Improved Efficiency of Perovskite Light-Emitting Diodes Using a Three-Step Spin-Coated CH3NH3PbBr3 Emitter and a PEDOT:PSS/MoO3-Ammonia Composite Hole Transport Layer
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
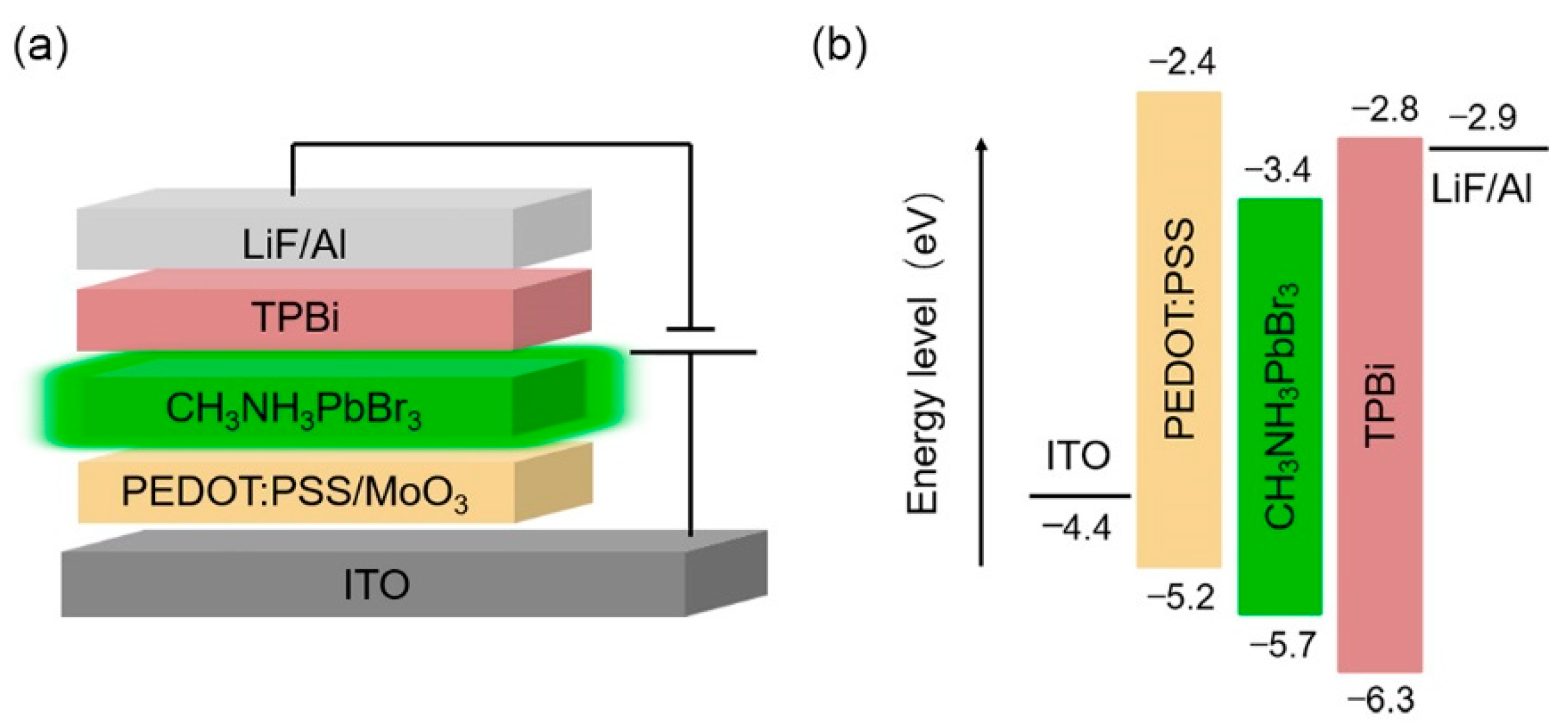
2.2. Device Fabrication
2.3. Device Characterization
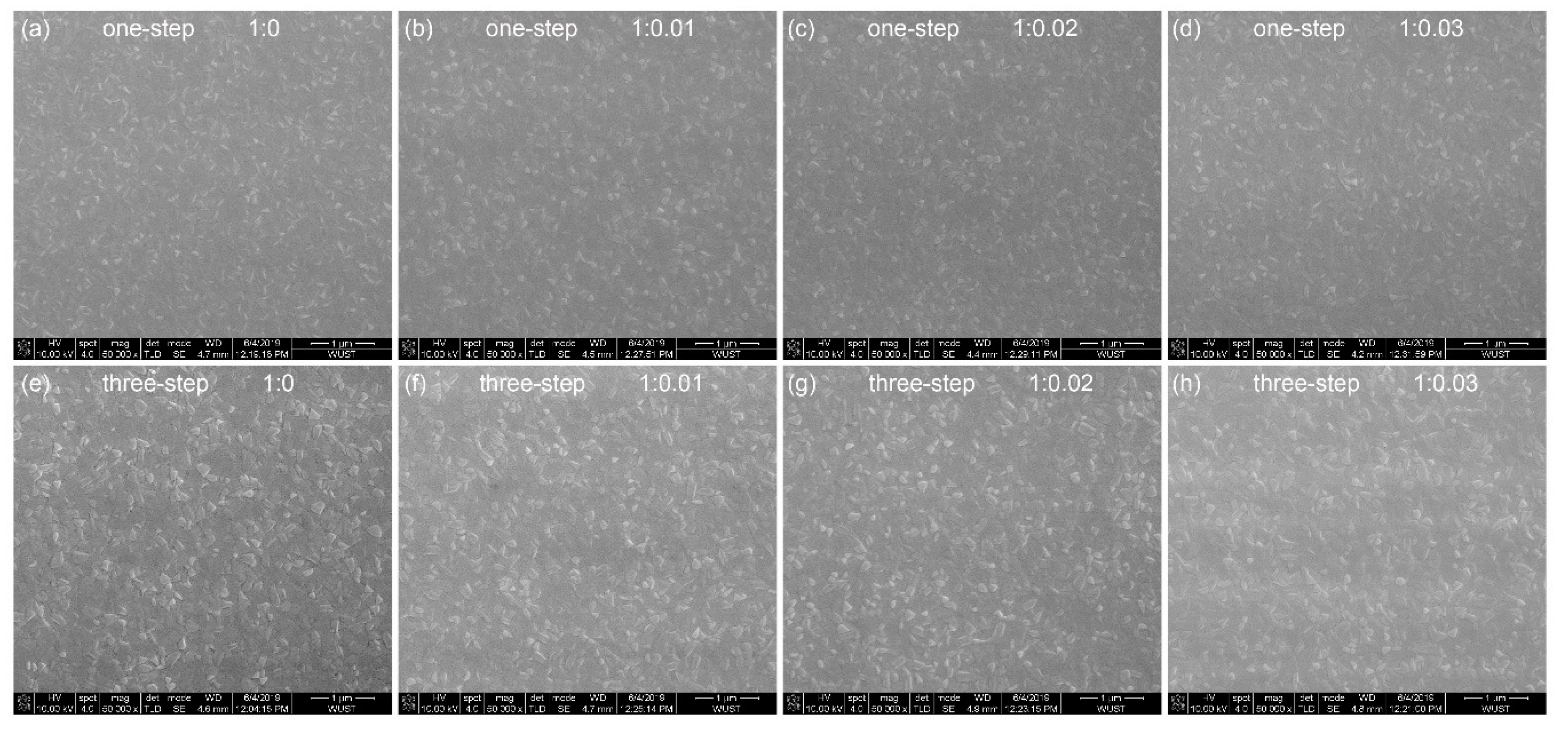
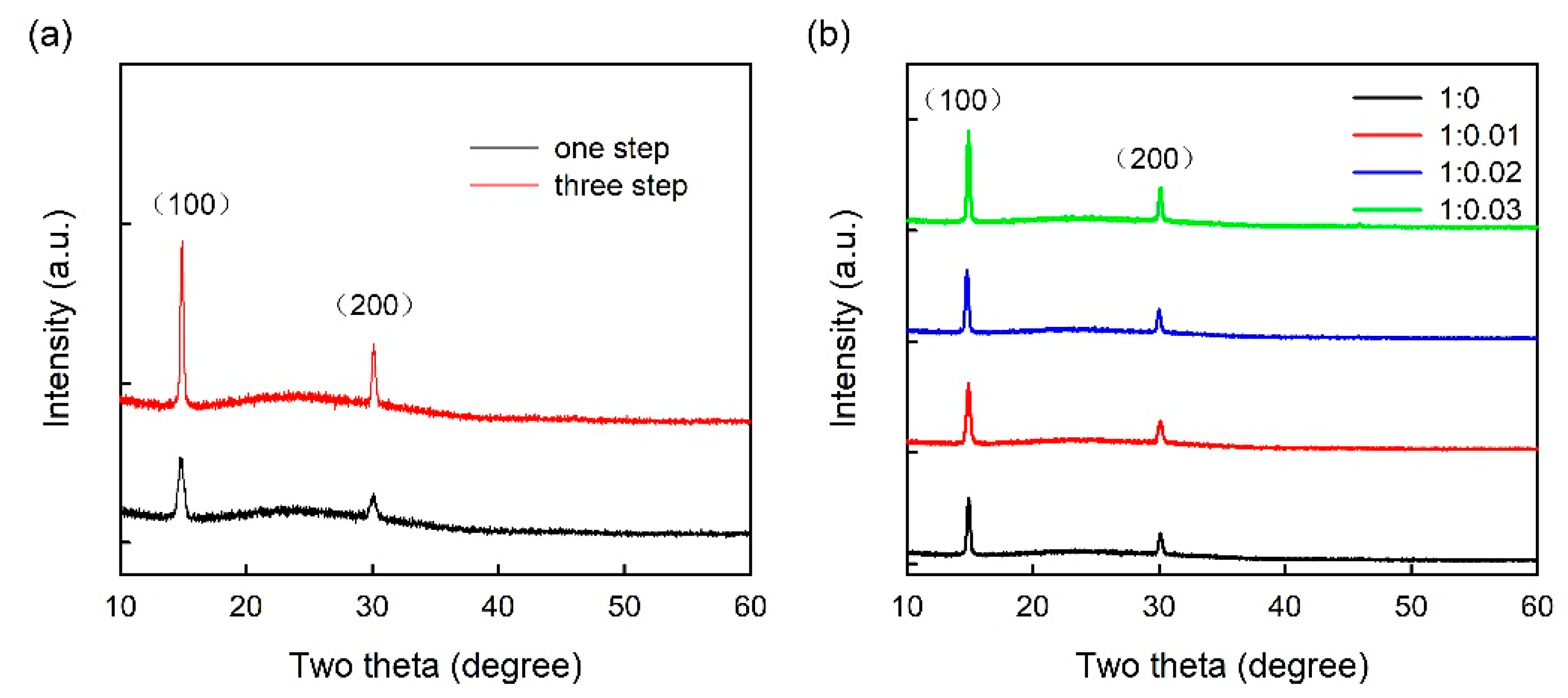
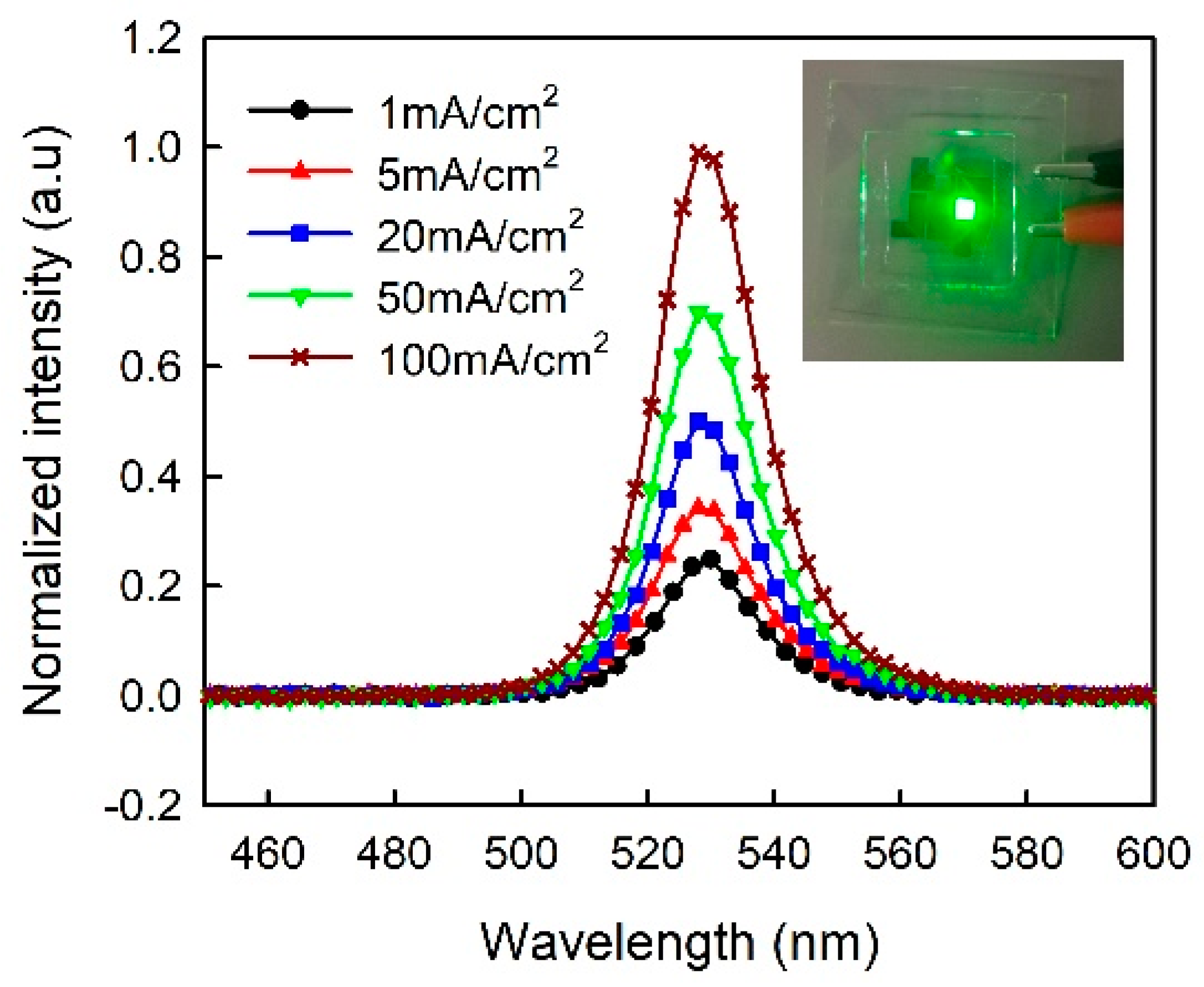
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Era, M.; Morimoto, S.; Tsutsui, T.; Saito, S. Organic-inorganic heterostructure electroluminescent device using a layered perovskite semiconductor (C6H5C2H4NH3)2PbI4. Appl. Phys. Lett. 1994, 65, 676. [Google Scholar] [CrossRef]
- Koutselas, I.; Bampoulis, P.; Maratou, E.; Evagelinou, T.; Pagona, G.; Papavassiliou, G.C. Some unconventional organic−inorganic hybrid low-dimensional semiconductors and related light-emitting devices. J. Phys. Chem. C 2011, 115, 8475–8483. [Google Scholar] [CrossRef]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.C.; Jeong, S.H.; Park, M.H.; Kim, Y.H.; Wolf, C.; Lee, C.L.; Heo, J.H.; Sadhanala, A.; Myoung, N.; Yoo, S.; et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Cho, H.; Heo, J.H.; Kim, T.S.; Myoung, N.; Lee, C.L.; Im, S.H.; Lee, T.W. Multicolored organic/inorganic hybrid perovskite light-emitting diodes. Adv. Mater. 2015, 27, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.B.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.W.; Lu, J.X.; Xie, L.Q.; Zhao, W.J.; Zhang, D.; Yan, C.Z.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, N.N.; Tian, H.; Guo, J.S.; Wei, Y.Q.; Chen, H.; Miao, Y.F.; Zou, W.; Pan, K.; He, Y.R.; et al. Perovskite light-emitting diodes based on spontaneously formed submicrometre-scale structures. Nature 2018, 562, 249–253. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.H.; Noh, J.; Han, S.H. Performance and stability of electroluminescent device with self-assembled layers of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) and polyelectrolytes. Thin Solid Films 2006, 510, 305–310. [Google Scholar] [CrossRef]
- Kim, J.S.; Friend, R.H.; Grizzi, I.; Burroughes, J.H. Spin-cast thin semiconducting polymer interlayer for improving device efficiency of polymer light-emitting diodes. Appl. Phys. Lett. 2005, 87, 023506. [Google Scholar] [CrossRef]
- Liu, N.; Mei, S.J.; Sun, D.W.; Shi, W.X.; Feng, J.H.; Zhou, Y.M.; Mei, F.; Xu, J.X.; Jiang, Y.; Cao, X.A. Effects of charge transport materials on blue fluorescent organic light-emitting diodes with a host-dopant system. Micromachines 2019, 10, 344. [Google Scholar] [CrossRef]
- You, H.; Dai, Y.F.; Zhang, Z.Q.; Ma, D.G. Improved performances of organic light-emitting diodes with metal oxide as anode buffer. J. Appl. Phys. 2007, 101, 026105. [Google Scholar] [CrossRef]
- Li, J.Z.; Yahiro, M.; Ishida, K.; Yamada, H.; Matsushige, K. Enhanced performance of organic light emitting device by insertion of conducting/insulating WO3 anodic buffer layer. Synth. Met. 2005, 151, 141. [Google Scholar] [CrossRef]
- Meyer, J.; Zilberberg, K.; Riedl, T.; Kahn, A. Electronic structure of Vanadium pentoxide: An efficient hole injector for organic electronic materials. J. Appl. Phys. 2011, 110, 033710. [Google Scholar] [CrossRef]
- Shrotriya, V.; Li, G.; Yao, Y.; Chu, C.W.; Yang, Y. Transition metal oxides as the buffer layer for polymer photovoltaic cells. Appl. Phys. Lett. 2006, 88, 073508. [Google Scholar] [CrossRef]
- Shi, W.X.; Liu, N.; Zhou, Y.M.; Cao, X.A. Effects of postannealing on the characteristics and reliability of polyfluorene organic light-emitting diodes. IEEE Trans. Electron Devices 2019, 66, 1057–1062. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Chen, J.S.; Chen, W.; Ma, D.G. Poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate)/MoO3 composite layer for efficient and stable hole injection in organic semiconductors. J. Appl. Phys. 2012, 111, 043716. [Google Scholar] [CrossRef]
- Meyer, J.; Khalandovsky, R.; Gorrn, P.; Kahn, A. MoO3 films spin-coated from a nanoparticle suspension for efficient hole-injection in organic electronics. Adv. Mater. 2011, 23, 70–73. [Google Scholar] [CrossRef]
- Wong, K.H.; Ananthanarayanan, K.; Luther, J.; Balaya, P. Origin of hole selectivity and the role of defects in low-temperature solution-processed molybdenum oxide interfacial layer for organic solar cells. J. Phys. Chem. C 2012, 116, 16346–16351. [Google Scholar] [CrossRef]
- Murase, S.; Yang, Y. Solution processed MoO3 interfacial layer for organic photovoltaics prepared by a facile synthesis method. Adv. Mater. 2012, 24, 2459–2462. [Google Scholar] [CrossRef]
- Sun, J.Y.; Tseng, W.H.; Lan, S.; Lin, S.H.; Yang, P.C.; Wu, C.I.; Lin, C.F. Performance enhancement in inverted polymer photovoltaics with solution-processed MoOx and air-plasma treatment for anode modification. Sol. Energy Mater. Sol. Cells 2013, 109, 178–184. [Google Scholar] [CrossRef]
- Liu, F.M.; Shao, S.Y.; Guo, X.Y.; Zhao, Y.; Xie, Z.Y. Efficient polymer photovoltaic cells using solution-processed MoO3 as anode buffer layer. Sol. Energy Mater. Sol. Cells 2010, 94, 842–845. [Google Scholar] [CrossRef]
- Hou, F.H.; Su, Z.S.; Jin, F.M.; Yan, X.W.; Wang, L.D.; Zhao, H.F.; Zhu, J.Z.; Chu, B.; Li, W.L. Efficient and stable planar heterojunction perovskite solar cells with an MoO3/PEDOT:PSS hole transporting layer. Nanoscale 2015, 7, 9427–9432. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ahn, N.; Jang, M.S.; Lee, J.K.; Yoon, H.; Park, N.G.; Choi, M. Transparent Conductive Oxide-Free Graphene-Based Perovskite Solar Cells with over 17% Efficiency. Adv. Energy Mater. 2016, 6, 1501873. [Google Scholar] [CrossRef]
- Zheng, Q.H.; Li, W.S.; Zhang, Y.; Xu, K.; Xu, J.W.; Wang, H.; Xiong, J.; Zhang, X.Y.; Zhang, X.W. Solution-processed composite interfacial layer of MoOx-doped graphene oxide for robust hole injection in organic light-emitting diode. Phys. Status Solidi RRL 2018, 12, 1700434. [Google Scholar] [CrossRef]
- Kim, D.B.; Yu, J.C.; Nam, Y.S.; Kim, D.W.; Jung, E.D.; Lee, S.Y.; Lee, S.; Park, J.H.; Lee, A.Y.; Lee, B.R.; et al. Improved performance of perovskite light-emitting diodes using a PEDOT:PSS and MoO3 composite layer. J. Mater. Chem. C 2016, 4, 8161–8165. [Google Scholar] [CrossRef]
- Meng, Y.; Ahmadi, M.; Wu, X.Y.; Xu, T.F.; Xu, L.; Xiong, Z.H.; Chen, P. High performance and stable all-inorganic perovskite light emitting diodes by reducing luminescence quenching at PEDOT:PSS/perovskites interface. Org. Electron. 2019, 64, 47–53. [Google Scholar] [CrossRef]
- Feng, Z.Q.; Wang, L.; Yu, H.T.; Ma, X.Q.; Zhang, Q.; Chen, S.F.; Liu, L.H.; Huang, W. Towards efficient perovskite light-emitting diodes: A multi-step spin-coating method for a dense and uniform perovskite film. Org. Electron. 2018, 61, 18–24. [Google Scholar] [CrossRef]
- Qasim, K.; Wang, B.P.; Zhang, Y.P.; Li, P.F.; Wang, Y.S.; Li, S.J.; Lee, S.T.; Liao, L.S.; Lei, W.; Bao, Q.L. Solution-processed extremely efficient multicolor perovskite light-emitting diodes utilizing doped electron transport layer. Adv. Funct. Mater. 2017, 27, 1606874. [Google Scholar] [CrossRef]
- Giebink, N.C.; Forrest, S.R. Quantum efficiency roll-off at high brightness in fluorescent and phosphorescent organic light emitting diodes. Phys. Rev. B 2008, 77, 235215. [Google Scholar] [CrossRef]
- Murawski, C.; Leo, K.; Gather, M.C. Efficiency roll-off in organic light-emitting diodes. Adv. Mater. 2013, 25, 6801–6827. [Google Scholar] [CrossRef]

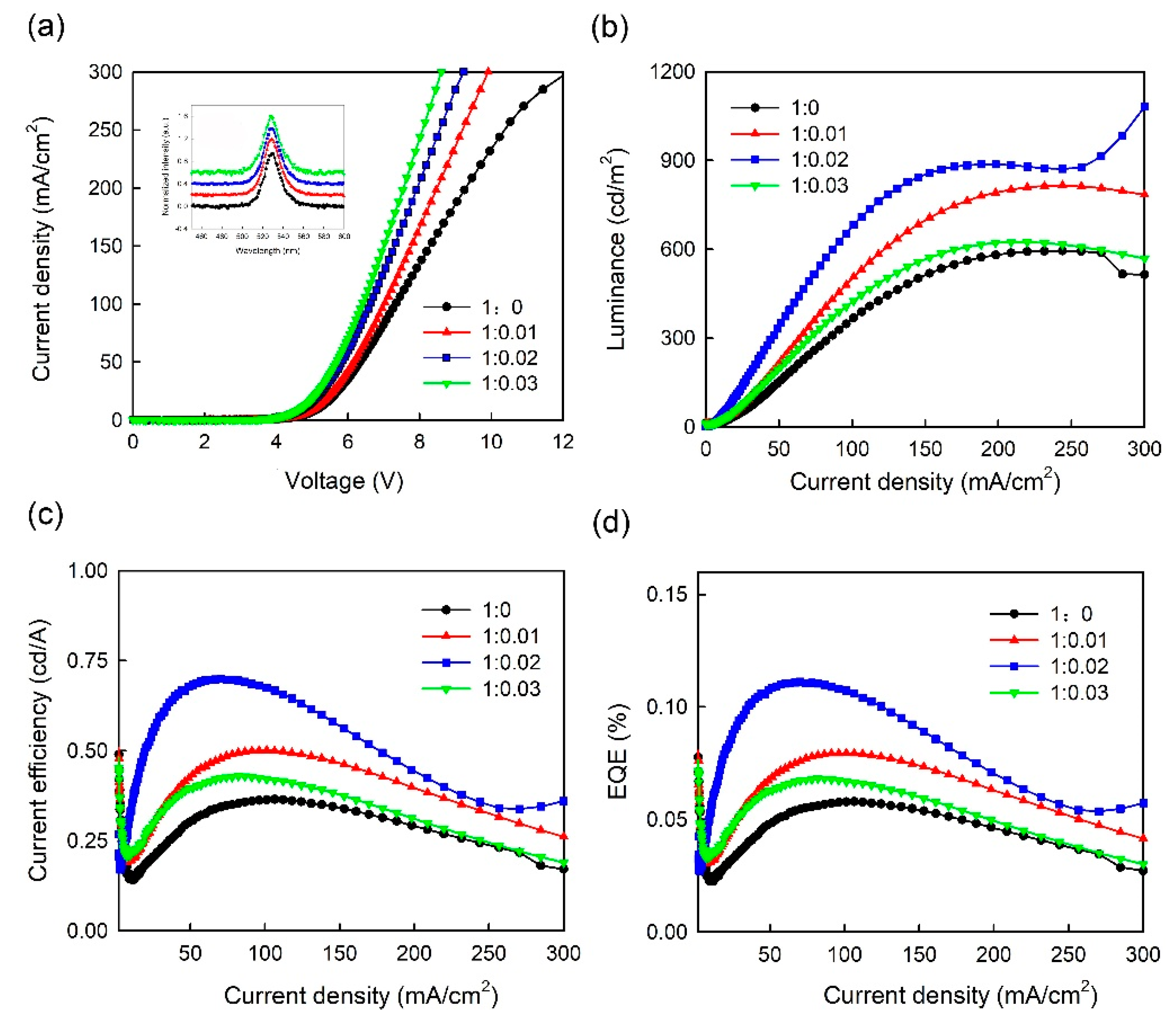
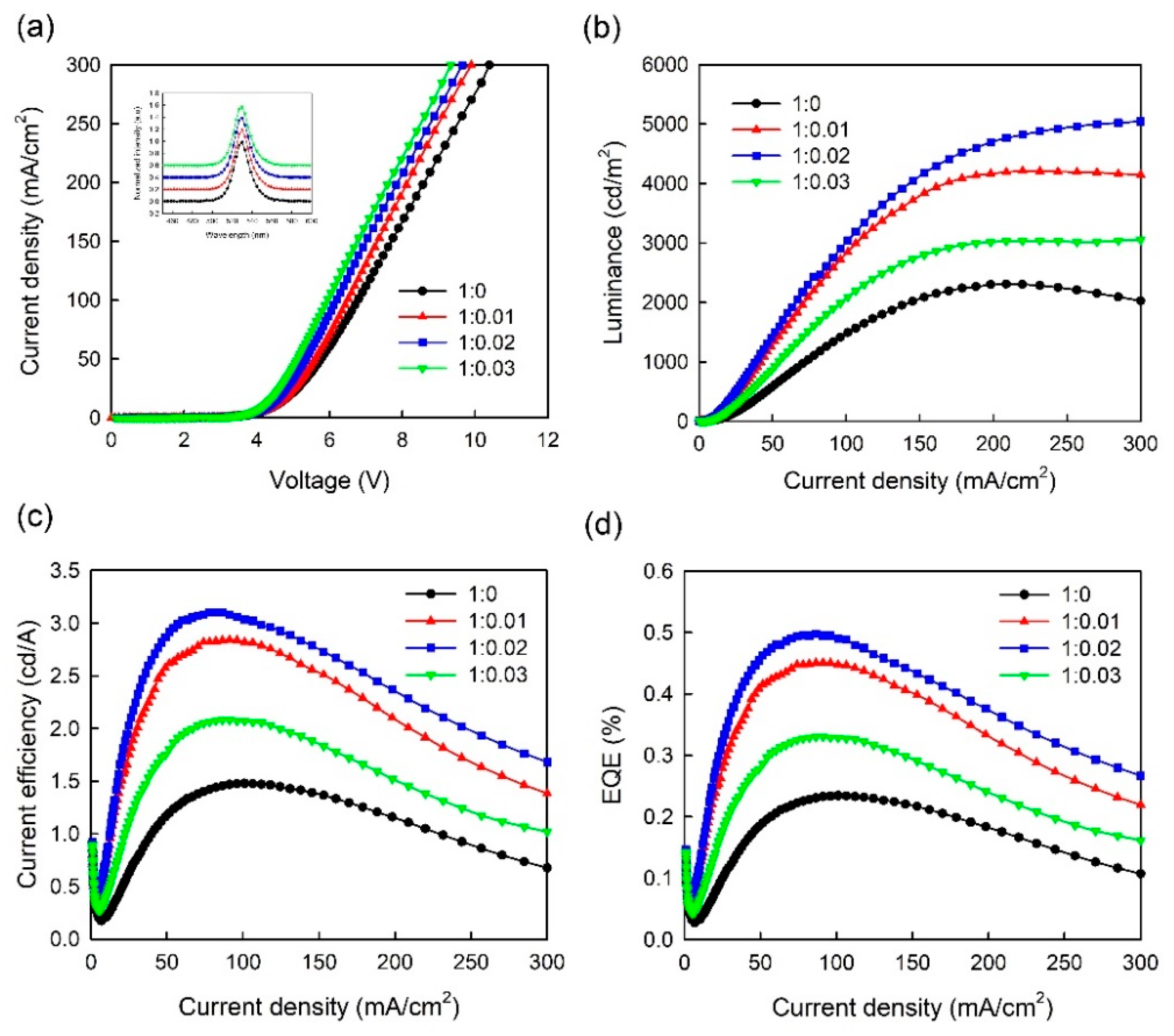
| Volume Ratio of PEDOT:PSS/MoO3-Ammonia | Lmax (cd/m2) | CEmax (cd/A) | EQEmax (%) | Turn-on Voltage (V) |
|---|---|---|---|---|
| 1:0 (S1) | 593 | 0.36 | 0.057 | 4.3 |
| 1:0.01 (S2) | 816 | 0.5 | 0.079 | 4.22 |
| 1:0.02 (S3) | 1082 | 0.7 | 0.11 | 4.18 |
| 1:0.03 (S4) | 624 | 0.42 | 0.068 | 4.14 |
| Volume Ratio of PEDOT:PSS/MoO3-Ammonia | Lmax (cd/m2) | CEmax (cd/A) | EQEmax (%) | Turn-on Voltage (V) |
|---|---|---|---|---|
| 1:0 (T1) | 2309 | 1.47 | 0.23 | 4.08 |
| 1:0.01 (T2) | 4215 | 2.84 | 0.45 | 3.84 |
| 1:0.02 (T3) | 5044 | 3.12 | 0.5 | 3.75 |
| 1:0.03 (T4) | 3055 | 2.08 | 0.33 | 3.68 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Mei, S.; Sun, D.; Liu, N.; Shi, W.; Feng, J.; Mei, F.; Xu, J.; Jiang, Y.; Cao, X. Improved Efficiency of Perovskite Light-Emitting Diodes Using a Three-Step Spin-Coated CH3NH3PbBr3 Emitter and a PEDOT:PSS/MoO3-Ammonia Composite Hole Transport Layer. Micromachines 2019, 10, 459. https://doi.org/10.3390/mi10070459
Zhou Y, Mei S, Sun D, Liu N, Shi W, Feng J, Mei F, Xu J, Jiang Y, Cao X. Improved Efficiency of Perovskite Light-Emitting Diodes Using a Three-Step Spin-Coated CH3NH3PbBr3 Emitter and a PEDOT:PSS/MoO3-Ammonia Composite Hole Transport Layer. Micromachines. 2019; 10(7):459. https://doi.org/10.3390/mi10070459
Chicago/Turabian StyleZhou, Yuanming, Sijiong Mei, Dongwei Sun, Neng Liu, Wuxing Shi, Jiahuan Feng, Fei Mei, Jinxia Xu, Yan Jiang, and Xianan Cao. 2019. "Improved Efficiency of Perovskite Light-Emitting Diodes Using a Three-Step Spin-Coated CH3NH3PbBr3 Emitter and a PEDOT:PSS/MoO3-Ammonia Composite Hole Transport Layer" Micromachines 10, no. 7: 459. https://doi.org/10.3390/mi10070459
APA StyleZhou, Y., Mei, S., Sun, D., Liu, N., Shi, W., Feng, J., Mei, F., Xu, J., Jiang, Y., & Cao, X. (2019). Improved Efficiency of Perovskite Light-Emitting Diodes Using a Three-Step Spin-Coated CH3NH3PbBr3 Emitter and a PEDOT:PSS/MoO3-Ammonia Composite Hole Transport Layer. Micromachines, 10(7), 459. https://doi.org/10.3390/mi10070459




