Robotic Micropipette Aspiration for Multiple Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Key Techniques
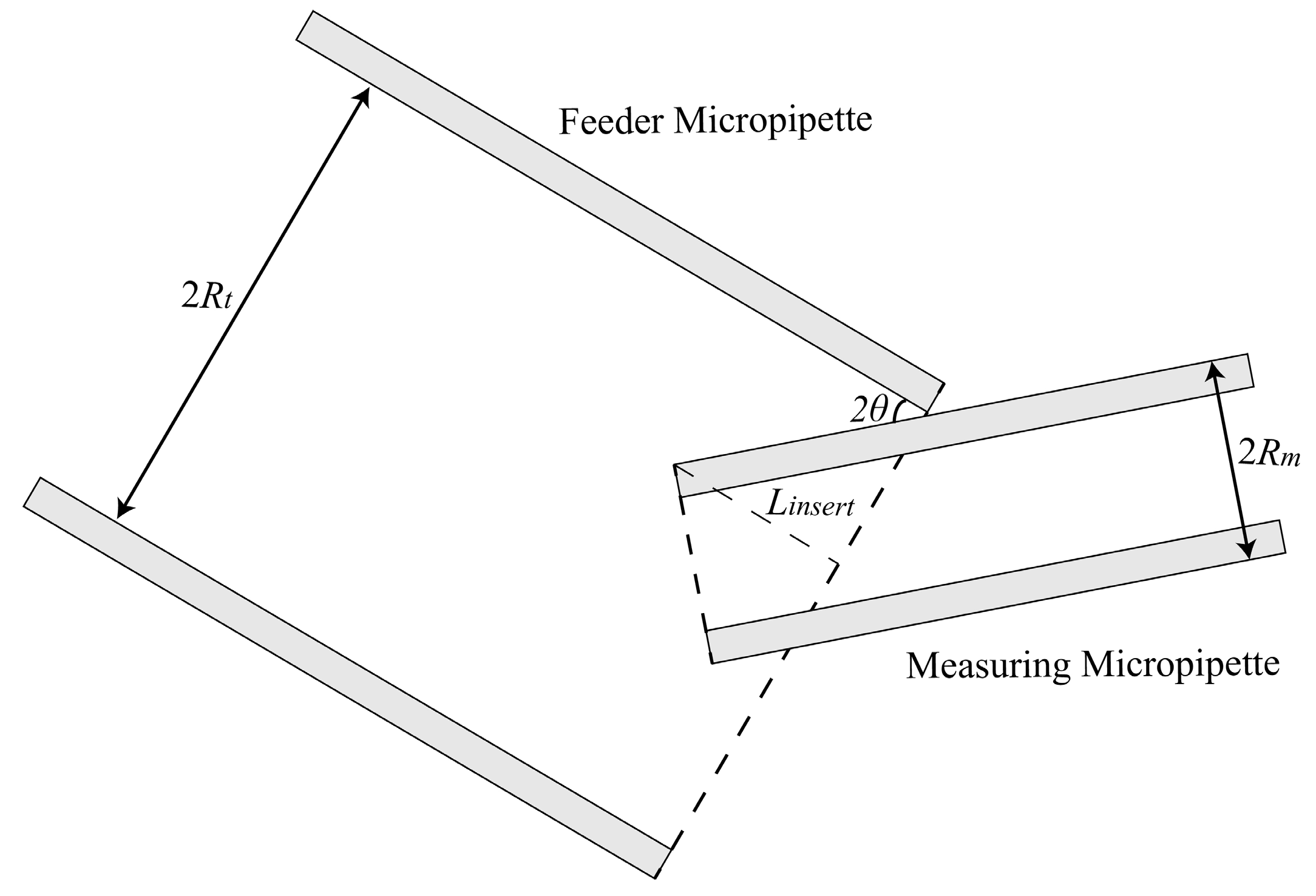
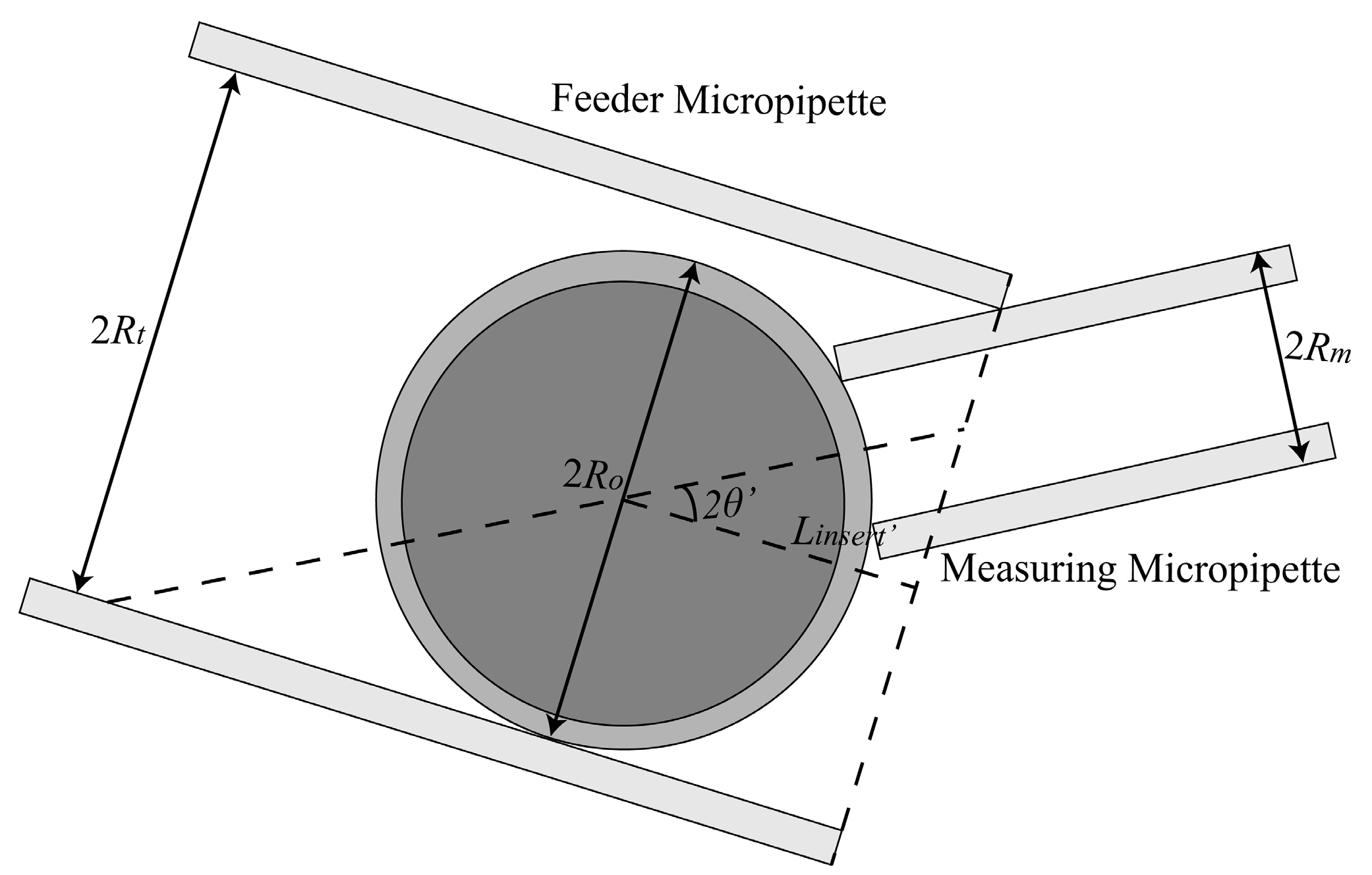
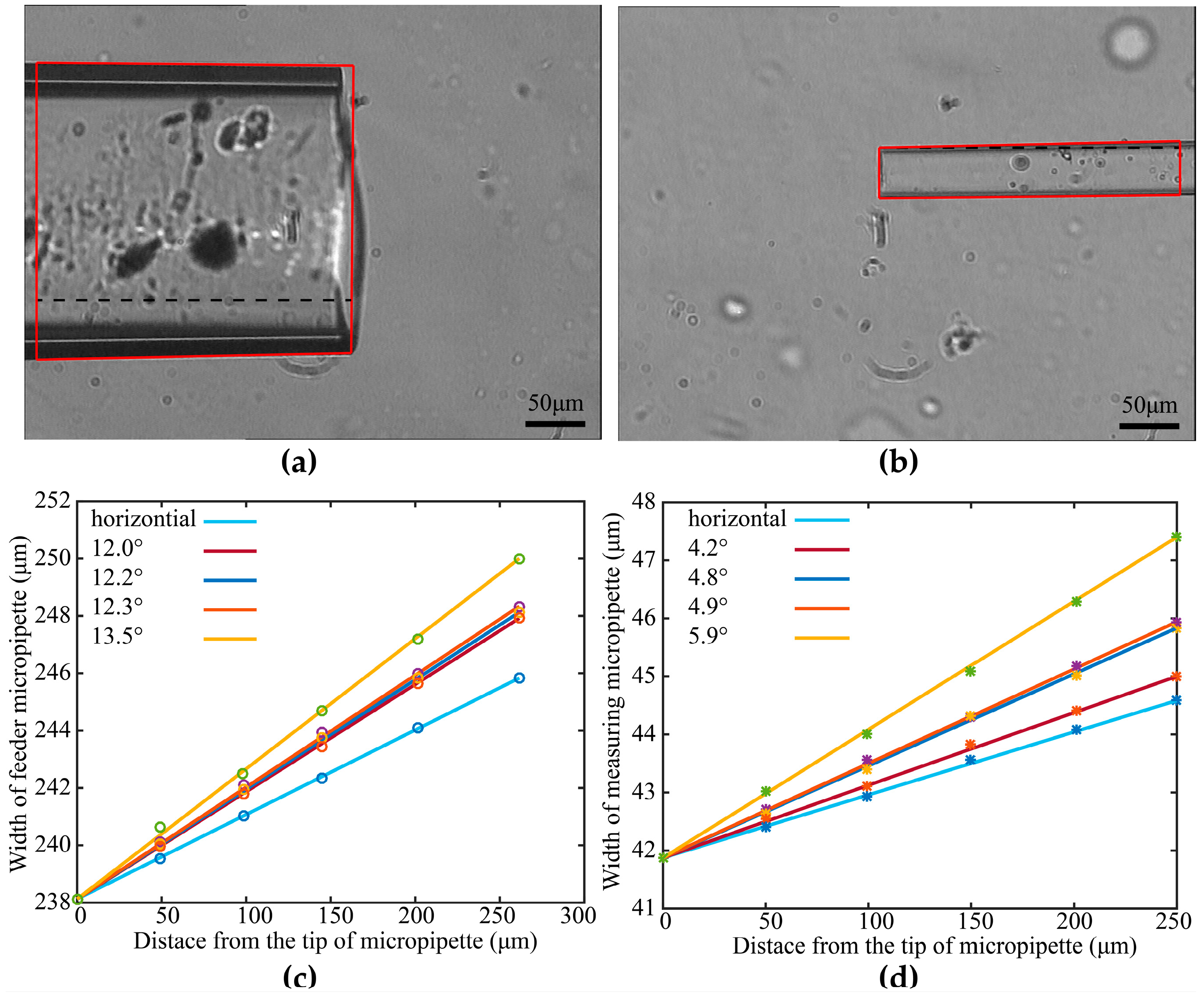
2.1.1. Maximum Permissible Tilt Angle Determination for Measuring Micropipette (MM) and Feeder Micropipette (FM)
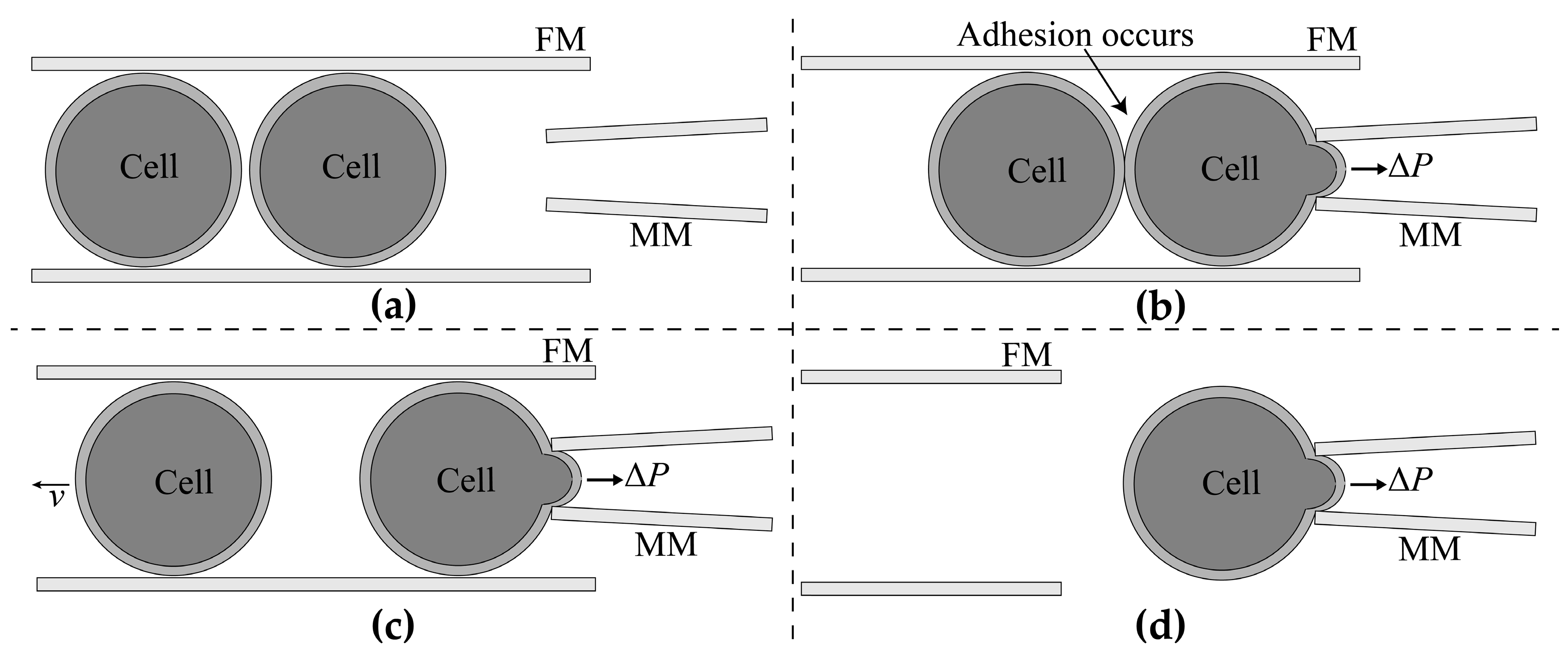
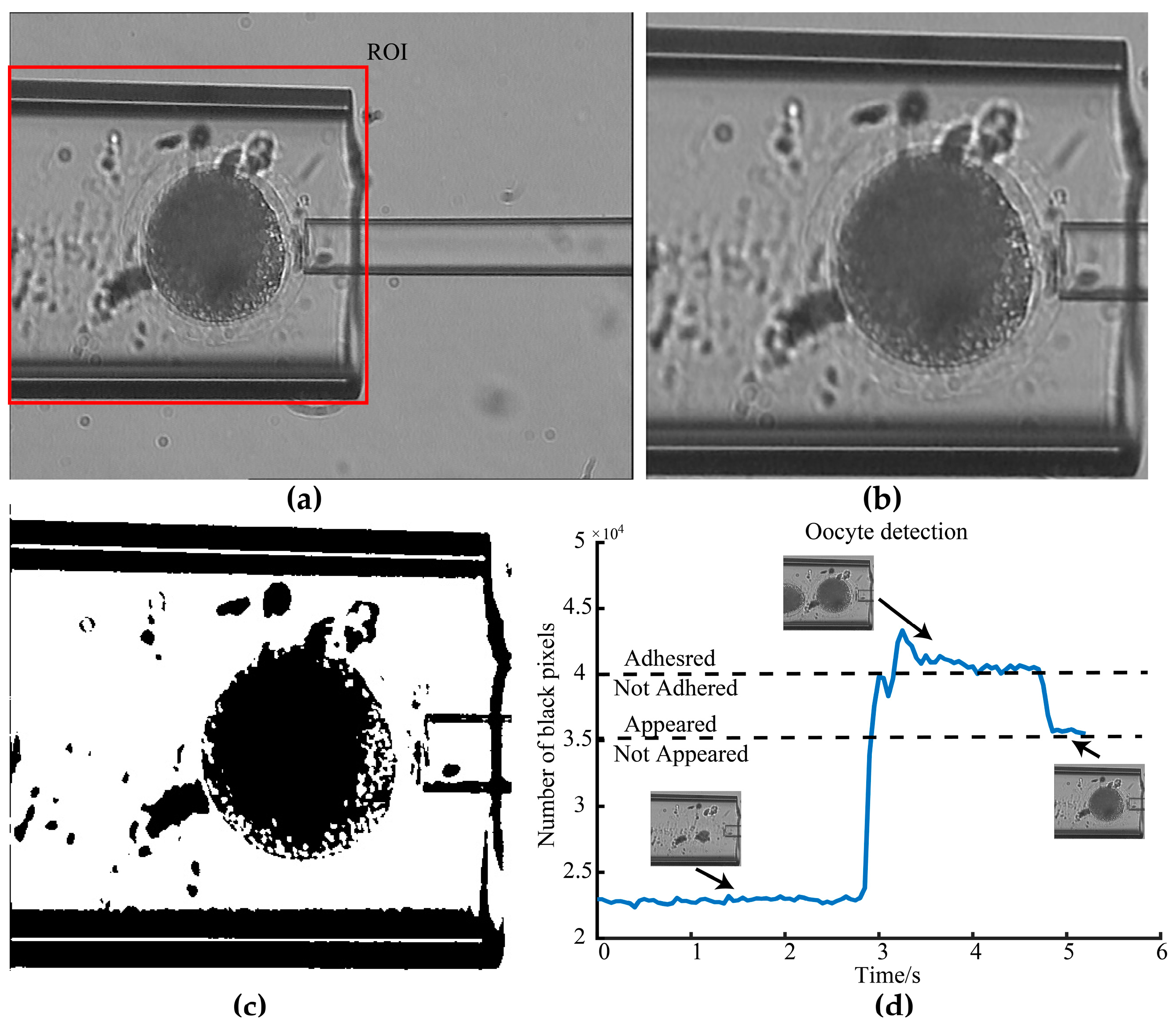
2.1.2. Adhesion Detection
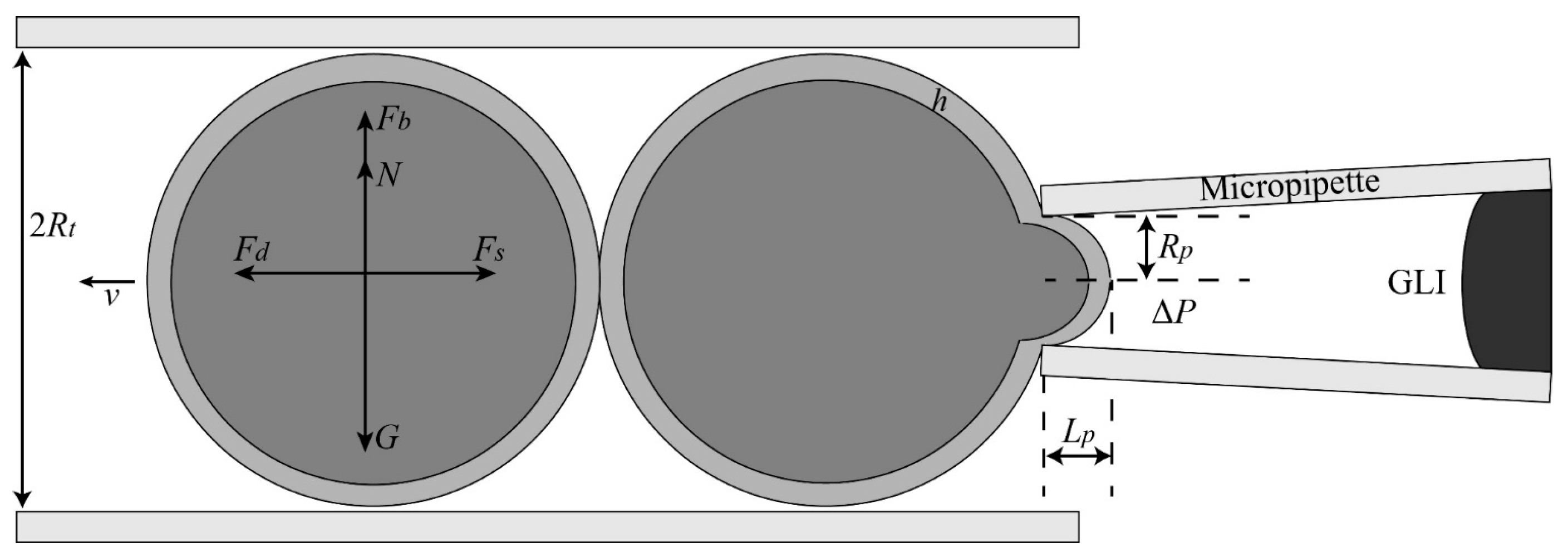
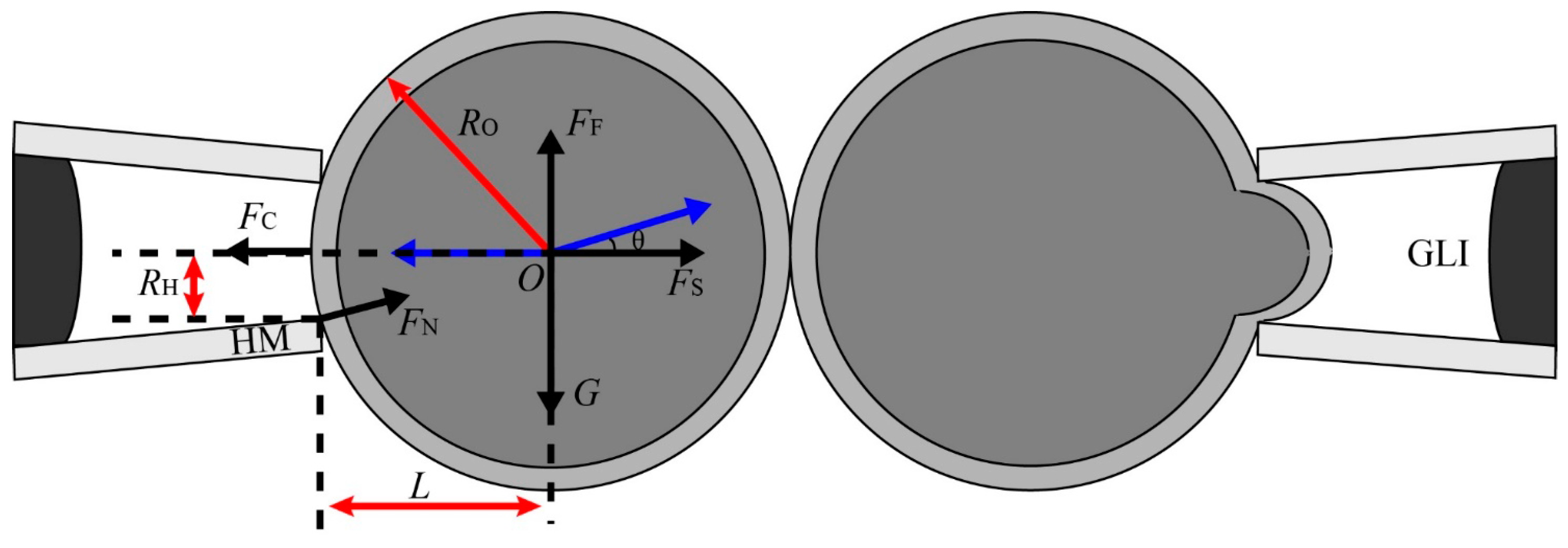
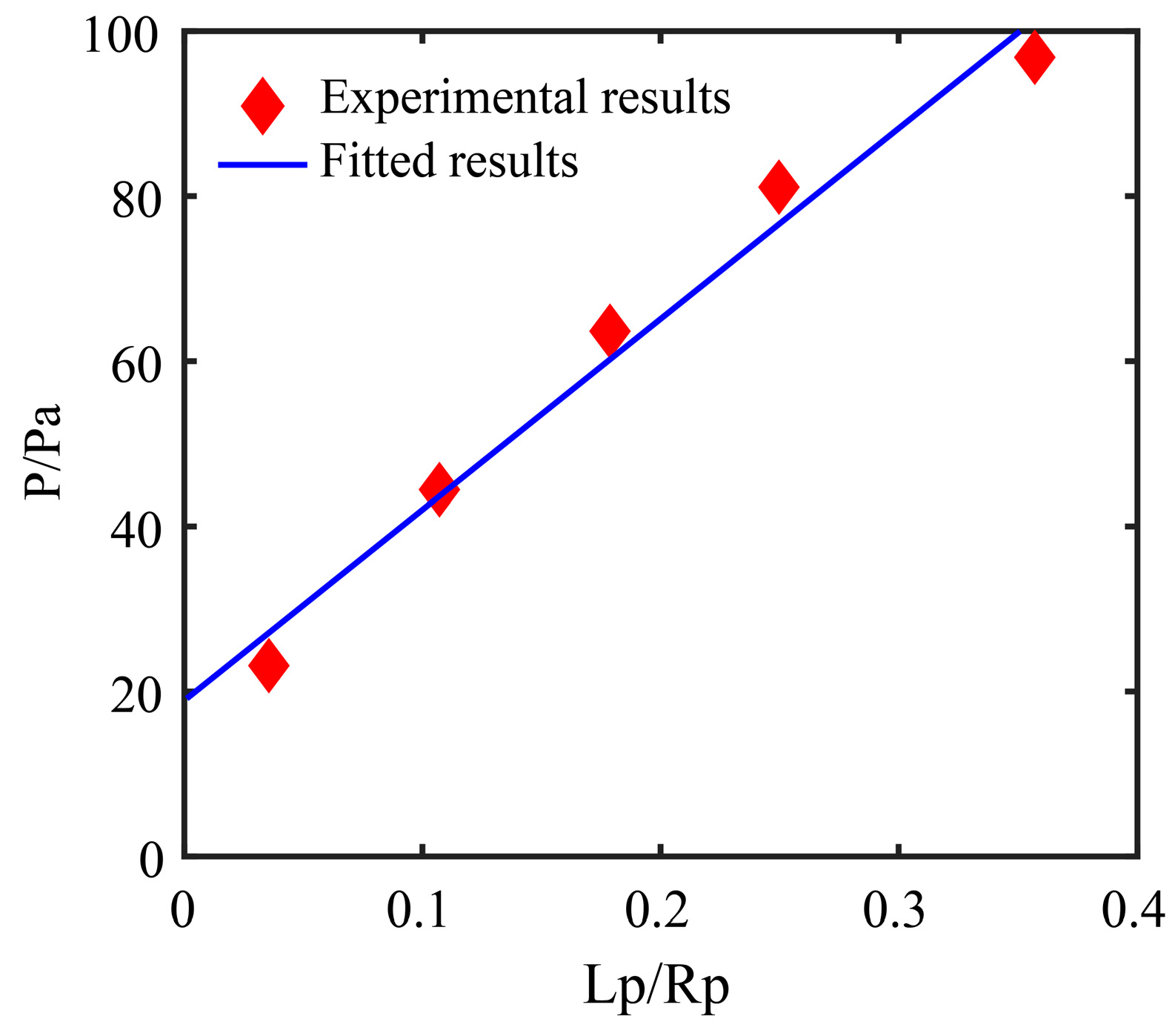
2.1.3. Aspiration Pressure to Break Cell Adhesion
2.1.4. Automated Aspiration of Cell
2.1.5. Oocytes Preparation
2.2. Robotic MA Process for Batch Cells
- Collect multiple oocytes into the FM.
- Insert MM into the FM automatically.
- Apply positive pressure in the FM until the oocyte is delivered to the microscopic field.
- Hold the oocyte with negative pressure in the MM.
- Draw back the adhesive oocytes with the calculated negative pressure in the FM and withdraw the FM.
- Measure the Young’s modulus of ZP by MM.
- Move MM to the “Measured Area”. Release the measured oocyte with positive pressure in MM.
- Move the MM back and move the FM back.
- Repeat steps 2–7 if there are oocytes to be measured, otherwise end the measurement process.
2.3. Statistical Analysis
2.4. Ethical Statement
3. Results
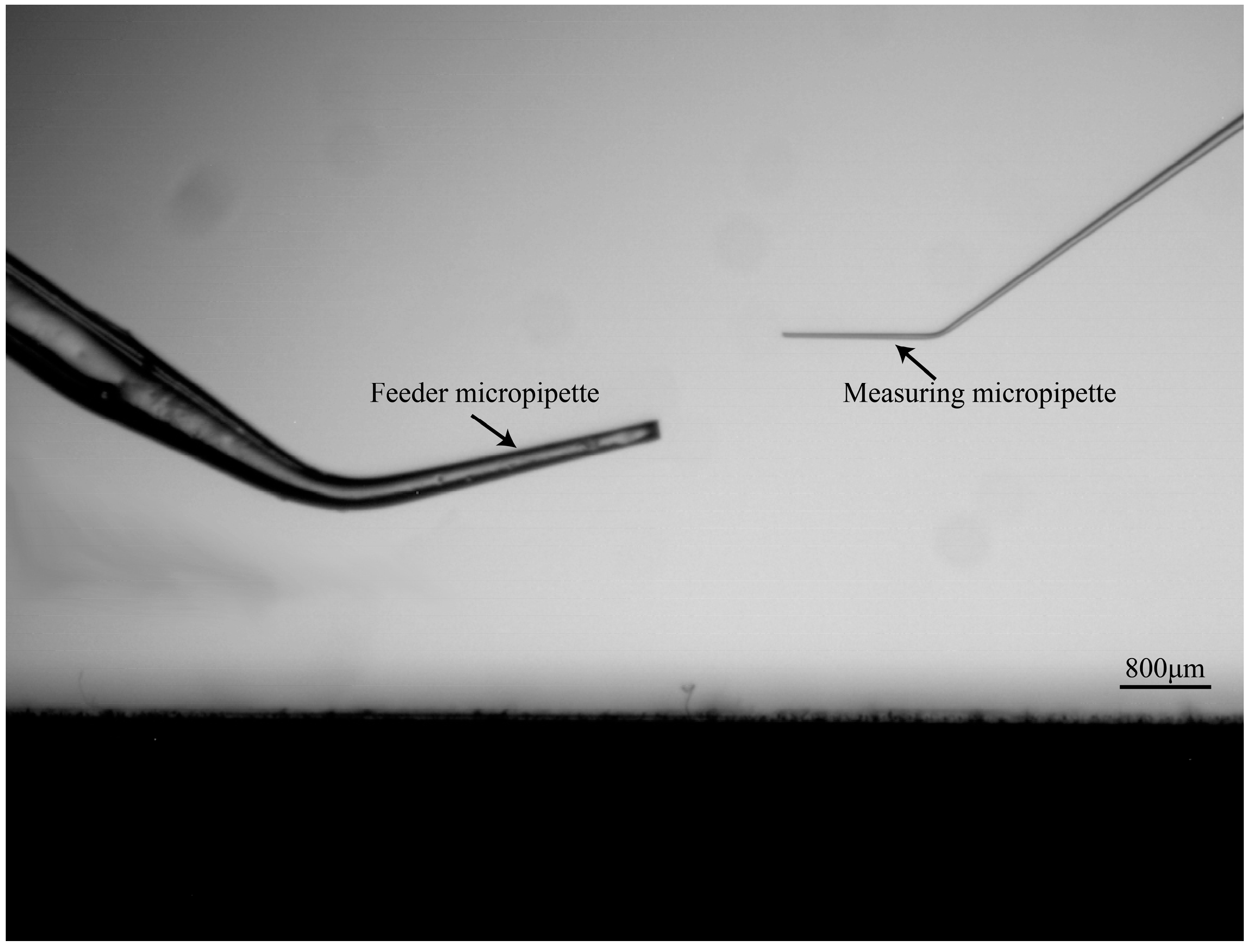
3.1. System Setup
3.2. Drawing Back Pressure Calculation
3.3. Young’s Modulus Detection Results
3.4. Oocyte Elasticity under Different Culture Environments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pu, H.; Liu, N.; Yu, J.; Yang, Y.; Sun, Y.; Peng, Y.; Xie, S.; Luo, J.; Sun, Y. Micropipette aspiration of single cells for both mechanical and electrical characterization. IEEE Trans. Biomed. Eng. 2019. [Google Scholar] [CrossRef] [PubMed]
- Faria, E.C.; Ma, N.; Gazi, E.; Gardner, P.; Brown, M.; Clarke, N.W.; Snook, R.D. Measurement of elastic properties of prostate cancer cells using AFM. Analyst 2008, 133, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Mater. 2007, 55, 3989–4014. [Google Scholar] [CrossRef]
- Cross, S.E.; Jin, Y.-S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Kol, N.; Gladnikoff, M.; Barlam, D.; Shneck, R.Z.; Rein, A.; Rousso, I. Mechanical Properties of Murine Leukemia Virus Particles: Effect of Maturation. Biophys. J. 2006, 91, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Shojaei-Baghini, E.; Zheng, Y.; Sun, Y. Automated micropipette aspiration of single cells. Ann. Biomed. Eng. 2013, 41, 1208–1216. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, M.; Cui, M.; Qin, Y.; Yu, J.; Sun, M.; Zhao, X.; Feng, X. A novel pneumatic micropipette aspiration method using a balance pressure model. Rev. Sci. Instrum. 2013, 84, 123703. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Feng, Z.; Wang, L.; Li, H.; Wang, H.; Xu, D.; Zhao, X.; Feng, D.; Feng, X. Resveratrol reverses the adverse effects of a diet-induced obese murine model on oocyte quality and zona pellucida softening. Food Funct. 2018, 9, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, J.; Zong, Z.; Wan, K.-T.; Sun, Y. Elastic and viscoelastic characterization of mouse oocytes using micropipette indentation. Ann. Biomed. Eng. 2012, 40, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Murayama, Y.; Mizuno, J.; Kamakura, H.; Fueta, Y.; Nakamura, H.; Akaishi, K.; Anzai, K.; Wantanabe, A.; Inui, H.; Omata, S. Mouse zona pellucida dynamically changes its elasticity during oocyte maturation, fertilization and early embryo development. Hum. Cell 2006, 19, 119–125. [Google Scholar] [CrossRef]
- Waldenstrom, U.; Engstrom, A.-B.; Hellberg, D.; Nilsson, S. Low-oxygen compared with high-oxygen atmosphere in blastocyst culture, a prospective randomized study. Fertil. Steril. 2009, 91, 2461–2465. [Google Scholar] [CrossRef]
- Lourens, A.; van den Brand, H.; Meijerhof, R.; Kemp, B. Effect of eggshell temperature during incubation on embryo development, hatchability, and posthatch development. Poult. Sci. 2005, 84, 914–920. [Google Scholar] [CrossRef]
- Popova, E.; Bader, M.; Krivokharchenko, A. Effect of Culture Conditions on Viability of Mouse and Rat Embryos Developed in Vitro. Genes 2011, 2, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Bow, H.; Pivkin, I.V.; Diez-Silva, M.; Goldfless, S.J.; Dao, M.; Niles, J.C.; Suresh, S.; Han, J. A microfabricated deformability-based flow cytometer with application to malaria. Lab. Chip 2011, 11, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Rosenbluth, M.J.; Lam, W.A.; Fletcher, D.A. Analyzing cell mechanics in hematologic diseases with microfluidic biophysical flow cytometry. Lab. Chip 2008, 8, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Shevkoplyas, S.S.; Yoshida, T.; Munn, L.L.; Bitensky, M.W. Biomimetic autoseparation of leukocytes from whole blood in a microfluidic device. Anal. Chem. 2005, 77, 933–937. [Google Scholar] [CrossRef]
- Vichare, S.; Sen, S.; Inamdar, M.M. Cellular mechanoadaptation to substrate mechanical properties: Contributions of substrate stiffness and thickness to cell stiffness measurements using AFM. Soft Matter 2014, 10, 1174–1181. [Google Scholar] [CrossRef]
- Delcea, M.; Schmidt, S.; Palankar, R.; Fernandes, P.A.; Fery, A.; Möhwald, H.; Skirtach, A.G. Mechanobiology: Correlation between mechanical stability of microcapsules studied by AFM and impact of cell-induced stresses. Small 2010, 6, 2858–2862. [Google Scholar] [CrossRef]
- Cai, X.; Gao, S.; Cai, J.; Wu, Y.; Deng, H. Artesunate induced morphological and mechanical changes of Jurkat cell studied by AFM. Scanning 2009, 31, 83–89. [Google Scholar] [CrossRef]
- Li, Y.; Wen, C.; Xie, H.; Ye, A.; Yin, Y. Mechanical property analysis of stored red blood cell using optical tweezers. Colloids Surf. B 2009, 70, 169–173. [Google Scholar] [CrossRef]
- Guo, H.-L.; Liu, C.-X.; Duan, J.-F.; Jiang, Y.-Q.; Han, X.-H.; Li, Z.-L.; Cheng, B.-Y.; Zhang, D.-Z. Mechanical properties of breast cancer cell membrane studied with optical tweezers. Chin. Phys. Lett. 2004, 21, 2543–2546. [Google Scholar]
- Laurent, V.M.; Henon, S.; Planus, E.; Fodil, R.; Balland, M.; Isabey, D.; Gallet, F. Assessment of mechanical properties of adherent living cells by bead micromanipulation: Comparison of magnetic twisting cytometry vs optical tweezers. J. Biomech. Eng. 2002, 124, 408–421. [Google Scholar] [CrossRef]
- Bausch, A.R.; Moller, W.; Sackmann, E. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys. J. 1999, 76, 573–579. [Google Scholar] [CrossRef]
- Sohail, T.; Tang, T.; Nadler, B. Micropipette aspiration of an inflated fluid-filled spherical membrane. Z. Angew. Math. Phys. 2012, 63, 737–757. [Google Scholar] [CrossRef]
- Mohammadalipour, A.; Choi, Y.E.; Benencia, F.; Buedick, M.M.; Tees, D.F.J. Investigation of mechanical properties of breast cancer cells using micropipette aspiration technique. FASEB J. 2012, 26. [Google Scholar] [CrossRef]
- Kamat, N.P.; Lee, M.H.; Lee, D.; Hammer, D.A. Micropipette aspiration of double emulsion-templated polymersomes. Soft Matter 2011, 7, 9863–9866. [Google Scholar] [CrossRef]
- Hochmuth, R.M. Micropipette aspiration of living cells. J. Biomech. 2000, 33, 15–22. [Google Scholar] [CrossRef]
- Evans, E.; Yeung, A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys. J. 1989, 56, 151–160. [Google Scholar] [CrossRef]
- Mattos, L.S.; Grant, E.; Thresher, R.; Kluckman, K. Blastocyst microinjection automation. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 822–831. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, C.; Ang, W.T.; Tan, S.Y.M.; Latt, W.T. Autofocusing and Polar Body Detection in Automated Cell Manipulation. IEEE Trans. Biomed. Eng. 2016, 64, 1099–1105. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Zhao, Q.; Sun, M.; Lu, G. Automatic somatic cell operating process for nuclear transplantation. In Proceedings of the 2012 7th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Kyoto, Japan, 5–8 March 2012. [Google Scholar]
- Zhao, Q.; Sun, M.; Cui, M.; Yu, J.; Qin, Y.; Zhao, X. Robotic Cell Rotation Based on the Minimum Rotation Force. IEEE Trans. Autom. Sci. Eng. 2014, 12, 1504–1515. [Google Scholar] [CrossRef]
- Zhao, Q.; Cui, M.; Zhao, X.; Sun, M.; Wang, Y.; Feng, J. Batch-operation Process. of Nuclear Transplatnion Based on Global Field of View. In Proceedings of the 30th Chinese Control. Conference, Yantai, China, 22–24 July 2011. [Google Scholar]
- Wang, X.; Liu, Y.; Li, S.; Cui, M.; Zhao, X. Automated cell transportation for batch-cell manipulation. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017. [Google Scholar]
- Zhao, Q.; Shirinzadeh, B.; Cui, M.; Sun, M.; Liu, Y.; Zhao, X. A novel cell weighing method based on the minimum immobilization pressure for biological applications. J. Appl. Phys. 2015, 118, 044301. [Google Scholar] [CrossRef]
- Khalilian, M.; Navidbakhsh, M.; Valojerdi, M.R.; Chizari, M.; Yazdi, P.E. Estimating Young’s modulus of zona pellucida by micropipette aspiration in combination with theoretical models of ovum. J. R. Soc. Interface 2009, 7. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, L.G.; Setton, L.A.; Guilak, F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005, 1, 317–325. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Sun, M.; Zhao, X. Robotic Cell Rotation Based on Optimal Poking Direction. Micromachines 2018, 9, 141. [Google Scholar] [CrossRef]
- Liu, Y.; Di, C.; Cui, M.; Sun, M.; Huang, J.; Zhao, X. Evaluation of the deformability of the cell’s zona pellucida based on the subpixel cell contour detection algorithm. In Proceedings of the 35th Chinese Control. Conference (CCC), Chengdu, China, 27–29 July 2016. [Google Scholar]
- Kim, N.-H.; Moon, S.J.; Prather, R.S.; Day, B.N. Cytoskeletal alteration in aged porcine oocytes and parthenogenesis. Mol. Reprod. Dev. 1996, 43, 513–518. [Google Scholar] [CrossRef]
- Dodson, M.G.; Minhas, B.S.; Curtis, S.K.; Palmer, T.V.; Robertson, J.L. Spontaneous Zona Reaction in the Mouse as a Limiting Factor for the Time in Which an Oocyte May Be Fertilized. J. In Vitro Fertil. Embryo Transf. 1989, 6, 101–106. [Google Scholar] [CrossRef]




| Groups | Amount | Elasticity (kPa) |
|---|---|---|
| New | 15 | 19.41 ± 4.33 |
| Traditional | 15 | 19.20 ± 5.43 |
| Groups | Localization | Hold | Measure | Release | Total |
|---|---|---|---|---|---|
| New method | 4 s | 4 s | 11 s | 11 s | 0.5 min |
| Traditional method | 58 s | 40 s | 11 s | 11 s | 2 min |
| Groups | Amount | Elasticity (kPa) |
|---|---|---|
| Mature oocytes | 15 | 10.26 ± 5.65 |
| Aged oocytes | 15 | 18.51 ± 3.41 |
| Groups | Amount | Elasticity (kPa) |
|---|---|---|
| A | 15 | 8.15 ± 4.93 |
| B | 15 | 5.55 ± 3.02 |
| C | 10 | 22.55 ± 10.65 |
| D | 12 | 9.45 ± 4.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Cui, M.; Huang, J.; Sun, M.; Zhao, X.; Zhao, Q. Robotic Micropipette Aspiration for Multiple Cells. Micromachines 2019, 10, 348. https://doi.org/10.3390/mi10050348
Liu Y, Cui M, Huang J, Sun M, Zhao X, Zhao Q. Robotic Micropipette Aspiration for Multiple Cells. Micromachines. 2019; 10(5):348. https://doi.org/10.3390/mi10050348
Chicago/Turabian StyleLiu, Yaowei, Maosheng Cui, Jingjing Huang, Mingzhu Sun, Xin Zhao, and Qili Zhao. 2019. "Robotic Micropipette Aspiration for Multiple Cells" Micromachines 10, no. 5: 348. https://doi.org/10.3390/mi10050348
APA StyleLiu, Y., Cui, M., Huang, J., Sun, M., Zhao, X., & Zhao, Q. (2019). Robotic Micropipette Aspiration for Multiple Cells. Micromachines, 10(5), 348. https://doi.org/10.3390/mi10050348




