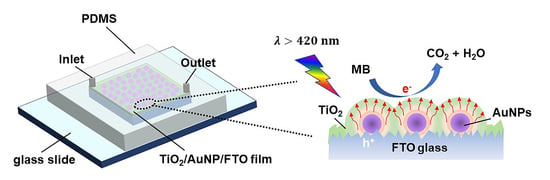
Microfluidic Reactors for Plasmonic Photocatalysis Using Gold Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
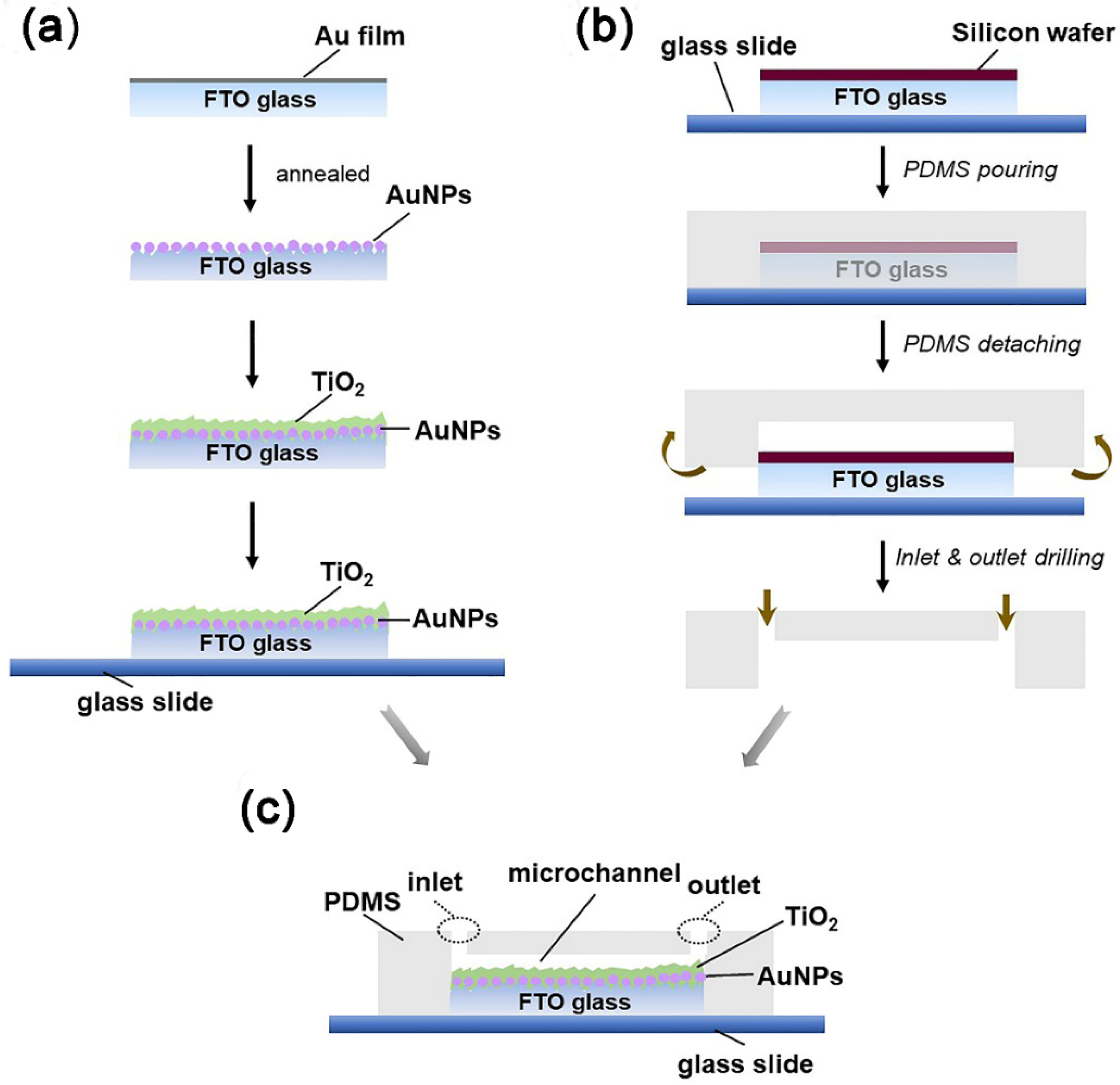
2.1. Fabrication of Au Nanoparticles and TiO2 Films
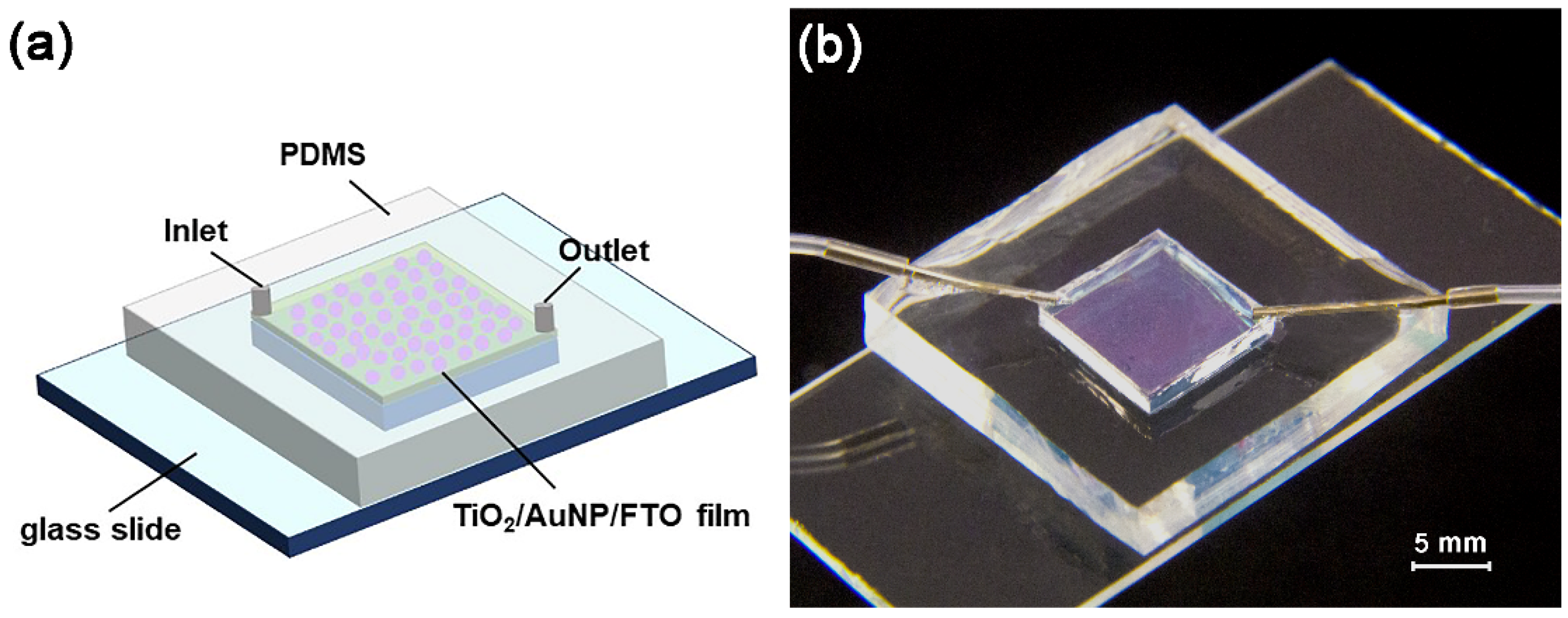
2.2. Fabrication of Microreactors
- A piece of FTO block (dimensions 10 × 10 × 2.2 mm) is cut from an FTO glass.
- Another piece of thin silicon wafer (thickness = 0.46 mm) of the same footprint is mounted on top of the FTO block by NOA81.
- The silicon/FTO block is further adhered to a glass slide by NOA81.
2.3. Photocatalytic Degradation Experiment
3. Results and Discussion
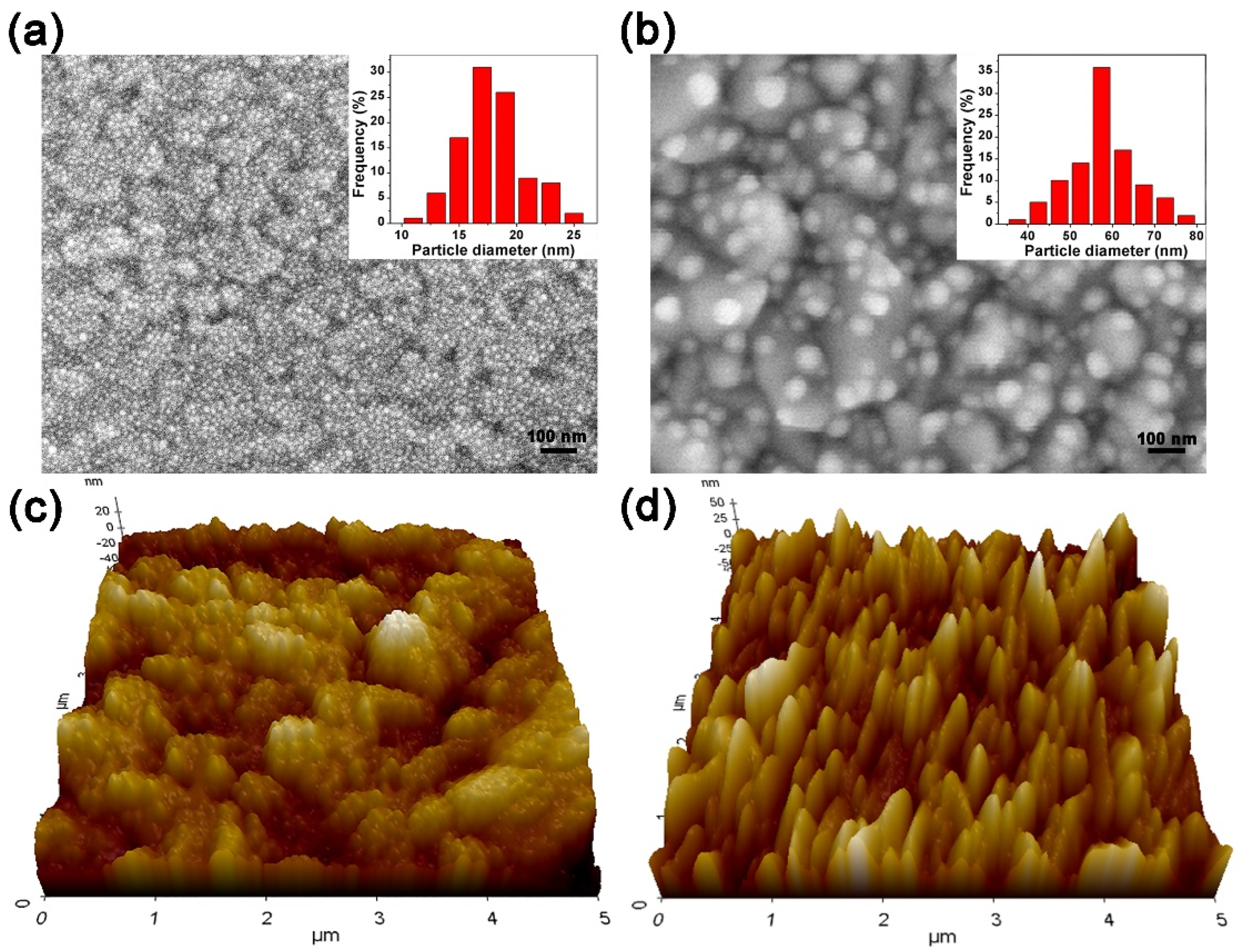
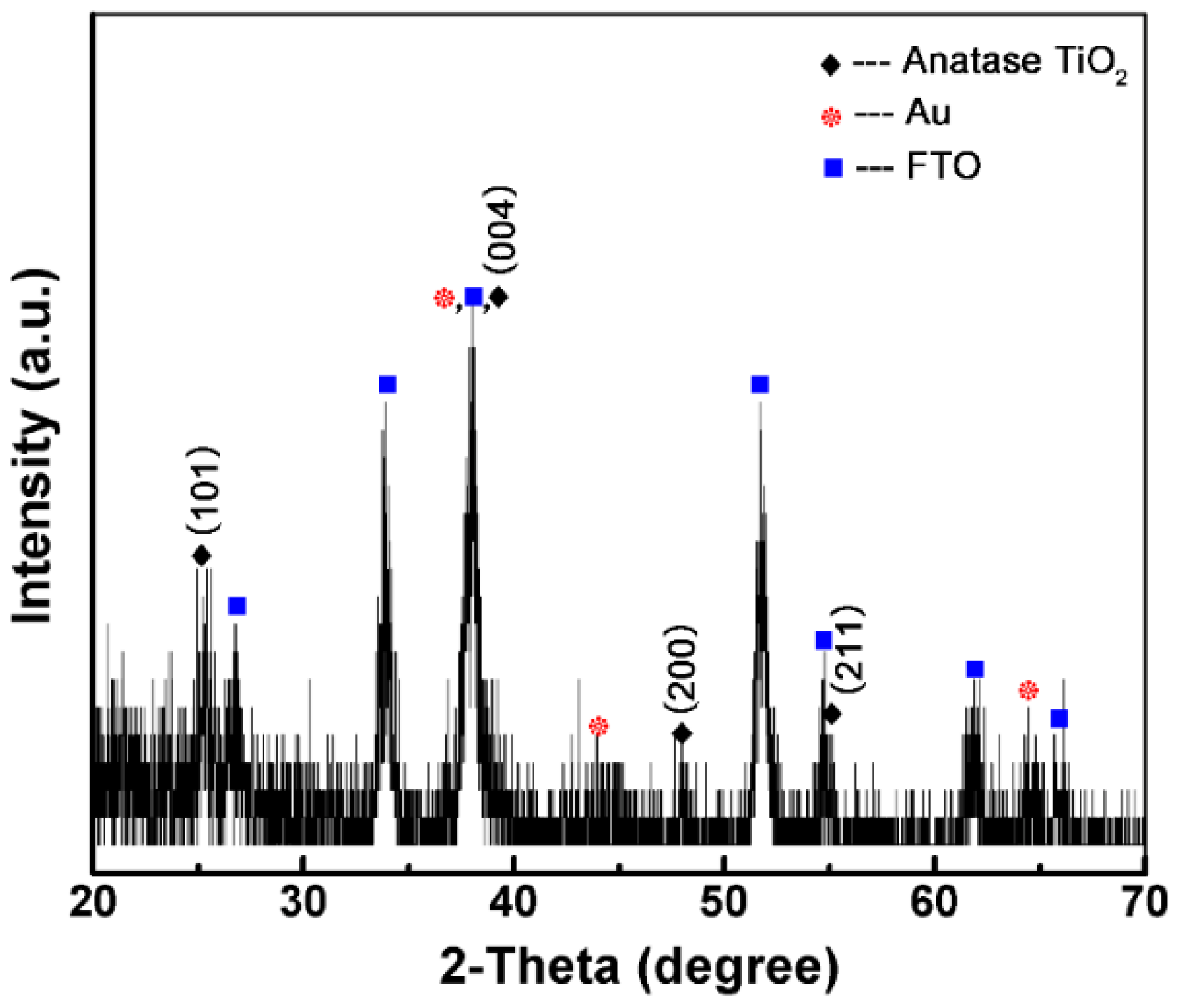
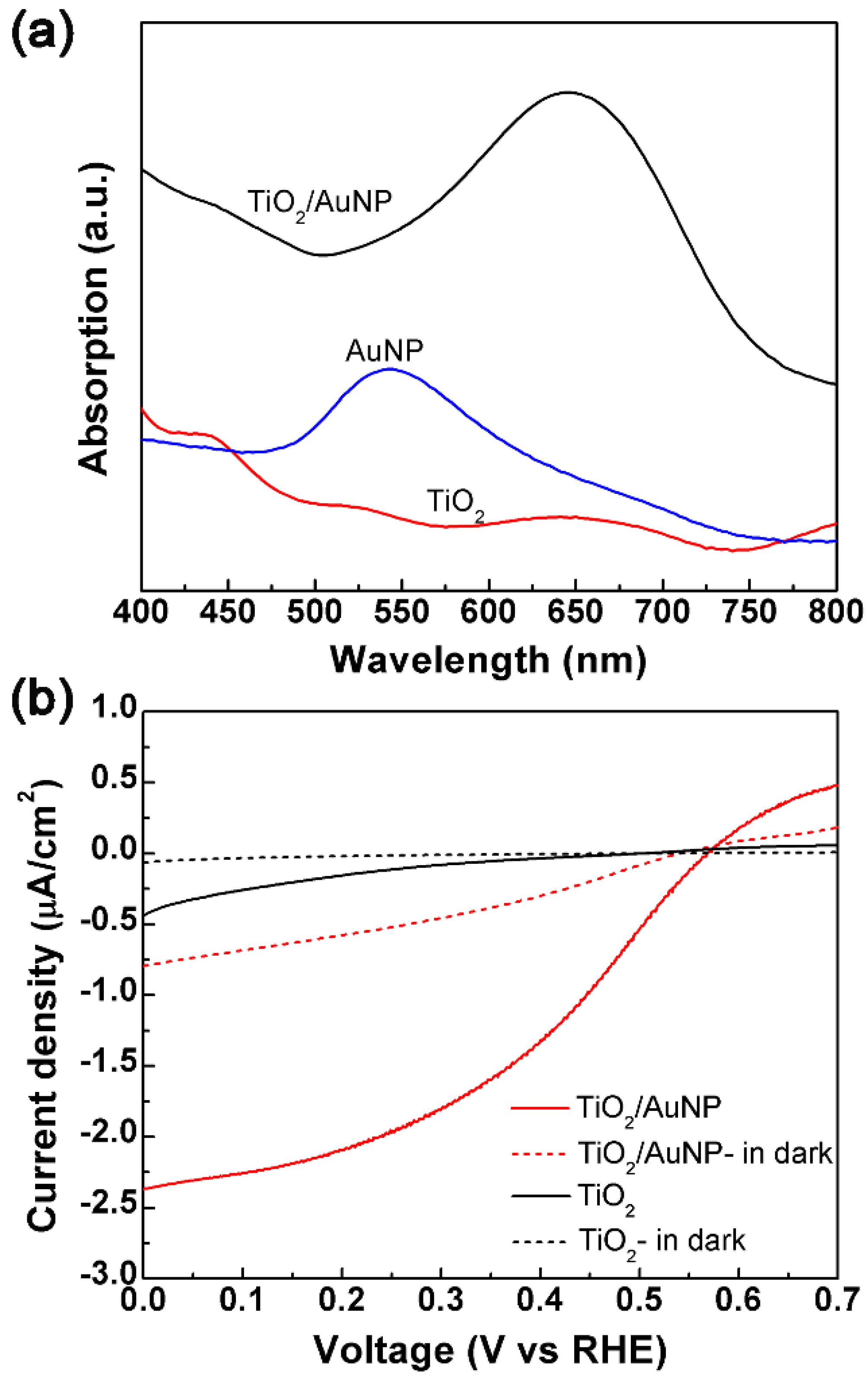
3.1. Material Characterization
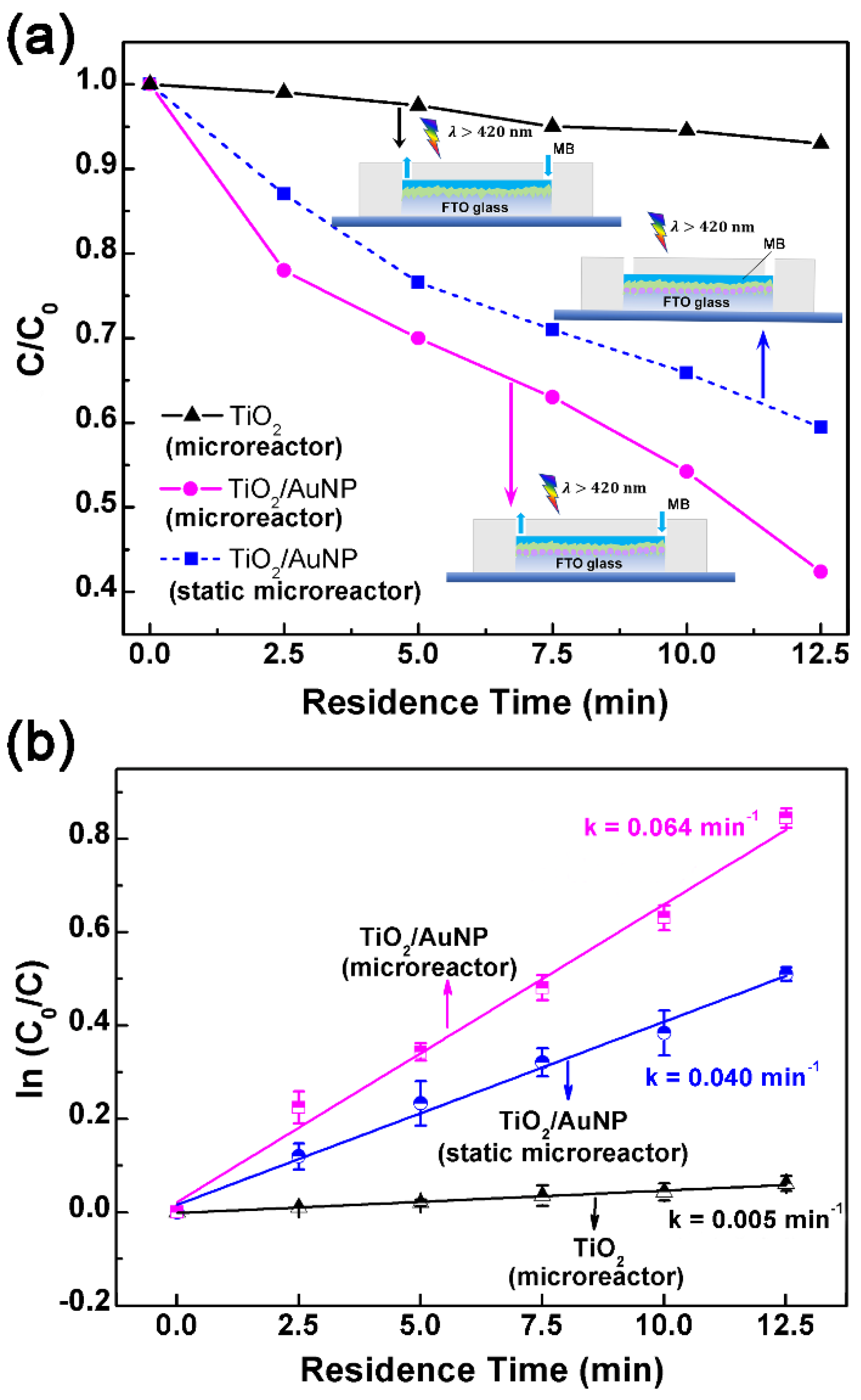
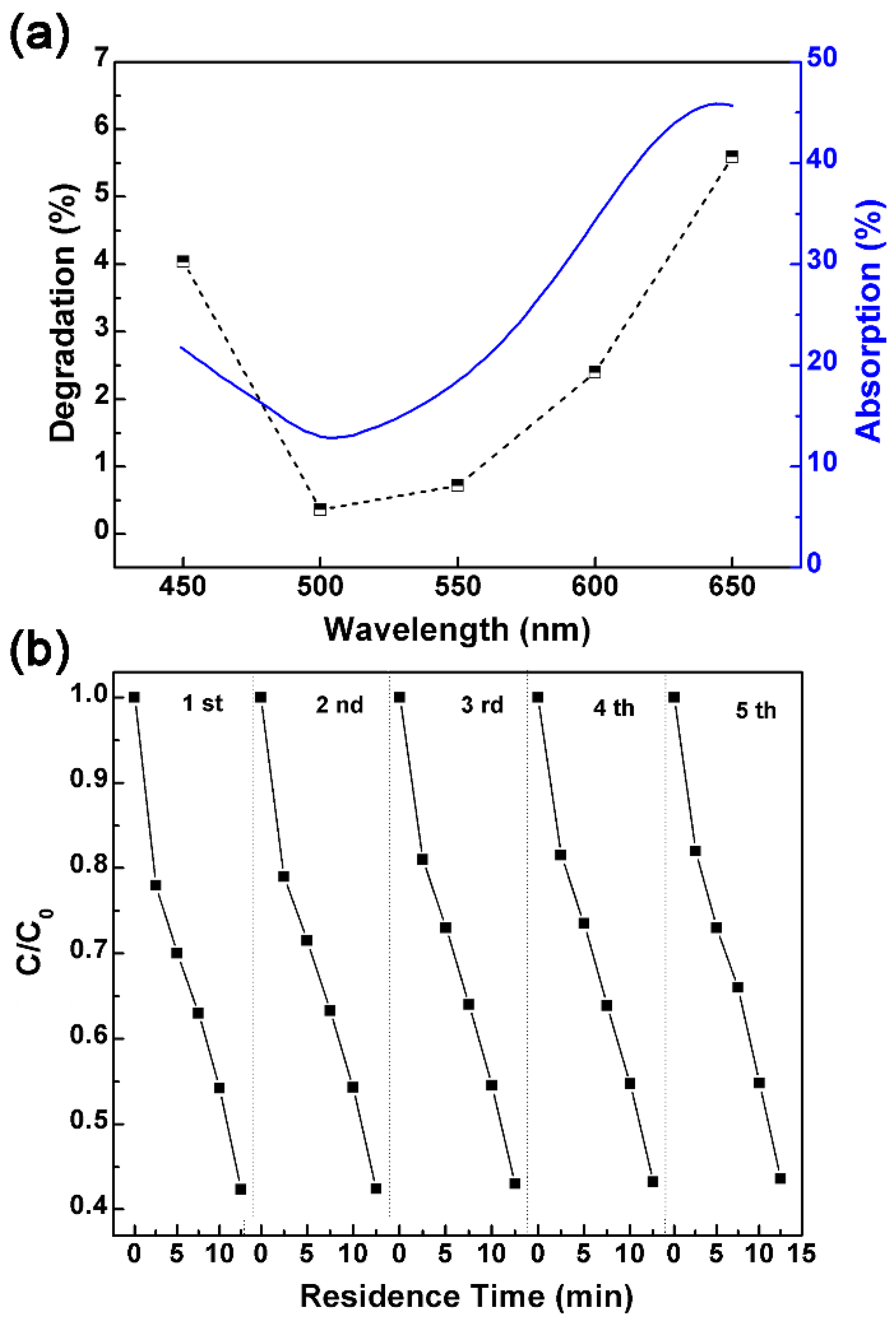
3.2. Photodegradation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shi, W.; Song, Y.; Zhang, X.; Duan, D.; Wang, H.; Sun, Z. Nanoporous Pt/TiO2 nanocomposites with greatly enhanced photocatalytic performance. J. Chin. Chem. Soc. 2018, 65, 1286–1292. [Google Scholar] [CrossRef]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A review of intensification of photocatalytic processes. Chem. Eng. Process. Process Intensif. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Parmar, J.; Jang, S.; Soler, L.; Kim, D.P.; Sánchez, S. Nano-photocatalysts in microfluidics, energy conversion and environmental applications. Lab Chip 2015, 15, 2352–2356. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lei, L.; Zhang, X.M.; Tsang, Y.H.; Chen, Y.; Chan, H.L.W. A comparative study of preparation methods of nanoporous TiO2 films for microfluidic photocatalysis. Microelectron. Eng. 2011, 88, 2797–2799. [Google Scholar] [CrossRef]
- Ling, C.M.; Mohamed, A.R.; Bhatia, S. Performance of photocatalytic reactors using immobilized TiO2 film for the degradation of phenol and methylene blue dye present in water stream. Chemosphere 2004, 57, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J. Photocatalytic activity of Ag/ZnO heterostructure nanocatalyst: Correlation between structure and property. J. Phys. Chem. C 2008, 112, 10773–10777. [Google Scholar] [CrossRef]
- Fragua, D.M.; Abargues, R.; Rodriguez-Canto, P.J.; Sanchez-Royo, J.F.; Agouram, S.; Martinez-Pastor, J.P. Au-ZnO Nanocomposite Films for Plasmonic Photocatalysis. Adv. Mater. Interfaces 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.-S.; Tsai, D.P. Plasmonic photocatalysis. Reports Prog. Phys. 2013, 76, 46401. [Google Scholar] [CrossRef]
- Ho, K.H.W.; Shang, A.; Shi, F.; Lo, T.W.; Yeung, P.H.; Yu, Y.S.; Zhang, X.; Wong, K.Y.; Lei, D.Y. Plasmonic Au/TiO2-dumbbell-on-film nanocavities for high-efficiency hot-carrier generation and extraction. Adv. Funct. Mater. 2018, 28, 1–10. [Google Scholar]
- Dinh, C.T.; Yen, H.; Kleitz, F.; Do, T.O. Three-dimensional ordered assembly of thin-shell Au/TiO2 hollow nanospheres for enhanced visible-light-driven photocatalysis. Angew. Chemie Int. Ed. 2014, 53, 6618–6623. [Google Scholar] [CrossRef]
- Tan, F.; Wang, N.; Lei, D.Y.; Yu, W.; Zhang, X. Plasmonic black absorbers for enhanced photocurrent of visible-light photocatalysis. Adv. Opt. Mater. 2017, 5, 1600399. [Google Scholar] [CrossRef]
- Dijkstra, M.F.J.; Panneman, H.J.; Winkelman, J.G.M.; Beenackers, A.A.C.M.; Kelly, J.J. Modeling the photocatalytic degradation of formic acid in a reactor with immobilized catalyst. Chem. Eng. Sci. 2002, 57, 4895–4907. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J. Photomineralisation of 4-chlorophenol sensitised by TiO2 thin films. J. Photochem. Photobiol. A Chem. 1998, 118, 53–63. [Google Scholar] [CrossRef]
- Huang, X.; Hao, H.; Liu, Y.; Zhu, Y.; Zhang, X. Rapid screening of graphitic carbon nitrides for photocatalytic cofactor regeneration using a drop reactor. Micromachines 2017, 8, 175. [Google Scholar] [CrossRef]
- Lindstrom, H.; Wootton, R.; Iles, A. High surface area titania photocatalytic microfluidic reactors. AIChE J. 2007, 53, 695–702. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Wang, Y.; Yu, W.; Chan, H.L.W. Microfluidic reactors for photocatalytic water purification. Lab Chip 2014, 14, 1074–1082. [Google Scholar] [CrossRef]
- Wang, N.; Tan, F.; Wan, L.; Wu, M.; Zhang, X. Microfluidic reactors for visible-light photocatalytic water purification assisted with thermolysis. Biomicrofluidics 2014, 8, 54122. [Google Scholar] [CrossRef]
- Wang, N.; Tan, F.; Tsoi, C.C.; Zhang, X. Photoelectrocatalytic microreactor for seawater decontamination with negligible chlorine generation. Microsyst. Technol. 2017, 23, 4495–4500. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Chen, B.; Song, W.; Chan, N.Y.; Chan, H.L.W. Microfluidic photoelectrocatalytic reactors for water purification with an integrated visible-light source. Lab Chip 2012, 12, 3983–3990. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Z.; Chen, Q.; Wu, Q.; Huang, X.; So, P.-K.; Shao, L.; Yao, Z.; Jia, Y.; Li, Z.; et al. Continuous artificial synthesis of glucose precursor using enzyme-immobilized microfluidic reactors. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Matsushita, Y.; Ichimura, T.; Ohba, N.; Kumada, S.; Sakeda, K.; Suzuki, T.; Tanibata, H.; Murata, T. Recent progress on photoreactions in microreactors. Pure Appl. Chem. 2007, 79, 1959–1968. [Google Scholar] [CrossRef]
- Liu, A.L.; Li, Z.Q.; Wu, Z.Q.; Xia, X.H. Study on the photocatalytic reaction kinetics in a TiO2 nanoparticles coated microreactor integrated microfluidics device. Talanta 2018, 182, 544–548. [Google Scholar] [CrossRef]
- Liao, W.; Wang, N.; Wang, T.; Xu, J.; Han, X.; Liu, Z.; Zhang, X.; Yu, W. Biomimetic microchannels of planar reactors for optimized photocatalytic efficiency of water purification. Biomicrofluidics 2016, 10, 14123. [Google Scholar] [CrossRef] [PubMed]
- Wootton, R.C.R.; Fortt, R.; De Mello, A.J. On-chip generation and reaction of unstable intermediates—Monolithic nanoreactors for diazonium chemistry: Azo dyes. Lab Chip 2002, 2, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, Q.; Shao, L.; Jia, Y.; Zhang, X. Microfluidic immobilized enzyme reactors for continuous biocatalysis. React. Chem. Eng. 2019, in press. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Li, T.; Wang, J.; Xu, M.; Yu, W.; El Abed, A.; Zhang, X. Review on optofluidic microreactors for artificial photosynthesis. Beilstein J. Nanotechnol. 2018, 9, 30–41. [Google Scholar] [CrossRef]
- Sakai, N.; Fujiwara, Y.; Takahashi, Y.; Tatsuma, T. Plasmon-Resonance-based generation of cathodic photocurrent at electrodeposited gold nanoparticles coated with TiO2 films. ChemPhysChem 2009, 10, 766–769. [Google Scholar] [CrossRef]
- Chen, H.; Liu, G.; Wang, L. Switched photocurrent direction in Au/TiO2 bilayer thin films. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Huang, J.; He, Y.; Wang, L.; Huang, Y.; Jiang, B. Bifunctional Au@TiO2 core–shell nanoparticle films for clean water generation by photocatalysis and solar evaporation. Energy Convers. Manag. 2017, 132, 452–459. [Google Scholar] [CrossRef]
- Naik, G.K.; Mishra, P.M.; Parida, K. Green synthesis of Au/TiO2 for effective dye degradation in aqueous system. Chem. Eng. J. 2013, 229, 492–497. [Google Scholar] [CrossRef]
- Della Gaspera, E.; Karg, M.; Baldauf, J.; Jasieniak, J.; Maggioni, G.; Martucci, A. Au nanoparticle monolayers covered with sol-gel oxide thin films: Optical and morphological study. Langmuir 2011, 27, 13739–13747. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chae, L.; Lee, K.C. Microstructure and surface plasmon absorption of sol-gel-prepared Au nanoclusters in TiO2 thin films. Nanostructured Mater. 1999, 11, 195–201. [Google Scholar] [CrossRef]
- Lei, L.; Wang, N.; Zhang, X.M.; Tai, Q.; Tsai, D.P.; Chan, H.L.W. Optofluidic planar reactors for photocatalytic water treatment using solar energy. Biomicrofluid. 2010, 4, 043004. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, A. Microfluidic photocatalytic device exploiting PDMS/TiO2 nanocomposite. Appl. Surf. Sci. 2015, 335, 50–54. [Google Scholar] [CrossRef]
- Su, Y.H.; Ke, Y.F.; Cai, S.L.; Yao, Q.Y. Surface plasmon resonance of layer-by-layer gold nanoparticles induced photoelectric current in environmentally-friendly plasmon-sensitized solar cell. Light Sci. Appl. 2012, 1, 2–6. [Google Scholar] [CrossRef]
- Xu, J.; Gu, P.; Birch, D.J.S.; Chen, Y. Plasmon-Promoted Electrochemical Oxygen Evolution Catalysis from Gold Decorated MnO2 Nanosheets under Green Light. Adv. Funct. Mater. 2018, 28, 1–7. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Wong, Y.L.; Jian, A.; Tsoi, C.C.; Wang, M.; Li, W.; Zhang, W.; Sang, S.; Zhang, X. Microfluidic Reactors for Plasmonic Photocatalysis Using Gold Nanoparticles. Micromachines 2019, 10, 869. https://doi.org/10.3390/mi10120869
Jia H, Wong YL, Jian A, Tsoi CC, Wang M, Li W, Zhang W, Sang S, Zhang X. Microfluidic Reactors for Plasmonic Photocatalysis Using Gold Nanoparticles. Micromachines. 2019; 10(12):869. https://doi.org/10.3390/mi10120869
Chicago/Turabian StyleJia, Huaping, Yat Lam Wong, Aoqun Jian, Chi Chung Tsoi, Meiling Wang, Wanghao Li, Wendong Zhang, Shengbo Sang, and Xuming Zhang. 2019. "Microfluidic Reactors for Plasmonic Photocatalysis Using Gold Nanoparticles" Micromachines 10, no. 12: 869. https://doi.org/10.3390/mi10120869
APA StyleJia, H., Wong, Y. L., Jian, A., Tsoi, C. C., Wang, M., Li, W., Zhang, W., Sang, S., & Zhang, X. (2019). Microfluidic Reactors for Plasmonic Photocatalysis Using Gold Nanoparticles. Micromachines, 10(12), 869. https://doi.org/10.3390/mi10120869