Characterization of a Fiber Bundle-Based Real-Time Ultrasound/Photoacoustic Imaging System and Its In Vivo Functional Imaging Applications
Abstract
1. Introduction
2. Materials and Methods
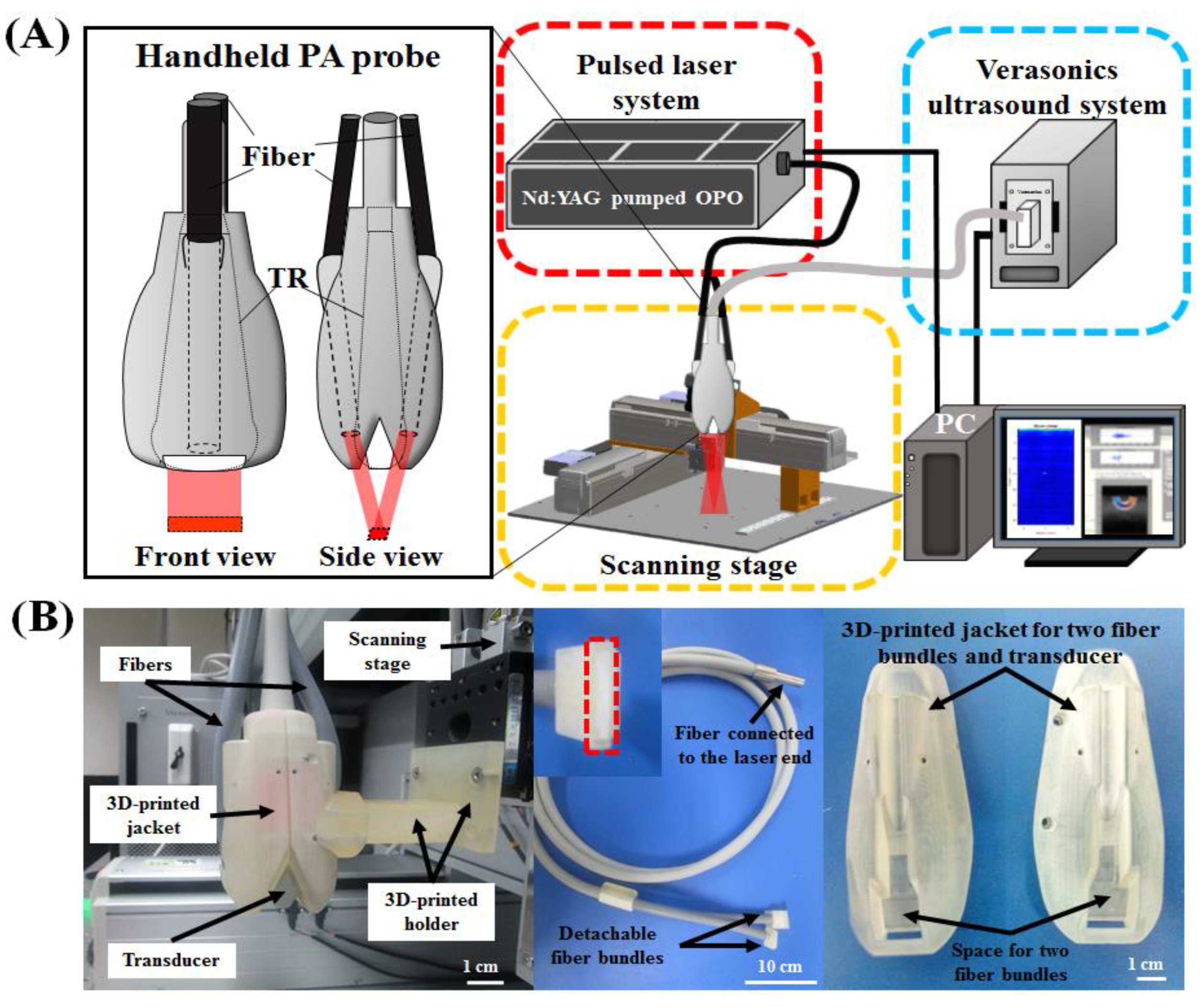
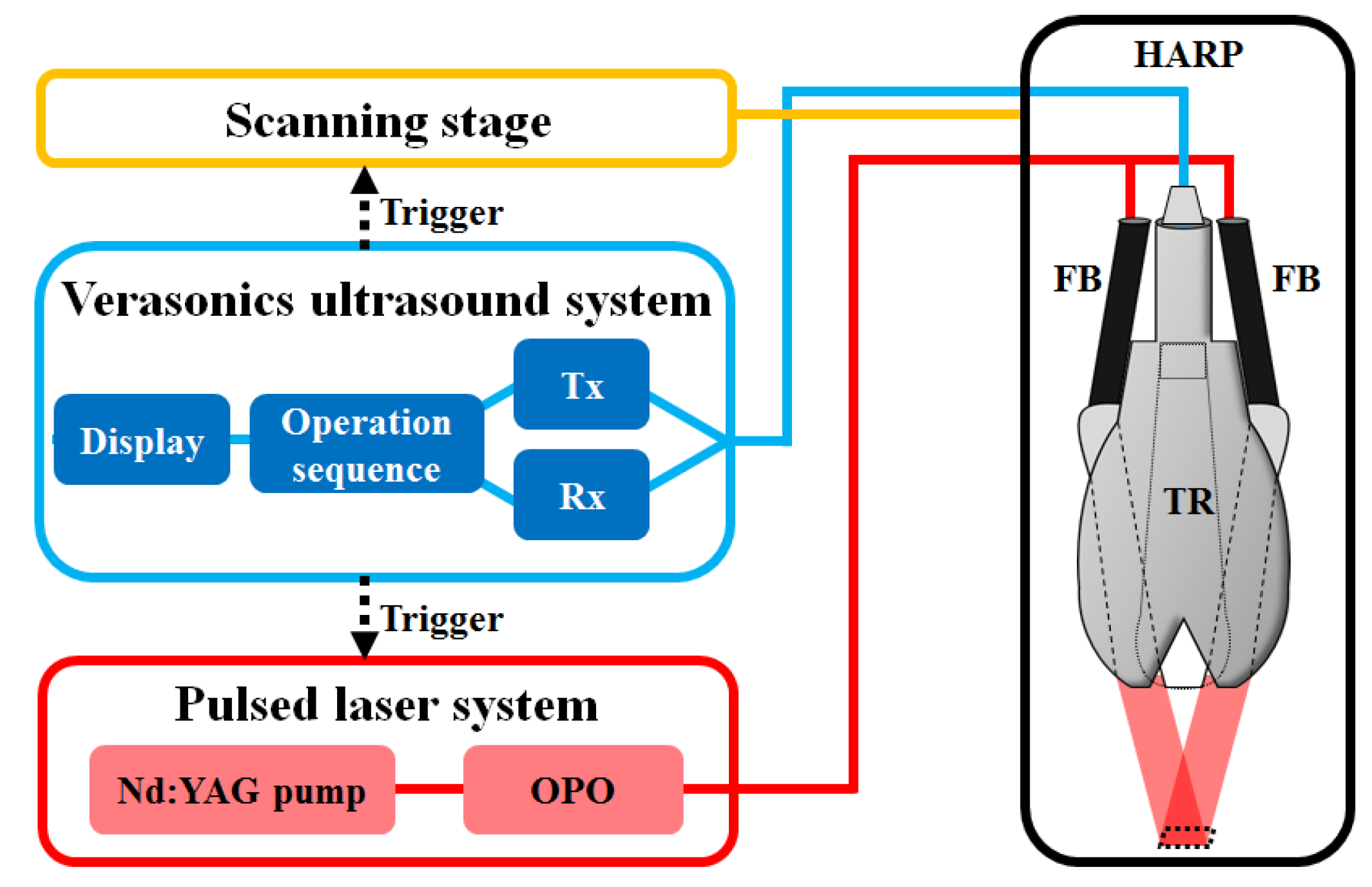
2.1. Handheld Dual-Modality Real-Time Ultrasound/Photoacoustic Imaging System with Fiber Bundle-Based Illumination
2.2. Design of the Photoacoustic Probe—Integration of the Fiber Bundle-Based Illumination System, 3-D-Printed Jacket and Ultrasound Transducer
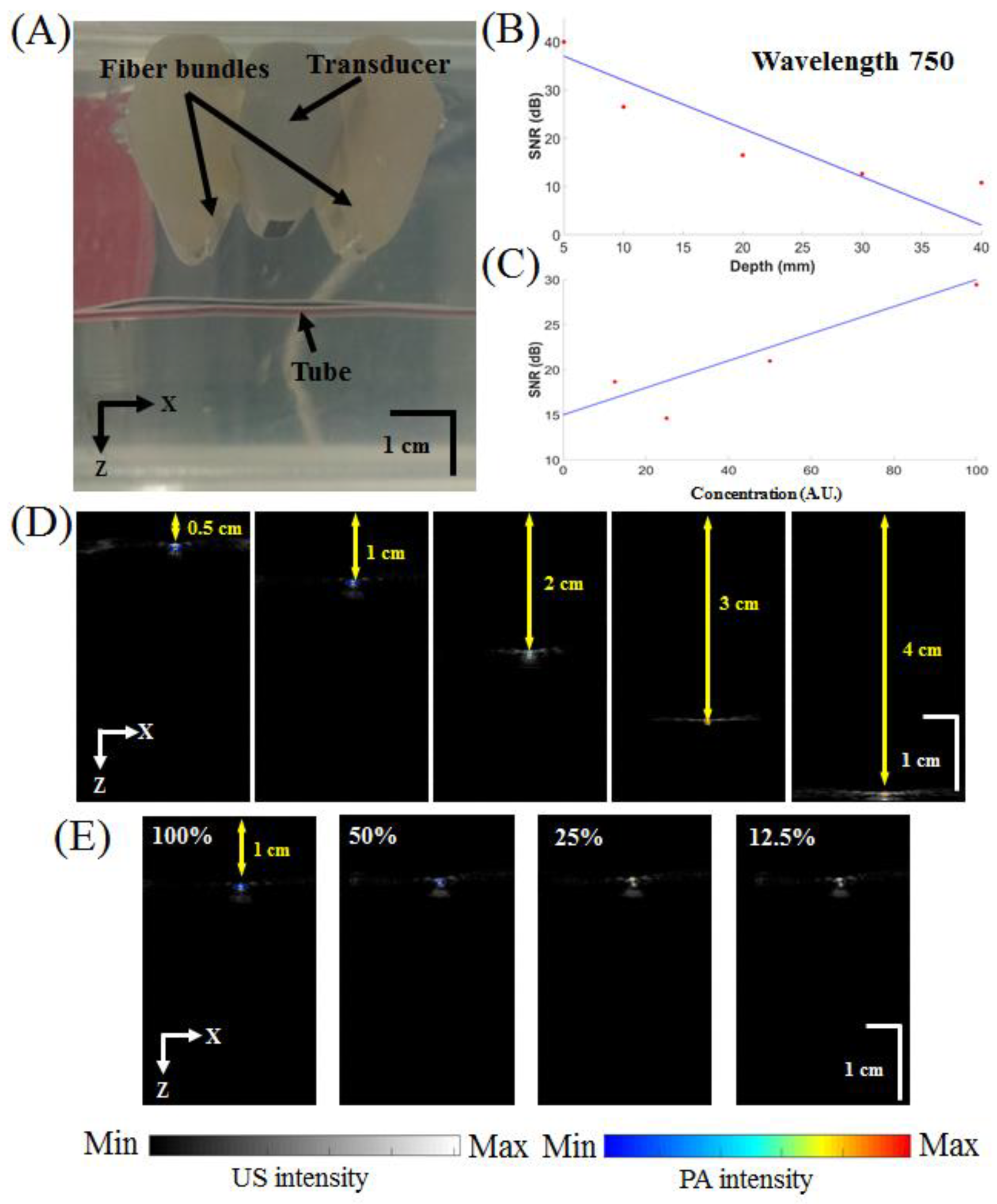
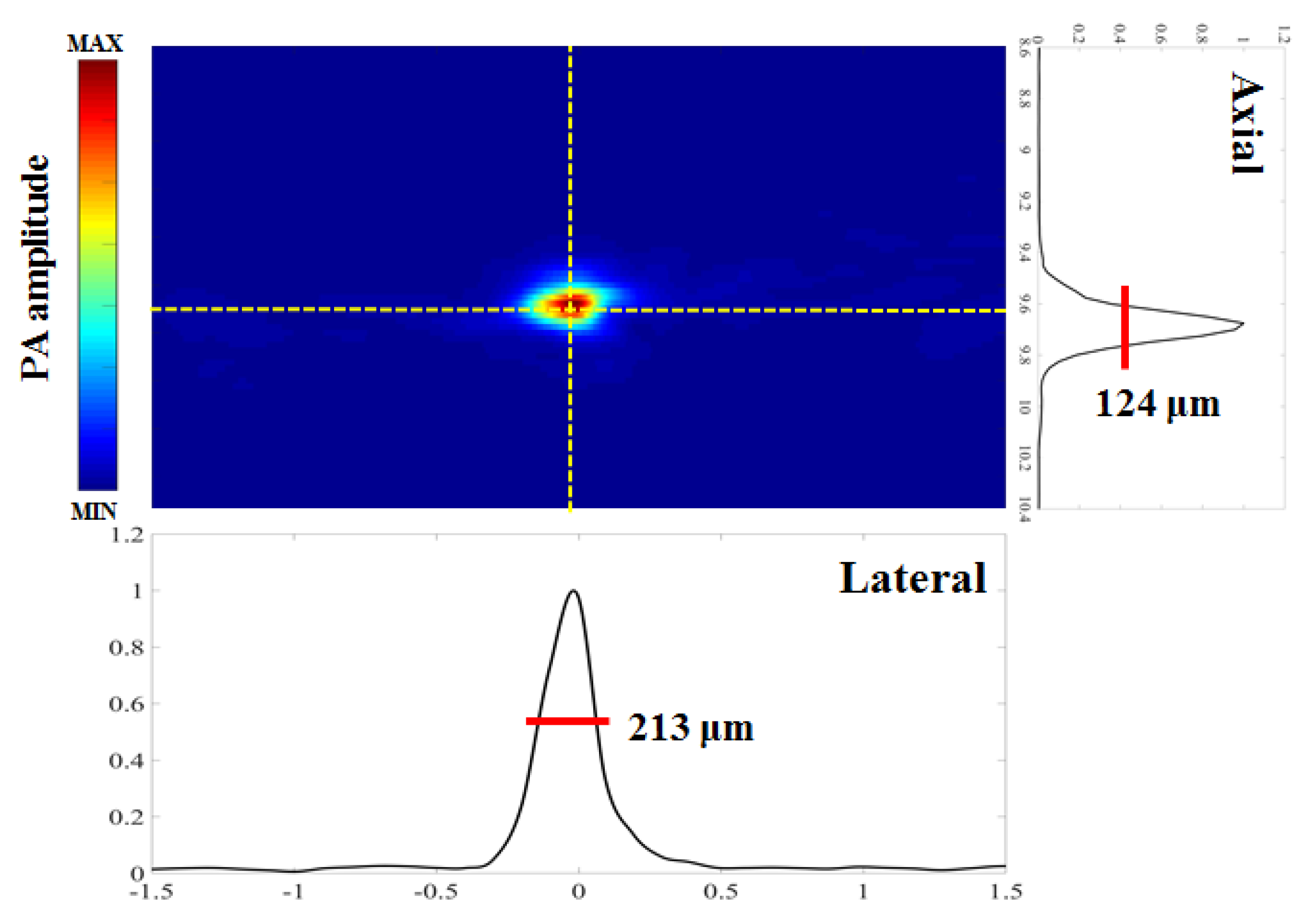
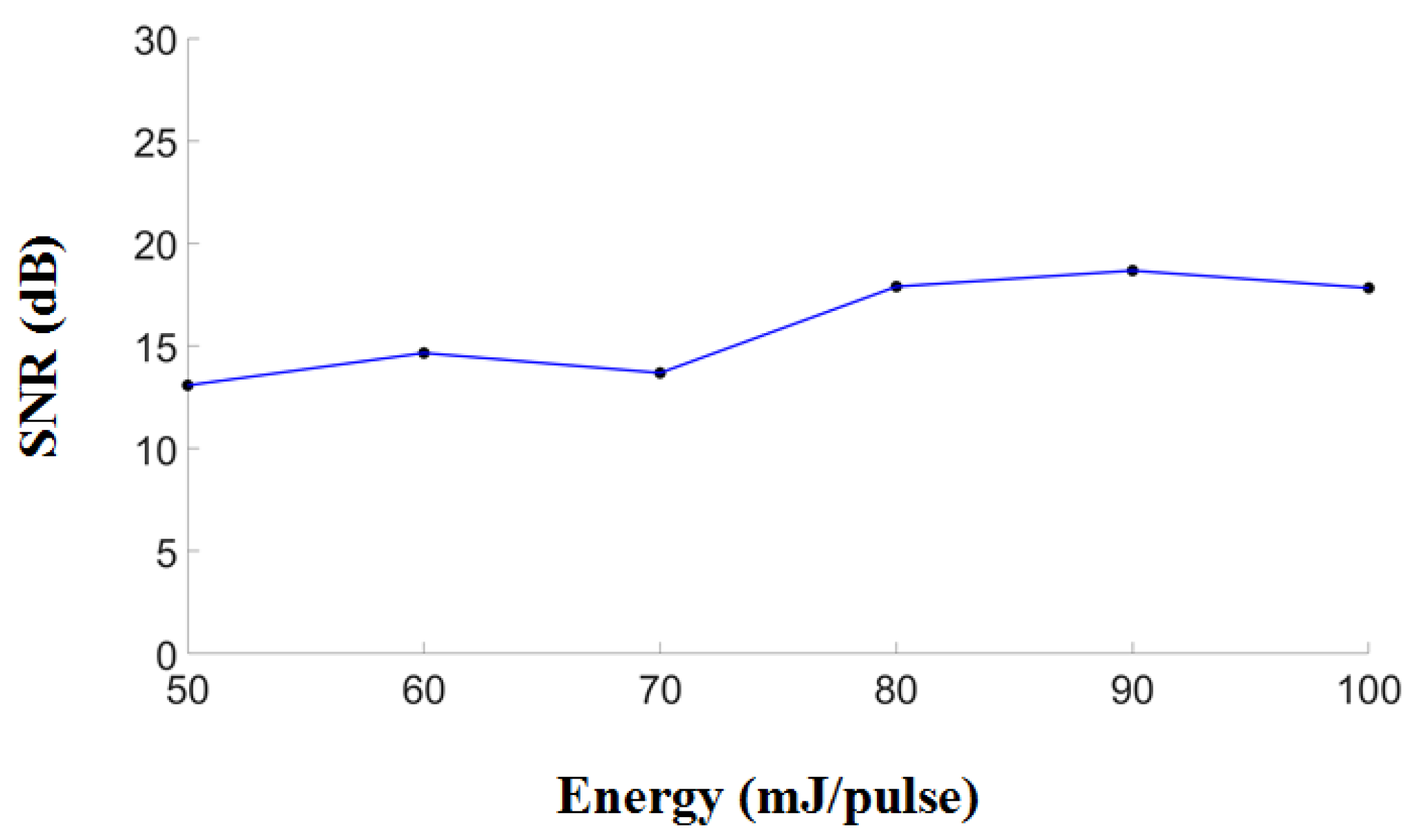
2.3. Performance Test of the Proposed Photoacoustic System
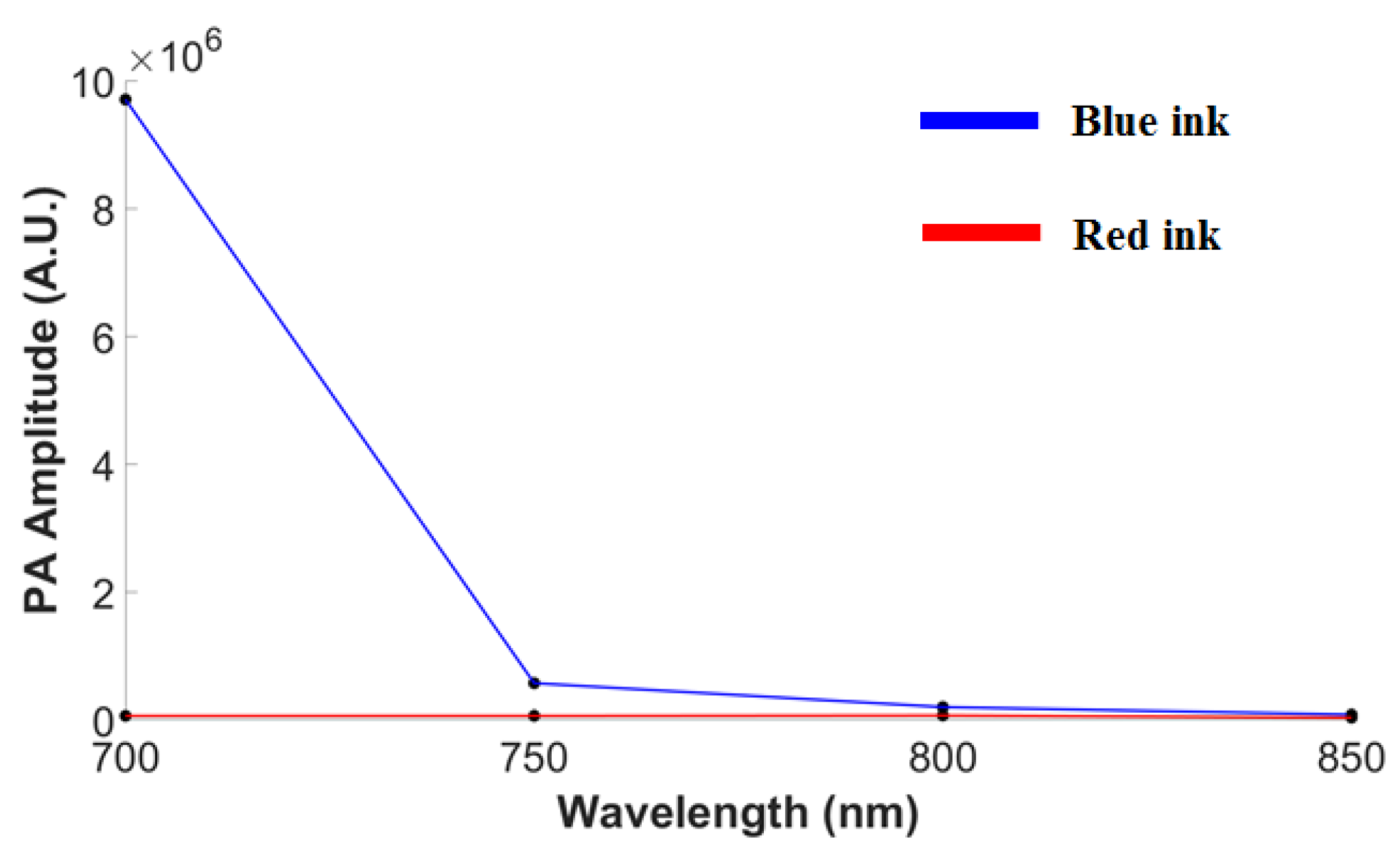
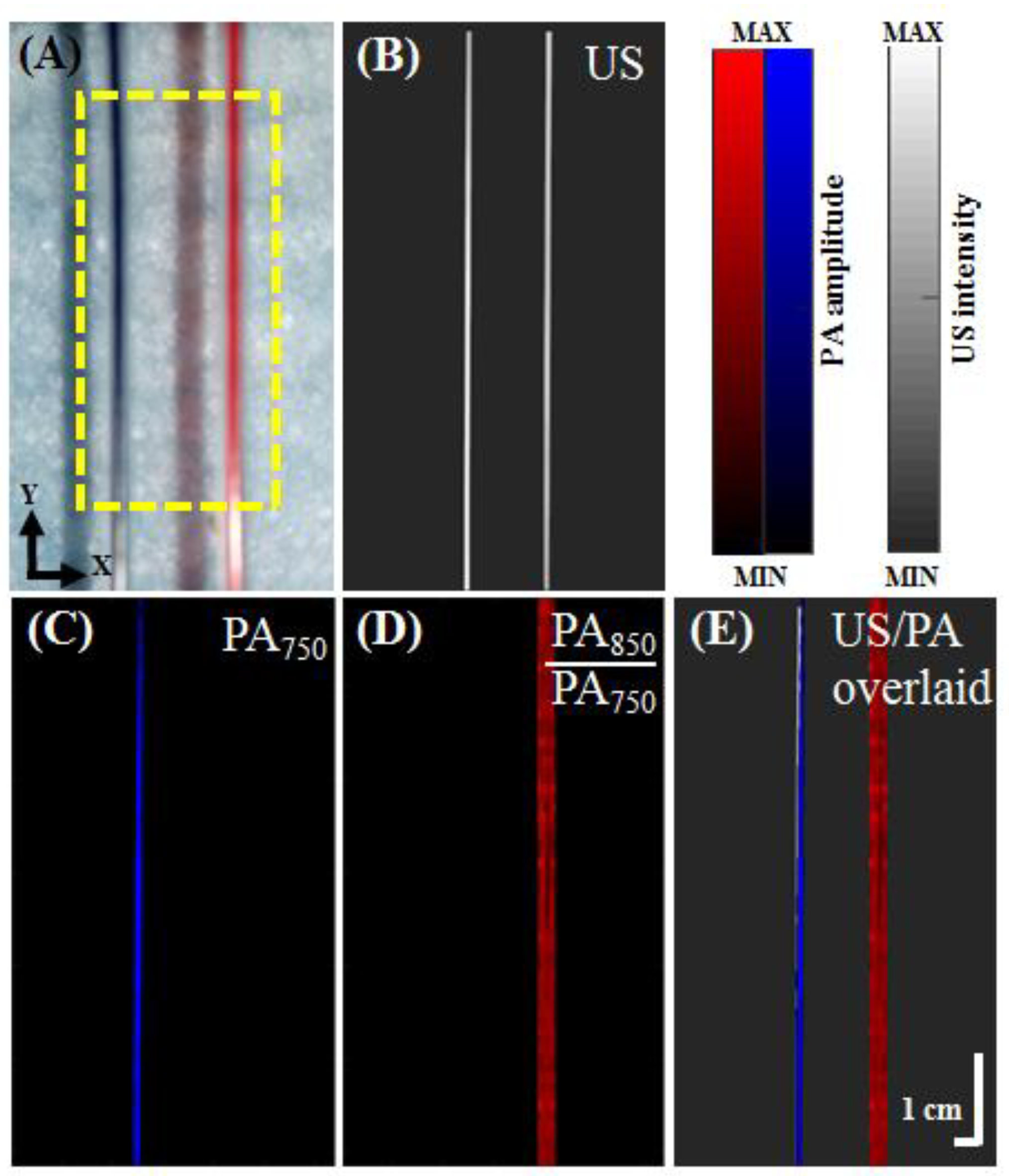
2.4. Photoacoustic Spectral Imaging of Blue and Red Inks
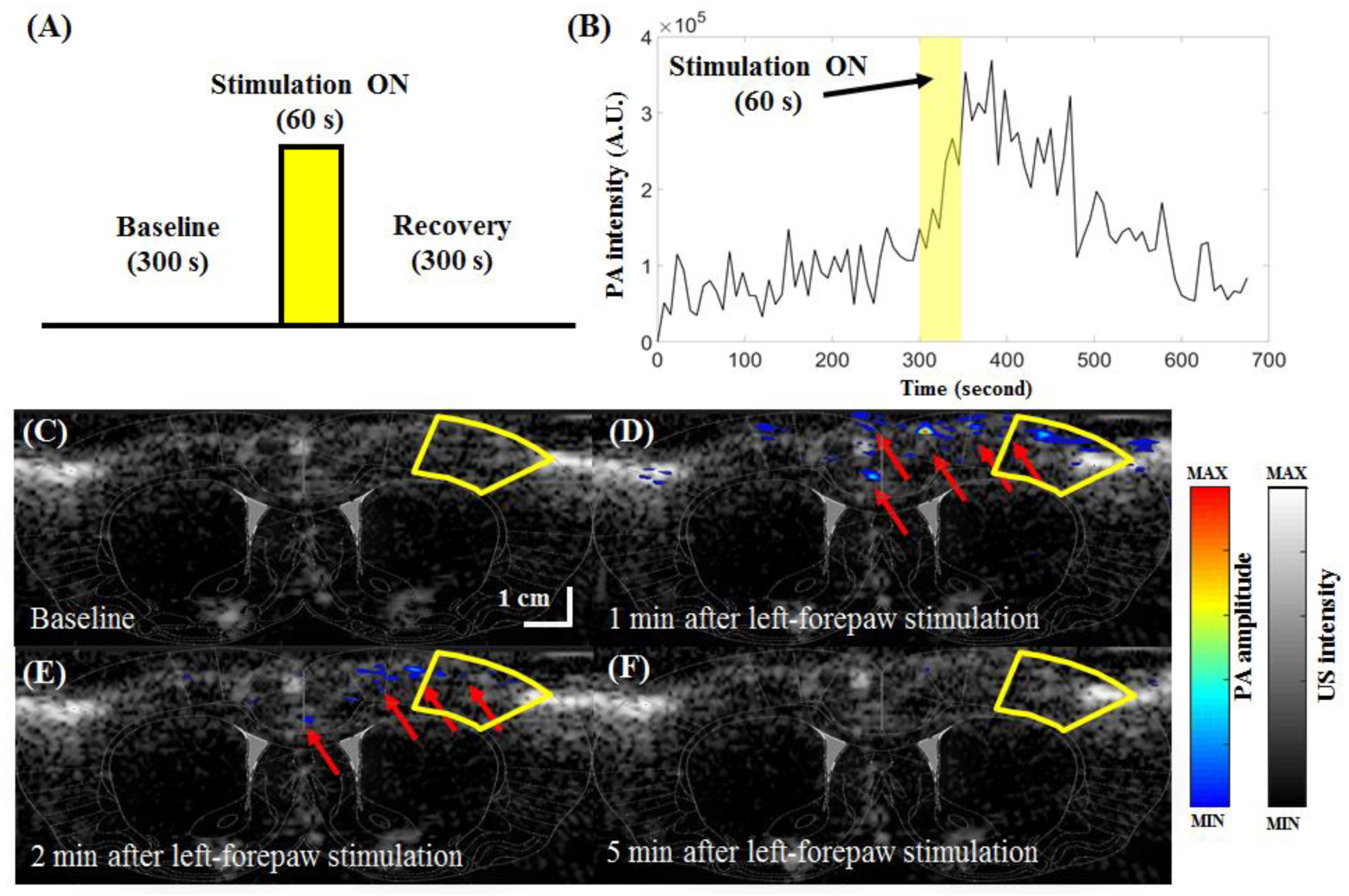
2.5. In Vivo Rat Brain Structure and Functional Imaging (Rat Left-Forepaw Electrical Stimulation)
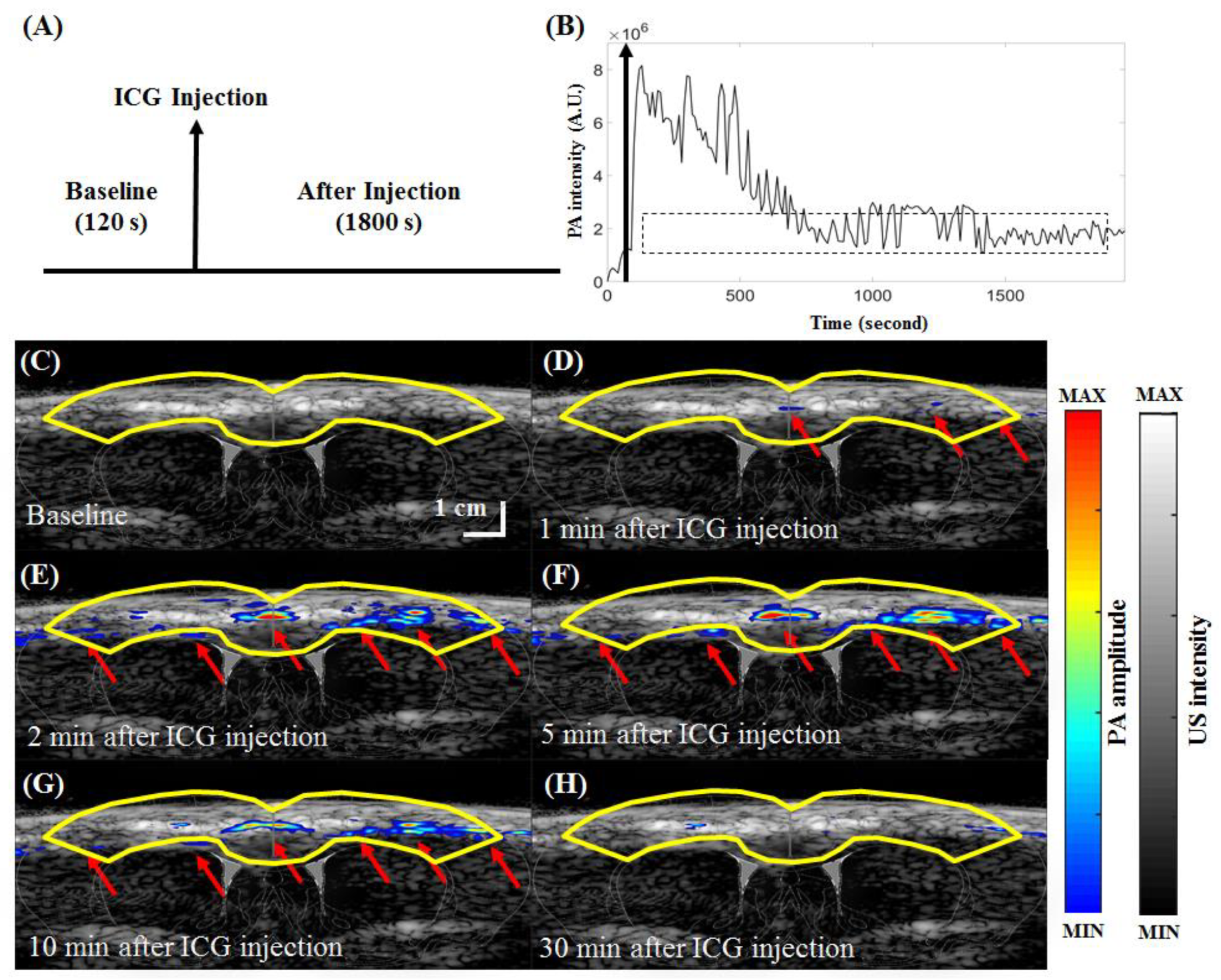
2.6. In Vivo Functional Indocyanine Green-Based Pharmacokinetic Imaging
3. Results and Discussion
3.1. Performance of the Developed Fully Programmable Ultrasound and Photoacoustic Imaging System
3.2. In Vitro Ink-Filled Tube Phantom Imaging
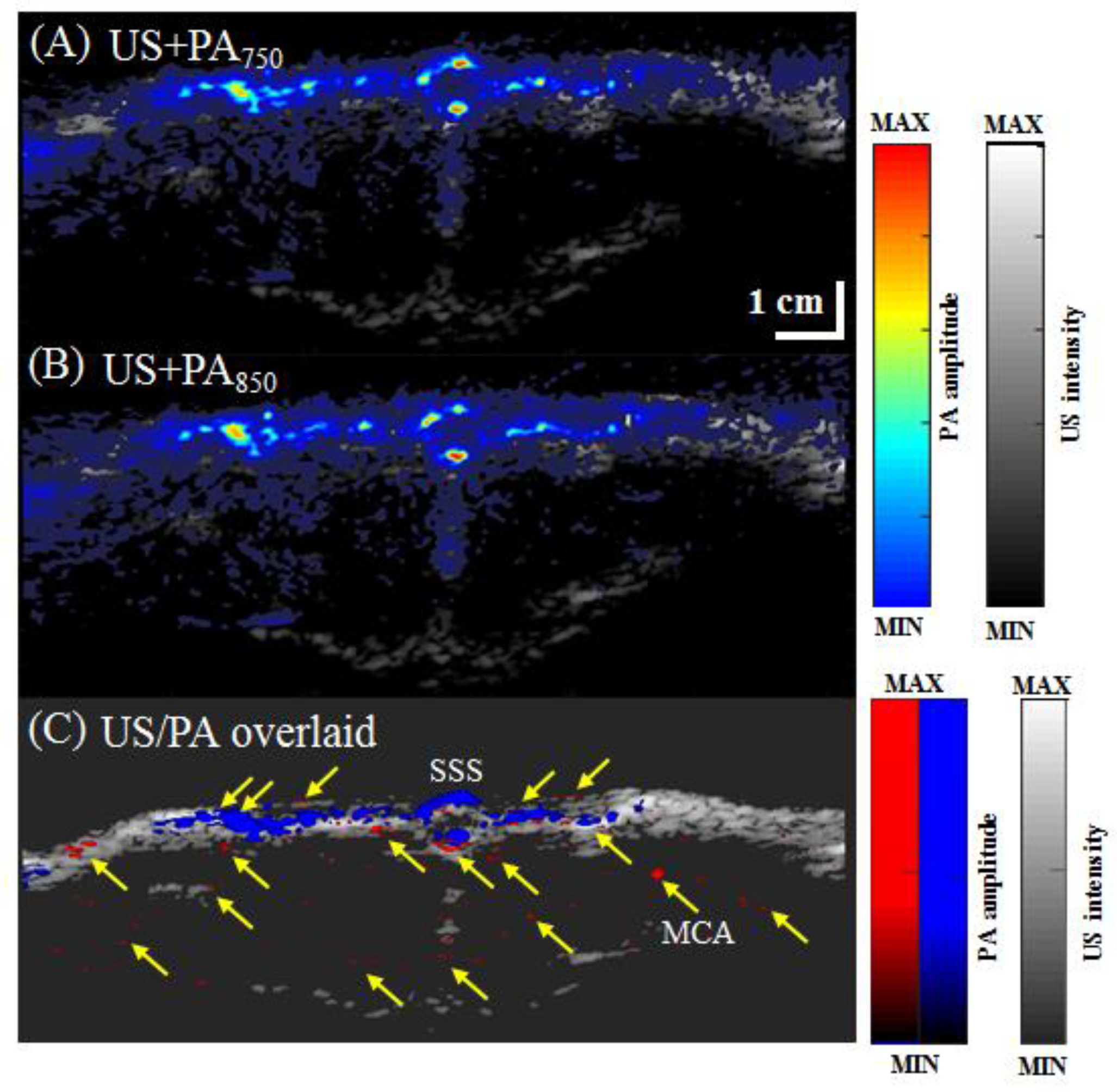
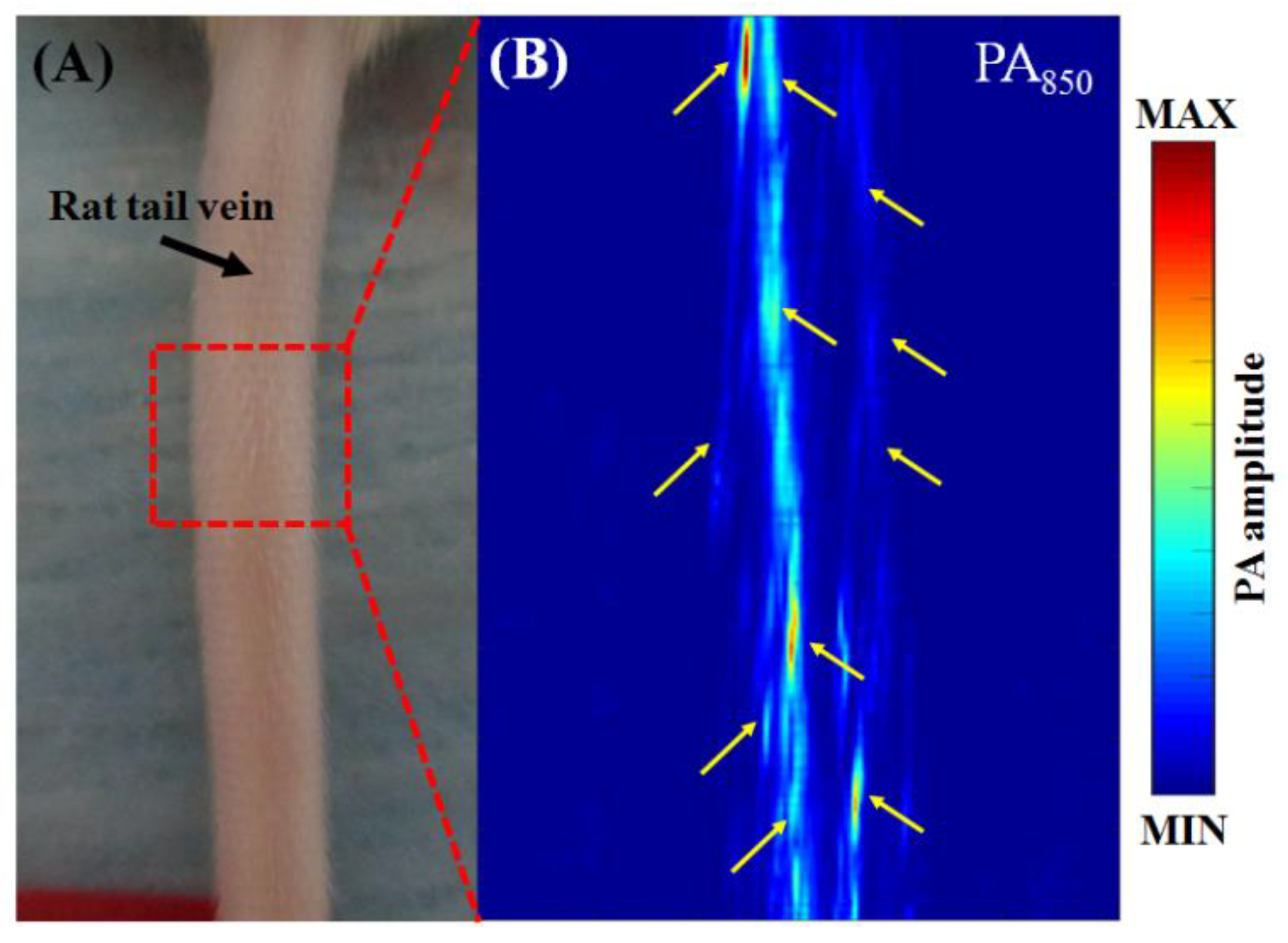
3.3. In Vivo Vascular Imaging of Rat Brain and Tail
3.4. In Vivo Functional Imaging of Changes in Cortical Brain Hemodynamics during Left-Forepaw Electrical Stimulation
3.5. In Vivo Functional Imaging of Indocyanine Green-based Cerebral Pharmacokinetics in Rat
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, C.; Kim, J.Y.; Kim, C. Recent Progress on Photoacoustic Imaging Enhanced with Microelectromechanical Systems (MEMS) Technologies. Micromachines 2018, 9, 584. [Google Scholar] [CrossRef]
- Wang, L.V. Tutorial on photoacoustic microscopy and computed tomography. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 171–179. [Google Scholar] [CrossRef]
- Wang, L.V. Photoacoustic Imaging and Spectroscopy; CRC: Boca Raton, FL, USA, 2009. [Google Scholar]
- Culver, J.P.; Siegel, A.M.; Franceschini, M.A.; Mandeville, J.B.; Boas, D.A. Evidence that cerebral blood volume can provide brain activation maps with better spatial resolution than deoxygenated hemoglobin. NeuroImage 2005, 27, 947–959. [Google Scholar] [CrossRef]
- Siegel, A.M.; Culver, J.P.; Mandeville, J.B.; Boas, D.A. Temporal comparison of functional brain imaging with diffuse optical tomography and fMRI during rat forepaw stimulation. Phys. Med. Biol. 2003, 48, 1391–1403. [Google Scholar] [CrossRef]
- Wang, L.V.; Wu, H.I. Biomedical Optics: Principles and Imaging; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Gratton, G.; Fabiani, M. Dynamic brain imaging: Event-related optical signals (EROS) measures of the time course and localization of cognitive-related activity. Psychon. Bull. Rev. 1998, 5, 535–563. [Google Scholar] [CrossRef]
- Wang, L.V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 2009, 3, 503–509. [Google Scholar] [CrossRef]
- Stein, E.W.; Maslov, K.; Wang, L.V. Noninvasive mapping of the electrically stimulated mouse brain using photoacoustic microscopy. Proc. SPIE 2008, 6856, 685654. [Google Scholar]
- Stein, E.W.; Maslov, K.; Wang, L.V. Noninvasive, in vivo imaging of blood-oxygenation dynamics within the mouse brain using photoacoustic microscopy. J. Biomed. Opt. 2009, 14, 020502. [Google Scholar] [CrossRef]
- Liao, L.D.; Li, M.L.; Lai, H.Y.; Shih, Y.Y.I.; Lo, Y.C.; Tsang, S.; Chao, P.C.P.; Lin, C.T.; Jaw, F.S.; Chen, Y.Y. Imaging brain hemodynamic changes during rat forepaw electrical stimulation using functional photoacoustic microscopy. NeuroImage 2010, 52, 562–570. [Google Scholar] [CrossRef]
- Liao, L.D.; Liu, Y.H.; Lai, H.Y.; Bandla, A.; Shih, Y.Y.; Chen, Y.Y.; Thakor, N.V. Rescue of cortical neurovascular functions during the hyperacute phase of ischemia by peripheral sensory stimulation. Neurobiol. Dis. 2015, 75, 53–63. [Google Scholar] [CrossRef]
- Liu, Y.H.; Liao, L.D.; Tan, S.S.H.; Kwon, K.Y.; Ling, J.M.; Bandla, A.; Lai, H.Y. Assessment of neurovascular dynamics during transient ischemic attack by the novel integration of micro-electrocorticography electrode array with functional photoacoustic microscopy. Neurobiol. Dis. 2015, 82, 455–465. [Google Scholar] [CrossRef]
- Xi, L.; Grobmyer, S.R.; Wu, L.; Chen, R.M.; Zhou, G.Y.; Gutwein, L.G.; Sun, J.J.; Liao, W.J.; Zhou, Q.F.; Xie, H.K.; et al. Evaluation of breast tumor margins in vivo with intraoperative photoacoustic imaging. Opt. Express 2012, 20, 8726–8731. [Google Scholar] [CrossRef]
- Wong, T.T.W.; Zhang, R.; Hai, P.; Zhang, C.; Pleitez, M.A.; Aft, R.L.; Novack, D.V.; Wang, L.V. Fast label-free multilayered histology-like imaging of human breast cancer by photoacoustic microscopy. Sci. Adv. 2017, 3, e1602168. [Google Scholar] [CrossRef]
- Cai, X.; Liu, X.; Liao, L.D.; Bandla, A.; Ling, J.M.; Liu, Y.H.; Thakor, N.; Bazan, G.C.; Liu, B. Encapsulated Conjugated Oligomer Nanoparticles for Real-Time Photoacoustic Sentinel Lymph Node Imaging and Targeted Photodynamic Therapy. Small 2016, 12, 4873–4880. [Google Scholar] [CrossRef]
- Chuah, S.Y.; Attia, A.B.; Long, V.; Ho, C.J.; Malempati, P.; Fu, C.Y.; Ford, S.J.; Lee, J.S.; Tan, W.P.; Razansky, D.; et al. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography. Ski. Res. Technol. 2017, 23, 221–226. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable Real-time Clinical Photoacoustic and Ultrasound Imaging System. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef]
- Grinvald, A.; Frostig, R.D.; Lieke, E.; Hildesheim, R. Optical imaging of neuronal activity. Physiol. Rev. 1988, 68, 1285–1366. [Google Scholar] [CrossRef]
- Sivasubramanian, K.; Periyasamy, V.; Wen, K.K.; Pramanik, M. Optimizing light delivery through fiber bundle in photoacoustic imaging with clinical ultrasound system: Monte Carlo simulation and experimental validation. J. Biomed. Opt. 2017, 22. [Google Scholar] [CrossRef]
- Wang, P.H.; Liu, H.L.; Hsu, P.H.; Lin, C.Y.; Wang, C.R.C.; Chen, P.Y.; Wei, K.C.; Yen, T.C.; Li, M.L. Gold-nanorod contrast-enhanced photoacoustic micro-imaging of focused-ultrasound induced blood-brain-barrier opening in a rat model. J. Biomed. Opt. 2012, 17. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Liu, Z.M.; Schmidt, K.F.; Sicard, K.M.; Duong, T.Q. Imaging oxygen consumption in forepaw somatosensory stimulation in rats under isoflurane anesthesia. Magn. Reson. Med. 2004, 52, 277–285. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, B.; Ji, L.; Jiang, H. 4-D photoacoustic tomography. Sci. Rep. 2013, 3, 1113. [Google Scholar] [CrossRef]
- Liao, L.D.; Tsytsarev, V.; Delgado-Martínez, I.; Li, M.-L.; Erzurumlu, R.; Vipin, A.; Orellana, J.; Lin, Y.-R.; Lai, H.-Y.; Chen, Y.-Y. Neurovascular coupling: in vivo optical techniques for functional brain imaging. BioMed. Eng. OnLine 2013, 12, 38. [Google Scholar] [CrossRef]
- Bandla, A.; Liao, L.D.; Chan, S.J.; Ling, J.M.; Liu, Y.H.; Shih, Y.I.; Pan, H.C.; Wong, P.T.; Lai, H.Y.; King, N.K.K.; et al. Simultaneous functional photoacoustic microscopy and electrocorticography reveal the impact of rtPA on dynamic neurovascular functions after cerebral ischemia. J. Cereb. Blood Flow Metab. 2017. [Google Scholar] [CrossRef]
- Suckow, M.; Weisbroth, S.; Franklin, C. The Laboratory Rat; Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- Liao, L.D.; Lin, C.T.; Shih, Y.Y.; Lai, H.Y.; Zhao, W.T.; Duong, T.Q.; Chang, J.Y.; Chen, Y.Y.; Li, M.L. Investigation of the cerebral hemodynamic response function in single blood vessels by functional photoacoustic microscopy. J. Biomed. Opt. 2012, 17, 061210. [Google Scholar] [CrossRef]
- Devor, A.; Hillman, E.M.C.; Tian, P.; Waeber, C.; Teng, I.C.; Ruvinskaya, L.; Shalinsky, M.H.; Zhu, H.; Haslinger, R.H.; Narayanan, S.N.; et al. Stimulus-induced changes in blood flow and 2-deoxyglucose uptake dissociate in ipsilateral somatosensory cortex. J. Neurosci. 2008, 31, 14347–14357. [Google Scholar] [CrossRef]
- Shih, Y.Y.I.; Chen, C.C.V.; Shyu, B.C.; Lin, Z.J.; Chiang, Y.C.; Jaw, F.S.; Chen, Y.Y.; Chang, C. A new scenario for negative functional magnetic resonance imaging signals: Endogenous neurotransmission. J. Neurosci. 2009, 29, 3036–3044. [Google Scholar] [CrossRef]
- Keilholz, S.D.; Silva, A.C.; Raman, M.; Merkle, H.; Koretsky, A.P. BOLD and CBV-weighted functional magnetic resonance imaging of the rat somatosensory system. Magn. Reson. Med. 2006, 55, 316–324. [Google Scholar] [CrossRef]
- Keilholz, S.D.; Silva, A.C.; Raman, M.; Merkle, H.; Koretsky, A.P. Functional MRI of the rodent somatosensory pathway using multislice echo planar imaging. Magn. Reson. Med. 2004, 52, 89–99. [Google Scholar] [CrossRef]
- Mandeville, J.B.; Marota, J.J.A.; Kosofsky, B.E.; Keltner, J.R.; Weissleder, R.; Rosen, B.R.; Weisskoff, R.M. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn. Reson. Med. 2005, 39, 615–624. [Google Scholar] [CrossRef]
- Hyder, F.; Behar, K.L.; Martin, M.A.; Blamire, A.M.; Shulman, R.G. Dynamic magnetic resonance imaging of the rat brain during forepaw stimulation. J. Cereb. Blood Flow Metab. 1994, 14, 649–655. [Google Scholar] [CrossRef]
- Haacke, E.M.; Lai, S.; Reichenbach, J.R.; Kuppusamy, K.; Hoogenraad, F.G.C.; Takeichi, H.; Lin, W. In vivo measurement of blood oxygen saturation using magnetic resonance imaging: A direct validation of the blood oxygen level-dependent concept in functional brain imaging. Hum. Brain Mapp. 1998, 5, 341–346. [Google Scholar] [CrossRef]
- Langham, M.C.; Magland, J.F.; Epstein, C.L.; Floyd, T.F.; Wehrli, F.W. Accuracy and precision of MR blood oximetry based on the long paramagnetic cylinder approximation of large vessels. Magn. Reson. Med. 2009, 62, 333–340. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Brzica, H.; Abdullahi, W.; Ibbotson, K.; Ronaldson, P.T. Role of Transporters in Central Nervous System Drug Delivery and Blood-Brain Barrier Protection: Relevance to Treatment of Stroke. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517693802. [Google Scholar] [CrossRef]
- Sheng, Y.; Liao, L.D.; Thakor, N.V.; Tan, M.C. Nanoparticles for Molecular Imaging. J. Biomed. Nanotechnol. 2014, 10, 2641–2676. [Google Scholar] [CrossRef]
- Yoo, S.W.; Jung, D.; Min, J.J.; Kim, H.; Lee, C. Biodegradable Contrast Agents for Photoacoustic Imaging. Appl. Sci. 2018, 8, 567. [Google Scholar] [CrossRef]
- Gurka, M.K.; Pender, D.; Chuong, P.; Fouts, B.L.; Sobelov, A.; McNally, M.W.; Mezera, M.; Woo, S.Y.; McNally, L.R. Identification of pancreatic tumors in vivo with ligand-targeted, pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography. J. Control. Release 2016, 231, 60–67. [Google Scholar] [CrossRef]
- Prabhakar, N.; Rosenholm, J.M. Nanodiamonds for advanced optical bioimaging and beyond. Curr. Opin. Colloid Interface Sci. 2019, 39, 220–231. [Google Scholar] [CrossRef]
- Fu, Q.R.; Zhu, R.; Song, J.B.; Yang, H.H.; Chen, X.Y. Photoacoustic Imaging: Contrast Agents and Their Biomedical Applications. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, H.; Wang, Y.; Jhang, D.-F.; Chu, T.-S.; Tsao, C.-H.; Tsai, C.-H.; Giamundo, S.; Chen, Y.-Y.; Liao, K.-W.; Chuang, C.-C.; et al. Characterization of a Fiber Bundle-Based Real-Time Ultrasound/Photoacoustic Imaging System and Its In Vivo Functional Imaging Applications. Micromachines 2019, 10, 820. https://doi.org/10.3390/mi10120820
Leng H, Wang Y, Jhang D-F, Chu T-S, Tsao C-H, Tsai C-H, Giamundo S, Chen Y-Y, Liao K-W, Chuang C-C, et al. Characterization of a Fiber Bundle-Based Real-Time Ultrasound/Photoacoustic Imaging System and Its In Vivo Functional Imaging Applications. Micromachines. 2019; 10(12):820. https://doi.org/10.3390/mi10120820
Chicago/Turabian StyleLeng, He, Yuhling Wang, De-Fu Jhang, Tsung-Sheng Chu, Chia-Hui Tsao, Chia-Hua Tsai, Steven Giamundo, You-Yin Chen, Kuang-Wen Liao, Chiung-Cheng Chuang, and et al. 2019. "Characterization of a Fiber Bundle-Based Real-Time Ultrasound/Photoacoustic Imaging System and Its In Vivo Functional Imaging Applications" Micromachines 10, no. 12: 820. https://doi.org/10.3390/mi10120820
APA StyleLeng, H., Wang, Y., Jhang, D.-F., Chu, T.-S., Tsao, C.-H., Tsai, C.-H., Giamundo, S., Chen, Y.-Y., Liao, K.-W., Chuang, C.-C., Ger, T.-R., Chen, L.-T., & Liao, L.-D. (2019). Characterization of a Fiber Bundle-Based Real-Time Ultrasound/Photoacoustic Imaging System and Its In Vivo Functional Imaging Applications. Micromachines, 10(12), 820. https://doi.org/10.3390/mi10120820






