Femtosecond Laser Micromachining of Soda–Lime Glass in Ambient Air and under Various Aqueous Solutions
Abstract
1. Introduction
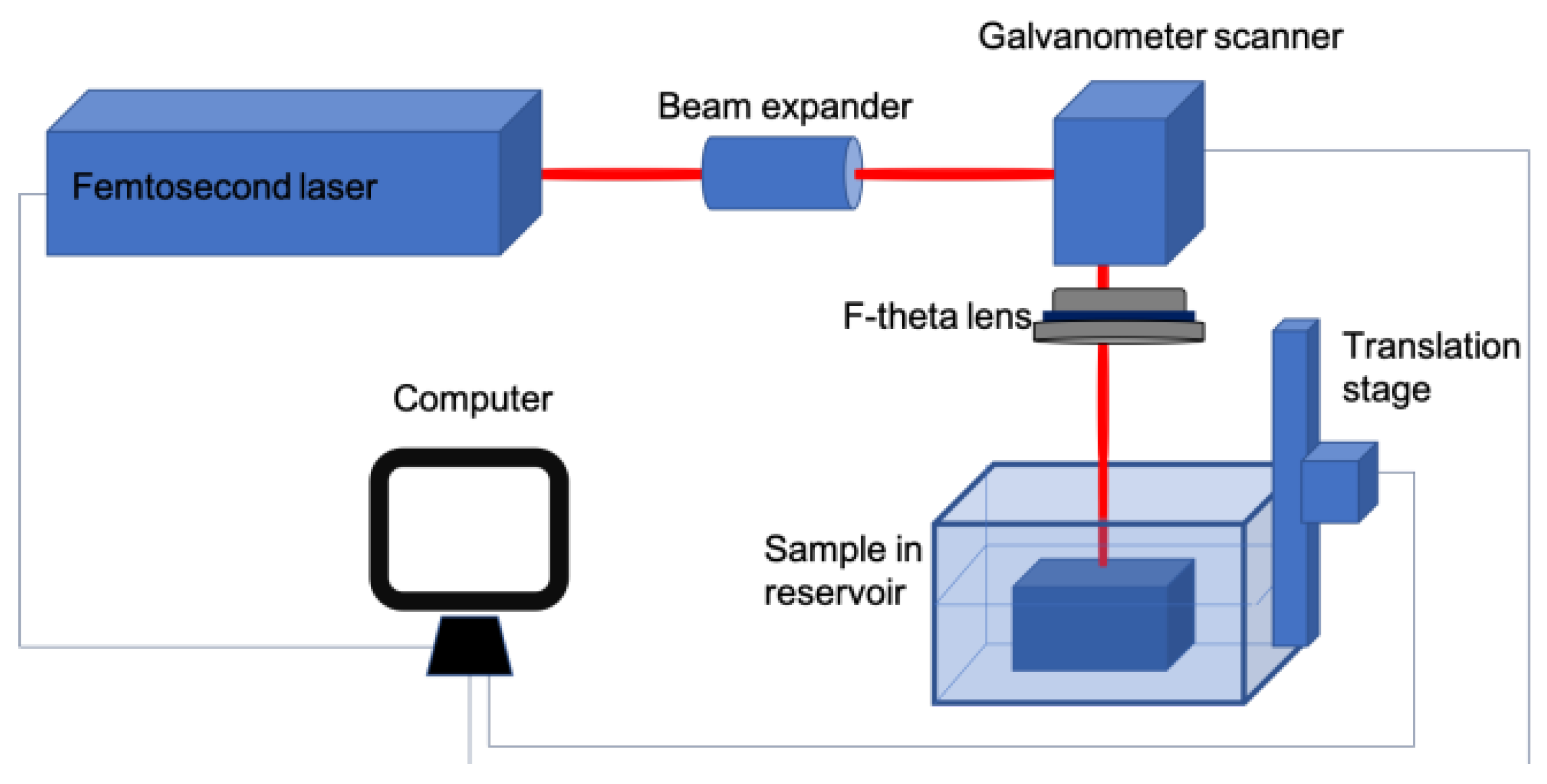
2. Experimental Setup
3. Results and Discussion
3.1. Preparation of Specimens
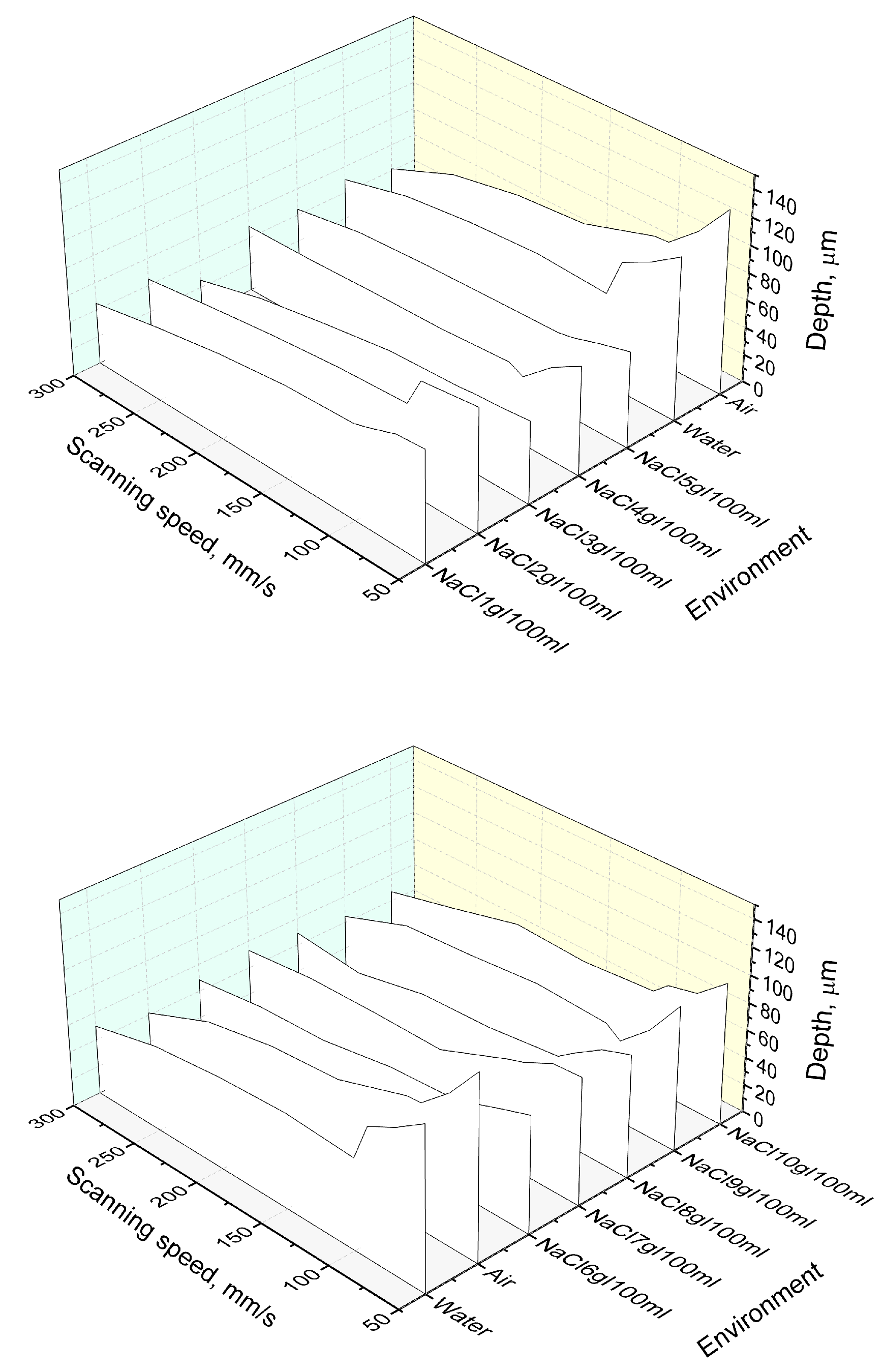
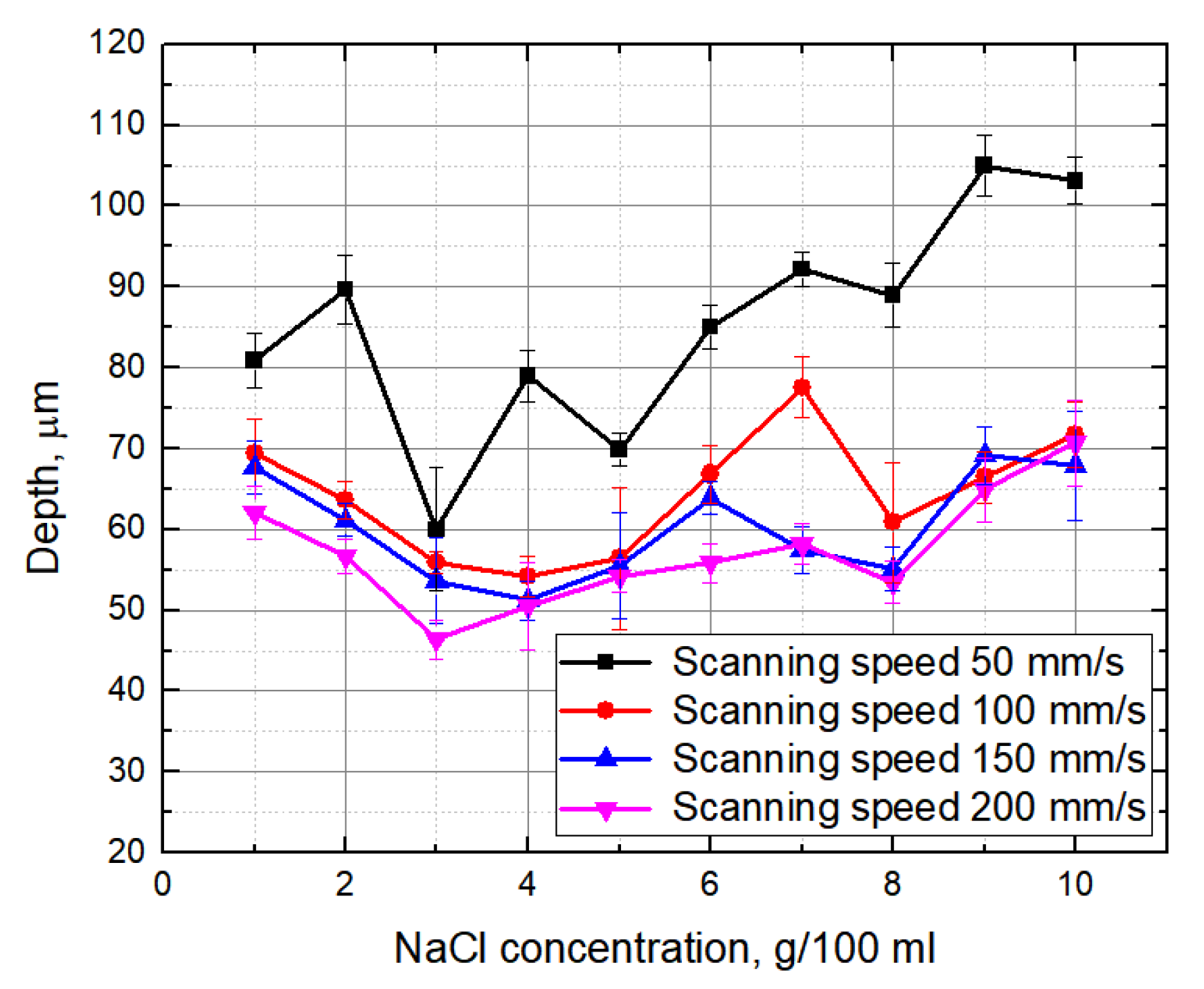
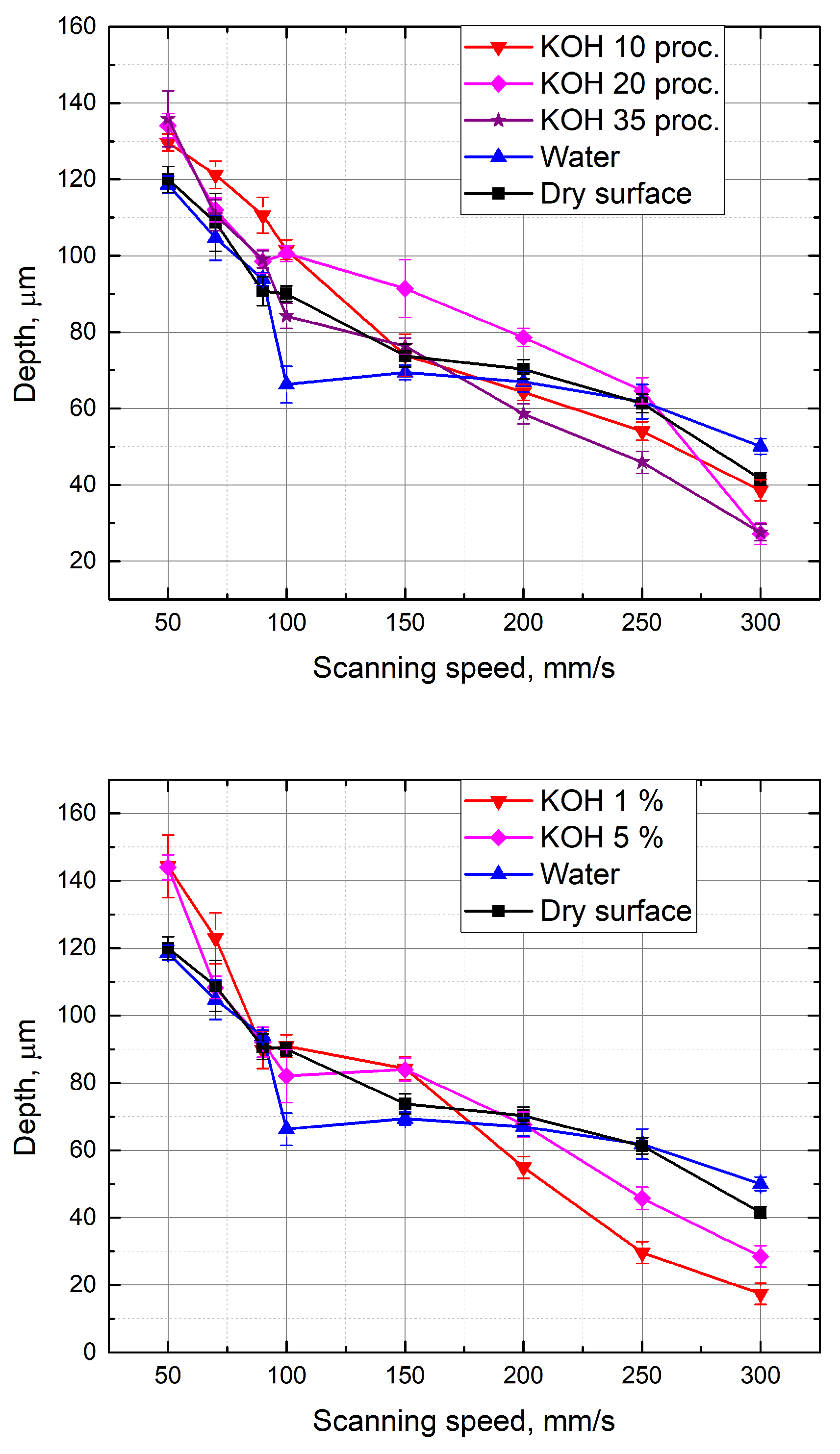
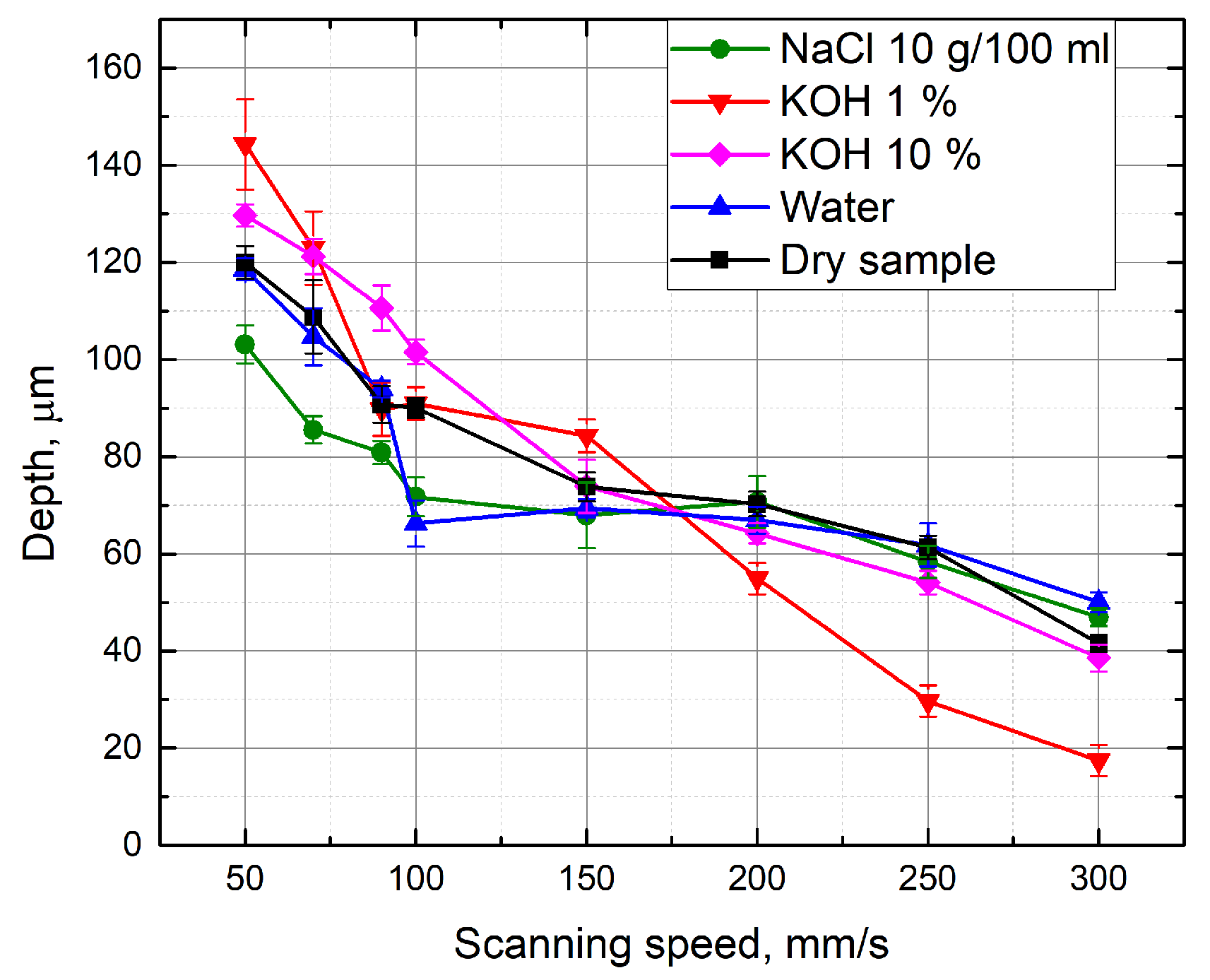
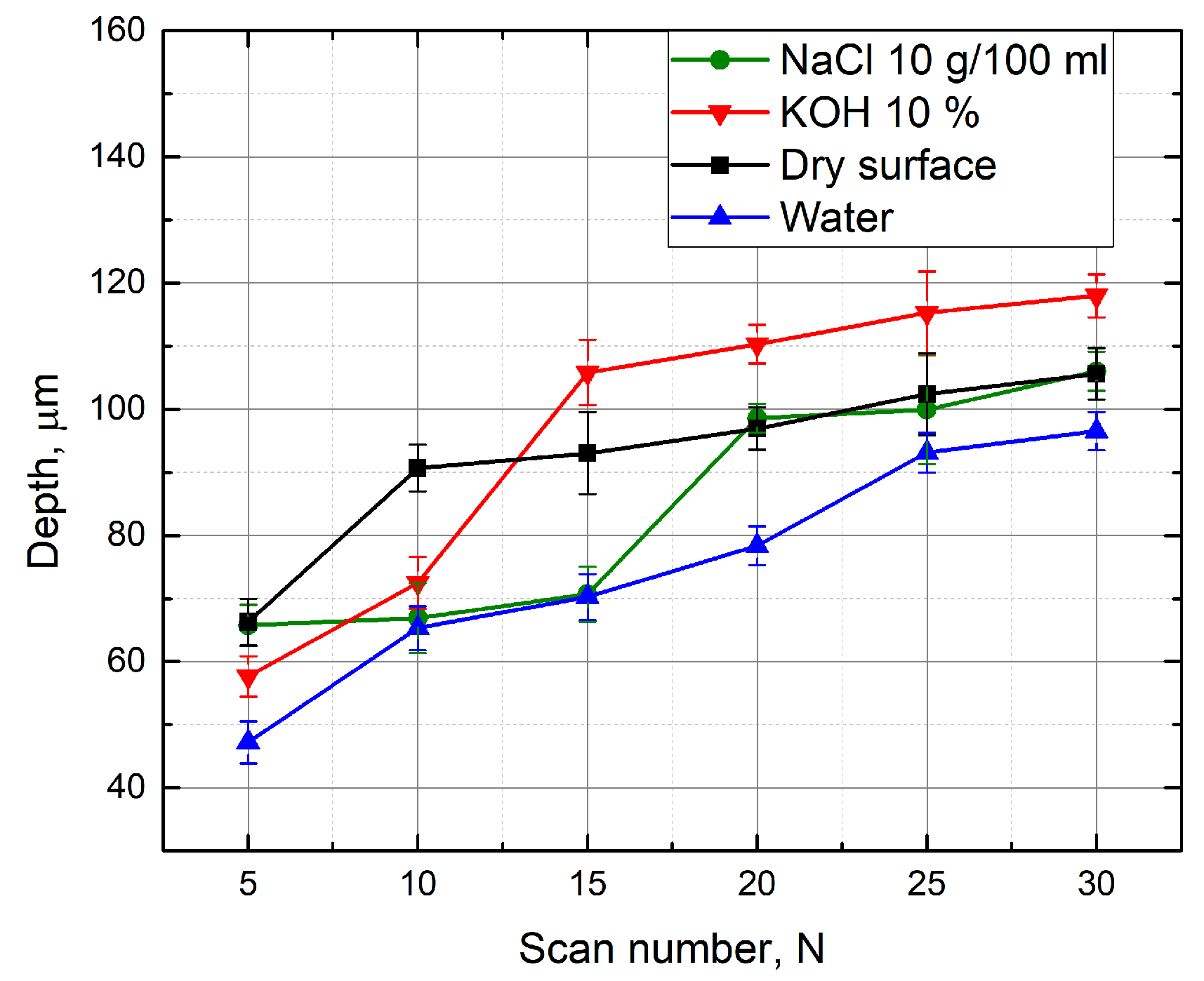
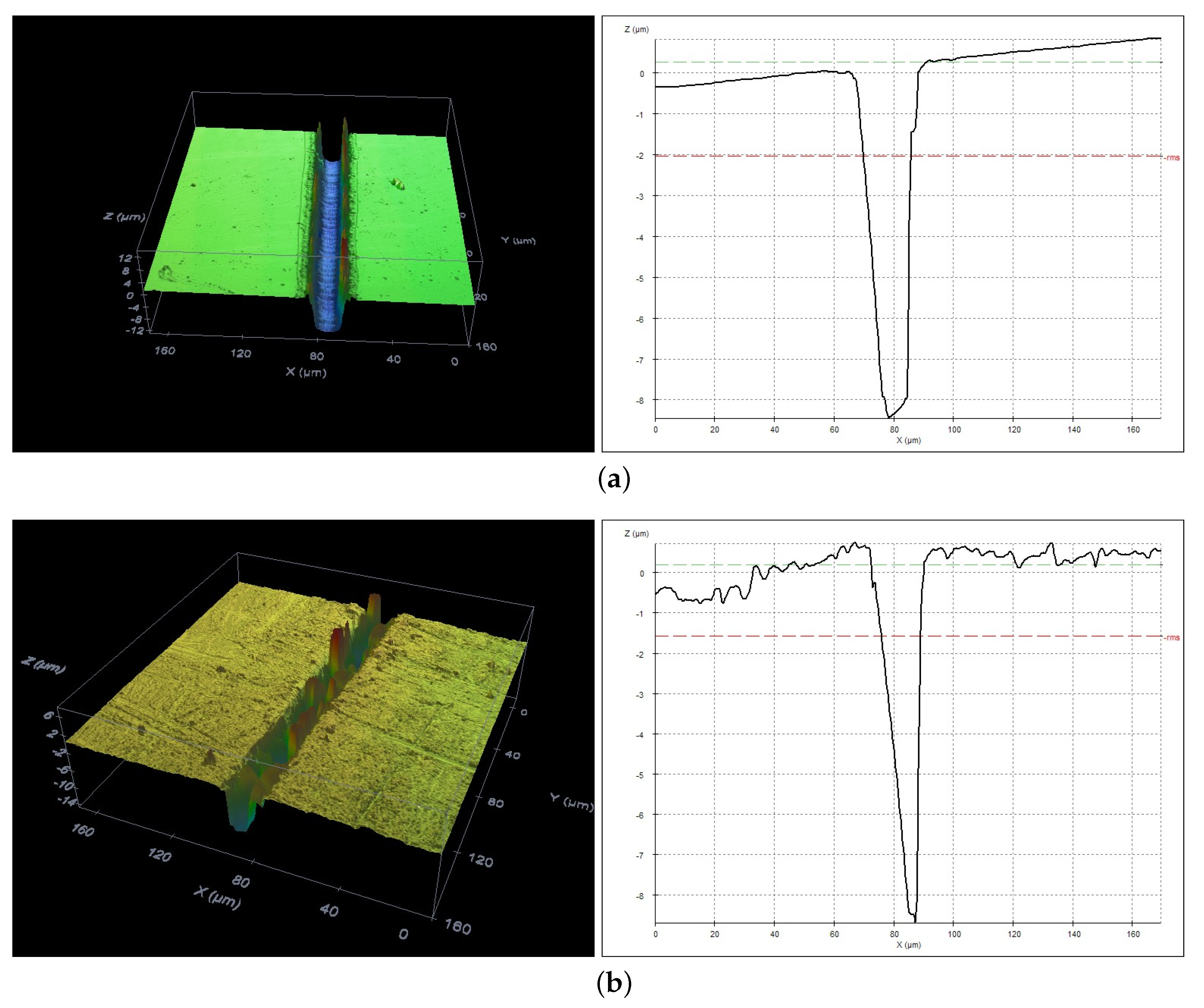
3.2. Evaluation of the Groove Depths
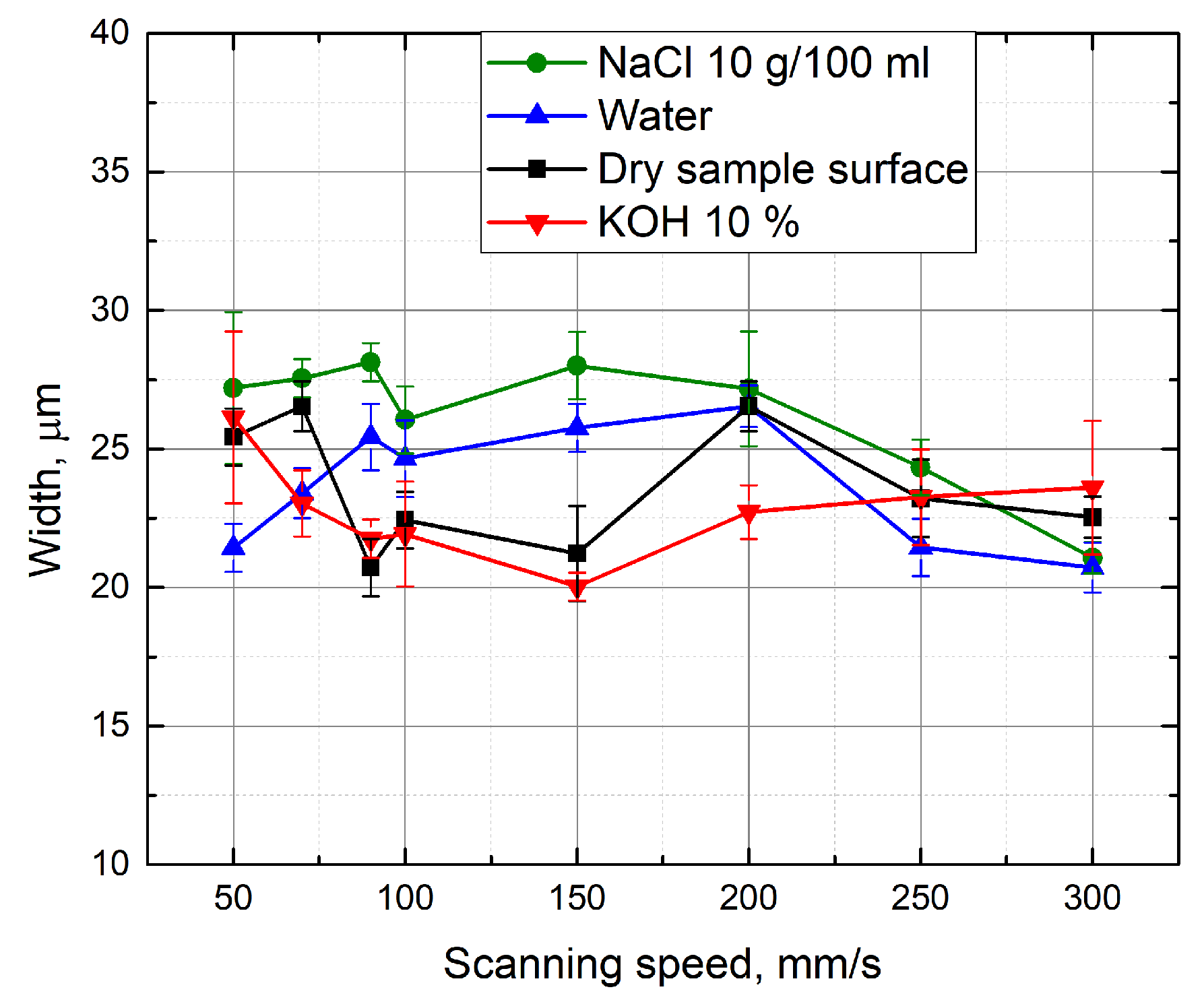
3.3. Evaluation of the Depth-To-Width Ratio of the Formed Grooves
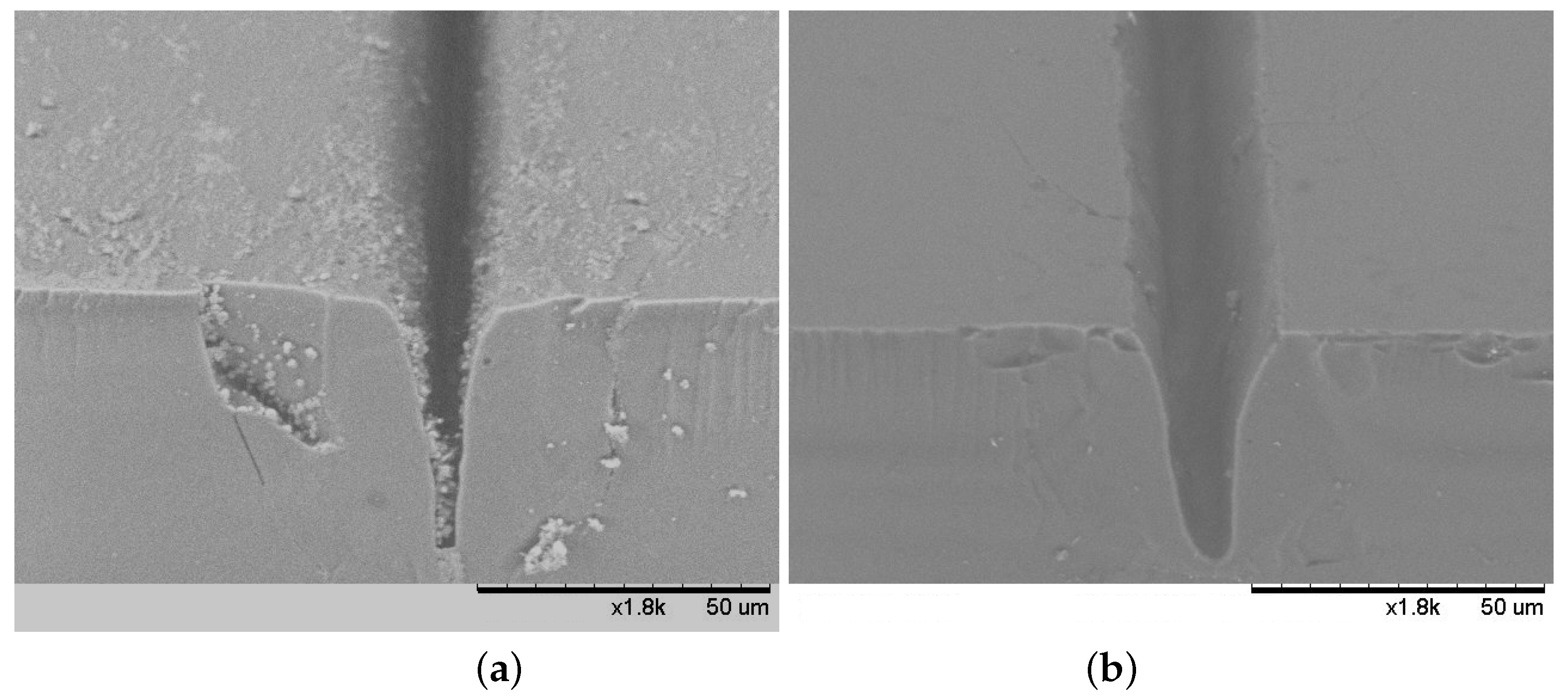
3.4. Evaluation of Grooves Quality
3.5. Comparison of the Presented Techniques with Other Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Momma, C.; Chichkov, B.N.; Nolte, S.; Alvensleben, F.V.; Tuennermann, A.; Welling, H.; Wellegehausen, B. Short-pulse laser ablation of solid targets. Opt. Commun. 1996, 129, 134–142. [Google Scholar] [CrossRef]
- Baersch, N.; Koerber, K.; Ostendorf, A.; Toenshoff, K.H. Ablation and cutting of planar silicon devices using femtosecond laser pulses. Appl. Phys. A 2003, 77, 237–242. [Google Scholar]
- Nakata, Y.; Okada, T.; Maeda, M. Fabrication of dot matrix, comb, and nanowire structures using laser ablation by interfered femtosecond laser beams. Appl. Phys. Lett. 2002, 81, 4239–4241. [Google Scholar] [CrossRef]
- Nolte, S.; Schrempel, F.; Dausinger, F. Ultrashort Pulse Laser Technology. In Laser Sources and Applications; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Sundaram, S.K.; Mazur, E. Inducing and probing non-thermal transitions in semiconductors using femtosecond laser pulses. Nat. Mater. 2002, 1, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Watanabe, W.; Nolte, S.; Schaffer, C.B. Ultrafast processes for bulk modification of transparent materials. MRS Bull. 2006, 31, 620–625. [Google Scholar] [CrossRef]
- Gattass, R.R.; Mazur, E. Femtosecond laser micromachining in transparent materials. Nat. Photon. 2008, 2, 219–225. [Google Scholar] [CrossRef]
- Shimotsuma, Y.; Hirao, K.; Kazansky, P.G.; Qiu, J. Three-dimensional micro- and nano-fabrication in transparent materials by femtosecond laser. Jpn. J. Appl. Phys. 2005, 44, 4735. [Google Scholar] [CrossRef]
- Della Valle, G.; Osellame, R.; Laporta, P. Micromachining of photonic devices by femtosecond laser pulses. J. Opt. A Pure Appl. Opt. 2009, 11, 013001. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Ultrafast lasers—reliable tools for advanced materials processing. Light Sci. Appl. 2014, 3, e149. [Google Scholar] [CrossRef]
- Kruusing, A. Handbook of Liquids—Assisted Laser Processing; Elsevier Ltd.: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Charee, W.; Tangwarodomnukun, V. Dynamic features of bubble induced by a nanosecond pulse laser in still and flowing water. Opt. Laser Technol. 2018, 100, 230–243. [Google Scholar] [CrossRef]
- Ren, J.; Kelly, M.; Hesselink, L. Laser ablation of silicon in water with nanosecond and femtosecond pulses. Opt. Lett. 2005, 30, 1740–1742. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Shiliang, Q.; Zhongyi, G. Fabrication of microfluidic devices in silica glass by water-assisted ablation with femtosecond laser pulses. J. Micromech. Microeng. 2011, 21, 075008. [Google Scholar] [CrossRef]
- Saxena, I.; Ehmann, K.; Cao, I. Productivity Enhancement in Laser Induced Plasma Micromachining by altering the Salinity of Dielectric Media. ICOMM-2014 2014, 93, 1–6. [Google Scholar]
- Luong, K.P.; Tanable, R.; Ito, Y. Machining on Rear Surface of Silicon Substrate by an Infrared Femtosecond Laser via Non-linear Absorption Processes. Procedia CIRP 2016, 42, 73–76. [Google Scholar] [CrossRef]
- Li, L.; Achara, C. Chemical Assisted Laser Machining for Minimisation of Recast and Heat Affected Zone. CIRP Ann. Manuf. Technol. 2004, 53, 175–178. [Google Scholar] [CrossRef]
- Muhammad, N.; Li, L. Underwater femtosecond laser micromachining of thin nitinol tubes for medical coronary stent manufacture. App. Phys. A 2012, 107, 849–861. [Google Scholar] [CrossRef]
- Kruusing, A. Underwater and water-assisted laser processing: Part 2—Etching, cutting and rarely used methods. Opt. Lasers Eng. 2004, 41, 329–352. [Google Scholar] [CrossRef]
- Sakka, T.; Iwanaga, S.; Ogata, Y.H.; Matsunawa, A.; Takemoto, T. Laser ablation at solid–liquid interfaces: An approach from optical emission spectra. J. Chem. Phys. 2000, 112, 8645–8653. [Google Scholar] [CrossRef]
- Shafeev, G.A.; Simakhin, A.V. Spatially confined laser-induced damage of Si under a liquid layer. Appl. Phys. A 1992, 54, 311–316. [Google Scholar] [CrossRef]
- Baskevicius, A.; Balachninaite, O.; Karpavicius, M.; Butkus, S.; Paipulas, D.; Sirutkaitis, V. Monitoring of the Femtosecond Laser Micromachining Process of Materials Immersed in Water by Use of Laser-Induced Breakdown Spectroscopy. J. Laser Micro/Nanoeng. 2016, 11, 381. [Google Scholar] [CrossRef]
- Ahmmed, K.M.T. Colin Grambow and Anne-Marie Kietzig, Fabrication of Micro/Nano Structures on Metals by Femtosecond Laser Micromachining. Micromachines 2014, 5, 1219–1253. [Google Scholar] [CrossRef]
- Von Gutfeld, R.J.; Hodgson, R.T. Laser enhanced etching in KOH. Appl. Phys. Lett. 1982, 40, 352. [Google Scholar] [CrossRef]
- Nowak, R.; Metev, S. Thermochemical laser etching of stainless steel and titanium in liquids. Appl. Phys. A 1996, 63, 133–138. [Google Scholar] [CrossRef]
- Shiby, S.; Nammi, S.; Vasa, N.J.; Krishnan, S. Pulsed laser micro-scribing of copper thin films on polyimide substrate in NaCl solution. Proc. SPIE 10520 2018. [Google Scholar] [CrossRef]
- Füle, M.; Gárdián, A.; Csontos, J.; Budai, J.; Tóth, Z. Ti:sapphire Laser Ablation of Silicon in Different Ambients. JLMN-J. Laser Micro/Nanoeng. 2014, 9, 119–125. [Google Scholar] [CrossRef]
- Cao, X.-W.; Chen, Q.-D.; Fan, H.; Zhang, L.; Juodkazis, S.; Sun, H.-B. Liquid-Assisted Femtosecond Laser Precision-Machining of Silica. Nanomaterials 2018, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Kanitz, A.; Hoppius, J.S.; Gurevich, E.L.; Ostendorf, A. Influence of the Liquid on Femtosecond Laser Ablation of Iron. Phys. Procedia 2016, 83, 114–122. [Google Scholar] [CrossRef]
- Hoppius, J.S.; Maragkaki, S.; Kanitz, A.; Gregorčič, P.; Gurevich, E.L. Optimization of femtosecond laser processing in liquids. Appl. Surface Sci. 2019, 467–468, 255–260. [Google Scholar] [CrossRef]
- Li, C.; Shi, X.; Si, J.; Chen, T.; Chen, F.; Liang, S.; Wu, Z.; Hou, X. Alcohol-assisted photoetching of silicon carbide with a femtosecond laser. Opt. Commun. 2009, 282, 78–80. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mačernytė, L.; Skruibis, J.; Vaičaitis, V.; Sirutkaitis, R.; Balachninaitė, O. Femtosecond Laser Micromachining of Soda–Lime Glass in Ambient Air and under Various Aqueous Solutions. Micromachines 2019, 10, 354. https://doi.org/10.3390/mi10060354
Mačernytė L, Skruibis J, Vaičaitis V, Sirutkaitis R, Balachninaitė O. Femtosecond Laser Micromachining of Soda–Lime Glass in Ambient Air and under Various Aqueous Solutions. Micromachines. 2019; 10(6):354. https://doi.org/10.3390/mi10060354
Chicago/Turabian StyleMačernytė, Lina, Julius Skruibis, Virgilijus Vaičaitis, Romualdas Sirutkaitis, and Ona Balachninaitė. 2019. "Femtosecond Laser Micromachining of Soda–Lime Glass in Ambient Air and under Various Aqueous Solutions" Micromachines 10, no. 6: 354. https://doi.org/10.3390/mi10060354
APA StyleMačernytė, L., Skruibis, J., Vaičaitis, V., Sirutkaitis, R., & Balachninaitė, O. (2019). Femtosecond Laser Micromachining of Soda–Lime Glass in Ambient Air and under Various Aqueous Solutions. Micromachines, 10(6), 354. https://doi.org/10.3390/mi10060354




