Rheological Issues in Carbon-Based Inks for Additive Manufacturing
Abstract
:1. Introduction
2. Background
2.1. Rheology
2.1.1. Linear Rheology
2.1.2. Non-Linear Rheology
2.2. Rheological Connection to Additive Manufacturing
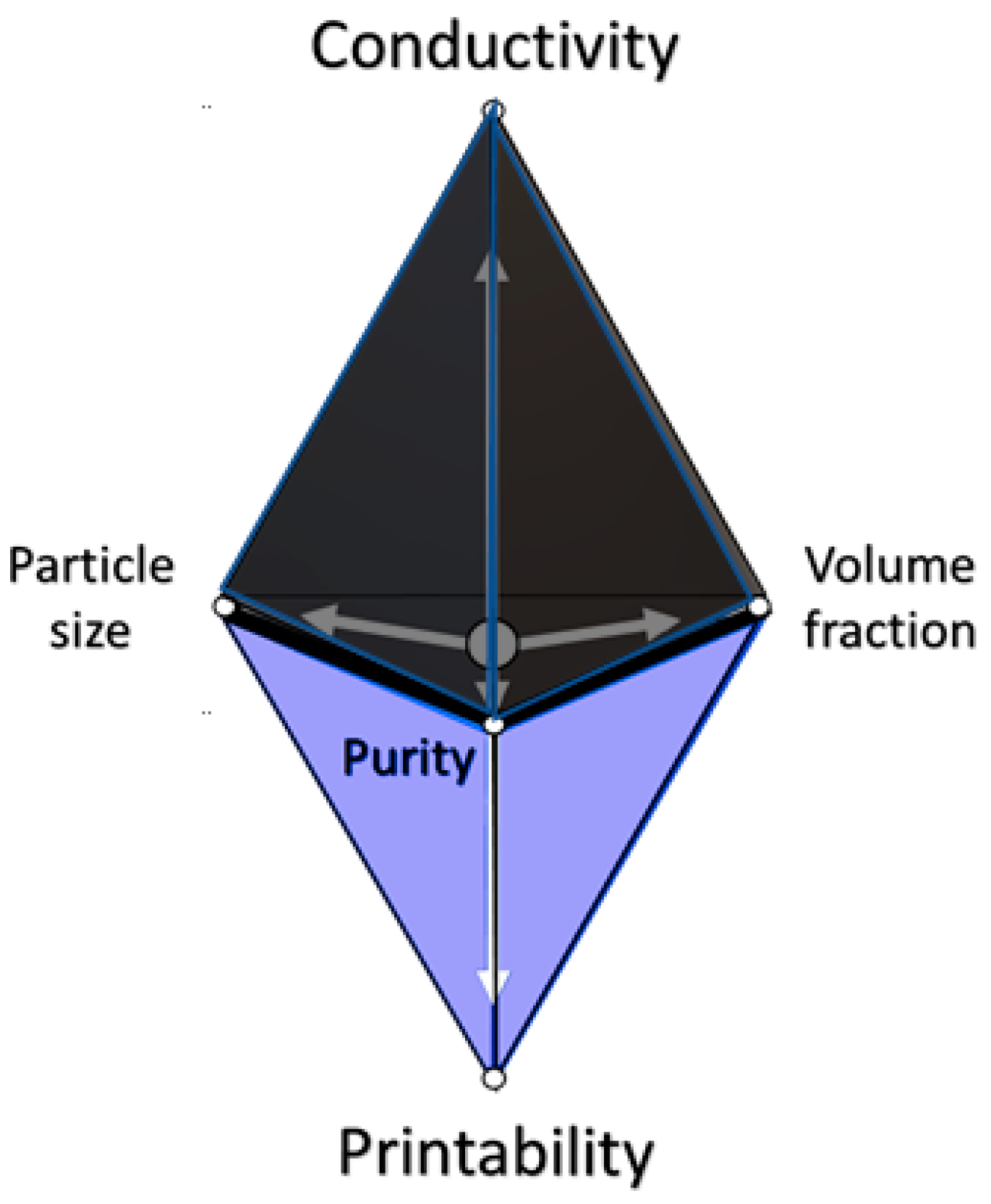
2.3. Factors Affecting Rheology
2.3.1. Temperature
2.3.2. Pressure
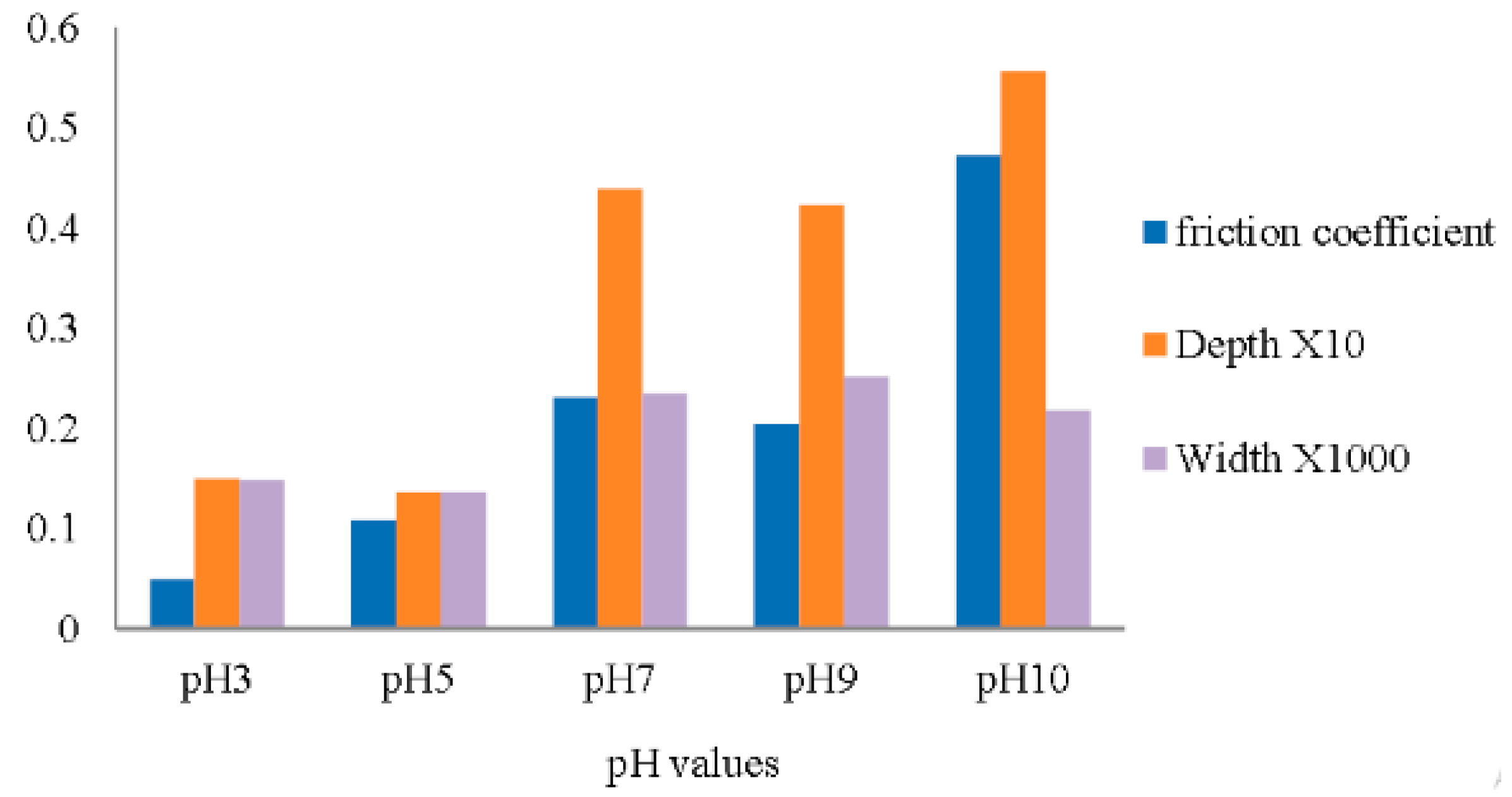
2.3.3. pH
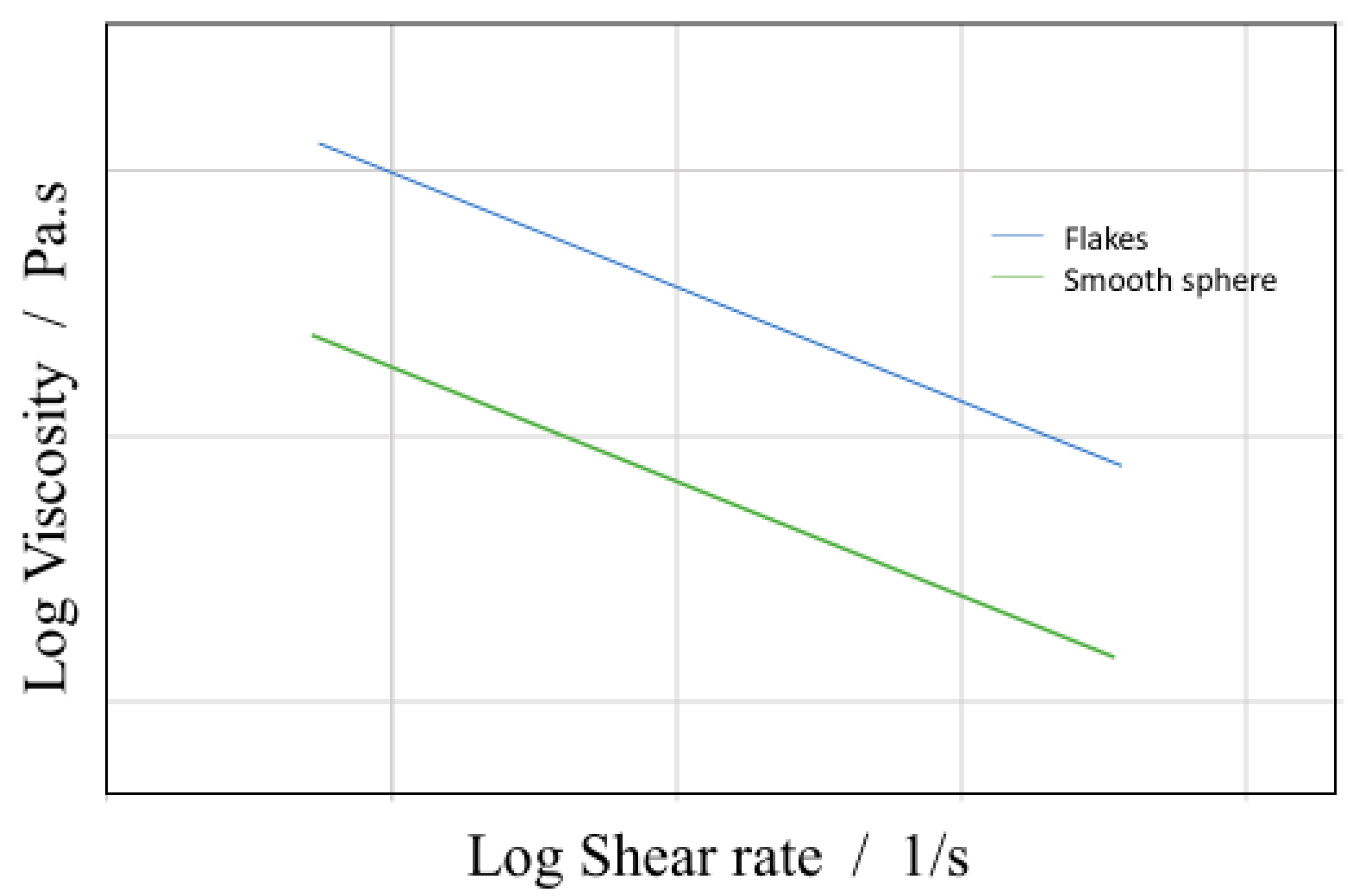
2.3.4. Topography & Shape of the Suspension
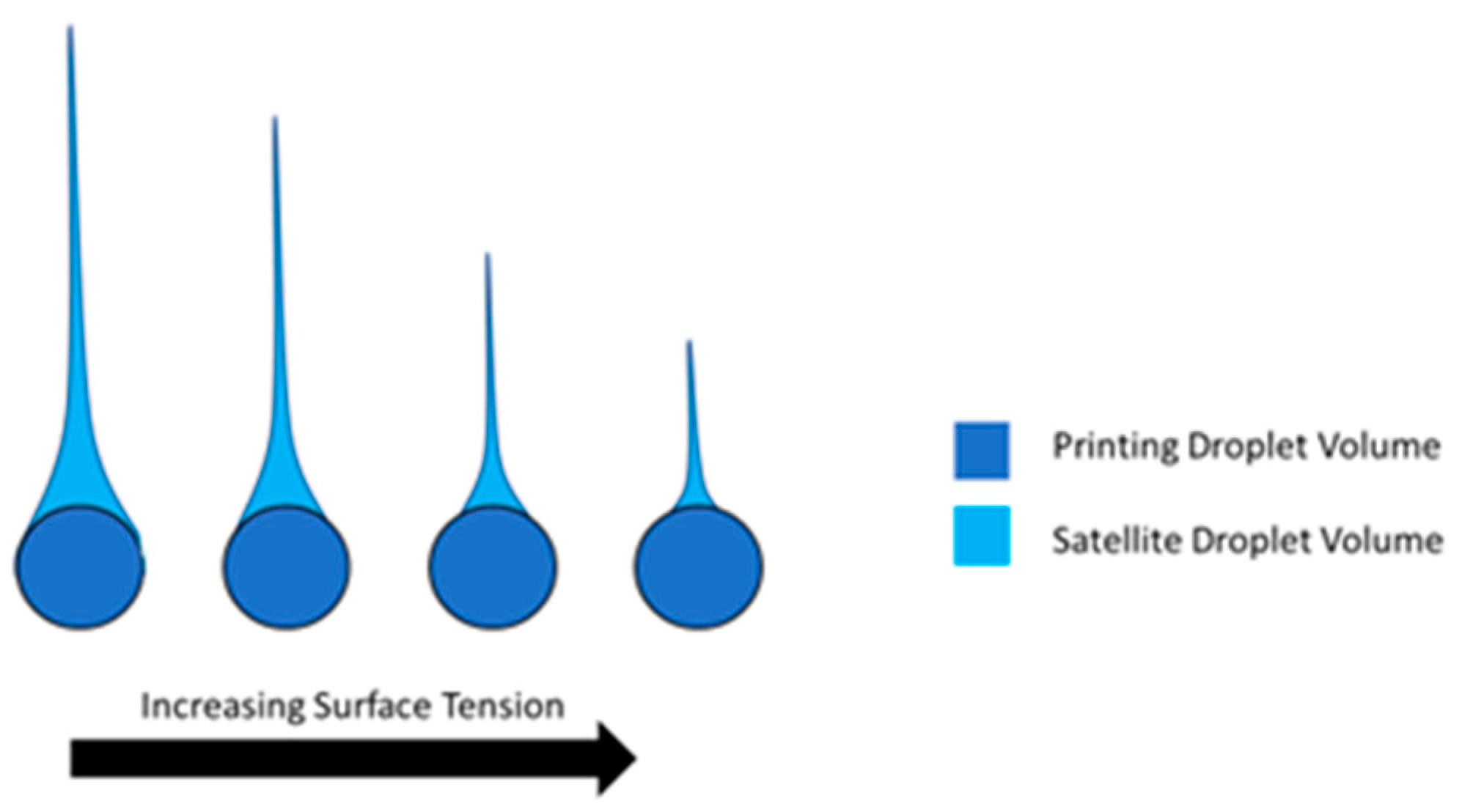
2.3.5. Surface Tension in Printing
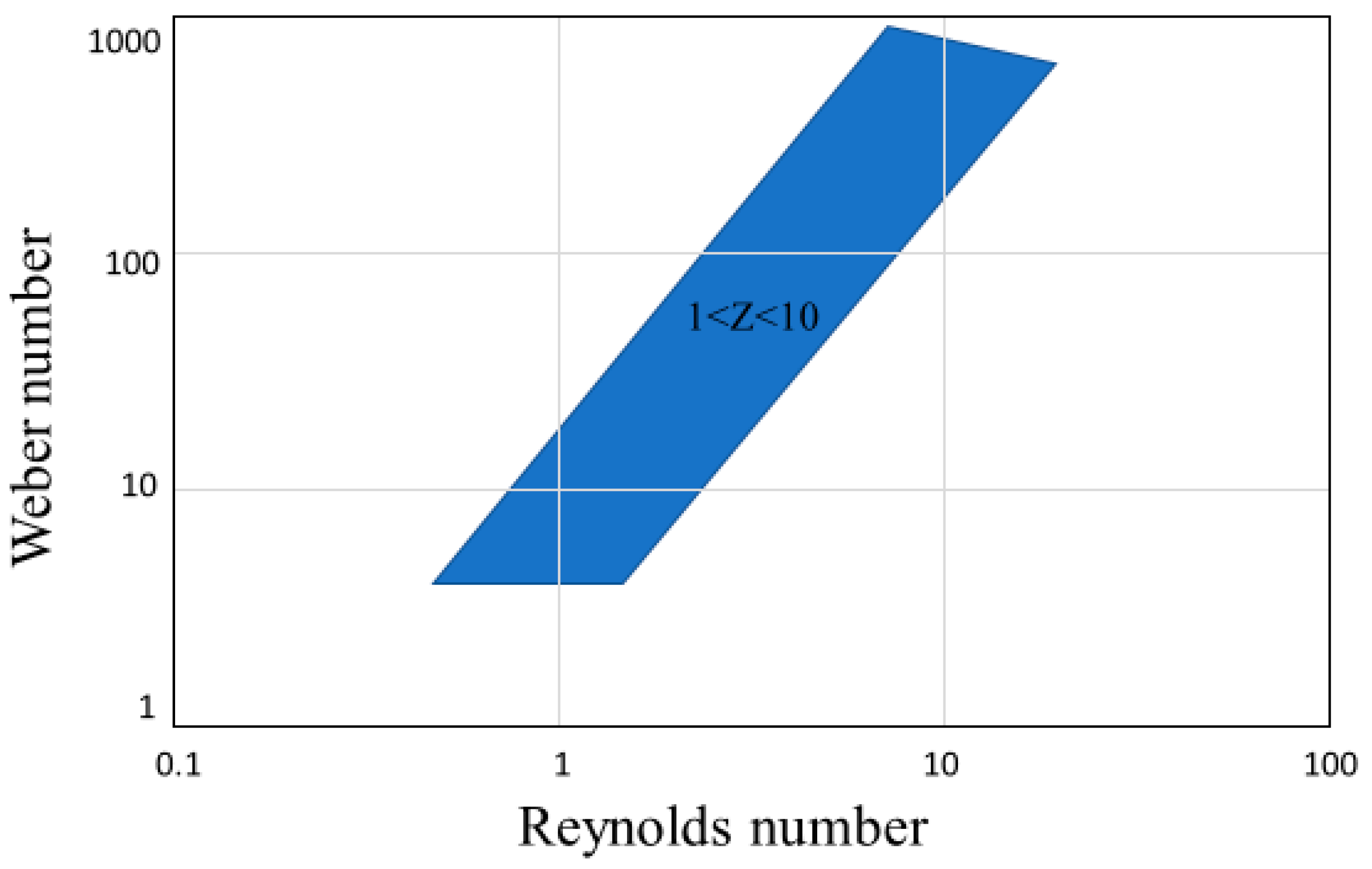
2.4. Relation to Inkjet Printing
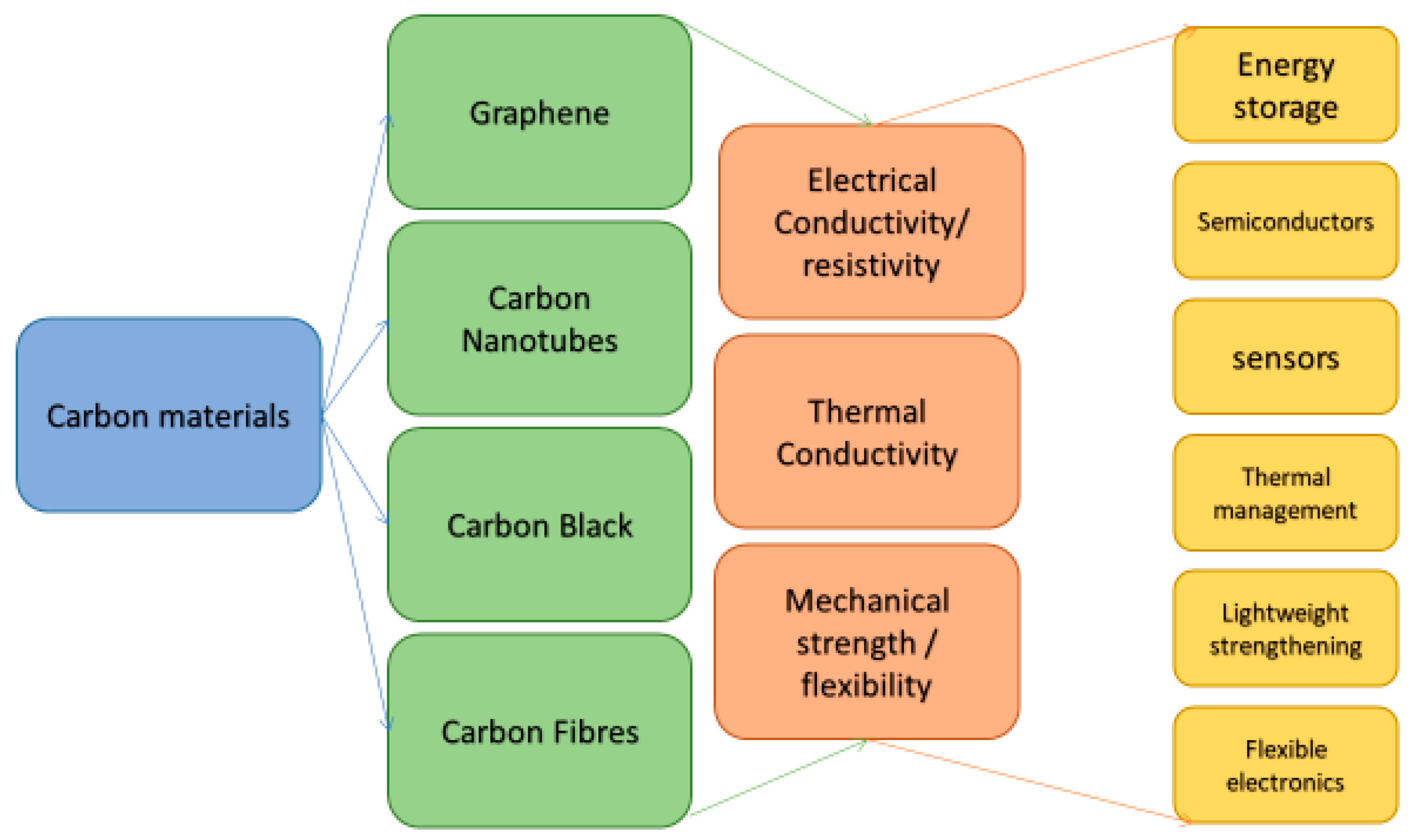
3. Carbon Based Inks
3.1. Carbon Based Inks—A Colloidal Suspension
3.1.1. Colloidal Systems
3.1.2. Einstein Viscosity & the Krieger–Dougherty Equation
3.2. Graphene
3.3. Graphene Oxide
3.4. Carbon Nanotubes
3.5. Carbon Black
3.6. Carbon Fiber
4. Problems Associated with Printing Carbon Based Inks
4.1. Agglomeration
4.2. Maintaining Suspension and Dispersion
4.3. Health, Safety, and Environmental Concerns
5. Applications of Printable Carbon Inks
5.1. Electronics
5.1.1. Transistors
5.1.2. Sensors
5.1.3. Electrodes
5.1.4. Supercapacitor
5.2. Biological Scaffolding
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- The Editors of Encyclopaedia Britannica. Carbon. In Encyclopædia Britannica; Encyclopædia Britannica, Inc.: Chicago, IL, USA, 2018. [Google Scholar]
- Wei, X.; Li, D.; Jiang, W.; Gu, Z.; Wang, X.; Zhang, Z.; Sun, Z. 3D Printable Graphene Composite. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Lee, A.; Sudau, K.; Ahn, K.H.; Lee, S.J.; Willenbacher, N. Optimization of Experimental Parameters to Suppress Nozzle Clogging in Inkjet Printing. Ind. Eng. Chem. Res. 2012, 51, 13195–13204. [Google Scholar] [CrossRef]
- Postiglione, G.; Natale, G.; Griffini, G.; Levi, M.; Turri, S. Conductive 3D microstructures by direct 3D printing of polymer/carbon nanotube nanocomposites via liquid deposition modeling. Compos. Part A Appl. Sci. Manuf. 2015, 76, 110–114. [Google Scholar] [CrossRef]
- Dybowska-Sarapuk, Ł.; Szalapak, J.; Wróblewski, G.; Wyżkiewicz, I.; Słoma, M.; Jakubowska, M. Rheology of inks for various techniques of printed electronics. In Advanced Mechatronics Solutions; Springer: New York, NY, USA, 2016; Volume 393. [Google Scholar]
- Maksud, M.I.; Yusof, M.; Embong, Z.; Nodin, M.; Rejab, N.A. Investigation on Printability of Carbon Nanotube (CNTs) Inks By Flexographic onto Various Substrates. Int. J. Mater. Sci. Eng. 2014, 2, 49–55. [Google Scholar] [CrossRef]
- Derby, B. Additive Manufacture of Ceramics Components by Inkjet Printing. Engineering 2015, 1, 113–123. [Google Scholar] [CrossRef]
- Guo, Y.; Patanwala, H.S.; Bognet, B.; Ma, A.W.K. Inkjet and inkjet-based 3D printing: Connecting fluid properties and printing performance. Rapid Prototyp. J. 2017, 23, 562–576. [Google Scholar] [CrossRef]
- Motyka, A.L. An Introduction to Rheology with an Emphasis on Application to Dispersions. J. Chem. Educ. 1996, 73, 374. [Google Scholar] [CrossRef]
- Deshpande, A. Techniques in Oscillatory Shear Rheology. Available online: http://www.physics.iitm.ac.in/~compflu/Lect-notes/abhijit.pdf (accessed on 29 January 2019).
- Marques, S.; Creus, G. Rheological Models: Integral and Differential Representations. In Computational Viscoelasticity; Springer: New York, NY, USA, 2012; pp. 11–21. [Google Scholar]
- Wallevik, O.H.; Feys, D.; Wallevik, J.E.; Khayat, K.H. Avoiding inaccurate interpretations of rheological measurements for cement-based materials. Cem. Concr. Res. 2015, 78, 100–109. [Google Scholar] [CrossRef]
- Atala, A.; Lanza, R.; Mikos, T.; Nerem, R. Principles of Regenerative Medicine; Elsevier Science: Amsterdam, The Netherlands, 2018; ISBN 9780128098936. [Google Scholar]
- Hoath, S.D.; Hsiao, W.-K.; Jung, S. Properties of PEDOT: PSS from Oscillating Drop Studies. In NIP & Digital Fabrication Conference; The Society for Imaging Science and Technology: Cambridge, MA, USA, 2014; pp. 299–303. [Google Scholar]
- Shenoy, A.V. Rheology of Filled Polymer Systems; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Alias, A.A.; Kinoshita, H.; Nishina, Y.; Fujii, M. Dependence of ph level on tribological effect of graphene oxide as an additive in water lubrication. Int. J. Automot. Mech. Eng. 2016, 13, 3150–3156. [Google Scholar] [CrossRef]
- Reinhardt, K.; Hofmann, N.; Eberstein, M. The importance of shear thinning, thixotropic and viscoelastic properties of thick film pastes to predict effects on printing performance. In Proceedings of the EMPC 2017 21st European Microelectronics and Packaging Conference (EMPC) & Exhibition, Warsaw, Poland, 10–13 September 2017; pp. 1–7. [Google Scholar]
- Niu, R.; Gong, J.; Xu, D.; Tang, T.; Sun, Z.-Y. The Effect of Particle Shape on the Structure and Rheological Properties of Carbon-Based Particle Suspensions. Chin. J. Polym. Sci. 2015, 33, 1550–1561. [Google Scholar] [CrossRef]
- Vafaei, S.; Tuck, C.; Ashcroft, I.; Wildman, R. Surface microstructuring to modify wettability for 3D printing of nano-filled inks. Chem. Eng. Res. Des. 2016, 109, 414–420. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, G.K. An Introduction to Fluid Dynamics; Cambridge University Press: Cambridge, UK, 2000; ISBN 9780521663960. [Google Scholar]
- Derby, B.; Reis, N. Inkjet Printing of Highly Loaded Particulate Suspensions. MRS Bull. 2003, 28, 815–818. [Google Scholar] [CrossRef]
- Kuscer, D.; Shen, J.Z. Chapter 18—Advanced Direct Forming Processes for the Future. In Advanced Ceramics for Dentistry; Shen, J.Z., Kosmač, T., Eds.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 375–390. ISBN 978-0-12-394619-5. [Google Scholar]
- Kovalchuk, N.M.; Nowak, E.; Simmons, M.J.H. Kinetics of liquid bridges and formation of satellite droplets: Difference between micellar and bi-layer forming solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 521, 193–203. [Google Scholar] [CrossRef]
- Kim, C.; Bernal, L. Density and viscosity ratio effects in droplet formation. In 38th Aerospace Sciences Meeting and Exhibit; Aerospace Sciences Meetings; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2000. [Google Scholar]
- Hoath, S.; Martin, G.D.; Hatchings, I.M. Effects of fluid viscosity on drop-on-demand ink-jet break-off. In NIP & Digital Fabrication Conference; Society for Imaging Science and Technology: Washington, DC, USA, 2010. [Google Scholar]
- Prudenziati, M.; Hormadaly, J. 1—Technologies for printed films. In Woodhead Publishing Series in Electronic and Optical Materials; Prudenziati, M., Hormadaly, J.B.T.-P.F., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 3–29. ISBN 978-1-84569-988-8. [Google Scholar]
- Dybowska-Sarapuk, L.; Kielbasinski, K.; Arazna, A.; Futera, K.; Skalski, A.; Janczak, D.; Sloma, M.; Jakubowska, M. Efficient Inkjet Printing of Graphene-Based Elements: Influence of Dispersing Agent on Ink Viscosity. Nanomater 2018, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Udawattha, D.S.; Narayana, M.; Wijayarathne, U.P.L. Predicting the effective viscosity of nanofluids based on the rheology of suspensions of solid particles. J. King Saud Univ. Sci. 2017, in press. [Google Scholar] [CrossRef]
- Naficy, S.; Jalili, R.; Aboutalebi, S.H.; Gorkin, R.A., III; Konstantinov, K.; Innis, P.C.; Spinks, G.M.; Poulin, P.; Wallace, G.G. Graphene oxide dispersions: Tuning rheology to enable fabrication. Mater. Horizons 2014, 1, 326–331. [Google Scholar] [CrossRef]
- Vallés, C.; Young, R.J.; Lomax, D.J.; Kinloch, I.A. The rheological behaviour of concentrated dispersions of graphene oxide. J. Mater. Sci. 2014, 49, 6311–6320. [Google Scholar] [CrossRef]
- Ma, A.; Mackley, M.; Chinesta, F. The Microstructure and Rheology of Carbon Nanotube Suspensions. Int. J. Mater. Form. 2008, 1, 75–81. [Google Scholar] [CrossRef]
- Hobbie, E.K.; Fry, D.J. Rheology of concentrated carbon nanotube suspensions. J. Chem. Phys. 2007, 126, 124907. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Ahir, S.V.; Terentjev, E. Dispersion rheology of carbon nanotubes in a polymer matrix. Phys. Rev. B 2006, 73, 125422. [Google Scholar] [CrossRef]
- Barrie, C.L.; Griffiths, P.C.; Abbott, R.J.; Grillo, I.; Kudryashov, E.; Smyth, C. Rheology of aqueous carbon black dispersions. J. Colloid Interface Sci. 2004, 272, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Ajinjeru, C.; Kishore, V.; Liu, P.; Hassen, A.A.; Lindahl, J.; Kunc, V.; Duty, C. Rheological evaluation of high temperature polymers to identify successful extrusion parameters. In Proceedings of the 27th Annual International Solid Freeform Fabrication Symposium, Additive Manufacturing Conference, Austin, TX, USA, 7–9 August 2017; pp. 485–494. [Google Scholar]
- Hoath, S.D. Fundamentals of Inkjet Printing: The Science of Inkjet and Droplets; Wiley: New York, NY, USA, 2016; ISBN 9783527337859. [Google Scholar]
- Taris, L.; Poirier, S.; Vinsonneau, S.; Mesnilgrente, F.; Temple-Boyer, P. Experimental Temperature Compensation on Drop-On-Demand Inkjet Printing. Micro Nanosyst. 2010, 2, 137–141. [Google Scholar] [CrossRef]
- Hutchings, G.D.M.; Hoath, S.D.; Hutchings, I.M. Inkjet printing—The physics of manipulating liquid jets and drops. J. Phys. Conf. Ser. 2008, 105, 12001. [Google Scholar]
- Barati, H.; Wu, M.; Kharicha, A.; Ludwig, A. A transient model for nozzle clogging. Powder Technol. 2018, 329, 181–198. [Google Scholar] [CrossRef]
- Barati, H.; Wu, M.; Kharicha, A.; Ludwig, A. A transient model for nozzle clogging—Part II: Validation and verification. Powder Technol. 2017. [Google Scholar] [CrossRef]
- Everett, D.H. Basic Principles of Colloid Science; Royal Society of Chemistry: Cambridge, UK, 1988. [Google Scholar]
- Barrie, C.L. Rheology of Carbon Black Dispersions; Cardiff University: Cardiff, UK, 2004. [Google Scholar]
- Willenbacher, N.; Georgieva, K. 1 Rheology of Disperse Systems; Wiley: New York, NY, USA, 2013; pp. 1–57. [Google Scholar]
- Mueller, S.; Llewellin, E.; Mader, H.M.; Mueller, B.S.; Mader, A.H.M. The rheology of suspensions of solid particles. Proc. R. Soc. A Math. Phys. Eng. Sci. 2009, 2010, 1201–1228. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Caia, W. Graphene: A versatile nanoplatform for biomedical applications. Nanoscale 2013, 4, 3833–3842. [Google Scholar] [CrossRef]
- Kim, F.; Cote, L.J.; Huang, J. Graphene oxide: Surface activity and two-dimensional assembly. Adv. Mater. 2010, 22, 1954–1958. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Wang, Y.; Yan, C.; Yao, Y.; Chen, Y.; Dai, J.; Lacey, S.; Wang, Y.; Wan, J.; Li, T.; et al. Graphene Oxide-Based Electrode Inks for 3D-Printed Lithium-Ion Batteries. Adv. Mater. 2016, 28, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Jakus, A.E.; Shah, R.N. Creating electronic and biomedical structures and devices. Mater. Matters 2016, 11, 43–48. [Google Scholar]
- Secor, E.B.; Prabhumirashi, P.L.; Puntambekar, K.; Geier, M.L.; Hersam, M.C. Inkjet printing of high conductivity, flexible graphene patterns. J. Phys. Chem. Lett. 2013, 4, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Merino, M.J.; Paredes, J.I.; Villar-Rodil, S.; Guardia, L.; Solís-Fernández, P.; Salinas-Torres, D.; Cazorla-Amorós, D.; Morallón, E.; Martínez-Alonso, A.; Tascón, J.M.D. Investigating the influence of surfactants on the stabilization of aqueous reduced graphene oxide dispersions and the characteristics of their composite films. Carbon 2012, 50, 3184–3194. [Google Scholar] [CrossRef]
- Uddin, M.E.; Kuila, T.; Nayak, G.C.; Kim, N.H.; Ku, B.-C.; Lee, J.H. Effects of various surfactants on the dispersion stability and electrical conductivity of surface modified graphene. J. Alloys Compd. 2013, 562, 134–142. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.; Khanra, P.; Kim, N.H.; Lee, J. Chemical Functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Lin, Z.; Le, T.; Song, X.; Yao, Y.; Li, Z.; Moon, K.; Tentzeris, M.M.; Wong, C. Preparation of Water-Based Carbon Nanotube Inks and Application in the Inkjet Printing of Carbon Nanotube Gas Sensors. J. Electron. Packag. 2013, 135, 011001. [Google Scholar] [CrossRef]
- Kordás, K.; Mustonen, T.; Tóth, G.; Jantunen, H.; Lajunen, M.; Soldano, C.; Talapatra, S.; Kar, S.; Vajtai, R.; Ajayan, P.M. Inkjet printing of electrically conductive patterns of carbon nanotubes. Small 2006, 2, 1021–1025. [Google Scholar] [CrossRef]
- Shin, S.R.; Farzad, R.; Tamayol, A.; Manoharan, V.; Mostafalu, P.; Zhang, Y.S.; Akbari, M.; Jung, S.M.; Kim, D.; Comotto, M.; et al. A Bioactive Carbon Nanotube-Based Ink for Printing 2D and 3D Flexible Electronics. Adv. Mater. 2016, 28, 3280–3289. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, H.; Qiu, J.; Zhou, C. Inkjet printing of single-walled carbon nanotube/RuO2 nanowire supercapacitors on cloth fabrics and flexible substrates. Nano Res. 2010, 3, 594–603. [Google Scholar] [CrossRef]
- Park, C.; Allaby, M. Oxford Dictionary of Environment and Conservation; University Press Oxford: Oxford, UK, 2013. [Google Scholar]
- Taylor, A.D.; Kim, E.Y.; Humes, V.P.; Kizuka, J.; Thompson, L. Inkjet Printing of Carbon Supported Platinum 3-D Catalyst Layers for Use in Fuel Cells. J. Power Sources 2007, 171, 101–106. [Google Scholar] [CrossRef]
- Tekinalp, H.L.; Kunc, V.; Velez-Garcia, G.M.; Duty, C.E.; Love, L.J.; Naskar, A.K.; Blue, C.A.; Ozcan, S. Highly oriented carbon fiber-polymer composites via additive manufacturing. Compos. Sci. Technol. 2014, 105, 144–150. [Google Scholar] [CrossRef]
- Matsuzaki, R.; Ueda, M.; Namiki, M.; Jeong, T.K.; Asahara, H.; Horiguchi, K.; Nakamura, T.; Todoroki, A.; Hirano, Y. Three-dimensional printing of continuous-fiber composites by in-nozzle impregnation. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, T.; Yang, C.; Qingrui, W.; Li, D. Interface and performance of 3D printed continuous carbon fiber reinforced PLA composites. Compos. Part A 2016, 88, 198–205. [Google Scholar] [CrossRef]
- Srinivasan, S.; Praveen, V.K.; Philip, R.; Ajayaghosh, A. Bioinspired superhydrophobic coatings of carbon nanotubes and linear π systems based on the “bottom-up” self-assembly approach. Angew. Chem. Int. Ed. 2008, 47, 5750–5754. [Google Scholar] [CrossRef] [PubMed]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through pKa Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Kahrizsangi, A.; Neshati, J.; Shariatpanahi, H.; Akbarinezhad, E. Improving the UV degradation resistance of epoxy coatings using modified carbon black nanoparticles. Prog. Org. Coat. 2015, 85, 199–207. [Google Scholar] [CrossRef]
- Suslick, K.S. Applications of Ultrasound to Materials Chemistry. MRS Bull. 1995, 20, 29–34. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Kharissova, O.V.; Méndez, U.O. Methods for Dispersion of Carbon Nanotubes in Water and Common Solvents. MRS Proc. 2014, 1700, 109–114. [Google Scholar] [CrossRef]
- Tortorich, R.; Choi, J.-W. Inkjet Printing of Carbon Nanotubes. Nanomaterials 2013, 3, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Woltornist, S.J.; Oyer, A.J.; Carrillo, J.-M.Y.; Dobrynin, A.V.; Adamson, D.H. Conductive Thin Films of Pristine Graphene by Solvent Interface Trapping. ACS Nano 2013, 7, 7062–7066. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Elimelech, M. Aggregation and deposition kinetics of fullerene (C60) nanoparticles. Langmuir 2006, 22, 10994–11001. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Yang, J.; Feng, Q.; Huang, K.; Zhou, H.; Hu, J.; Dong, S. Three-dimensional graphene-based materials by direct ink writing method for lightweight application. Int. J. Light. Mater. Manuf. 2018, 1, 96–101. [Google Scholar] [CrossRef]
- Popescu, M.T.; Tasis, D.; Papadimitriou, K.D.; Gkermpoura, S.; Galiotis, C.; Tsitsilianis, C. Colloidal stabilization of graphene sheets by ionizable amphiphilic block copolymers in various media. RSC Adv. 2015, 5, 89447–89460. [Google Scholar] [CrossRef]
- Su, Y.; Yang, G.; Lu, K.; Petersen, E.J.; Mao, L. Colloidal properties and stability of aqueous suspensions of few-layer graphene: Importance of graphene concentration. Environ. Pollut. 2017, 220, 469–477. [Google Scholar] [CrossRef]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.D.; Hersam, M.C.; Bouchard, D. Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ. Sci. Technol. 2013, 47, 6288–6296. [Google Scholar] [CrossRef]
- Huang, L.; Huang, Y.; Liang, J.; Wan, X.; Chen, Y. Graphene-based conducting inks for direct inkjet printing of flexible conductive patterns and their applications in electric circuits and chemical sensors. Nano Res. 2011, 4, 675–684. [Google Scholar] [CrossRef]
- Torrisi, F.; Hasan, T.; Wu, W.; Sun, Z.; Lombardo, A.; Kulmala, T.S.; Hsieh, G.W.; Jung, S.; Bonaccorso, F.; Paul, P.J.; et al. Inkjet-printed graphene electronics. ACS Nano 2012, 6, 2992–3006. [Google Scholar] [CrossRef]
- Pan, K.; Fan, Y.; Leng, T.; Li, J.; Xin, Z.; Zhang, J.; Hao, L.; Gallop, J.; Novoselov, K.S.; Hu, Z. Sustainable production of highly conductive multilayer graphene ink for wireless connectivity and IoT applications. Nat. Commun. 2018, 9, 5197. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Pasta, M.; La Mantia, F.; Cui, L.; Jeong, S.; Deshazer, H.D.; Choi, J.W.; Han, S.M.; Cui, Y. Stretchable, Porous, and Conductive Energy Textiles. Nano Lett. 2010, 10, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.; Kwon, Y.; Jeon, S.-Y.; Yu, W.-R. Optimally conductive networks in randomly dispersed CNT:graphene hybrids. Sci. Rep. 2015, 5, 16568. [Google Scholar] [CrossRef] [PubMed]
- Hof, F.; Kampioti, K.; Huang, K.; Jaillet-Bartholome, C.; Derr, A.; Poulin, P.; Yusof, H.; White, T.; Koziol, K.; Paukner, C.; et al. Conductive inks of graphitic nanoparticles from a sustainable carbon feedstock. Carbon 2016, 111, 142–149. [Google Scholar] [CrossRef]
- Liang, J.; Tong, K.; Pei, Q. A Water-Based Silver-Nanowire Screen-Print Ink for the Fabrication of Stretchable Conductors and Wearable Thin-Film Transistors. Adv. Mater. 2016, 28, 5986–5996. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.; Murphy, F.; Baublyte, L.; McAlea, E.M.; Tofail, S.A. The insurability of nanomaterial production risk. Nat Nanotechnol. 2013, 8, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, R.; Molander, S.; Sandén, B. Review of Potential Environmental and Health Risks of the Nanomaterial Graphene. Hum. Ecol. Risk Assess. 2013, 19, 873–887. [Google Scholar] [CrossRef]
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A concise review of carbon nanotube’s toxicology. Nano Rev. 2013, 4. [Google Scholar] [CrossRef]
- Roy, W.R. The environmental fate and movement of organic solvents in water, soil, and air. In Handbook of Solvents Vol.3, s; Illinois State Geological Survey: Richland, WA, USA, 2019; Volume 16.1, pp. 1149–2000. [Google Scholar]
- Yu, S.; Yin, Y.; Liu, J. Silver nanoparticles. Environ. Sci. Process. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef]
- Saad, A.A.E.E. Environmental Pollution Reduction by Using VOC-Free Water-Based Gravure Inks and Drying them with a New Drying System Based on Dielectric Heating. Ph.D. Thesis, Universität Wuppertal, Wuppertal, Germany, 2007. [Google Scholar]
- Ha, M.; Xia, Y.; Green, A.A.; Zhang, W.; Renn, M.J.; Kim, C.H.; Hersam, M.C.; Frisbie, C.D. Printed, Sub-3V Digital Circuits on Inks. ACS Nano 2010, 4, 4388–4395. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, W.S.; Kim, D.; Yang, J.R.; Han, J.T.; Lee, G.W.; Kim, J.T.; Seol, S.K. 3D printing of reduced graphene oxide nanowires. Adv. Mater. 2015, 27, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Carey, T.; Cacovich, S.; Divitini, G.; Ren, J.; Mansouri, A.; Kim, J.M.; Wang, C.; Ducati, C.; Sordan, R.; Torrisi, F. Fully inkjet-printed two-dimensional material field-effect heterojunctions for wearable and textile electronics. Nat. Commun. 2017, 8, 1202. [Google Scholar] [CrossRef] [PubMed]
- Monne, M.A.; Enuka, E.; Wang, Z.; Chen, M.Y. Inkjet printed graphene-based field-effect transistors on flexible substrate. In Proceedings of the SPIE, San Diego, CA, USA, 25 August 2017; Volume 10349. [Google Scholar]
- Xiang, L.; Wang, Z.; Liu, Z.; Weigum, S.E.; Yu, Q.; Chen, M.Y. Inkjet-Printed Flexible Biosensor Based on Graphene Field Effect Transistor. IEEE Sens. J. 2016, 16, 8359–8364. [Google Scholar] [CrossRef]
- Ji, A.; Chen, Y.; Wang, X.; Xu, C. Inkjet printed flexible electronics on paper substrate with reduced graphene oxide/carbon black ink. J. Mater. Sci. 2018, 29, 1–11. [Google Scholar] [CrossRef]
- Friedman, J.S.; Girdhar, A.; Gelfand, R.M.; Memik, G.; Mohseni, H.; Taflove, A.; Wessels, B.W.; Leburton, J.-P.; Sahakian, A.V. Cascaded spintronic logic with low-dimensional carbon. Nat. Commun. 2017, 8, 15635. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, F.; Del Mauro, A.D.G.; Burrasca, G.; La Ferrara, V.; Quercia, L.; Massera, E.; Di Francia, G.; Sala, D. Della Ink-jet printing technique in polymer/carbon black sensing device fabrication. Sens. Actuators B Chem. 2009, 143, 421–429. [Google Scholar] [CrossRef]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Mengüç, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D printing of strain sensors within highly stretchable elastomers. Adv. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef]
- Wang, T.; Cook, C.C.; Serban, S.; Ali, T.; Drago, G.; Derby, B. Fabrication of glucose biosensors by inkjet printing. arXiv, 2012; arXiv:1207.1190v1. [Google Scholar]
- Browne, M.P.; Novotný, F.; Sofer, Z.; Pumera, M. 3D Printed Graphene Electrodes’ Electrochemical Activation. ACS Appl. Mater. Interfaces 2018, 10, 40294–40301. [Google Scholar] [CrossRef]
- Schlatter, S.; Rosset, S.; Shea, H. Inkjet printing of carbon black electrodes for dielectric elastomer actuators. In Proceedings of the SPIE, San Diego, CA, USA, 25 August 2017; Volume 10163. [Google Scholar]
- Kwon, O.-S.; Kim, H.; Ko, H.; Lee, J.; Lee, B.; Jung, C.-H.; Choi, J.-H.; Shin, K. Fabrication and characterization of inkjet-printed carbon nanotube electrode patterns on paper. Carbon 2013, 58, 116–127. [Google Scholar] [CrossRef]
- Tortorich, R.P.; Song, E.; Choi, J.-W. Inkjet-Printed Carbon Nanotube Electrodes with Low Sheet Resistance for Electrochemical Sensor Applications. J. Electrochem. Soc. 2014, 161, B3044–B3048. [Google Scholar] [CrossRef]
- Yao, B.; Chandrasekaran, S.; Zhang, J.; Xiao, W.; Qian, F.; Zhu, C.; Duoss, E.B.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Efficient 3D Printed Pseudocapacitive Electrodes with Ultrahigh MnO2 Loading. Joule 2018, in press. [Google Scholar] [CrossRef]
- Yu, G.; Hu, L.; Liu, N.; Wang, H.; Vosgueritchian, M.; Yang, Y.; Cui, Y.; Bao, Z. Enhancing the supercapacitor performance of graphene/MnO2 nanostructured electrodes by conductive wrapping. Nano Lett. 2011, 11, 4438–4442. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Hu, H.; Liang, G.; Ye, C. Carbon-Based Flexible and All-Solid-State Micro-supercapacitors Fabricated by Inkjet Printing with Enhanced Performance. Nano-Micro Lett. 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Jiang, Y.; Tang, X.; Pan, Y.; Hu, S. Fully Packaged Carbon Nanotube Supercapacitors by Direct Ink Writing on Flexible Substrates. ACS Appl. Mater. Interfaces 2017, 9, 28433–28440. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Zhu, W.; Nowicki, M.; Lee, G.; Heo, D.N.; Kim, J.; Zuo, Y.Y.; Zhang, L.G. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J. Neural Eng. 2018, 15, 016018. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Mishra, A.; Lin, P.T.P.; Ng, S.H.; Yeong, W.Y.; Kim, Y.J.; Yoon, Y.J. 3D Printed Polycaprolactone Carbon Nanotube Composite Scaffolds for Cardiac Tissue Engineering. Macromol. Biosci. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Jiang, X.; Hong, X.; Jiang, Y.; Shao, Z.; Zhu, D. Recent Developments Concerning the Dispersion Methods and Mechanisms of Graphene. Coatings 2013, 8, 33. [Google Scholar] [CrossRef]
- Gnanasekaran, K.; Heijmans, T.; van Bennekom, S.; Woldhuis, H.; Wijnia, S.; de With, G.; Friedrich, H. 3D printing of CNT- and graphene-based conductive polymer nanocomposites by fused deposition modeling. Appl. Mater. Today 2017, 9, 21–28. [Google Scholar] [CrossRef]
- Foster, C.W.; Down, M.P.; Zhang, Y.; Ji, X.; Rowley-Neale, S.J.; Smith, G.C.; Kelly, P.J.; Banks, C.E. 3D Printed Graphene Based Energy Storage Devices. Sci. Rep. 2017, 7, 42233. [Google Scholar] [CrossRef]
- Jost, K.; Stenger, D.; Perez, C.R.; McDonough, J.K.; Lian, K.; Gogotsi, Y.; Dion, G. Knitted and screen printed carbon-fiber supercapacitors for applications in wearable electronics. Energy Environ. Sci. 2013, 6, 2698–2705. [Google Scholar] [CrossRef]
- Compton, B.G.; Lewis, J.A. 3D-Printing of Lightweight Cellular Composites. Adv. Mater. 2014, 26, 5930–5935. [Google Scholar] [CrossRef] [PubMed]
| Solvent | Surface Tension (mN/m) | GO Solubility (μg/mL) | rGO Solubility (μg/mL) |
|---|---|---|---|
| De-ionized water | 72.8 | 6.6 | 4.74 |
| Acetone | 25.2 | 0.8 | 0.9 |
| Methanol | 22.7 | 0.16 | 0.52 |
| Ethanol | 22.1 | 0.25 | 0.91 |
| 2-propanol | 21.66 | 1.82 | 1.2 |
| Ethylene glycol | 47.7 | 5.5 | 4.9 |
| Tetrahydrofuran (THF) | 26.4 | 2.15 | 1.44 |
| N,N-dimethyformamide (DMF) | 37.1 | 1.96 | 1.73 |
| N-methyl-2-pyrrolidone (NMP) | 40.1 | 8.7 | 9.4 |
| n-Hexane | 18.43 | 0.1 | 0.61 |
| Dichloromethane (DCM) | 26.5 | 0.21 | 1.16 |
| Chloroform | 27.5 | 1.3 | 4.6 |
| Toluene | 28.4 | 1.57 | 4.14 |
| Chlorobenzene (CB) | 33.6 | 1.62 | 3.4 |
| o-Dichlorobenzene (o-DCB) | 36.7 | 1.91 | 8.94 |
| 1-Chloronaphthalene (CN) | 41.8 | 1.8 | 8.1 |
| Acetylaceton | 31.2 | 1.5 | 1.02 |
| Diethyl ether | 17 | 0.72 | 0.4 |
| Carbon Form | Ink Method | Zero Shear Viscosity/Pa·s | Reference |
|---|---|---|---|
| Graphene | Organic solvent | ~18.5 | [29] |
| Organic solvent + dispersant | ~6 | - | |
| Water 0.5% | 0.478 mPa·s | [30] | |
| Water 1% | ~ 0.52 mPa·s | - | |
| Water 1.5% | ~ 0.56 mPa·s | - | |
| Graphene Oxide | LC viscoelastic gel 2 mg/mL | ~3.8 | [31] |
| LC viscoelastic gel 9 mg/mL | ~100 | - | |
| PMMA matrix 0.05% GO | ~80 | [32] | |
| PMMA matrix 1.2% GO | ~20,000 | - | |
| Carbon Nanotubes | epoxy resin 0.3% treated CNT | 20 | [33] |
| PIB 1.7% MWCNT | ~1 | [34] | |
| PIB 3.0% MWCNT | ~7 | - | |
| PIB 6.0% MWCNT | ~900 | - | |
| PDMS 1% MWCNT | ~10 | [35] | |
| PDMS 4% MWCNT | 30–40 | - | |
| Carbon Black | Poly-acrylate, 5.3% spherical | ~0.6 | [36] |
| Poly-acrylate, 11% spherical | ~6000 | - |
| Film | rGO (wt%) | Conductivity (S·m−1) | Specific Capacitance (F·g−1) |
|---|---|---|---|
| rGO | 100 | 7548 | 38 |
| rGO/PBA | 36 | 13.31 | 1 |
| rGO/DOC | 47 | 0.06 | 1 |
| rGO/TDOC | 36 | 2.18 | 3 |
| rGO/PSS | 41 | 10.51 | 114 |
| rGO/SDBS | 29 | 0.87 | 7 |
| rGO/SDS | 87 | 4679 | 46 |
| rGO/CHAPS | 36 | 0.92 | 2 |
| rGO/DBDM | 11 | 0.01 | 3 |
| rGO/P-123 | 38 | 5.53 | 12 |
| rGO/Brij 700 | 10 | 1.08 | 6 |
| rGO/Tween 80 | 13 | 0.41 | 95 |
| Sample | Adsorbed Surfactant (%) | Conductivity (S·m−1) |
|---|---|---|
| GO | 34.34 | 0.002 |
| CR-G | - | 4760 |
| SDBS-0.25-G | 7.02 | 108 |
| SDBS-0.5-G | 6.13 | 106 |
| SDBS-1-G | 9.31 | 97 |
| SDS-0.25-G | 17.41 | 94 |
| SDBS-0.5-G | 17.93 | 93 |
| SDBS-1-G | 21.62 | 95 |
| TRX-0.25-G | 9.63 | 98 |
| TRX-0.5-G | 9.63 | 92 |
| TRX-1-G | 9.37 | 89 |
| Modification Techniques | Modifying Agent | Dispersing Medium | Dispersibility (mg/mL) | Electrical Conductivity (S·m−1) |
|---|---|---|---|---|
| Nucleophilic Substitution | Alkyl amine/amino acid | CHCl3, THF, toluene, DCM | - | - |
| 4-Aminobenzene sulfonic acid | Water | 0.2 | - | |
| 4,4’-Diaminodiphenyl ether | Xylene, methanol | 0.1 | - | |
| POA | THF | 0.2 | - | |
| Allylamine | Water, DMF | 1.55 | - | |
| APTS | Water, ethanol, DMF, DMSO | 0.5 | - | |
| IL-NH2 | Water, DMF, DMSO | 0.5 | - | |
| PLL | Water | 0.5 | - | |
| Dopamine | Water | 0.05 | - | |
| Polyglycerol | Water | 3 | - | |
| Poly(norepinephrine) | Water, methanol, acetone, DMF, NMP, THF | 0.1 | - | |
| Electrophilic Substitution | ANS | Water | 3 | 145 |
| 4-Bromo aniline | DMF | 0.02 | - | |
| Sulfanilic acid | Water | 2 | 1250 | |
| NMP | Ethanol, DMF, NMP, PC, THF | 0.2–1.4 | 21,600 | |
| Condensation Reaction | Organic isocyanate | DMF, NMP, DMSO, HMPA | 1 (DMF) | - |
| Organic diisocyanate | DMF | - | 1.9 × 104 | |
| ODA | THF, CCl4, 1,2-dichloroethane | 0.5 (THF) | - | |
| TMEDA | THF | 0.2 | - | |
| PEG-NH2 | Water | 1 | - | |
| CS | Water | 2 | ||
| TPAPAM | THF | - | - | |
| β-CD | Water, acetone, DMF | 1 (DMF) | - | |
| α-CD, β-CD, γ-CD | Water, ethanol, DMF, DMSO | >2.5 | - | |
| PVA | Water, DMSO | - | - | |
| TPP-NH2 | DMF | - | - | |
| Adenine, cystine, nicotamide, OVA | Water | 0.1 | - | |
| Addition Reaction | POA | THF | 0.2 | - |
| Polyacetylene | Ortho dichlorobenzene (O-DCB) | 0.1 | - | |
| Aryne | DMF, O-DCB | 0.4 | - | |
| Cyclopropanated malonate | Toluene, O-DCB, DMF, DCM | 0.5 | - |
| Carbon Form | Ink | Conductivity (S·m−1) | Thickness of Prints | Reference |
|---|---|---|---|---|
| Graphene | Pristine | ~40,000 | - | [72] |
| GO + water | ~400 | 20 prints | [78] | |
| Few layer GO + water | ~875 | 20 prints | [78] | |
| G + NMP (Substrate O2 plasma treated) | ~0.08 | 50 nm | [79] | |
| G + NMP (Substrate Pristine) | ~30 | 50 nm | [79] | |
| G + NMP (Substrated HMDS-coated) | ~95 | 50 nm | [79] | |
| G + Cyrene | 37,000 | 7.8 μm | [80] | |
| Carbon Nanotube | SWNT + water + SDBS (substrate paper) | ~550 | 50 nm | [81] |
| MWCNT 12% + PAN + DMF | ~100 | 300 nm | [82] | |
| MWCNT 89% + PAN + DMF | ~333 | 300 nm | [82] | |
| MWCNT + aqueous solution | 2400 ± 180 | 10 μm | [59] | |
| Carbon Black | Cold microwave plasma, CO2 1.7% | 256 | - | [83] |
| Silver | Ag microparticles + Organic binder + solvent (Substrate PET/glass) | 46,700 | Screen printed | [84] |
| Dispersion Method | Mechanism | Advantage | Disadvantage |
|---|---|---|---|
| Physical methods | Applying physical force to separate agglomerated graphene | Simple operation | Low dispersion rate and possible damage to nanoparticles |
| Covalent bonding methods | Introducing various active groups by chemical reaction on the surface or edge of the graphene | Making the graphene more workable and operable | Causing damage to the initial structure of the graphene |
| Noncovalent bonding methods | Modifying the graphene’s structure with functionalized molecules through non-covalent interaction | Functionalizing carbon forms, allowing ease of use | Introduces other components and impurities to the carbon forms |
| Carbon Form | Ink Method | Application | Reference |
|---|---|---|---|
| Graphene | rGO + water | Nanowire arches | [92] |
| Graphene/h-BN + NMP + ethanol | Transistor | [93] | |
| N-Methylpyrrolidone | Transistor | [79] | |
| PBT/Graphene composite | Conductive polymer | [113] | |
| GO + water | Lithium ion battery electrodes | [50] | |
| Graphene/PLA | Electrodes | [101] | |
| Graphene/PLA | Energy storage | [114] | |
| Graphene + Hypromellose, aerogel suspension | Pseudocapacitive Electrodes | [105] | |
| Graphene + poly-lactide-co-glycolide | Electrical and biomedical scaffolding | - | |
| Carbon Nanotubes | PEDOT:PSS | Digital circuit | [91] |
| PBT/CNT composite | Conductive polymer | [113] | |
| Amine functionalization MWCNT/PEGDA matrix | Nerve regeneration scaffolding | [109] | |
| CNT + PCL in chloroform | Cardiac tissue scaffolding | [110] | |
| PCL-hydroxyapatite scaffold + CNT | Stimulate bone cell growth | [111] | |
| Carbon Black | Polymer-carbon black | Chemical sensor | [99] |
| Conductive carbon grease (Dimethylpolysiloxane) | Strain sensor | [99] | |
| Carbon Fibers | Active carbon + water | supercapacitor | [115] |
| Epoxy | Lightweight cellular composites, controlled alignment | [116] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’ Mahony, C.; Haq, E.U.; Silien, C.; Tofail, S.A.M. Rheological Issues in Carbon-Based Inks for Additive Manufacturing. Micromachines 2019, 10, 99. https://doi.org/10.3390/mi10020099
O’ Mahony C, Haq EU, Silien C, Tofail SAM. Rheological Issues in Carbon-Based Inks for Additive Manufacturing. Micromachines. 2019; 10(2):99. https://doi.org/10.3390/mi10020099
Chicago/Turabian StyleO’ Mahony, Charlie, Ehtsham Ul Haq, Christophe Silien, and Syed A. M. Tofail. 2019. "Rheological Issues in Carbon-Based Inks for Additive Manufacturing" Micromachines 10, no. 2: 99. https://doi.org/10.3390/mi10020099
APA StyleO’ Mahony, C., Haq, E. U., Silien, C., & Tofail, S. A. M. (2019). Rheological Issues in Carbon-Based Inks for Additive Manufacturing. Micromachines, 10(2), 99. https://doi.org/10.3390/mi10020099





