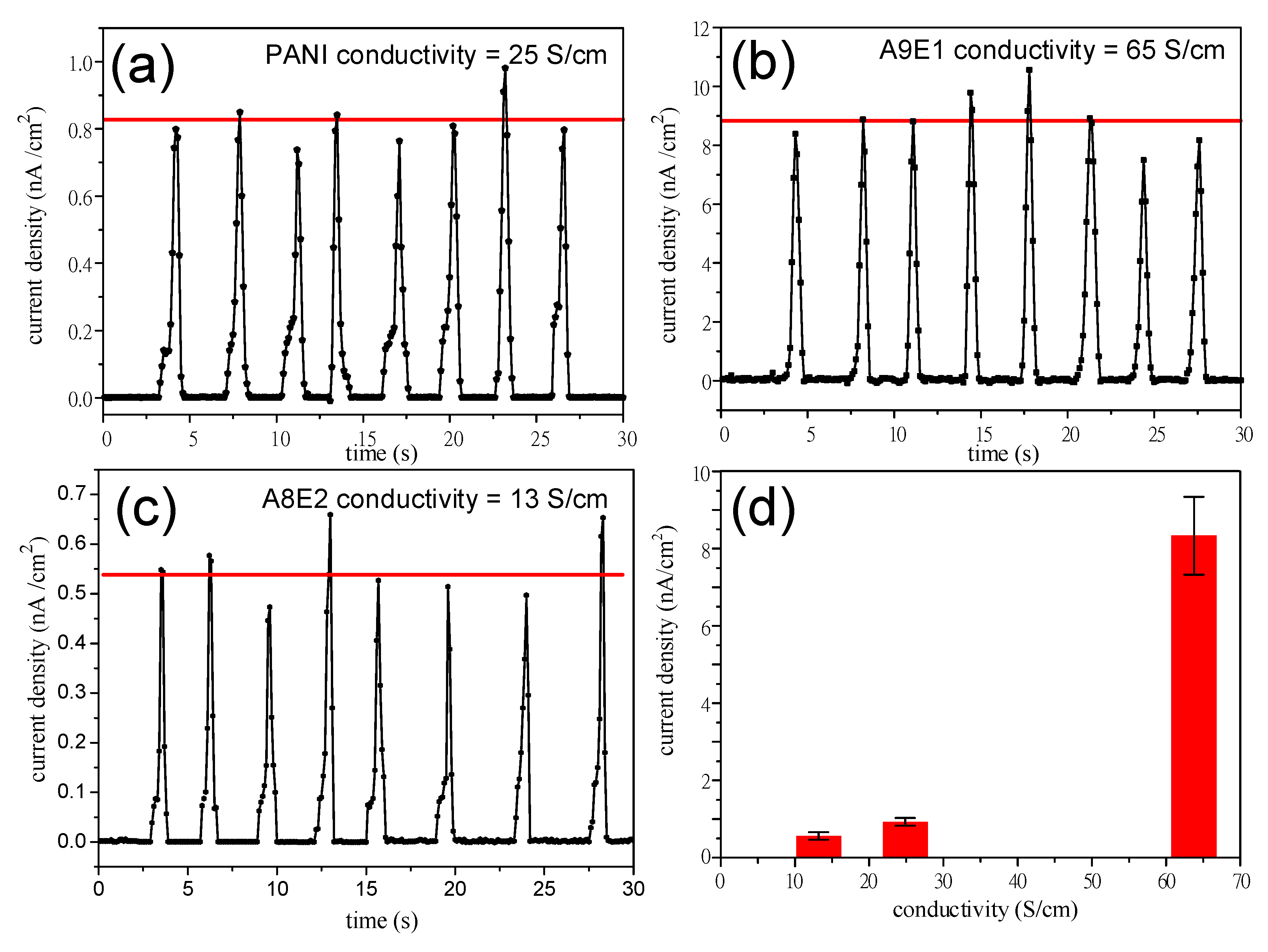
| Sample | Fe(OTs)3/Monomers | Aniline: EDOT | Roughness (RMS/nm) | Conductivity (S/cm) |
|---|---|---|---|---|
| A10E0 (PANI) | 1.75 | 10:0 | 70.86 | 13 |
| A9E1 | 1.75 | 9:1 | 38.30 | 65 |
| A8E2 | 1.75 | 8:2 | 58.69 | 25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-P.; Lin, C.-C.; Hsiao, C.-C.; Chou, P.-A.; Cheng, Y.-Y.; Hsieh, C.-C.; Dai, C.-A. Nanopiezoelectric Devices for Energy Generation Based on ZnO Nanorods/Flexible-Conjugated Copolymer Hybrids Using All Wet-Coating Processes. Micromachines 2020, 11, 14. https://doi.org/10.3390/mi11010014
Lee Y-P, Lin C-C, Hsiao C-C, Chou P-A, Cheng Y-Y, Hsieh C-C, Dai C-A. Nanopiezoelectric Devices for Energy Generation Based on ZnO Nanorods/Flexible-Conjugated Copolymer Hybrids Using All Wet-Coating Processes. Micromachines. 2020; 11(1):14. https://doi.org/10.3390/mi11010014
Chicago/Turabian StyleLee, Yu-Ping, Chieh-Chuan Lin, Chih-Chung Hsiao, Po-An Chou, Yao-Yi Cheng, Chih-Chen Hsieh, and Chi-An Dai. 2020. "Nanopiezoelectric Devices for Energy Generation Based on ZnO Nanorods/Flexible-Conjugated Copolymer Hybrids Using All Wet-Coating Processes" Micromachines 11, no. 1: 14. https://doi.org/10.3390/mi11010014
APA StyleLee, Y.-P., Lin, C.-C., Hsiao, C.-C., Chou, P.-A., Cheng, Y.-Y., Hsieh, C.-C., & Dai, C.-A. (2020). Nanopiezoelectric Devices for Energy Generation Based on ZnO Nanorods/Flexible-Conjugated Copolymer Hybrids Using All Wet-Coating Processes. Micromachines, 11(1), 14. https://doi.org/10.3390/mi11010014





