Coaxial Electrohydrodynamic Atomization for the Production of Drug-Loaded Micro/Nanoparticles
Abstract
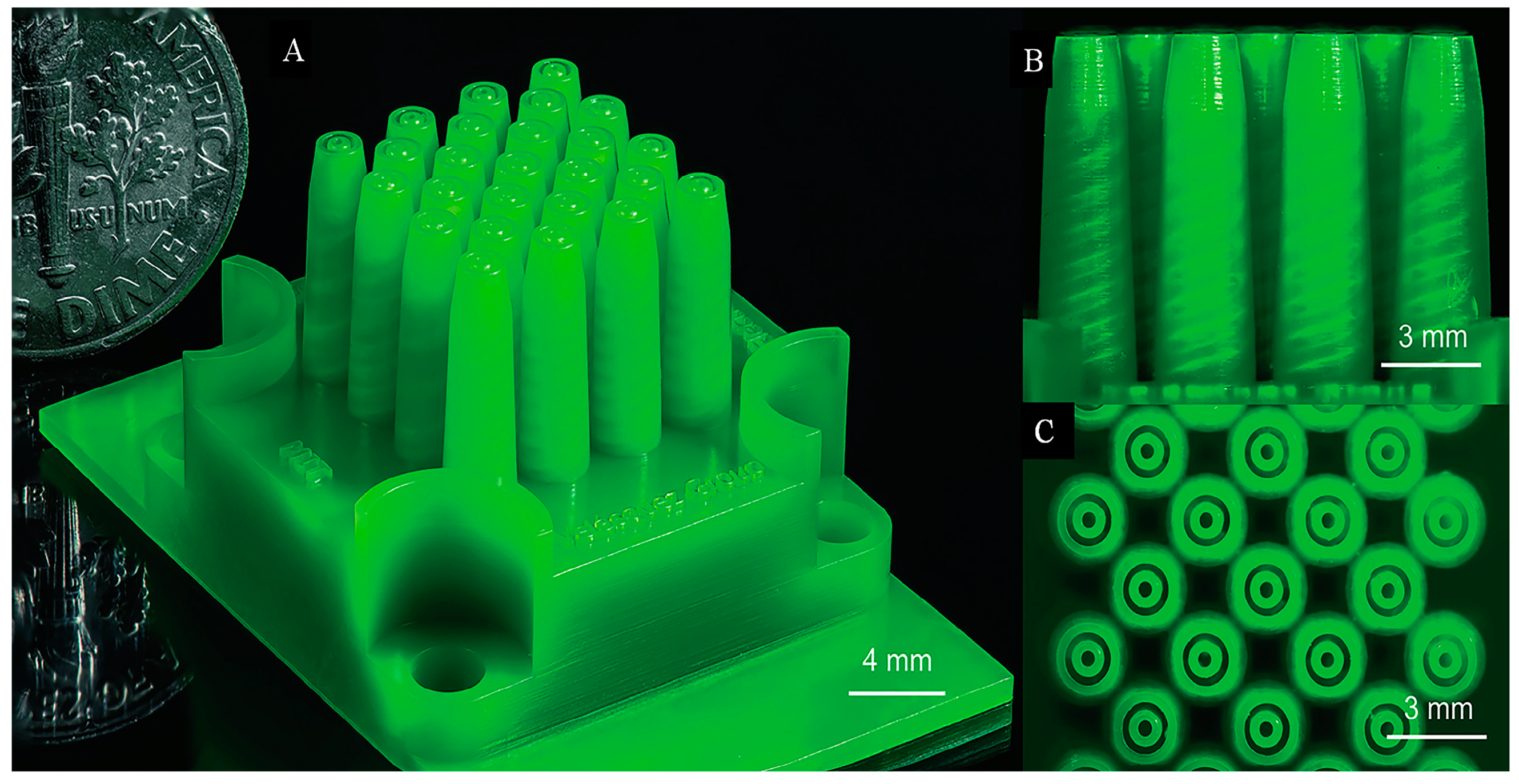
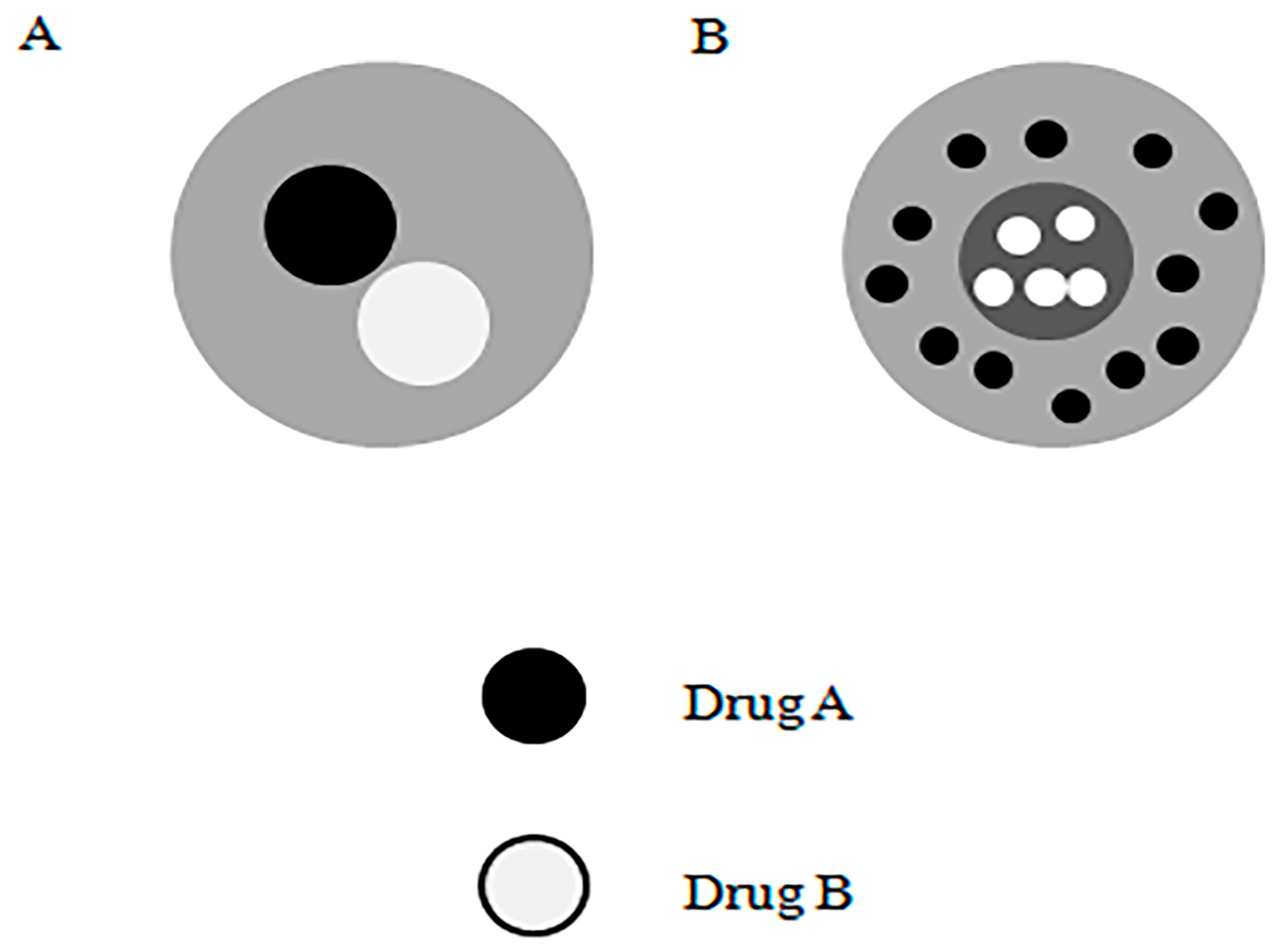
1. Introduction
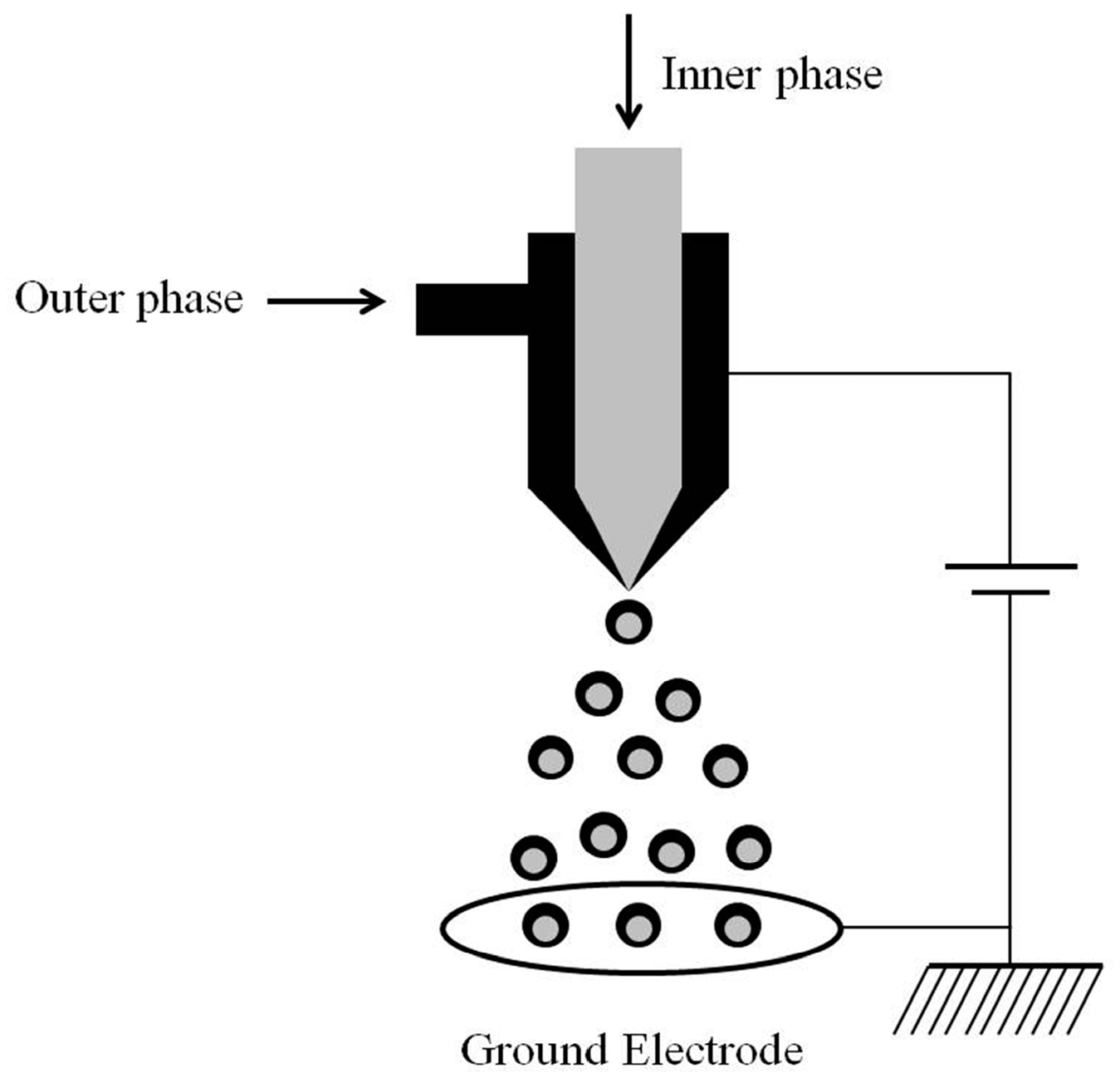
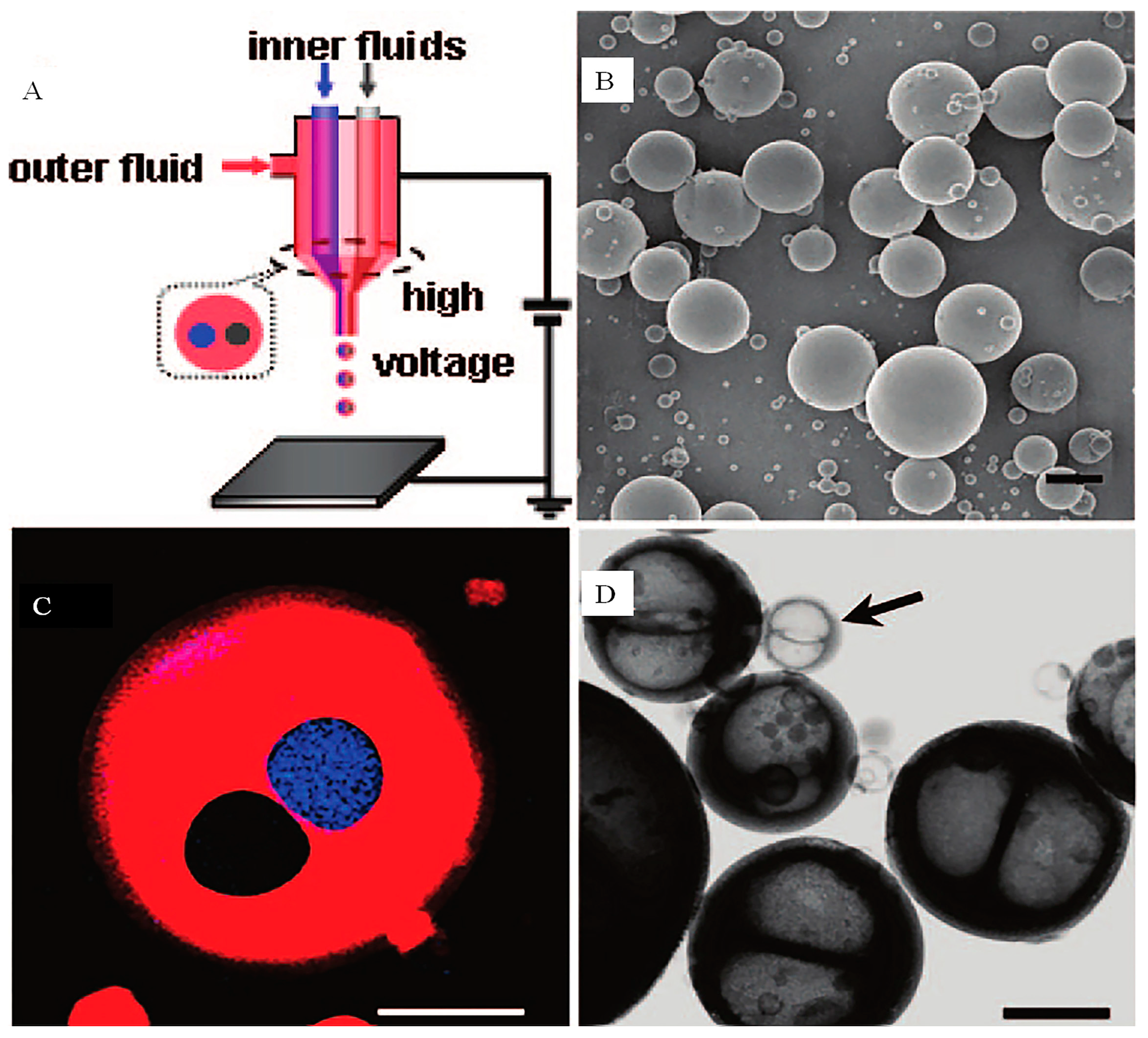
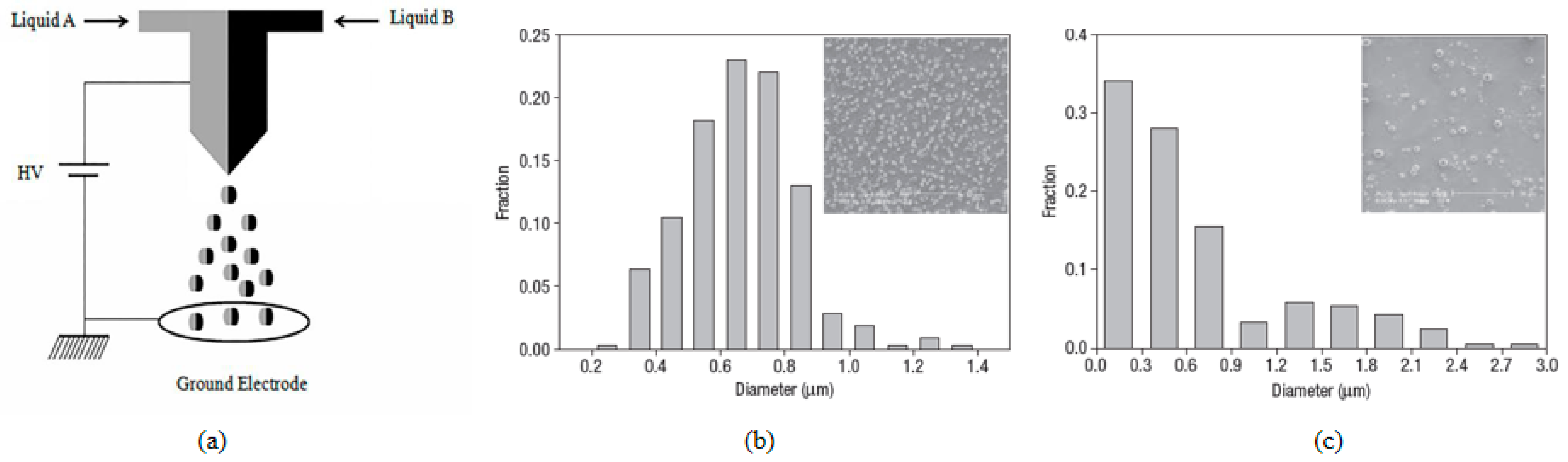
2. Concept of CEHDA
2.1. Double-layer Structure Encapsulation
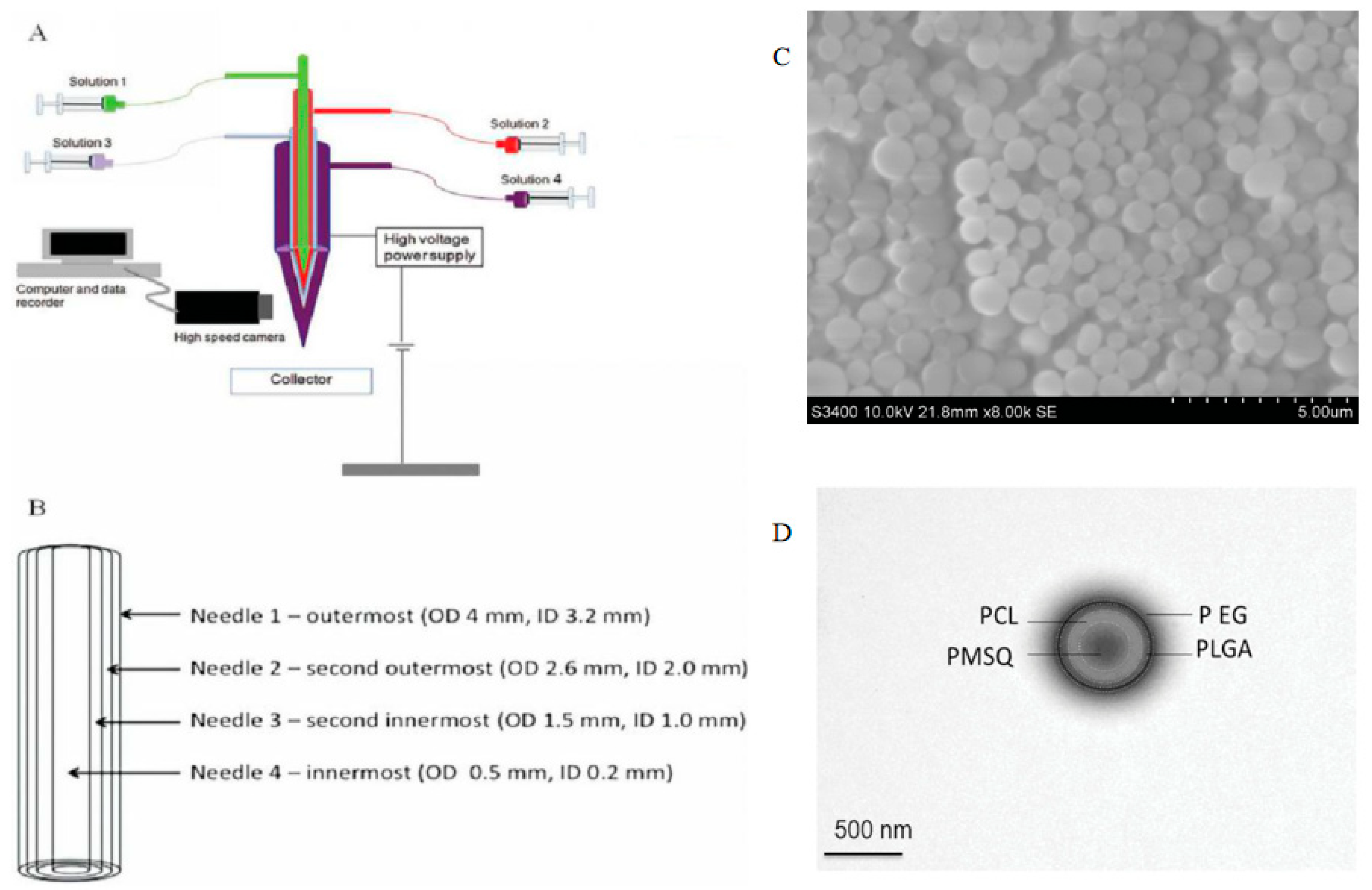
2.2. Multilayer Structure Encapsulation
2.3. Multicomponent Encapsulation
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Liao, I.C.; Adler, A.; Leong, K.W. Electrohydrodynamics: A facile technique to fabricate drug delivery systems. Adv. Drug Deliv. Rev. 2009, 61, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; MacKay, J.A.; McDaniel, J.R.; Chilkoti, A.; Clark, R.L. Fabrication of elastin-like polypeptide nanoparticles for drug delivery by electrospraying. Biomacromolecules 2009, 10, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Waits, C.M.; Morgan, B.; Gomez, A. Compact multiplexing of monodisperse electrosprays. J. Aerosol. Sci. 2009, 40, 907–918. [Google Scholar] [CrossRef]
- Bhatnagar, P. Multiplexed electrospray deposition for protein microarray with micromachined silicon device. Appl. Phys. Lett. 2007, 91, 014102. [Google Scholar] [CrossRef]
- Xie, J.; Wang, C. Encapsulation of proteins in biodegradable polymeric microparticles using electrospray in the Taylor Cone-Jet mode. Biotechnol. Bioeng. 2007, 97, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliver. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.D.; Selomulya, C. On the spray drying of uniform functional microparticles. Particuology 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Mehta, P.; Haj-Ahmad, R.; Rasekh, M.; Arshad, M.S.; Smith, A.; van der Merwe, S.M.; Li, X.; Chang, M.; Ahmad, Z. Pharmaceutical and biomaterial engineering via electrohydrodynamic atomization technologies. Drug Discov. Today 2017, 22, 157–165. [Google Scholar] [CrossRef]
- Liu, Z.P.; Zhang, Y.Y.; Yu, D.G.; Wu, D.; Li, H.L. Fabrication of sustained-release zein nanoparticles via modified coaxial electrospraying. Chem. Eng. J. 2018, 334, 807–816. [Google Scholar] [CrossRef]
- Wan, F.; Yang, M. Design of PLGA-based depot delivery systems for biopharmaceuticals prepared by spray drying. Int. J. Pharmaceut. 2016, 498, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A. Production of drug-loaded polymeric nanoparticles by electrospraying technology. J Biomed. Nanotechnol. 2014, 10, 2200–2217. [Google Scholar] [CrossRef]
- Sridhar, R.; Ramakrishna, S. Electrosprayed nanoparticles for drug delivery and pharmaceutical applications. Biomatter 2013, 3, e24281. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.L.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 2005, 436, 568–572. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Q.; Chow, P.K.; Wang, D.; Wang, C. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials 2013, 34, 8511–8520. [Google Scholar] [CrossRef]
- Eltayeb, M.; Stride, E.; Edirisinghe, M.; Harker, A. Electrosprayed nanoparticle delivery system for controlled release. Mat. Sci. Eng. C-Mater 2016, 66, 138–146. [Google Scholar] [CrossRef]
- Davoodi, P.; Feng, F.; Xu, Q.; Yan, W.; Tong, Y.W.; Srinivasan, M.P.; Sharma, V.K.; Wang, C. Coaxial electrohydrodynamic atomization: Microparticles for drug delivery applications. J. Control Release 2015, 205, 70–82. [Google Scholar] [CrossRef]
- Rasekh, M.; Ahmad, Z.; Cross, R.; Hernández-Gil, J.; Wilton-Ely, J.D.E.T.; Miller, P.W. Facile preparation of drug-loaded tristearin encapsulated superparamagnetic iron oxide nanoparticles using coaxial electrospray processing. Mol. Pharmaceutics 2017, 14, 2010–2013. [Google Scholar] [CrossRef]
- Yan, W.C.; Ong, X.J.; Pun, K.T.; Tan, D.Y.; Sharma, V.K.; Tong, Y.W.; Wang, C.H. Preparation of tPA-loaded microbubbles as potential theranostic agents: A novel one-step method via coaxial electrohydrodynamic atomization technique. Chem. Eng. J. 2017, 307, 168–180. [Google Scholar] [CrossRef]
- Raut, J.S.; Akella, S.; Singh, A.K.; Naik, V.M. Catastrophic drop breakup in electric field. Langmuir 2009, 25, 4829–4834. [Google Scholar] [CrossRef] [PubMed]
- Regele, J.D.; Papac, M.J.; Rickard, M.; Dunn-Rankin, D. Effects of capillary spacing on EHD spraying from an array of cone jets. J. Aerosol Sci. 2002, 33, 1471–1479. [Google Scholar] [CrossRef]
- Deng, W.W.; Klemic, J.F.; Li, X.H.; Reed, M.A.; Gomez, A. Increase of electrospray throughput using multiplexed microfabricated sources for the scalable generation of monodisperse droplets. J. Aerosol. Sci. 2006, 37, 696–714. [Google Scholar] [CrossRef]
- Deng, W.; Gomez, A. Influence of space charge on the scale-up of multiplexed electrosprays. J. Aerosol. Sci. 2007, 38, 1062–1078. [Google Scholar] [CrossRef]
- Almeria, B.; Deng, W.; Fahmy, T.M.; Gomez, A. Controlling the morphology of electrospray-generated PLGA microparticles for drug delivery. J. Colloid Interf. Sci. 2010, 343, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, R.; Galan, D.; Marquez, M.; Loscertales, I.G.; Barrero, A. Multiple electrosprays emitted from an array of holes. J. Aerosol. Sci. 2005, 36, 1387–1399. [Google Scholar] [CrossRef]
- Parhizkar, M.; Reardon, P.J.T.; Knowles, J.C.; Browning, R.J.; Stride, E.; Pedley, R.B.; Grego, T.; Edirisinghe, M. Performance of novel high throughput multi electrospray systems for forming of polymeric micro/nanoparticles. Mater. Design 2017, 126, 73–84. [Google Scholar] [CrossRef]
- Almeria, B.; Fahmy, T.M.; Gomez, A. A multiplexed electrospray process for single-step synthesis of stabilized polymer particles for drug delivery. J. Control Release 2011, 154, 203–210. [Google Scholar] [CrossRef]
- Lhernould, M.S.; Lambert, P. Compact polymer multi-nozzles electrospray device with integrated microfluidic feeding system. J. Electrostat. 2011, 69, 313–319. [Google Scholar] [CrossRef]
- Yang, W.; Lojewski, B.; Wei, Y.; Deng, W. Interactions and deposition patterns of multiplexed electrosprays. J. Aerosol. Sci. 2012, 46, 20–33. [Google Scholar] [CrossRef]
- Lenguito, G.; Gomez, A. Development of a multiplexed electrospray micro-thruster with post-acceleration and beam containment. J. Appl. Phys. 2013, 114, 154901. [Google Scholar] [CrossRef]
- Hill, F.A.; Heubel, E.V.; de Leon, P.P.; Velasquez-Garcia, L.F. High-throughput ionic liquid ion sources using arrays of microfabricated electrospray emitters with integrated extractor grid and carbon nanotube flow control structures. J. Microelectromech. S 2014, 23, 1237–1248. [Google Scholar] [CrossRef]
- Dandavino, S.; Ataman, C.; Ryan, C.N.; Chakraborty, S.; Courtney, D.; Stark, J.P.W.; Shea, H. Microfabricated electrospray emitter arrays with integrated extractor and accelerator electrodes for the propulsion of small spacecraft. J. Micromech. Microeng. 2014, 24, 075011. [Google Scholar] [CrossRef]
- Lojewski, B.; Yang, W.; Duan, H.; Xu, C.; Deng, W. Design, Fabrication, and Characterization of Linear Multiplexed Electrospray Atomizers Micro- Machined from Metal and Polymers. Aerosol. Sci. Tech. 2013, 47, 146–152. [Google Scholar] [CrossRef]
- Olvera-Trejo, D.; Velasquez-Garcia, L.F. Additively manufactured MEMS multiplexed coaxial electrospray sources for high-throughput, uniform generation of core-shell microparticles. Lab Chip 2016, 16, 4121–4132. [Google Scholar] [CrossRef] [PubMed]
- Loscertales, I.G.; Barrero, A.; Guerrero, I.; Cortijo, R.; Marquez, M.; Ganan-Calvo, A.M. Micro/nano encapsulation via electrified coaxial liquid jets. Science 2002, 295, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ng, W.J.; Lee, L.Y.; Wang, C. Encapsulation of protein drugs in biodegradable microparticles by co-axial electrospray. J. Colloid Interf. Sci. 2008, 317, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, B.; Jackson, A.; Zha, W.; Lee, L.J.; Wyslouzil, B.E. Coaxial electrohydrodynamic spraying: A novel one-step technique to prepare oligodeoxynucleotide encapsulated lipoplex nanoparticles. Mol. Pharmaceut. 2009, 6, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, R.; Ahmad, Z.; Soric, M.; Stride, E.; Edirisinghe, M. Nanoparticle delivery systems formed using electrically sprayed co-flowing excipients and active agent. J. Biomed. Nanotechnol. 2011, 7, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Seremeta, K.P.; Hoecht, C.; Taira, C.; Cortez Tornello, P.R.; Abraham, G.A.; Sosnik, A. Didanosine-loaded poly(epsilon-caprolactone) microparticles by a coaxial electrohydrodynamic atomization (CEHDA) technique. J. Mater. Chem. B 2015, 3, 102–111. [Google Scholar] [CrossRef]
- Rasekh, M.; Young, C.; Roldo, M.; Lancien, F.; Le Mevel, J.; Hafizi, S.; Ahmad, Z.; Barbu, E.; Gorecki, D. Hollow-layered nanoparticles for therapeutic delivery of peptide prepared using electrospraying. J. Mater. Sci-Mater M 2015, 26, 256. [Google Scholar] [CrossRef] [PubMed]
- Gallovic, M.D.; Schully, K.L.; Bell, M.G.; Elberson, M.A.; Palmer, J.R.; Darko, C.A.; Bachelder, E.M.; Wyslouzil, B.E.; Keane-Myers, A.M.; Ainslie, K.M. Acetalated dextran microparticulate vaccine formulated via Coaxial Electrospray preserves toxin neutralization and enhances murine survival following inhalational Bacillus anthracis exposure. Adv. Healthc. Mater. 2016, 5, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Prabhakaran, M.P.; Thian, E.S.; Ramakrishna, S. Protein encapsulated core-shell structured particles prepared by coaxial electrospraying: Investigation on material and processing variables. Int. J. Pharmaceut. 2014, 473, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Laelorspoen, N.; Wongsasulak, S.; Yoovidhya, T.; Devahastin, S. Microencapsulation of Lactobacillus acidophilus in zein-alginate core-shell microcapsules via electrospraying. J. Funct. Foods 2014, 7, 342–349. [Google Scholar] [CrossRef]
- Sharma, B.; Takamura, Y.; Shimoda, T.; Biyani, M. A bulk sub-femtoliter in vitro compartmentalization system using super-fine electrosprays. Sci. Rep. 2016, 6, 26257. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Pack, D.W. Pulsatile protein release from monodisperse liquid-core microcapsules of controllable shell thickness. Pharm. Res-Dordr. 2014, 31, 3201–3210. [Google Scholar] [CrossRef]
- Lee, Y.; Mei, F.; Bai, M.; Zhao, S.; Chen, D. Release profile characteristics of biodegradable-polymer-coated drug particles fabricated by dual-capillary electrospray. J. Control Release 2010, 145, 58–65. [Google Scholar] [CrossRef]
- Xu, S.; Xu, Q.; Zhou, J.; Wang, J.; Zhang, N.; Zhang, L. Preparation and characterization of folate-chitosan-gemcitabine core-shell nanoparticles for potential tumor-targeted drug delivery. J. Nanosci. Nanotechno. 2013, 13, 129–138. [Google Scholar] [CrossRef]
- Xu, Q.; Chin, S.E.; Wang, C.; Pack, D.W. Mechanism of drug release from double-walled PDLLA (PLGA) microspheres. Biomaterials 2013, 34, 3902–3911. [Google Scholar] [CrossRef]
- Cao, L.; Luo, J.; Tu, K.; Wang, L.; Jiang, H. Generation of nano-sized core-shell particles using a coaxial tri-capillary electrospray-template removal method. Colloid Surface B 2014, 115, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cui, L.; Yu, D.; Zhao, Z.; Chen, L. Electrosprayed core-shell solid dispersions of acyclovir fabricated using an epoxy-coated concentric spray head. Int. J. Nanomed. 2014, 9, 1967–1977. [Google Scholar]
- Enayati, M.; Ahmad, Z.; Stride, E.; Edirisinghe, M. One-step electrohydrodynamic production of drug-loaded micro- and nanoparticles. J. R. Soc. Interface 2010, 7, 667–675. [Google Scholar] [CrossRef]
- Jing, Y.; Zhu, Y.; Yang, X.; Shen, J.; Li, C. Ultrasound-triggered smart drug release from multifunctional core-shell capsules one-step fabricated by coaxial electrospray method. Langmuir 2011, 27, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Qin, H.; Yin, Z.; Hua, J.; Pack, D.W.; Wang, C. Coaxial electrohydrodynamic atomization process for production of polymeric composite microspheres. Chem. Eng. Sci. 2013, 104, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.H.; Laidmae, I.; Kogermann, K.; Lust, A.; Meos, A.; Chien, N.N.; Heinamaki, J. Development of electrosprayed artesunate-loaded core-shell nanoparticles. Drug Dev. Ind. Pharm. 2017, 43, 1134–1142. [Google Scholar]
- Ghayempour, S.; Mortazavi, S.M. Fabrication of micro–nanocapsules by a new electrospraying method using coaxial jets and examination of effective parameters on their production. J. Electrostat. 2013, 71, 717–727. [Google Scholar] [CrossRef]
- Hao, S.; Wang, B.; Wang, Y. Porous hydrophilic core/hydrophobic shell nanoparticles for particle size and drug release control. Mat. Sci. Eng C-Mater. 2015, 49, 51–57. [Google Scholar] [CrossRef]
- Ahmad, Z.; Zhang, H.B.; Farook, U.; Edirisinghe, M.; Stride, E.; Colombo, P. Generation of multilayered structures for biomedical applications using a novel tri-needle coaxial device and electrohydrodynamic flow. J. R. Soc. Interface 2008, 5, 1255–1261. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers-Basel 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Allemann, E.; Fessi, H.; Doelker, E. Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers. Drug Dev. Ind. Pharm. 1998, 24, 1113–1128. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhang, L.; Wang, Z.; Yu, X.; Long, Y.; Zhang, X.; Zhao, N.; Xu, J. Multifunctional polymethylsilsesquioxane (PMSQ) surfaces prepared by electrospinning at the sol-gel transition: Superhydrophobicity, excellent solvent resistance, thermal stability and enhanced sound absorption property. J. Colloid. Interf. Sci. 2011, 359, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Labbaf, S.; Deb, S.; Cama, G.; Stride, E.; Edirisinghe, M. Preparation of multicompartment sub-micron particles using a triple-needle electrohydrodynamic device. J. Colloid Interf. Sci. 2013, 409, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Labbaf, S.; Ghanbar, H.; Stride, E.; Edirisinghe, M. Preparation of multilayered polymeric structures using a novel four-needle coaxial electrohydrodynamic device. Macromol. Rapid Comm. 2014, 35, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Pina, M.F.; Lau, W.; Scherer, K.; Parhizkar, M.; Edirisinghe, M.; Craig, D. The generation of compartmentalized nanoparticles containing siRNA and cisplatin using a multi-needle electrohydrodynamic strategy. Nanoscale 2017, 9, 5975–5985. [Google Scholar] [CrossRef] [PubMed]
- Okushima, S.; Nisisako, T.; Torii, T.; Higuchi, T. Controlled production of monodisperse double emulsions by two-step droplet breakup in microfluidic devices. Langmuir 2004, 20, 9905–9908. [Google Scholar] [CrossRef]
- Peyratout, C.S.; Dahne, L. Tailor-made polyelectrolyte microcapsules: From multilayers to smart containers. Angew Chem. Int. Edit 2004, 43, 3762–3783. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long-circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef]
- Larsen, G.; Velarde-Ortiz, R.; Minchow, K.; Barrero, A.; Loscertales, I.G. A method for making inorganic and hybrid (organic/inorganic) fibers and vesicles with diameters in the submicrometer and micrometer range via sol-gel chemistry and electrically forced liquid jets. J. Am. Chem. Soc. 2003, 125, 1154–1155. [Google Scholar] [CrossRef]
- Marin, A.G.; Loscertales, I.G.; Marquez, M.; Barrero, A. Simple and double emulsions via coaxial jet electrosprays. Phys. Rev. Lett. 2007, 98, 014502. [Google Scholar] [CrossRef] [PubMed]
- Dror, Y.; Salalha, W.; Avrahami, R.; Zussman, E.; Yarin, A.L.; Dersch, R.; Greiner, A.; Wendorff, J.H. One-step production of polymeric microtubes by co-electrospinning. Small 2007, 3, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Bazilevsky, A.V.; Yarin, A.L.; Megaridis, C.M. Co-electrospinning of core-shell fibers using a single-nozzle technique. Langmuir 2007, 23, 2311–2314. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Shao, C.L.; Liu, Y.C. A simple method for controllable preparation of polymer nanotubes via a single capillary electrospinning. Langmuir 2007, 23, 10920–10923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cao, X.; Jiang, L. Bio-mimic multichannel microtubes by a facile method. J. Am. Chem. Soc. 2007, 129, 764–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, Y.; Song, Y.; Jiang, L. One-step multicomponent encapsulation by compound-fluidic electrospray. J. Am. Chem. Soc. 2008, 130, 7800–7801. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, Y.; Jiang, L. Compound-fluidic electrospray: An efficient method for the fabrication of microcapsules with multicompartment structure. Chin. Sci. Bull. 2009, 54, 3147–3153. [Google Scholar] [CrossRef]
- Si, T.; Yin, C.; Gao, P.; Li, G.; Ding, H.; He, X.; Xie, B.; Xu, R.X. Steady cone-jet mode in compound-fluidic electro-flow focusing for fabricating multicompartment microcapsules. Appl. Phys. Lett. 2016, 108, 021601. [Google Scholar] [CrossRef]
- Ahmed, F.; Pakunlu, R.I.; Brannan, A.; Bates, F.; Minko, T.; Discher, D.E. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J. Control Release 2006, 116, 150–158. [Google Scholar] [CrossRef]
- Chang, P.C.; Dovban, A.S.; Lim, L.P.; Chong, L.Y.; Kuo, M.Y.; Wang, C.H. Dual delivery of PDGF and simvastatin to accelerate periodontal regeneration in vivo. Biomaterials 2013, 34, 9990–9997. [Google Scholar] [CrossRef]
- Chang, P.C.; Chong, L.Y.; Dovban, A.S.; Lim, L.P.; Lim, J.C.; Kuo, M.Y.; Wang, C.H. Sequential platelet-derived growth factor-simvastatin release promotes dentoalveolar regeneration. Tissue Eng. A 2014, 20, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Kruger, E.A.; Figg, W.D. Protein binding alters the activity of suramin, carboxyamidotriazole, and UCN-01 in an ex vivo rat aortic ring angiogenesis assay. Clin. Cancer Res. 2001, 7, 1867–1872. [Google Scholar] [PubMed]
- Song, S.H.; Yu, B.; Wei, Y.; Wientjes, M.G.; An, J. Low-dose suramin enhanced paclitaxel activity in chemotherapy-naive and paclitaxel-pretreated human breast xenograft tumors. Clin. Cancer Res. 2004, 10, 6058–6065. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Fu, Y.; Wang, C. Paclitaxel and suramin-loaded core/shell microspheres in the treatment of brain tumors. Biomaterials 2010, 31, 8732–8740. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Dong, Z.; Arifin, D.Y.; Hu, Y.; Wang, C. Core/shell microspheres via coaxial electrohydrodynamic atomization for sequential and parallel release of drugs. J. Biomed. Mater. Res. 2010, 95, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, E.; Karavasilis, V.; Tzamakou, E.; Rammou, D.; Soulti, K.; Piperidou, C.; Pavlidis, N. Interaction pharmacokinetics of pegylated liposomal doxorubicin (Caelyx) on coadministration with paclitaxel or docetaxel. Cancer Chemoth. Pharm. 2004, 53, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J.; Boesgaard, M.; Paaske, T.; Vittrup Jensen, B.; Dombernowsky, P. Combined doxorubicin and paclitaxel in advanced breast cancer: effective and cardiotoxic. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1996, 7, 687–693. [Google Scholar] [CrossRef]
- Gustafson, D.L.; Merz, A.L.; Long, M.E. Pharmacokinetics of combined doxorubicin and paclitaxel in mice. Cancer Lett. 2005, 220, 161–169. [Google Scholar] [CrossRef]
- Mavroudis, D.; Kouroussis, C.; Kakolyris, S.; Agelaki, S.; Kalbakis, K.; Androulakis, N.; Souglakos, J.; Samonis, G.; Georgoulias, V. Phase I study of paclitaxel (taxol) and pegylated liposomal doxorubicin (Caelyx) administered every 2 weeks in patients with advanced solid tumors. Oncol.-Basel 2002, 62, 216–222. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.S. Synthesis of biodegradable triple-layered capsules using a triaxial electrospray method. Polymer 2011, 52, 3325–3336. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, B.; Cao, Y.; Yu, Q.; Yin, T. Fabrication of core-shell micro/nanoparticles for programmable dual drug release by emulsion electrospraying. J. Nanopart. Res. 2013, 15, 1726. [Google Scholar] [CrossRef]
- Roh, K.H.; Martin, D.C.; Lahann, J. Biphasic Janus particles with nanoscale anisotropy. Nat. Mater. 2005, 4, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.H.; Martin, D.C.; Lahann, J. Triphasic nanocolloids. J. Am. Chem. Soc. 2006, 128, 6796–6797. [Google Scholar] [CrossRef] [PubMed]
- Roh, K.H.; Yoshida, M.; Lahann, J. Water-stable biphasic nanocolloids with potential use as anisotropic imaging probes. Langmuir 2007, 23, 5683–5688. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.; Roh, K.H.; Jiang, X.W.; Baker, G.L.; Lahann, J. Spatioselective modification of bicompartmental polymer particles and bers via huisgen 1, 3-dipolar cycloaddition. Macromol. Rapid Comm. 2008, 29, 1655–1660. [Google Scholar] [CrossRef]
- Bhaskar, S.; Pollock, K.M.; Yoshida, M.; Lahann, J. Towards designer microparticles: Simultaneous control of anisotropy, shape, and size. Small 2010, 6, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Roh, K.; Lim, D.W.; Wang, G.; Uher, C.; Lahann, J. Anisotropic hybrid particles based on electrohydrodynamic co-jetting of nanoparticle suspensions. Phys. Chem. 2010, 12, 11894–11899. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Hwang, S.; Uzun, O.; Stellacci, F.; Lahann, J. Compartmentalization of gold nanocrystals in polymer microparticles using electrohydrodynamic co-jetting. Macromol Rapid Commun. 2010, 31, 176–182. [Google Scholar] [CrossRef]
- Yoshida, M.; Roh, K.H.; Mandal, S.; Bhaskar, S.; Lim, D.; Nandivada, H.; Deng, X.; Lahann, J. Structurally controlled bio-hybrid materials based on unidirectional association of anisotropic microparticles with human endothelial cells. Adv. Mater. 2009, 21, 4920–4925. [Google Scholar] [CrossRef]
- Rahmani, S.; Villa, C.H.; Dishman, A.F.; Grabowski, M.E.; Pan, D.C.; Durmaz, H.; Misra, A.C.; Colon-Melendez, L.; Solomon, M.J.; Muzykantov, V.R.; Lahann, J. Long-circulating Janus nanoparticles made by electrohydrodynamic Co-jetting for systemic drug delivery applications. J. Drug Target 2015, 23, 750–758. [Google Scholar] [CrossRef]
- George, M.C.; Braun, P.V. Multicompartmental materials by electrohydrodynamic cojetting. Angew Chem. Int. Ed. Engl. 2009, 48, 8606–8609. [Google Scholar] [CrossRef] [PubMed]
- Lahann, J. Recent progress in nano-biotechnology: Compartmentalized micro- and nanoparticles via electrohydrodynamic Co-jetting. Small 2011, 7, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Liu, W.; Jiang, P.; Hong, T. Coaxial Electrohydrodynamic Atomization for the Production of Drug-Loaded Micro/Nanoparticles. Micromachines 2019, 10, 125. https://doi.org/10.3390/mi10020125
Chen C, Liu W, Jiang P, Hong T. Coaxial Electrohydrodynamic Atomization for the Production of Drug-Loaded Micro/Nanoparticles. Micromachines. 2019; 10(2):125. https://doi.org/10.3390/mi10020125
Chicago/Turabian StyleChen, Chuanpin, Wenfang Liu, Ping Jiang, and Tingting Hong. 2019. "Coaxial Electrohydrodynamic Atomization for the Production of Drug-Loaded Micro/Nanoparticles" Micromachines 10, no. 2: 125. https://doi.org/10.3390/mi10020125
APA StyleChen, C., Liu, W., Jiang, P., & Hong, T. (2019). Coaxial Electrohydrodynamic Atomization for the Production of Drug-Loaded Micro/Nanoparticles. Micromachines, 10(2), 125. https://doi.org/10.3390/mi10020125





