Occurrence of Regulated Mycotoxins and Other Microbial Metabolites in Dried Cassava Products from Nigeria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mycotoxins and Microbial Metabolites in Dried Cassava Products
2.1.1. Regulated Mycotoxins
2.1.2. Other Microbial Metabolites
3. Conclusions
4. Materials and Methodology
4.1. Sampling of Dried Cassava Products Traded in Nigeria
4.2. Determination of Mycotoxins and Other Microbial Metabolites
4.3. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Practical Applications
References
- Githunguri, C.M.; Mwiti, S.; Migwa, Y. Cyanogenic potentials of early bulking cassava planted at Katumani, a semi-arid area of Eastern Kenya. In Proceedings of the 8th African Crop Science Society Conference, El-Minia, Egypt, 27–31 October 2007; Volume 8, pp. 925–927. [Google Scholar]
- FAOSTAT. Database. 2014. Available online: http://faostat.fao.org (accessed on 30 September 2016).
- Wenham, J.E. Post-Harvest Deterioration of Cassava: A Biotechnology Perspective; FAO Plant Production and Protection Paper 130; NRI/FAO: Rome, Italy, 1995; p. 90. [Google Scholar]
- Nweke, F.I.; Okorji, E.C.; Njoku, J.E.; King, D.J. Expenditure elasticities of demand for major food items in south-east Nigeria. Trop. Agric. (Trinidad) 1994, 71, 229–234. [Google Scholar]
- Westby, A. Cassava utilization, storage and small-scale processing. In Cassava Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 281–300. [Google Scholar]
- Nyirenda, D.B.; Chiwona-Karltun, L.; Chitundu, M.; Haggblade, S.; Brimer, L. Chemical safety of cassava products in regions adopting cassava production and processing—Experience from Southern Africa. Food Chem. Toxicol. 2011, 49, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Onitilo, M.O.; Sanni, L.O.; Oyewole, O.B.; Maziya-Dixon, B. Physicochemical and functional properties of sour starches from different cassava varieties. Int. J. Food Prop. 2007, 10, 607–620. [Google Scholar] [CrossRef]
- Etudaiye, H.; Nwabueze, T.; Sanni, L. Quality of fufu processed from cassava mosaic disease (CMD) resistant varieties. Afr. J. Food Sci. 2009, 3, 061–067. [Google Scholar]
- Awoyale, W.; Abass, A.B.; Ndavi, M.; Maziya-Dixon, B.; Sulyok, M. Assessment of the potential industrial applications of commercial dried cassava products in Nigeria. Food Meas. 2017, 11, 598–609. [Google Scholar] [CrossRef]
- Oyewole, O.B.; Odunfa, S.O. Characterization and distribution of lactic acid bacteria in cassava fermentation during fufu production. J. Appl. Biotechnol. 1990, 68, 145–152. [Google Scholar] [CrossRef]
- Obadina, A.O.; Oyewole, O.B.; Odusami, A.O. Microbiological safety and quality assessment of some fermented cassava products (lafun, fufu, gari). Sci. Res. Essay 2009, 4, 432–435. [Google Scholar]
- Sanni, L.O.; Onitilo, M.; Oyewole, O.B.; Dipeolu, A.O.; Adebayo, K.; Ayinde, I.A.; Tomlins, K.; Westby, A. Effects of Cassava Varieties and Processing Methods on the Qualities of Tapioca in Southwest Nigeria. Paper presented at the Food Africa Initiative held at the Palais du Congress, Yaounde, Cameroon, 5–9 May 2003. [Google Scholar]
- Darkwa, N.A.; Jetuah, F.K.; Sekyere, D. Utilization of Cassava Flour for Production of Adhesive for the Manufacture of Paperboards; Sustainable Industrial Markets for Cassava Project, Final Reports on Project Output 2.2.2.; Forestry Research Institute of Ghana: Kumasi, Ghana, 2003; p. 16. Available online: http://www.researchintouse.com/nrk/RIUinfo/outputs/R8268_FTR_2.pdf (accessed on 20 April 2015).
- Ogunnike, A.M.; Adepoju, P.A.; Longe, A.O.; Elemo, G.N.; Oke, O.V. Effects of submerged and anaerobic fermentation on cassava flour (lafun). Afr. J. Biotechnol. 2015, 14, 961–970. [Google Scholar]
- Otegbayo, B.O.; Samuel, F.O.; Alalade, T. Functional properties of soy-enriched tapioca. Afr. J. Biotechnol. 2013, 12, 3583–3589. [Google Scholar]
- Sanni, L.O.; Adebowale, A.A.; Awoyale, W.; Fetuga, G.O. Quality of gari (roasted cassava mash) in Lagos State, Nigeria. Niger. Food J. 2008, 26, 125–130. [Google Scholar] [CrossRef]
- Nwancho, S.O.; Ekwu, F.C.; Mgbebu, P.O.; Njoku, C.K.; Okoro, C. Effect of Particle Size on the Functional, Pasting and Textural Properties of Gari Produced from Fresh Cassava Roots and Dry Chips. Int. J. Eng. Sci. 2014, 3, 50–55. [Google Scholar]
- Klich, M.A. Identification of Common Aspergillus Species; Centraalbureau Voor Schimmecultures: Utrecht, The Netherlands, 2002; p. 263. [Google Scholar]
- Datsugwai, M.S.; Ezekiel, B.; Audu, Y.; Legbo, M.I.; Azeh, Y.; Gogo, M.A. Mycotoxins: Toxigenic Fungal Compounds—A Review. ARPN J. Agric. Biol. Sci. 2013, 3, 687–692. [Google Scholar]
- Kaaya, A.N.; Eboku, D. Mould and aflatoxin contamination of dried cassava chips in Eastern Uganda: Association with traditional processing and storage practices. J. Biol. Sci. 2010, 10, 718–729. [Google Scholar] [CrossRef]
- Wild, C.P.; Gong, Y.Y. Mycotoxin and human disease: a largely ignored global health issue. Carcinogenesis 2010, 31, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Atanda, S.A.; Pessu, P.O.; Agoda, S.; Isong, I.U.; Adekalu, O.A.; Echendu, M.A.; Falade, T.C. Fungi and mycotoxins in stored foods. Afr. J. Microbiol. Res. 2011, 5, 4373–4382. [Google Scholar] [CrossRef]
- Juan, C.; Ritieni, A.; Mañes, J. Determination of trichothecenes and zearalenones in grain cereal, flour and bread by liquid chromatography tandem mass spectrometry. Food Chem. 2012, 134, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, G.O.; Alamu, A.E.; Akingbala, J.O.; Akanni, A.O. Influence of sun drying on the chemical composition, aflatoxin content and fungal counts of two pepper varieties—Capsicum annum and Capsicum frutescens. Plant Food Hum. Nutr. 1993, 49, 113–117. [Google Scholar] [CrossRef]
- Wareing, P.W.; Westby, A.; Gibbs, J.A.; Allotey, L.T.; Halm, M. Consumer preferences and fungal and mycotoxin contamination of dried cassava products from Ghana. Int. J. Food Sci. Technol. 2001, 36, 1–10. [Google Scholar] [CrossRef]
- Gnonlonfin, G.J.B.; Hell, K.; Fandohan, P.; Siame, A.B. Mycoflora and natural occurrence of aflatoxins and fumonisin B1 in cassava and yam chips from Benin, West Africa. Int. J. Food Microbiol. 2008, 122, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Manjula, K.; Hell, K.; Fandohan, P.; Abass, A.; Bandyopadhyay, R. Aflatoxin and fumonisin contamination of cassava products and maize grain from markets in Tanzania and Republic of the Congo. Toxin Rev. 2009, 28, 263–269. [Google Scholar] [CrossRef]
- Ediage, E.N.; Di Mavungu, J.D.; Monbaliu, S.; Van Peteghem, C.; De Saeger, S. A Validated Multianalyte LC-MS/MS Method for Quantification of 25 Mycotoxins in Cassava Flour, Peanut Cake and Maize Samples. J. Agric. Food Chem. 2011, 59, 5173–5180. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Beed, F.; Boni, S.; Abass, A.; Mukunzi, A.; Krska, R. Quantitation of multiple mycotoxins and cyanogenic glucosides in cassava samples from Tanzania and Rwanda by an LC-MS/MS-based multi-toxin method. Food Addit. Contam. Part A 2015, 32, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Sibanda, L.; Marovatsanga, L.T.; Pestka, J.J. Review of mycotoxin work in sub-Saharan Africa. Food Control 1997, 8, 21–29. [Google Scholar] [CrossRef]
- Bankole, S.A.; Adebanjo, A. Mycotoxins in food in West Africa: current situation and possibilities of controlling it. Afr. J. Biotechnol. 2003, 2, 254–263. [Google Scholar]
- Sanni, L.O.; Maxiya-Dixon, B.; Akanya, J.; Okoro, C.I.; Alaya, Y.; Egwuonwu, C.V.; Okechukwu, R.; Dixon, A. Standards for Cassava Products and Guidelines for Export; IITA: Ibadan, Nigeria, 2005; p. 23. [Google Scholar]
- Vogeser, M.; Parhofer, K. Liquid Chromatography Tandem-mass Spectrometry (LC-MS/MS)—Technique and Applications in Endocrinology. Exp. Clin. Endocrinol. Diabetes 2007, 115, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Nweke, F.I.; Spencer, D.S.C.; Lynam, J.K. The Cassava Transformation: Africa’s Best-Kept Secret; Michigan State University Press: Lansing, MI, USA, 2002; p. 273. [Google Scholar]
- FAO. A review of cassava in Africa with country case studies on Niger, Ghana, the United Republic of Tanzania, Uganda and Benin. In Proceedings of the Validation Forum on the Global Cassava Development Strategy, Rome, Italy, 26–28 April 2003. [Google Scholar]
- Chandra, R.; Sarbhoy, A.K. Production of Aflatoxins and Zearalenone by the toxigenic fungal isolates obtained from stored food grains of commercial crops. Indian Phytopathol. 1997, 50, 458–468. [Google Scholar]
- Creppy, E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002, 127, 9–28. [Google Scholar] [CrossRef]
- Masheshwar, P.K.; Moharram, S.A.; Janardhana, G.R. Detection of fumonisin producing Fusarium verticillioides in paddy (Oryza sativa. L) using polymerase chain reaction (PCR). Braz. J. Microbiol. 2009, 40, 134–138. [Google Scholar] [CrossRef]
- Brtko, J.; Rondahl, L.; Fickova, M.; Hudecova, D.; Eybl, V.; Uher, M. Kojic acid and its derivatives: History and present state of art. Cent. Eur. J. Public Health 2004, 12, 16–18. [Google Scholar]
- Balint, S.; Forsthoffer, J.; Brtko, J.; Dobias, J. Enlargement of Yield of Kojic Acid Production. CS Patent No. 252881, 1988. [Google Scholar]
- Wei, C.I.; Huang, T.S.; Fernando, S.Y.; Chung, K.T. Mutagenicity studies of kojic acid. Toxicol. Lett. 1991, 59, 213–220. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Kirkland, D.; Marzin, D.; Toutain, H.; Leclerc-Ribaud, C.; Jinnai, H. An assessment of the genotoxicity and human health risk of topical use of kojic acid [5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one]. Food Chem. Toxicol. 2004, 42, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Nyhan, W.L.; Gerritsen, T.; Gong, L.; Heiner, D.C.; Bray, P.F. Metabolism of glycine in the nonketotic form of hyperglycinemia. Pediatr. Res. 1968, 2, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Kocsubé, S.; Tóth, B.; Frisvad, J.C.; Perrone, G.; Susca, A.; Meijer, M.; Samson, R.A. Aspergillus brasiliensis sp. nov., a biseriate black Aspergillus species with world-wide distribution. Int. J. Syst. Evol. Microbiol. 2007, 57, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.S.; Weng, S.W.; Lin, M.W.; Lu, C.C.; Chiang, J.H.; Yang, J.S.; Lai, K.C.; Lin, J.P.; Tang, N.Y.; Lin, J.G.; et al. Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem. Toxicol. 2012, 50, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.O.; Schmitt, M.; Dekant, W.; Stopper, H.; Schlatter, J.; Schreier, P.; Lutz, W.K. Occurrence of emodin, chrysophanol and physcion in vegetables, herbs and liquors. Genotoxicity and anti-genotoxicity of the anthraquinones and of the whole plants. Food Chem. Toxicol. 1999, 37, 481–491. [Google Scholar] [CrossRef]
- Stierle, A.; Cardellina, J.H.; Strobel, G.A. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8013. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Stewart, M.; Ratnayake, R.; Lacey, E.; Gill, J.H. Citromycetins and bilains A-C: New aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. J. Nat. Prod. 2007, 70, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.; Visconti, A. Metabolism of alternariol monomethylether by porcine liver and intestinal mucosa in vitro. Toxicol. In Vitro 1987, 2, 27–29. [Google Scholar] [CrossRef]
- Scott, P.M.; Zhao, W.; Feng, S.; Lau, B.P.Y. Alternariatoxins alternariol and alternariol monomethyl ether in grain foods in Canada. Mycotoxin Res. 2012, 28, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M. Analysis of agricultural commodities and foods for Alternaria mycotoxins. J. AOAC Int. 2001, 84, 1809–1817. [Google Scholar] [PubMed]
- Kocher, U. Determination of 7 Alternaria-Toxins in edible oil and oilseeds by LC-MS/MS. In Proceedings of the 29th mycotoxin workshop, Stuttgart-Fellbach, Germany, 14–16 May 2007; p. 92. [Google Scholar]
- Cornford, E.M.; Bocash, W.D.; Braun, L.D.; Crane, P.D.; Oldendorf, W.H. Rapid distribution of tryptophol (3-indole ethanol) to the brain and other tissues. J. Clin. Investig. 1979, 63, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, A.G.; Vinokurova, N.G.; Zhelifonova, V.P. Mycotoxin production profiles of Penicillium vulpinum (Cooke & Massee) Seifert & Samson strains. Microbiology 2000, 69, 36–39. [Google Scholar]
- Malachova, A.; Sulyok, M.; Beltran, E.; Berthiller, F.; Krska, R. Multi-Toxin Determination in Food—The Power of “Dilute and Shoot” Approaches in LC-MS-MS. LC GC Eur. 2015, 28, 542–555. [Google Scholar]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, V.; Sulyok, M.; Labuda, R.; Bicker, W.; Krska, R. Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 395, 1355–1372. [Google Scholar] [CrossRef] [PubMed]
- EU. European Union Commission Decision No. 2002/657/EC. Commission decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (2002/657/EC). 2002. Available online: http://eur-lex.europa. eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:221:0008:0036:EN:PDF (accessed on 31 October 2014).
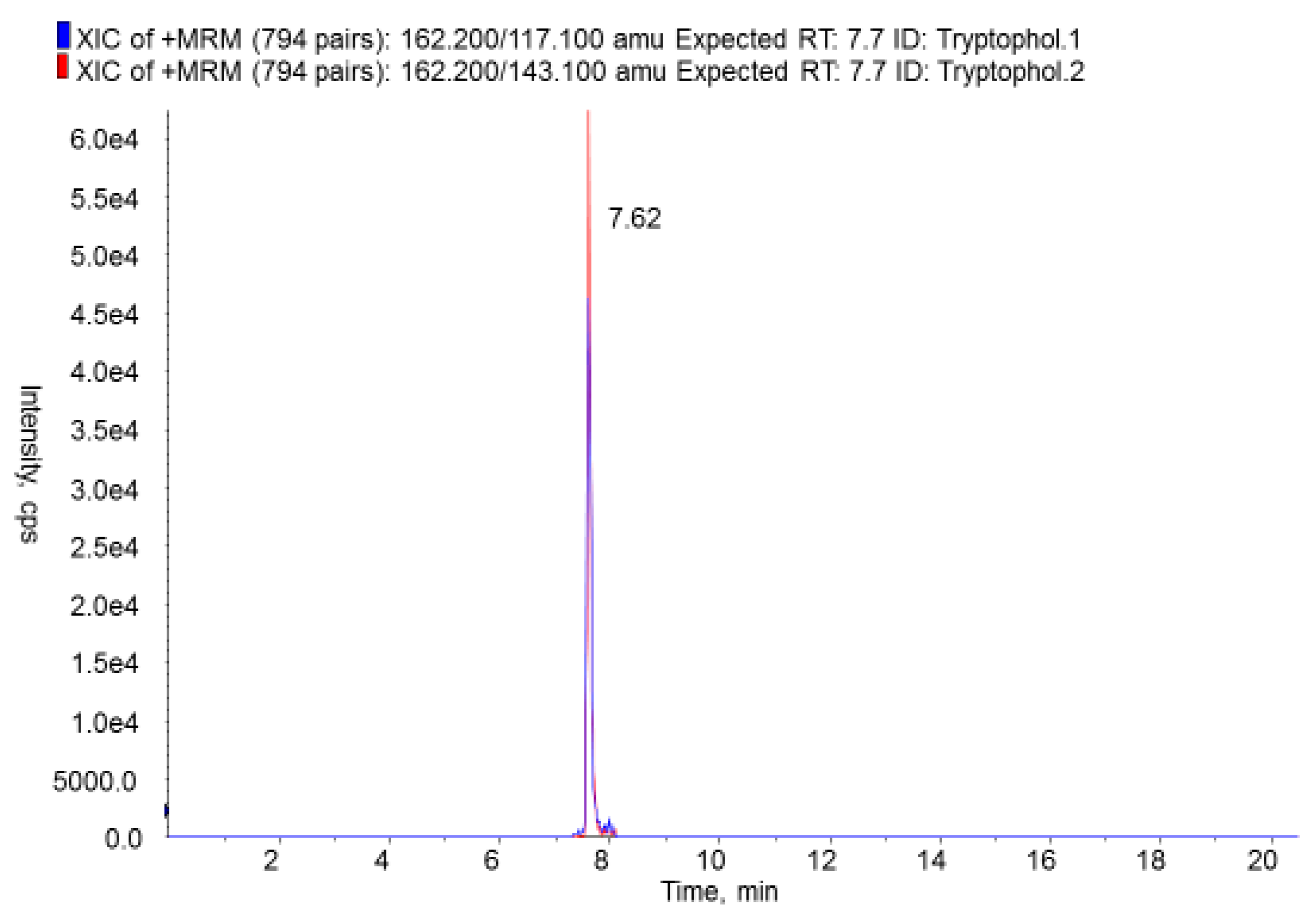
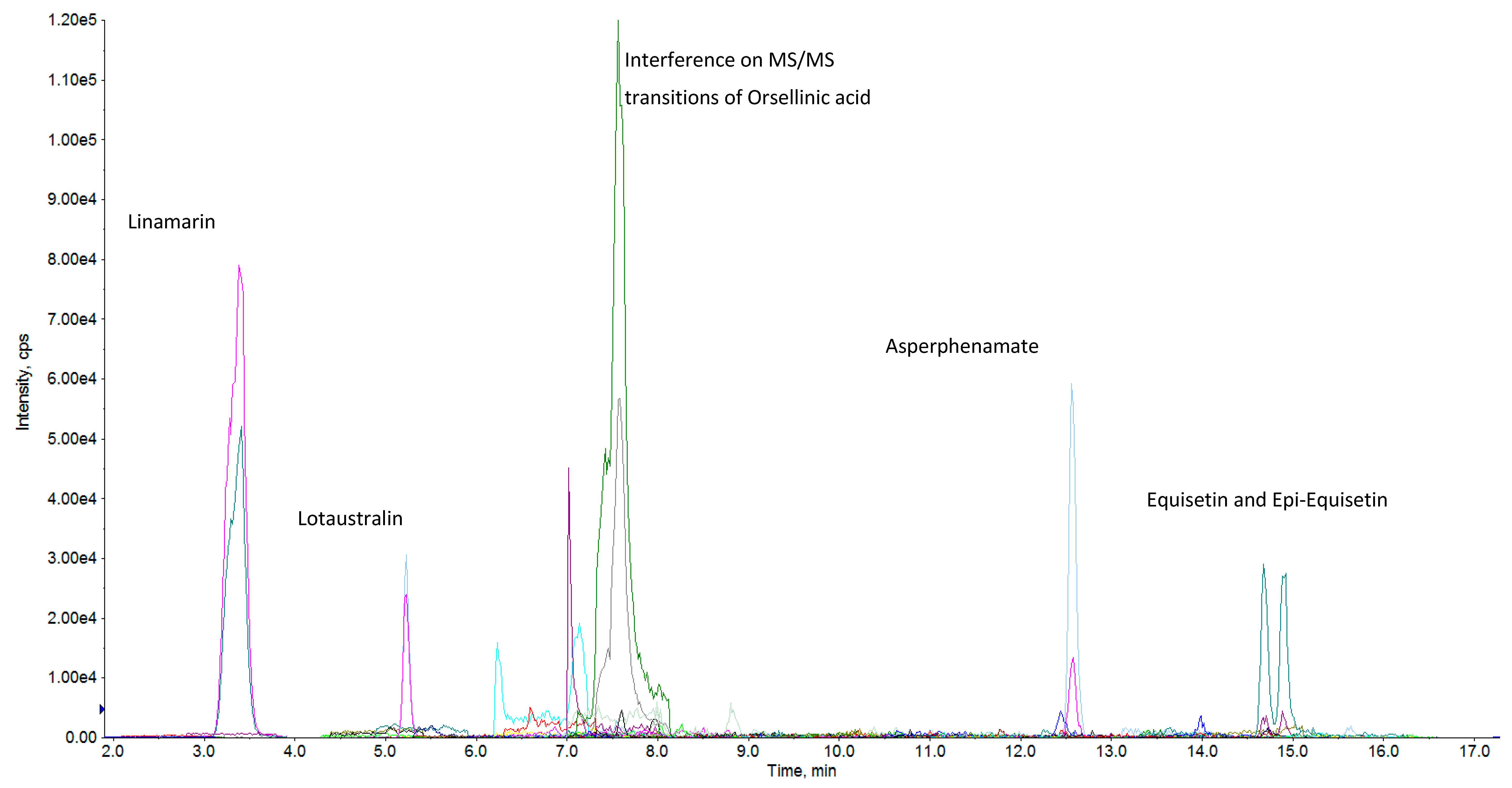
| Products | N | Aflatoxins | Other Mycotoxins | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Aflatoxin B1 (µg/kg) | Aflatoxin G1 (µg/kg) | Prevalence (%) | Fumonisin B1 (µg/kg) | Fumonisin B2 (µg/kg) | Fumonisin B3 (µg/kg) | Zearalenone (µg/kg) | Prevalence (%) | ||
| R (%) | 82.90 | 80.50 | 86.30 | 92.20 | 93.40 | 101.70 | |||
| LOD (µg/kg) | 0.20 | 0.20 | 3.00 | 1.50 | 2.00 | 0.30 | |||
| Cassava starch | 15 | + | + | 0.00 | + | + | + | + | 0.00 |
| HQCF | 29 | + | 2.94 (1) | 3.45 | + | + | + | 1.10 (2) | 6.90 |
| Lafun | 30 | + | + | 0.00 | 88.09 (1) | 10.70 (2) | + | 7.60 (6) | 30.00 |
| Fufu flour | 36 | 1.16 (3) | + | 8.33 | 102.71 (1) | 21.28 (2) | 14.49 (1) | 1.89(2) | 16.67 |
| Tapioca | 36 | + | + | 0.00 | + | + | + | + | 2.78 |
| Fine yellow gari | 50 | + | + | 0.00 | + | + | + | 90.40 (1) | 2.78 |
| Fine white gari | 113 | + | + | 0.00 | + | 218.12(1) | + | 0.92 (2) | 2.65 |
| Yellow kpo-kpo gari | 12 | + | + | 0.00 | + | + | + | + | 0.00 |
| White kpo-kpo gari | 52 | + | + | 0.00 | + | + | + | 11.01(3) | 5.77 |
| Range (all products) | 373 | 0.00–1.16 (3) | 0.00–2.94 (1) | 3.45–8.33 | 88.33–02.71 (2) | 10.70–18.12 (5) | 0.00–14.49 (1) | 0.92–90.40 (16) | 0.00–30.00 |
| Serial Number | Analyte | P/N | Prevalence (%) | LOD (µg/kg) | R (%) | Serial Number | Analyte | P/N | Prevalence (%) | LOD (µg/kg) | R (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Averufin | 27/373 | 7.24 | 0.06 | 71.2 | 18 | Asperphenamate | 371/373 | 99.46 | 0.04 | 100 |
| 2 | 3-Nitropropionic acid | 52/373 | 13.94 | 1.00 | 36 | 19 | Brevianamid F | 297/373 | 79.62 | 0.50 | 95.8 |
| 3 | Kojic acid | 266/373 | 71.31 | 15.00 | 100 | 20 | Citreorosein | 26/373 | 6.97 | 0.60 | 100 |
| 4 | Quinolactacin A | 27/373 | 7.24 | 0.08 | 100 | 21 | Tryptophol | 330/373 | 88.47 | 15.00 | 96.7 |
| 5 | Quinocitrinine A | 22/373 | 5.90 | 0.15 | 100 | 22 | Rugulusovin | 140/373 | 37.53 | 0.40 | 100 |
| 6 | Beauvericin | 20/373 | 5.36 | 0.002 | 97.6 | 23 | Cyclo (L-Pro-L-Tyr) | 319/373 | 85.52 | 1.50 | 100 |
| 7 | Epiequisetin | 45/373 | 12.06 | 0.20 | 136 | 24 | Cyclo (L-Pro-L-Val) | 343/373 | 91.96 | 0.50 | 100 |
| 8 | Equisetin | 39/373 | 10.46 | 0.20 | 136 | 25 | N-benzoyl-phenylalanine | 206/373 | 55.23 | 0.80 | 100 |
| 9 | Moniliformin | 30/373 | 8.04 | 0.40 | 82.4 | 26 | Emodin † | 156/373 | 41.82 | 0.20 | 105.8 |
| 10 | LL-Z 1272e | 32/373 | 8.58 | 0.06 | 100 | 27 | Isorhodoptilometrin | 22/373 | 5.90 | 0.06 | 100 |
| 11 | Alternariol methyl ether | 96/373 | 25.74 | 0.02 | 97.9 | 28 | Skyrin | 33/373 | 8.85 | 0.30 | 87 |
| 12 | Ilicicolin A | 68/373 | 18.23 | 0.15 | 100 | 29 | Usnic acid | 20/373 | 5.36 | 0.03 | 100 |
| 13 | Ilicicolin B | 90/373 | 24.13 | 0.30 | 100 | 30 | Fellutanine A | 180/373 | 48.26 | 0.60 | 100 |
| 14 | Ilicicolin C | 63/373 | 16.89 | 0.30 | 100 | 31 | Neoechinulin A | 49/373 | 13.14 | 0.60 | 100 |
| 15 | Ascochlorin | 58/373 | 15.55 | 0.30 | 100 | 32 | Unugisin E | 25/373 | 6.70 | 1.20 | 1000 |
| 16 | Chloramphenicol | 39/373 | 10.46 | 0.03 | 92 | 33 | Neoechinulin A | 32/373 | 8.58 | 0.40 | 100 |
| 17 | Asperglaucide | 370/373 | 99.20 | 0.40 | 100 | ||||||
| Product | N | Kojic Acid (µg/kg) | Asperphenamate (µg/kg) | N-Benzoyl-Phenylalanine (µg/kg) | Emodin (µg/kg) | Asperglaucide (µg/kg) |
|---|---|---|---|---|---|---|
| LOD (µg/kg) | 15.00 | 0.04 | 0.80 | 0.19 | 0.40 | |
| Cassava starch | 15 | 8.35 ± 23.75 b | 39.07 ± 92.51 b | 10.21 ± 25.14 b | 0.17 ± 0.26 a | 41.77 ± 65.73 b |
| HQCF | 29 | 632.68 ± 1616.54 b | 27.08 ± 50.74 b | 6.45 ± 13.20 b | 0.34 ± 0.83 a | 119.87 ± 298.89 b |
| Lafun | 30 | 1754.80 ± 7196.41 a | 71.90 ± 189.70 b | 12.66 ± 30.58 b | 0.30 ± 0.42 a | 385.83 ± 1117.12 a |
| Fufu flour | 36 | 32.61 ± 46.85 b | 63.98 ± 236.38 b | 8.60 ± 30.24 b | 31.17 ± 185.98 a | 52.72 ± 138.25 b |
| Tapioca | 36 | 13.95 ± 30.50 b | 34.93 ± 80.19 b | 3.92 ± 6.00 b | 0.19 ± 0.53 a | 100.81 ± 254.94 b |
| Fine yellow gari | 50 | 183.39 ± 184.70 b | 6.75 ± 8.36 b | 0.99 ± 1.11 b | 17.72 ± 114.32 a | 59.17 ± 194.50 b |
| Fine white gari | 113 | 167.49 ± 102.65 b | 9.51 ± 18.18.52 b | 1.55 ± 5.71 b | 1.57 ± 14.35 a | 25.40 ± 40.21 b |
| Yellow kpo-kpo gari | 12 | 59.67 ± 82.69 b | 270.19 ± 654.82 a | 141.05 ± 384.32 a | 2.50 ± 4.43 a | 358.68 ± 793.03 a |
| White kpo-kpo gari | 52 | 53.73 ± 58.68 b | 13.36 ± 22.44 b | 1.99 ± 3.73 b | 1.44 ± 8.70 a | 38.59 ± 39.59 b |
| Product | N | Alternaria spp. | Fusarium spp. | Penicillium spp. | ||||
|---|---|---|---|---|---|---|---|---|
| Cyclo (L-Pro-L-Tyr) (µg/kg) | Cyclo (L-Pro-L-Val) (µg/kg) | Alternariol methyl ether (µg/kg) | Tryptophol (µg/kg) | Brevianamid F (µg/kg) | Fellutanine A (µg/kg) | Rugulusovin (µg/kg) | ||
| Cassava starch | 15 | 27.28 ± 62.87 b | 88.50 ± 241.32 b | + | 202.30 ± 272.09 b | 8.45 ± 20.37 b | + | 0.93 ± 2.42 a |
| HQCF | 29 | 43.46 ± 69.02 b | 94.47 ± 206.88 b | 0.10 ± 0.21 b | 234.85 ± 578.44 b | 11.49 ± 19.11 b | 3.68 ± 5.61 a b | <LOD |
| Lafun | 30 | 31.93 ± 38.65 b | 85.81 ± 134.86 b | 0.05 ± 0.17 b | 1121.88 ± 2027.59 a | 8.44 ± 11.13 b | 0.99 ± 1.87c | 0.72 ± 2.64 a |
| Fufu flour | 36 | 22.43 ± 41.80 b | 128.17 ± 480.03 b | 0.02 ± 0.06 b | 718.35 ± 1101.42 a b | 7.09 ± 16.85 b | 1.20 ± 2.60c | 1.02 ± 3.74 a |
| Tapioca | 36 | 30.84 ± 117.80 b | 105.61 ± 341.60 b | + | 264.65 ± 689.61 b | 10.36 ± 37.98 b | 0.02 ± 0.12c | 2.05 ± 9.38 a |
| Fine yellow gari | 50 | 56.76 ± 69.66 b | 184.88 ± 292.37 b | 1.49 ± 1.84 a | 121.31 ± 166.12 b | 18.61 ± 24.33 b | 1.86 ± 3.33 bc | 0.06 ± 0.40 a |
| Fine white gari | 113 | 132.91 ± 146.49 b | 625.33 ± 672.51 a | 0.27 ± 1.07 b | 543.93 ± 4.85 a b | 43.95 ± 51.64 a | 4.14 ± 5.12 a | 0.18 ± 1.65 a |
| Yellow kpo-kpo gari | 12 | 199.94 ± 18.48 a | 57.18 ± 56.40 b | 1.06 ± 1.21 a | 980.97 ± 2234.56 a | 7.35 ± 6.31 b | 0.37 ± 0.90c | 1.16 ± 2.18 a |
| White kpo-kpo gari | 52 | 58.19 ± 61.28 b | 294.73 ± 370.81 b | 0.03 ± 0.10 b | 614.19 ± 1268.11 a b | 18.77 ± 21.51 b | 1.81 ± 3.80 bc | 1.06 ± 2.73 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abass, A.B.; Awoyale, W.; Sulyok, M.; Alamu, E.O. Occurrence of Regulated Mycotoxins and Other Microbial Metabolites in Dried Cassava Products from Nigeria. Toxins 2017, 9, 207. https://doi.org/10.3390/toxins9070207
Abass AB, Awoyale W, Sulyok M, Alamu EO. Occurrence of Regulated Mycotoxins and Other Microbial Metabolites in Dried Cassava Products from Nigeria. Toxins. 2017; 9(7):207. https://doi.org/10.3390/toxins9070207
Chicago/Turabian StyleAbass, Adebayo B., Wasiu Awoyale, Michael Sulyok, and Emmanuel O. Alamu. 2017. "Occurrence of Regulated Mycotoxins and Other Microbial Metabolites in Dried Cassava Products from Nigeria" Toxins 9, no. 7: 207. https://doi.org/10.3390/toxins9070207
APA StyleAbass, A. B., Awoyale, W., Sulyok, M., & Alamu, E. O. (2017). Occurrence of Regulated Mycotoxins and Other Microbial Metabolites in Dried Cassava Products from Nigeria. Toxins, 9(7), 207. https://doi.org/10.3390/toxins9070207





