Technological Assessment of MEMS Alkali Vapor Cells for Atomic References
Abstract
1. Introduction
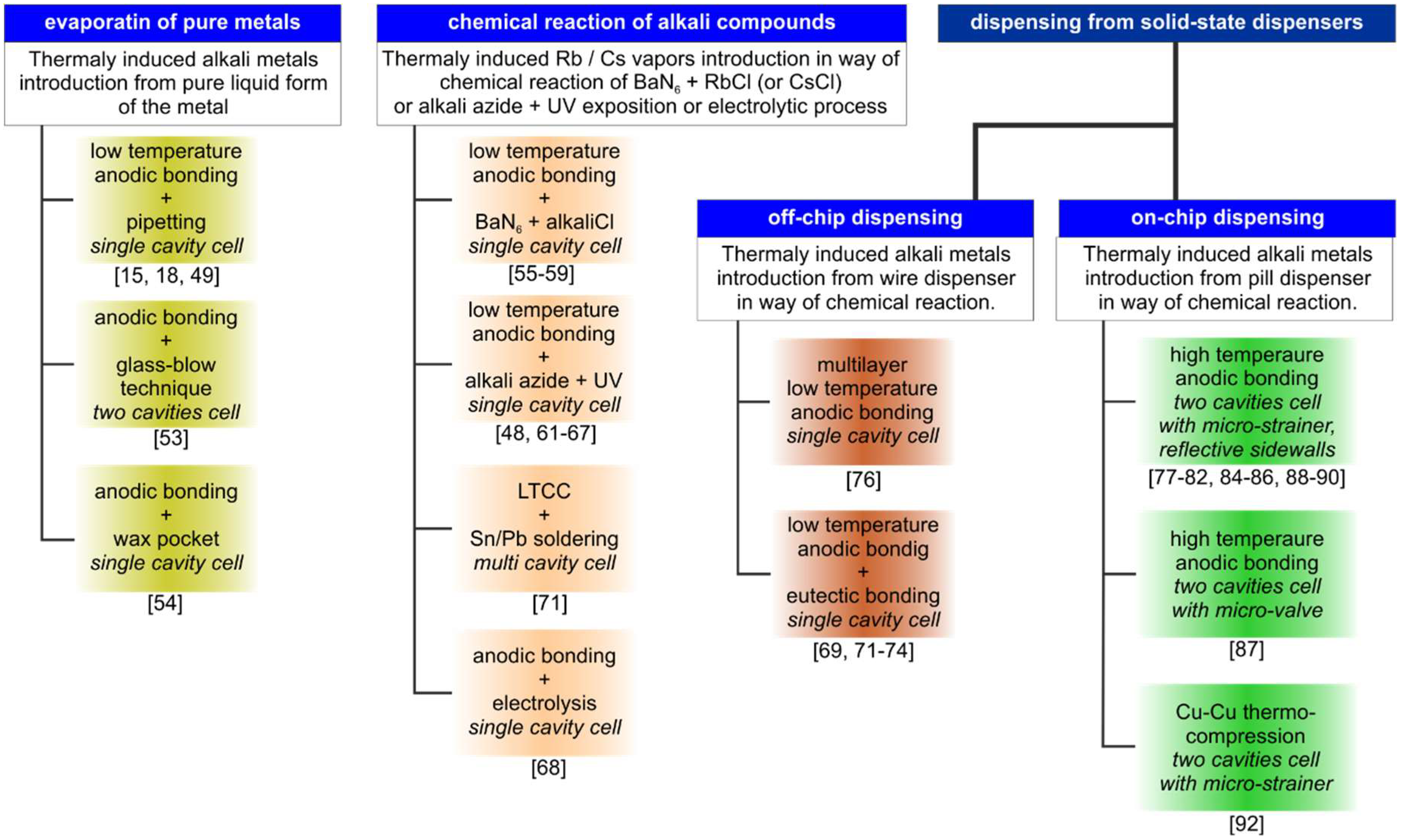
2. Methods of MEMS Vapor Cell Fabrication
2.1. Introduction of Pure Alkali Metals
2.1.1. Pipetting
- (1)
- fabrication of glass–silicon preform (deep reactive-ion etching, high-temperature anodic bonding),
- (2)
- direct alkali metal introduction through pipetting, and
- (3)
- final encapsulation by low-temperature, long-lasting anodic bonding.
2.1.2. Glassblowing in Microscale
2.1.3. Wax Pockets
2.2. Chemical Compound Reactions
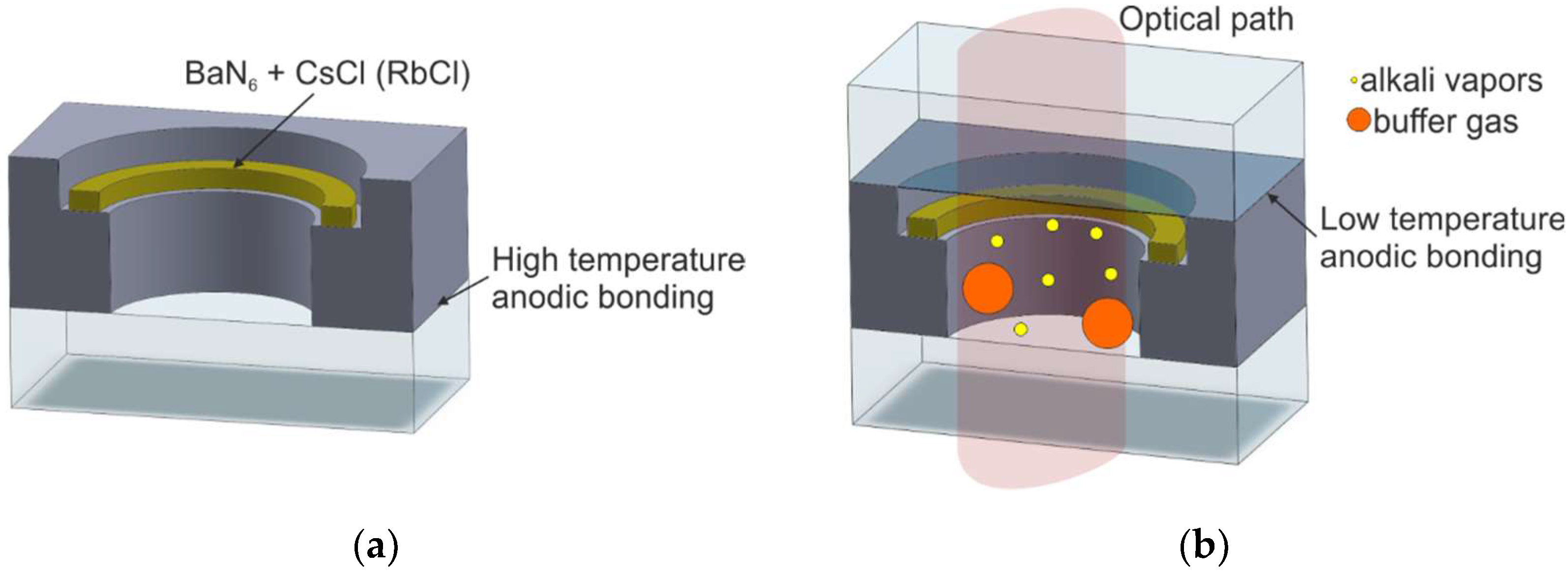
2.2.1. On-Chip Chemical Reactions of Alkali Metal Chloride and Barium Azide
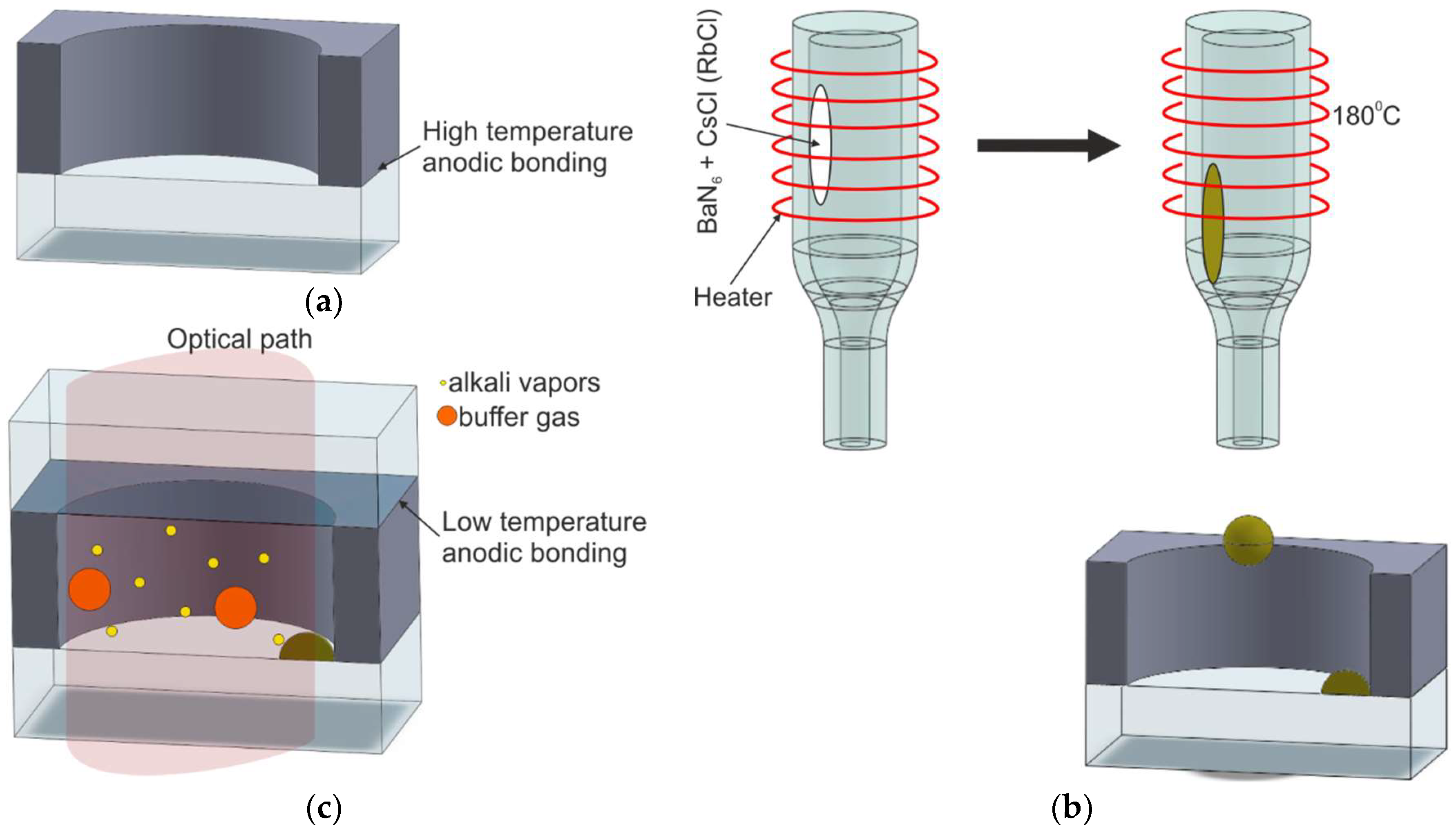
2.2.2. Off-Chip Chemical Reaction of Alkali Metal Chloride and Barium Azide
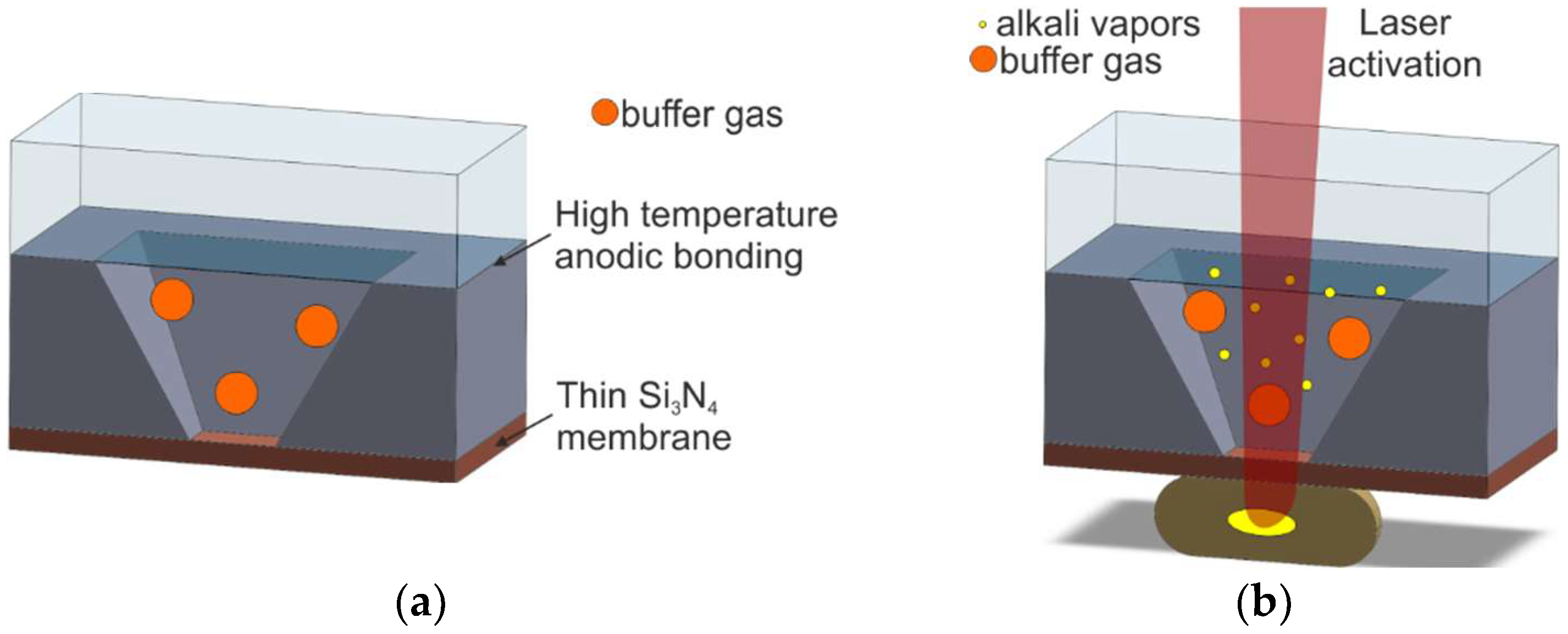
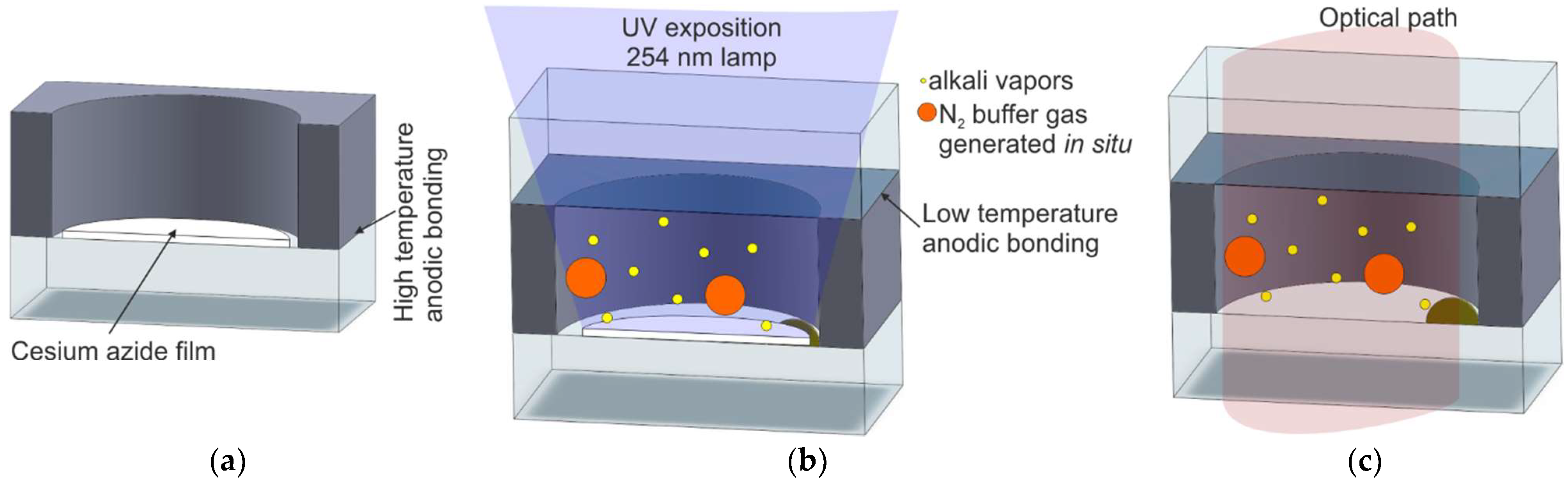
2.2.3. UV-Induced Chemical Reaction of Alkali Azide On-Chip
2.3. Electrolytic Alkali Metal Introduction
2.4. MEMS Vapor Cells by Off-Chip Dispensing of Alkali Vapors
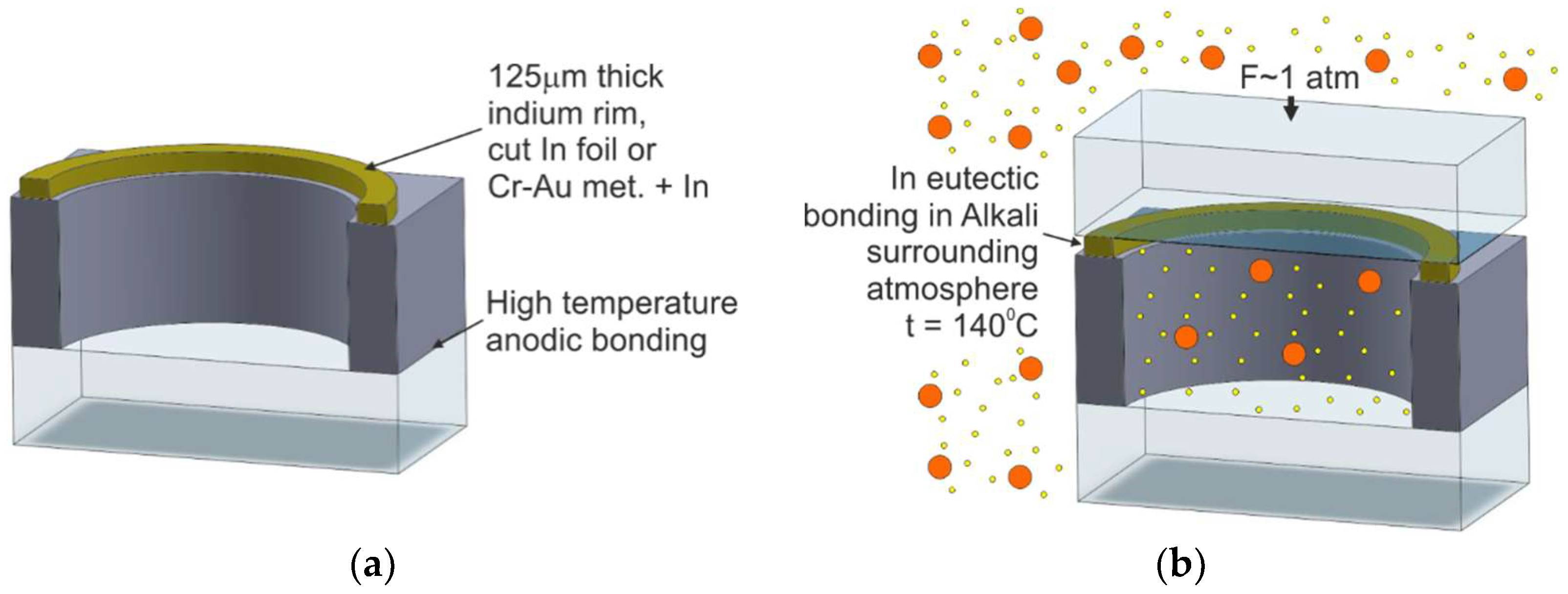
2.4.1. Off-Chip Dispensing and Eutectic Bonding
2.4.2. Off-Chip Dispensing and Anodic Bonding
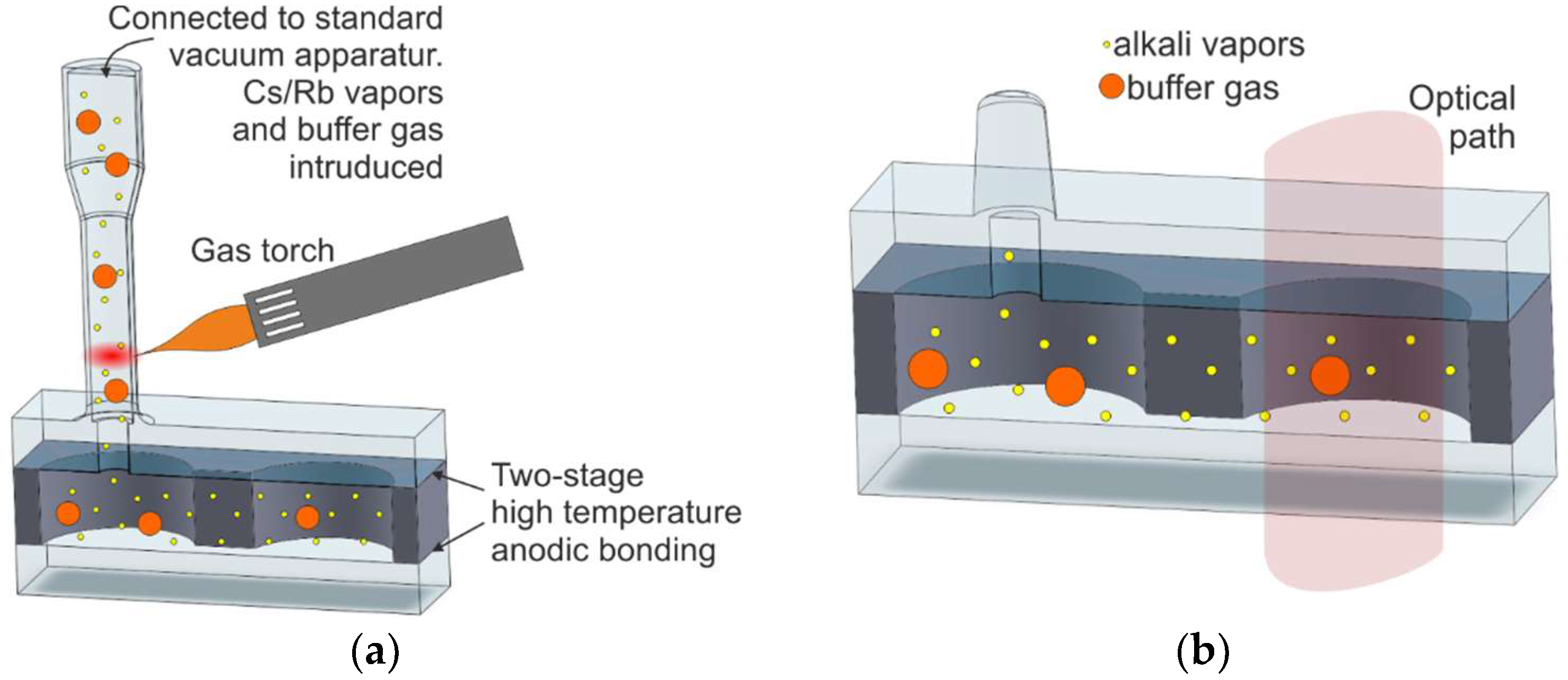
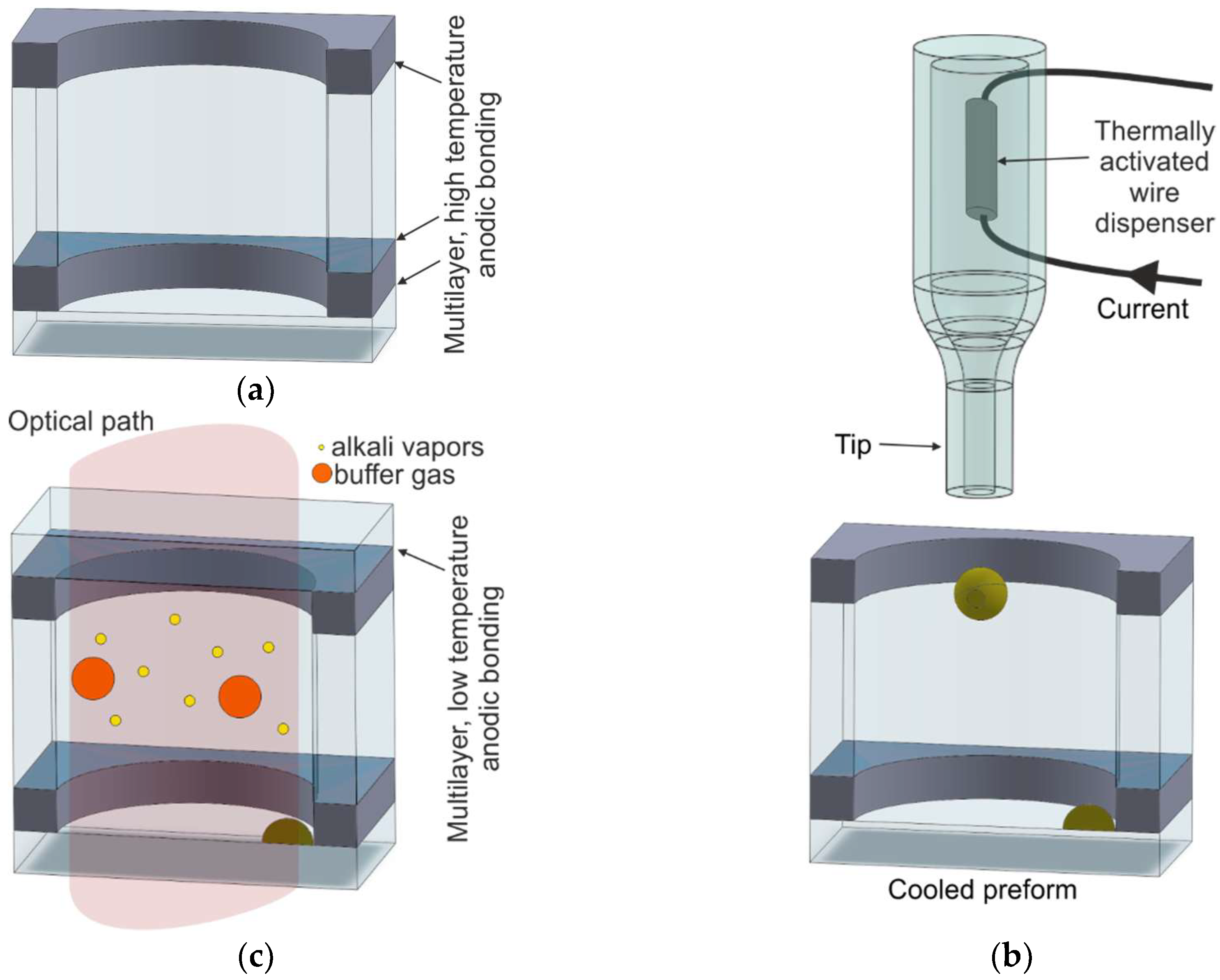
2.5. MEMS Vapor Cells by On-Chip Dispensing of Alkali Vapors
3. Conclusions and Final Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Sullivan, D.B. Time and frequency measurements at NIST: The first 100 years. In Proceedings of the International Frequency Control Symposium and PDA Exhibition, Seattle, WA, USA, 5–8 June 2001; pp. 4–17. [Google Scholar]
- Lombardi, M.A.; Heavner, T.P.; Jefferts, S.R. NIST Primary Frequency Standards and the Realization of the SI Second. J. Meas. Sci. 2007, 2, 74–89. [Google Scholar] [CrossRef]
- Chantry, P.J.; Liberman, I.; Verbanets, W.R.; Petronio, C.F.; Cather, R.L.; Partlow, W.D. Miniature laser-pumped cesium cell atomic clock oscillator. In Proceedings of the 1996 IEEE International Frequency Control Symposium, Honolulu, HI, USA, 5–7 June 1996; pp. 1002–1010. [Google Scholar]
- Allan, D.W. The Measurement of Frequency and Frequency Stability of Precision Oscillators; National Berau of Standards, Technical Note 669; NTRS: Boulder, CO, USA, May 1975. [Google Scholar]
- Howe, D.A. Frequency Domain Stability Measurements: A Tutorial Introduction; National Berau of Standards, Technical Note 679; NTRS: Boulder, CO, USA, March 1976. [Google Scholar]
- Dalton, B.J.; McDuff, R. Coherent population trapping. Two unequal phase fluctuating laser fields. Opt. Acta 1985, 32, 61–70. [Google Scholar] [CrossRef]
- Knappe, S.; Wynands, R.; Kitching, J.; Robinson, H.G.; Hollberg, L. Characterization of coherent population-trapping resonance as atomic frequency references. J. Opt. Soc. Am. B 2001, 85, 1545–1553. [Google Scholar] [CrossRef]
- Kitching, J.; Knappe, S.; Vukicevic, N.; Hollberg, L.; Wynands, R.; Weidmann, W. A microwave frequency reference based on VCSEL-driven dark line resonances in Cs vapor. IEEE Trans. Instrum. Meas. 2000, 49, 1313–1317. [Google Scholar] [CrossRef]
- Knappe, S.; Shah, V.; Schwindt, P.D.D.; Hollberg, L.; Kitching, J. A microfabricated atomic clock. Appl. Phys. Lett. 2004, 85, 1460–1462. [Google Scholar] [CrossRef]
- Knappe, S.; Schwindt, P.; Shah, V.; Hollberg, L.; Kitching, J.; Liew, L.; Moreland, J. Microfabricated atomic frequency references. In Proceedings of the IEEE International Ultrasonics, Ferroelectrics, and Frequency Control Joint 50th Anniversary Conference, Montreal, QC, Canada, 23–27 August 2004; pp. 87–91. [Google Scholar]
- Gerginov, V.; Knappe, S.; Schwindt, P.D.D.; Shah, V.; Liew, L.; Moreland, J.; Robinson, H.G.; Hollberg, L.; Kitching, J.; Brannon, A.; et al. Component-level demonstration of a microfabricated atomic frequency reference. In Proceedings of the IEEE International Frequency Control Symposium and Exposition, Vancouver, BC, Canada, 29–31 August 2005; pp. 758–766. [Google Scholar]
- Kitching, J.; Knappe, S.; Liew, L.; Schwindt, P.; Shah, V.; Moreland, J.; Hollberg, L. Microfabricated atomic clock. In Proceedings of the 18th IEEE International Conference on Micro Electro Mechanical Systems, MEMS 2005, Miami, FL, USA, 30 January–3 February 2005; pp. 1–7. [Google Scholar]
- Knappe, S.; Schwindt, P.; Hollberg, L.; Kitching, J. The performance of chip-scale atomic clocks. In Proceedings of the International Quantum Electronics Conference 2005 and the Pacific Rim Conference on Lasers and Electro-Optics 2005 (IQEC/CLEO-PR 2005), Tokyo, Japan, 22–27 May 2005; pp. 479–481. [Google Scholar]
- Knappe, S.; Schwindt, P.D.D.; Gerginov, V.; Shah, V.; Liew, L.; Moreland, J.; Robinson, H.G.; Hollberg, L.; Kitching, J. Microfabricated atomic clocks and magnetometers. J. Opt. A Pure Appl. Opt. 2006, 8, 318–322. [Google Scholar] [CrossRef]
- Brannon, A.; Gerginov, V.; Knappe, S.; Popovic, Z.; Kitching, J. System-level integration of a chip-scale atomic clock-microwave oscillator and physics package. In Proceedings of the MEP 2006 Multiconference on Electronics and Photonics, Guanajuato, Mexico, 7–11 November 2006; pp. 118–121. [Google Scholar]
- Kitching, J. An atomic ckock on a chip. Horol. J. Febr. 2005, 54–55. [Google Scholar]
- Kitching, J.; Knappe, S.; Liew, L.; Schwindt, P.D.D.; Gerginov, V.; Shah, V.; Moreland, J.; Brannon, A.; Breitbarth, J.; Popovic, Z.; et al. Chip-scale atomic frequency references. In Proceedings of the 18th International Technical Meeting of the Satellite Division, Long Beach, CA, USA, 13–16 September 2005; pp. 1662–1669. [Google Scholar]
- Lutwak, R.; Deng, J.; Riley, W.; Varghese, M.; Leblanc, J.; Tepolt, G.; Mescher, M.; Serkland, D.K.; Geib, K.M.; Peake, G.M. The chip-scale atomic clock—Low-power physics package. In Proceedings of the 36th Annual Precise Time and Time Interval (PTTI) Meeting, Washington, DC, USA, 7–9 December 2004; pp. 339–354. [Google Scholar]
- Lutwak, R. The chip-scale atomic clock–recent developments. In Proceedings of the 22nd International Conference on Micro Electro Mechanical Systems (MEMS 2009), Sorrento, Italy, 25–29 January 2009; pp. 573–577. [Google Scholar]
- Lutwak, R.; Rashed, A.; Varghese, M.; Tepolt, G.; LeBlanc, J.; Mescher, M.; Serkland, D.K.; Geib, K.M.; Peake, G.M.; Römisch, S. The chip-scale atomic clock–prototype evaluation. In Proceedings of the 39th Annual Precise Time and Time Interval (PTTI) Meeting, Long Beach, CA, USA, 26–29 November 2007; pp. 269–290. [Google Scholar]
- Lutwak, R.; Emmons, D.; Riley, W.; Garvey, R.M. The chip-scale atomic clock–coherent population trapping vs. conventional interrogation. In Proceedings of the 39th Annual Precise Time and Time Interval (PTTI) Meeting, Long Beach, CA, USA, 26–29 November 2007; pp. 233–248. [Google Scholar]
- Lutwak, R.; Vlitas, P.; Varghese, M.; Mescher, M.; Serkland, D.K.; Peake, G.M. The MAC—A miniature atomic clock. In Proceedings of the 2005 IEEE International Frequency Control Symposium and Exposition, Vancouver, BC, Canada, 29–31 August 2005. [Google Scholar]
- Lutwak, R.; Rashed, A.; Varghese, M.; Tepolt, G.; Leblanc, J.; Mescher, M.; Serkland, D.K.; Peake, G.M. The miniature atomic clock–pre-production results. In Proceedings of the IEEE International Frequency Control Symposium, 2007 Joint with the 21st European Frequency and Time Forum, Geneva, Switzerland, 29 May–1 June 2007. [Google Scholar]
- Deng, J.; Vlitas, P.; Taylor, D.; Perletz, L.; Lutwak, R. A Commercial CPT Rubidium Clock. In Proceedings of the European Frequency and Time Forum (EFTF), Toulouse, FR, USA, 22–25 April 2008. [Google Scholar]
- Braun, A.M.; Davis, T.J.; Kwakernaak, M.H.; Michalchuk, J.J.; Ulmer, A.; Chan, W.K.; Abeles, J.H.; Shellenbarger, Z.A.; Jau, Y.; Happer, W.; et al. RF-integrated and-state chip-scale atomic clock. In Proceedings of the 39th Annual Precise Time and Time Interval (PTTI) Meeting, Long Beach, CA, USA, 26–29 November 2007; pp. 233–248. [Google Scholar]
- Youngner, D.W.; Lust, L.M.; Carlson, D.R.; Lu, S.T.; Forner, L.J.; Chanhvongsak, H.M.; Stark, T.D. A manufacturable chip-scale atomic clock. In Proceedings of the 14th International Conference on Solid-State Sensors, Actuators and Microsystems, Transducers ’07 & Eurosensors XXI, Lyon, France, 10–14 June 2007; pp. 39–44. [Google Scholar]
- DeNatale, J.F.; Borwick, R.L.; Tsai, C.; Stupar, P.A.; Lin, Y.; Newgard, R.A.; Berquist, R.W.; Zhu, M. Compact, low-power chip-scale atomic clock. In Proceedings of the 2008 IEEE/ION Position, Location and Navigation Symposium, Monterey, CA, Canada, 5–8 May 2008; pp. 67–70. [Google Scholar]
- DeNatale, J.F.; Borwick, R.L.; Stupar, P.A. Compact, low-power atomic time and frequency standards. In Proceedings of the 26th Army Science Conference, Orlando, FL, USA, 1–4 December 2008. Report Number A647505. [Google Scholar]
- DeNatale, J.F.; Borwick, R.L.; Stupar, P.A.; Tsai, C. Compact Optical Assembly for Chip-Scale Atomic Clock. U.S. Patent US 2009/0251224 A1, 8 October 2009. [Google Scholar]
- Gorecki, C. Development of first European chip-scale atomic clocks: Technologies, assembling and metrology. Procedia Eng. 2012, 47, 898–903. [Google Scholar] [CrossRef]
- Yoshida, H. A New Compact High-Stability Oscillator. In Proceedings of the International Timing and Sync Forum ITSF 2015, Edinburgh, UK, 2–5 November 2015. [Google Scholar]
- Wynands, R. The atomic wrist-watch. Nature 2004, 429, 509. [Google Scholar] [CrossRef]
- Belcher, N.; Novikova, M.I. Optimization of a Prototype Atomic Clock Based on Coherent Population Trapping. In Proceedings of the 75th Annual Meeting of the Southeastern Section of APS, Raleigh, NC, USA, 30 October–1 November 2008. [Google Scholar]
- Dong, H.; Fang, J.; Zhou, B.; Qin, J.; Wan, S. Review of atomic MEMS: Driving technology and challenges. Microsyst. Technol. 2010, 16, 1683–1689. [Google Scholar] [CrossRef]
- Knappe, S. MEMS atomic clocks. In Comprehensive Microsystems; Elsevier B.V.: Amsterdam, The Netherlands, 2008; pp. 571–612. [Google Scholar]
- Kitching, J.; Knappe, S.; Hollberg, L. Miniature vapor-cell atomic-frequency references. Appl. Phys. Lett. 2002, 81, 553–555. [Google Scholar] [CrossRef]
- Boudot, R.; Miletic, D.; Dziuban, P.; Affolderbach, C.; Knapkiewicz, P.; Dziuban, J.; Mileti, G.; Girodano, V.; Gorecki, C. First-order cancellation of the Cs clock frequency temperature-dependence in Ne-Ar buffer gas mixture. Opt. Express 2011, 19, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Chutani, R.K.; Gorecki, C.; Boudot, R.; Dziuban, P.; Galliou, S.; Passilly, N.; Giordano, V.; Jornod, A. Microfabrication and thermal behavior of miniature cesium-vapor cells for atomic clock operations. In Proceedings of the MEMS 2011, Cancum, Mexico, 23–27 January 2011; pp. 712–715. [Google Scholar]
- Liu, X.; Mérolla, J.; Guérandel, S.; Gorecki, C.; de Clercq, E.; Boudot, R. Coherent-population-trapping resonances in buffer-gas-filled Cs-vapor cells with push-pull optical pumping. Phys. Rev. A 2013, 87, 013416. [Google Scholar] [CrossRef]
- Boudot, R.; Dziuban, P.; Hasegawa, M.; Chutani, R.K.; Galliou, S.; Giordano, V.; Gorecki, C. Coherent population trapping resonances in Cs–Ne vapor microcells for miniature clocks applications. J. Appl. Phys. 2011, 109, 014912. [Google Scholar] [CrossRef]
- Kitching, J. Chip-scale atomic devices. Appl. Phys. Rev. 2018, 5, 031302. [Google Scholar] [CrossRef]
- Reference Gas Cells Portfolio and Technical Description. Available online: http://www.precisionglassblowing.com (accessed on 28 December 2018).
- Reference Gas Cells Portfolio and Technical Description. Available online: https://www.sacher-laser.com (accessed on 28 December 2018).
- Reference Gas Cells Portfolio and Technical Description. Available online: http://www.triadtechno.com (accessed on 28 December 2018).
- Liew, L.; Knappe, S.; Moreland, J.; Robinson, H.; Hollberg, L.; Kitching, J. Microfabricated alkali atom vapor cells. Appl. Phys. Lett. 2004, 84, 2694–2696. [Google Scholar] [CrossRef]
- Kwakernaak, M.H.; Lipp, S.; McBride, S.; Zanzucchi, P.; Chan, W.K.; Khalfin, V.B.; An, H.; Whaley, R.D., Jr.; Willner, B.I.; Ulmer, A.; et al. Components for batch-fabricated chip-scale atomic-clocks. In Proceedings of the 36th Annual Precise Time and Time Interval (PTTI) Meeting, Washington, DC, USA, 7–9 December 2004; pp. 355–368. [Google Scholar]
- Dziuban, J. Bonding in Microsystem Technology; Springer: Berlin, Germany, 2006. [Google Scholar]
- Knapkiewicz, P.; Augustyniak, I. Sealing of Silicon-Glass Microcavities with Polymer Filling. Bull. Pol. Acad. Sci. Tech. Sci. 2016, 64, 283–286. [Google Scholar] [CrossRef]
- Losev, S.S.; Sevostianov, D.I.; Vassiliev, V.V.; Velishansky, V.L. Production of miniature glass cells with rubidium for chip scale atomic clock. Phys. Procedia 2015, 71, 242–246. [Google Scholar] [CrossRef]
- Lutwak, R.; Emmons, D.; English, T.; Riley, W.; Duwel, A.; Varghese, M.; Serkland, D.K.; Peake, G.M. The chip-scale atomic clock-recent development progress. In Proceedings of the 35th Annual Precise Time and Time Interval (PTTI) Meeting, San Diego, CA, USA, 2–4 December 2003; pp. 467–478. [Google Scholar]
- Lal, A.; Radhakrishnan, S. Alkali Metal-Wax Micropackets for Alkali Metal Handling. U.S. Patent US 7,666,485 B2, 23 February 2010. [Google Scholar]
- Rutkowski, J. Investigation of Thermal Activation of Caesium Dispensers for Micro-Atomic Clock. MsC Thesis, Wroclaw University of Science and Technology, Wroclaw, Poland, 2010. (In Polish). [Google Scholar]
- Liew, L.; Knappe, S.; Moreland, J.; Robinsod, H.; Hollberrg, L.; Kitchin, J. Micromachined alkali atom vapor cells for chip-scale atomic clocks. In Proceedings of the MEMS 2004 Technical Digest, Maastricht, The Netherlands, 25–29 January 2004; pp. 113–116. [Google Scholar]
- Knappe, S.; Schwindt, P.D.D.; Gerginov, V.; Shah, V.; Hollberg, L.; Kitching, J.; Liew, L.; Moreland, J. Microfabricated atomic clocks at NIST. In Proceedings of the 36th Annual Precise Time and Time Interval (PTTI) Meeting, Washington, DC, USA, 7–9 December 2004; pp. 355–368. [Google Scholar]
- Eklund, E.J.; Shkel, A.M.; Knappe, S.; Donley, E.; Kitching, J. Glass-blown spherical microcells for chip-scale atomic devices. Sens. Actuators A 2008, 143, 175–180. [Google Scholar] [CrossRef]
- Noor, R.M.; Gundeti, V.; Shkel, A.M. A status on components development for folded micro NMR gyro. In Proceeding of the 2017 IEEE International Symposium on Inertial Sensors and Systems (INERTIAL), Kauai, HI, USA, 28–30 March 2017; pp. 156–159. [Google Scholar]
- Liew, L.; Moreland, J.; Gerginov, V. Wafer-level filling of microfabricated atomic vapor cells based on thin-film deposition and photolysis of cesium azide. Appl. Phys. Lett. 2007, 90, 114106. [Google Scholar] [CrossRef]
- Liew, L.; Moreland, J.; Gerginov, V. Wafer-Level Fabrication and Filling of Cesium-Vapor Cells for Chip-Scale Atomic Devices; Contribution of the National Institute of Standards and Technology: Boulder, CO, USA, 2005. [Google Scholar]
- Woetzel, S.; Talkenberg, F.; Scholtes, T.; Jsselsteijn, R.I.; Schultze, V.; Meyer, H.-G. Lifetime improvement of micro-fabricated alkali vapor cells by atomic layer deposition wall coatings. Surf. Coat. Technol. 2013, 221, 158–162. [Google Scholar] [CrossRef]
- Woetzel, S.; Kessler, E.; Diegel, M.; Schultze, V.; Meyer, H.G. Low-temperature anodic bonding using thin films of lithium-niobate-phosphate glass. J. Micromech. Microeng. 2014, 24, 095001. [Google Scholar] [CrossRef]
- Woetzel, S.; Schultze, V.; Jsselsteijn, R.I.; Schulz, T.; Anders, S.; Stolz, R.; Meyer, H.-G. Microfabricated atomic vapor cell arrays for magnetic field measurements. Rev. Sci. Instrum. 2011, 82, 033111. [Google Scholar] [CrossRef] [PubMed]
- Overstolz, T.; Haesler, J.; Bergonzi, G.; Pezous, A.; Clerc, P.-A.; Ischer, S.; Kaufmann, J.; Despont, M. Wafer scale fabrication of highly integrated rubidium vapor cells. In Proceedings of the MEMS 2014, San Francisco, CA, USA, 26–30 January 2014; pp. 552–555. [Google Scholar]
- Karlen, S.; Gobet, J.; Overstolz, T.; Haesler, J.; Lecomte, S. Lifetime assessment of RbN3–filled MEMS atomic vapor cells with Al2O3 coating. Opt. Express 2017, 25, 2187–2194. [Google Scholar] [CrossRef]
- Gong, F.; Jau, Y.; Jensen, K.; Happer, W. Electrolytic fabrication of atomic clock cells. Rev. Sci. Instrum. 2006, 77, 076101. [Google Scholar] [CrossRef]
- Pétremand, Y.; Schori, C.; Straessle, R.; Mileti, G.; de Rooij, N.; Thomann, P. Low temperature indium-based sealing of microfabricated alkali cells for chip scale atomic clocks. In Proceedings of the EFTF 2010—24th European Frequency and Time Forum ESA/ESTAC, Noordwijk, The Netherlands, 13–16 April 2010. [Google Scholar]
- Schori, C.; Mileti, G.; Rochat, B.L.P. CPT Atomic Clock based on Rubidium 85. In Proceedings of the 24th European Frequency and Time Forum EFTF, ESA-ESTEC, Noordwijk, The Netherlands, 13–16 April 2010. [Google Scholar]
- Vecchio, F.; Venkatraman, V.; Shea, H.R.; Maeder, T.; Ryser, P. Dispensing and hermetic sealing Rb in a miniature reference cell for integrated atomic clocks. Sens. Actuators A 2011, 172, 330–335. [Google Scholar] [CrossRef]
- Straessle, R.; Pellaton, M.; Affolderbach, C.; Pétremand, Y.; Briand, D.; Mileti, G.; de Rooij, N.F. Low-temperature indium-bonded alkali vapour cell for chip-scale atomic clocks. J. Appl. Phys. 2013, 113, 064501. [Google Scholar] [CrossRef]
- Straessle, R.; Pellaton, M.; Affolderbach, C.; Pétremand, Y.; Briand, D.; Mileti, G.; de Rooij, N.F. Microfabricated alkali vapor cell with anti-relaxation wall coating. Appl. Phys. Lett. 2014, 105, 043502. [Google Scholar] [CrossRef]
- Violetti, M.; Pellaton, M.; Affolderbach, C.; Merli, F.; Zürcher, J.; Mileti, G.; Skrivervik, A.K. The Microloop-Gap Resonator: A Novel Miniaturized Microwave Cavity for Double-Resonance Rubidium Atomic Clocks. IEEE Sens. J. 2014, 14, 3193–3200. [Google Scholar] [CrossRef]
- Rushton, J.A.; Aldous, M.; Himsworth, M.D. The feasibility of a fully miniaturized magneto-optical trap for portable ultracold quantum technology. Rev. Sci. Instrum. 2014, 85, 121501. [Google Scholar] [CrossRef]
- Pétremand, Y.; Affolderbach, C.; Straessle, R.; Pellaton, M.; Briand, D.; Mileti, G.; de Rooij, N. Microfabricated rubidium vapous cell with a thick glass core for small-scale atomic clock applications. J. Micromach. Microeng. 2012, 22, 025013. [Google Scholar] [CrossRef]
- Dziuban, J.; Gorecki, C.; Giordano, V.; Nieradko, L.; Maillotte, H.; Moraja, M. Procédé de Fabrication d’une Cellule à gaz Active Pour L’horloge Atomique à gaz Ainsi Obtenue. French Patent 06/09089, 17 Octobre 2006. [Google Scholar]
- Knapkiewicz, P.; Dziuban, J.; Walczak, R.; Mauri, L.; Dziuban, P.; Gorecki, C. MEMS caesium vapour cell for European micro-atomic-clock. Procedia Eng. 2010, 5, 721–724. [Google Scholar] [CrossRef]
- Nieradko, L.; Gorecki, C.; Dziuban, J.; Douahi, A.; Giordano, V.; Beugnot, J.C.; Guérandel, S.; Moraja, M. From the implementation to the characterization and assembling of microfabricated optical al cali vapour cell for MEMS atomic clocks. In Proceedings of the 14th International Conference on Solid-State Sensors, Actuators and Microsystems, Transducers ’07 & Eurosensors XXI, Lyon, France, 10–14 June 2007; pp. 45–48. [Google Scholar]
- Douahi, A.; Nieradko, L.; Beugnot, J.C.; Dziuban, J.; Maillote, H.; Boudot, R.; Guérandel, S.; Moraja, M.; Gorecki, C.; Giordano, V. New vapor cell technology for chip scale atomic clock. In Proceedings of the IEEE International Frequency Control Symposium 2007 Joint with the 21st European Frequency and Time Forum, Geneva, Switzerland, 29 May–1 June 2007; pp. 58–61. [Google Scholar]
- Douahi, A.; Nieradko, L.; Beugnot, J.C.; Dziuban, J.; Maillote, H.; Gue´randel, S.; Moraja, M.; Gorecki, C.; Giordano, V. Vapour microcell for chip scale atomic frequency standard. Electron. Lett. 2007, 43, 33–34. [Google Scholar] [CrossRef]
- Knapkiewicz, P.; Dziuban, J.A.; Gorecki, C.; Dziuban, P.; Walczak, R.; Mauri, L. Komórka cezowa MEMS dla mikrozegara atomowego. Elektronika 2010, 51, 82–85. [Google Scholar]
- Hallier, S.J. Alkali Metal Generation and Gas Evolution from Alkali Metal Dispensers. Technical Report TR 18. Available online: www.saesgetters.com (accessed on 28 December 2018).
- Hasegawa, M.; Chutani, R.K.; Gorecki, C.; Boudot, R.; Dziuban, P.; Giordano, V.; Clatot, S.; Mauri, L. Microfabrication of cesium vapor cells with buffer gas for MEMS atomic clocks. Sens. Actuators A 2011, 167, 594–601. [Google Scholar] [CrossRef]
- Hasegawa, M.; Chutani, R.K.; Boudot, R.; Mauri, L.; Gorecki, C.; Liu, X.; Passilly, N. Effects of getters on hermetically sealed micromachined cesium–neon cells for atomic clocks. J. Micromech. Microeng. 2013, 23, 055022. [Google Scholar] [CrossRef]
- Maurice, V.; Rutkowski, J.; Kroemer, E.; Bargiel, S.; Passilly, N.; Boudot, R.; Gorecki, C.; Mauri, L.; Moraja, M. Microfabricated vapor cells filled with a cesium dispensing paste for miniature atomic clocks. Appl. Phys. Lett. 2017, 110, 164103. [Google Scholar] [CrossRef]
- Maurice, V. Design, Microfabrication and Characterization of Alkali Vapor Cells for Miniature Atomic Frequency References. Ph.D. Thesis, Université de Franche-Comté, Besancon, France, 2016. [Google Scholar]
- Ermak, S.V.; Semenov, V.V.; Piatyshev, E.N.; Kazakin, A.N.; Komarevtsev, I.M.; Velichko, E.N.; Davidov, V.V.; Petrenko, M.V. Microfabricated cells for chip-scale atomic clock based on coherent population trapping: Fabrication and investigation. St. Petersb. Polytech. Univ. J. Phys. Math. 2015, 1, 37–41. [Google Scholar] [CrossRef][Green Version]
- Han, R.; You, Z.; Zhang, F.; Xue, H.; Ruan, Y. Microfabricated Vapor Cells with Reflective Sidewalls for Chip Scale Atomic Sensors. Micromachines 2018, 9, 175. [Google Scholar] [CrossRef]
- Chutani, R.; Maurice, V.; Passilly, N.; Gorecki, C.; Boudot, R.; Hafiz, M.A.; Abbe, P.; Galliou, S.; Rauch, J.; de Clercq, E. Laser light routing in an elongated micromachined vapoer cell with diffraction gratings for atomic clock applications. Sci. Rep. 2015, 5, 14001. [Google Scholar] [CrossRef]
- Knapkiewicz, P. Alkali Vapor MEMS Cells Technology toward High-Vacuum Self-Pumping MEMS Cell for Atomic Spectroscopy. Micromachines 2018, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Karlen, S. Fabrication and Characterization of MEMS Alkali Vapor Cells Used in Chip-Scale Atomic Clocks and Other Atomic Devices. Ph.D. Thesis, Thèse présentée à la Faculté de Sciences de l’Université de Neuchâtel, Neuchatel, Switzerland, 2017. [Google Scholar]
- Vicarini, R.; Maurice, V.; Hafiz, M.A.; Rutkowski, J.; Gorecki, C.; Passilly, N.; Ribetto, L.; Gaff, V.; Volant, V.; Galliou, S.; et al. Demonstration of the mass-producible feature of a Cs vapor microcell technology for miniature atomic clocks. Sens. Actuators A 2018, 280, 99–106. [Google Scholar] [CrossRef]
- Grzebyk, T.; Gorecka-Drzazga, A.; Dziuban, J.A. Vacuum and residual gas composition MEMS sensor. Procedia Eng. 2015, 120, 671–674. [Google Scholar] [CrossRef]
- Grzebyk, T.; Gorecka-Drzazga, A. MEMS type ionization vacuum sensor. Sens. Actuators A 2016, 246, 148–155. [Google Scholar] [CrossRef]
- Grzebyk, T.; Knapkiewicz, P.; Szyszka, P.; Gorecka-Drzazga, A.; Dziuban, J. MEMS ion-sorption high vacuum pump. J. Phys. Conf. Ser. 2016, 773, 012047. [Google Scholar] [CrossRef]
- Dziuban, J.; Gorecka-Drzazga, A.; Knapkiewicz, P.; Grzebyk, T. From CPT to cooled-atom MEMS atomic microclock. In Proceedings of the COE 2016: 14th International Conference on Optical and Electronics Sensors, Gdańsk, Poland, 19–22 June 2016; p. 22, Book of Abstracts. [Google Scholar]
- Knapkiewicz, P.; Dziuban, J.; Grzebyk, T. Dynamically stabilized high vacuum inside MEMS optical cells for atomic spectroscopy. In Proceedings of the 2018 31st International Vacuum Nanoelectronics Conference (IVNC), Kyoto, Japan, 9–13 July 2018. [Google Scholar]



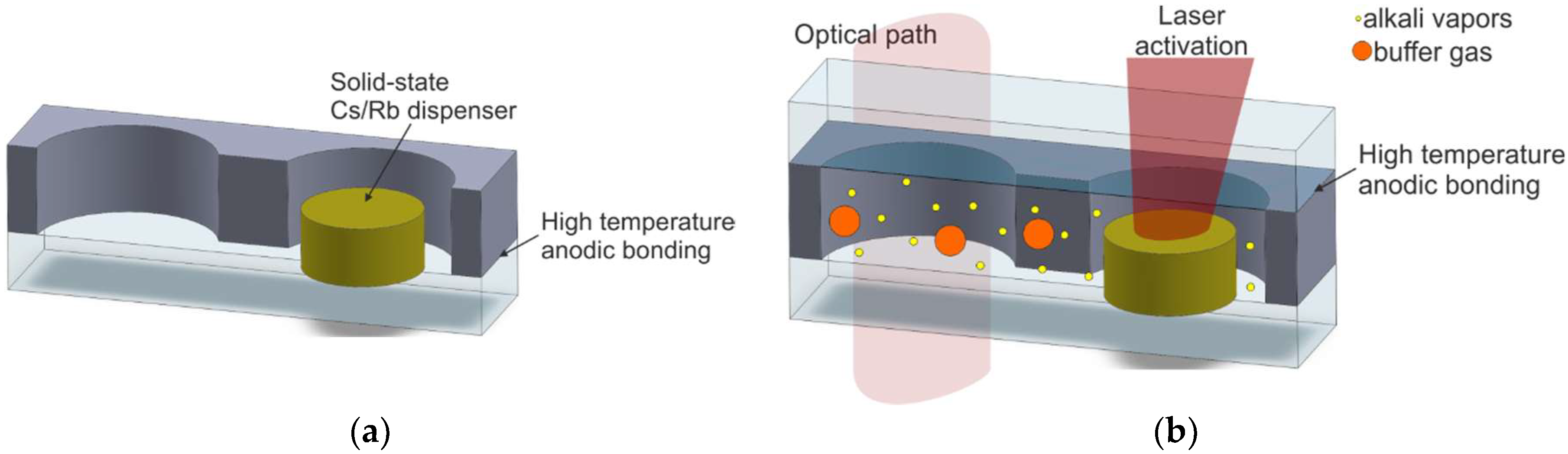
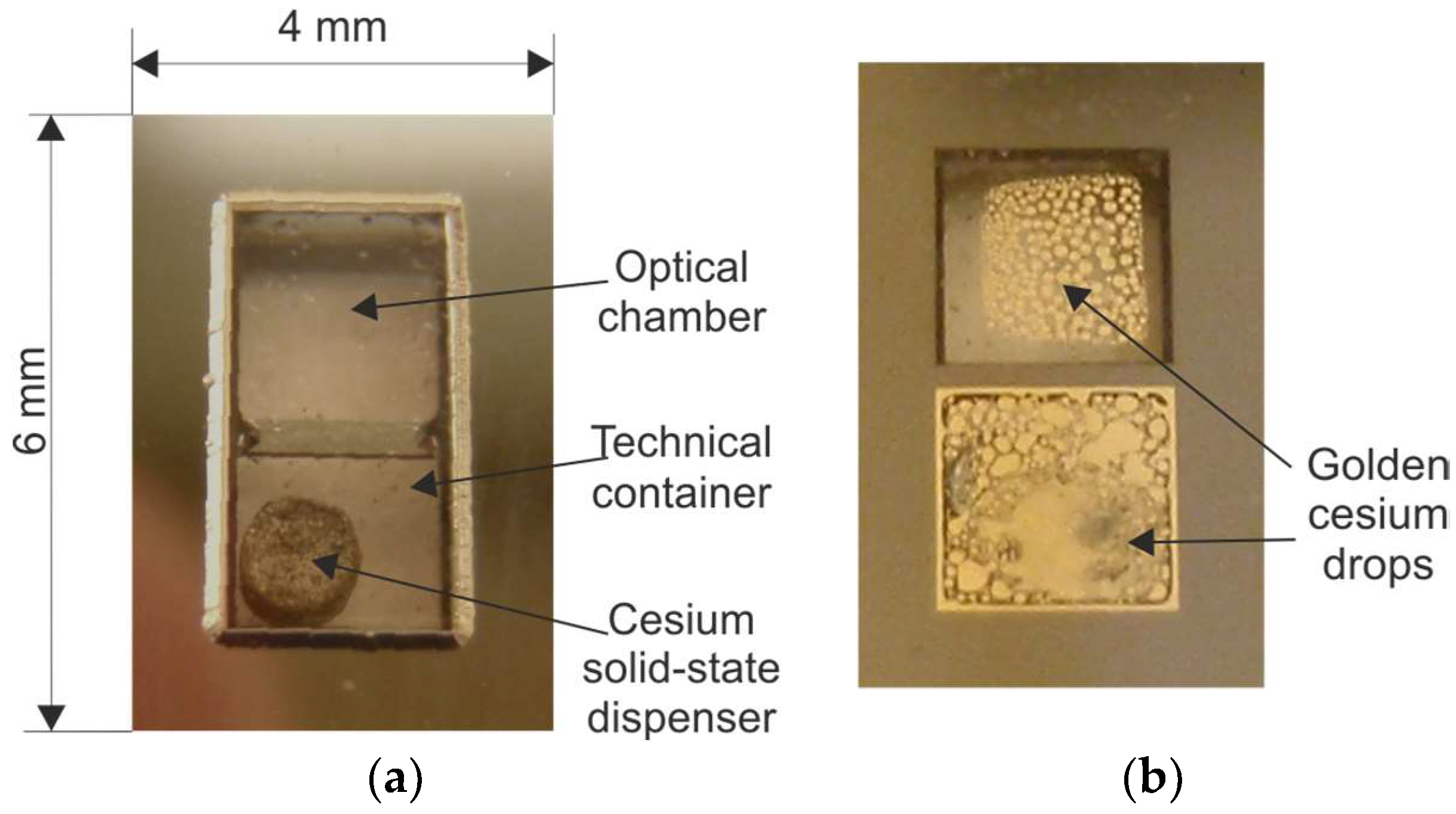

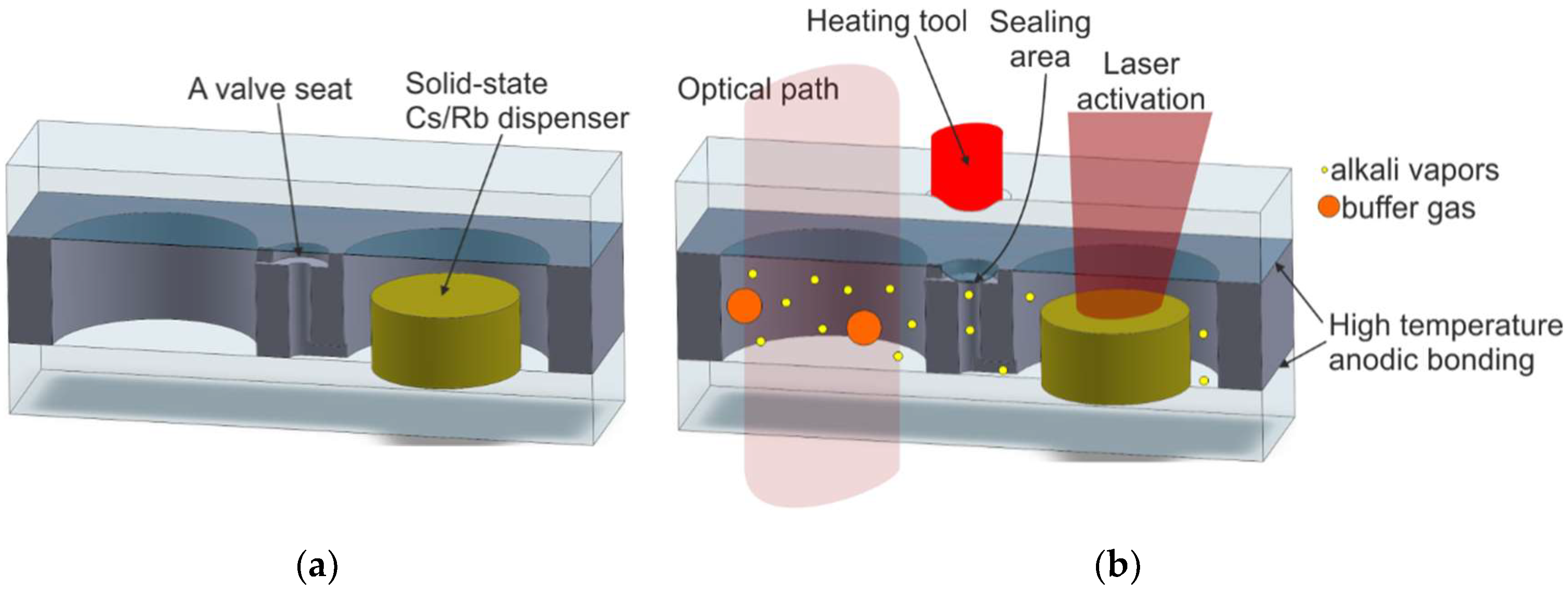

| Method Symbol/Method Description | Reference | MEMS Compatibility | Alkali Vapor Source | Activation Mechanism | Internal Atmosphere Quality | Process Repeatability |
|---|---|---|---|---|---|---|
| M1/Pipetting of pure alkali metal | [15,18,46] | Possible | Pure alkali metal | - | Excellent | Excellent |
| M2/Hybrid process of glassblowing and microfabrication | [50] | None | Pure alkali metal | - | Excellent | Excellent |
| M3/Alkali-wax micropackets | [51] | Possible | Evaporation of metallic alkali metal | Laser ablation, λ = 355 nm | Poor | Good |
| M4/Alkali compound introduction | [45,52,53] | Possible | Reaction between barium azide and alkali chloride | Thermal, T = 200–300 °C | Poor | Poor |
| M5/Dropping of pure alkali metal obtained by external chemical reaction | [54,55] | Possible | Reaction between barium azide and alkali chloride | Thermal, T = 180 °C | Good | Good |
| M6/Alkali azide deposition and UV photolysis | [45,57,58,59,60,61,62,63] | Excellent | Photolysis of alkali azide | Long UV lamp activation and shorter UV laser activation | Bad | Bad |
| M7/Electrolytic | [64] | Probably none | Electrolysis | Electrolytic, T = 540 °C U = 700 V | Poor | Good |
| M8/Off-chip dispensing and eutectic bonding | [65,67,68,69,70] | Possible | Pure alkali metal or solid-state dispenser | - | Poor | Excellent |
| M9/Off-chip dispensing and multilayer anodic bonding | [72] | Possible | Pure alkali metal or solid-state dispenser | - | Good | Excellent |
| M10/On-chip dispensing and high-temperature anodic bonding; structure with microstrainer or microvalve | [73,74,75,76,77,78,80,81,82,84,85,86] | Excellent | Solid-state dispenser | Heating of the dispenser by NIR laser | Excellent | Excellent |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapkiewicz, P. Technological Assessment of MEMS Alkali Vapor Cells for Atomic References. Micromachines 2019, 10, 25. https://doi.org/10.3390/mi10010025
Knapkiewicz P. Technological Assessment of MEMS Alkali Vapor Cells for Atomic References. Micromachines. 2019; 10(1):25. https://doi.org/10.3390/mi10010025
Chicago/Turabian StyleKnapkiewicz, Pawel. 2019. "Technological Assessment of MEMS Alkali Vapor Cells for Atomic References" Micromachines 10, no. 1: 25. https://doi.org/10.3390/mi10010025
APA StyleKnapkiewicz, P. (2019). Technological Assessment of MEMS Alkali Vapor Cells for Atomic References. Micromachines, 10(1), 25. https://doi.org/10.3390/mi10010025





